Summary
During gastrulation, chicken primordial germ cells (PGCs) are present in an extraembryonic region of the embryo from where they migrate towards the genital ridges. This is also observed in mammals, but in chicken the vehicle used by the migratory PGCs is the vascular system. We have analysed the migratory pathway of chicken PGCs, focusing on the period of transition from the extraembryonic region to the intraembryonic vascular system.
Our findings show that at Hamburger and Hamilton developmental stage HH12–HH14 the majority of PGCs concentrate axially in the sinus terminalis and favour transport axially via the anterior vitelline veins into the embryonic circulation. Moreover, directly blocking the blood flow through the anterior vitelline veins resulted in an accumulation of PGCs in the anterior region and a decreased number of PGCs in the genital ridges. We further confirmed the key role for the anterior vitelline veins in the correct migration of PGCs using an ex ovo culture method that resulted in defective morphogenetic development of the anterior vitelline veins.
We propose a novel model for the migratory pathway of chicken PGCs whereby the anterior vitelline veins play a central role at the extraembryonic and embryonic interface. The chicken model of PGC migration through the vasculature may be a powerful tool to study the process of homing (inflammation and metastasis) due to the striking similarities in regulatory signaling pathways (SDF1–CXCR4) and the transient role of the vasculature.
Key words: Primordial germ cells, Chicken, Migration, Vasculatory system, Extraembryonic tissue, Embryo
Introduction
Early during the development of amniotes, the germline is segregated from the somatic cell lineages. This is an important event because the primordial germ cells (PGCs), the precursors of the oocytes and sperm, carry the genetic information throughout generations and are therefore the engine of evolution, contributing to genetic variability in sexually reproducing animals (Johnson et al., 2011). Even though PGCs can be formed by two distinct mechanisms, epigenesis and preformation, they show some common characteristics, including early segregation, similar morphology cross-species (de Sousa Lopes and Roelen, 2010) and a distinct migratory period from a peripheral or extraembryonic location to the place where the somatic gonad compartments are formed. Understanding the details surrounding the migration of PGCs is important because an aberrant migration can cause cancer and infertility (reviewed by Chuva de Sousa Lopes and Roelen, 2010). Interestingly, in Gallus gallus the PGCs migrate from an anterior location towards the genital ridge compartment, whereas in Mus musculus the PGCs migrate from a posterior/caudal location towards the genital ridges (Nieuwkoop and Sutasurya, 1979).
In chicken, the staining method classically used to distinguish PGCs from the somatic cells was the periodic acid-Schiff (PAS) staining (Fujimoto et al., 1976). There are also immunological markers against cell-surface glycoproteins present in PGCs, like SSEA1, which is commonly used to identify mammalian and chicken PGCs. However, SSEA1 is not restricted to chicken or mammalian PGCs, but is found in several types of undifferentiated multipotent mouse and chicken cells (Pain et al., 1996; Solter and Knowles, 1978). More recently, Tsunekawa and colleagues identified the chicken vasa homolog (Cvh) gene and have shown its germline-specific expression (Tsunekawa et al., 2000). The function of vasa is not well understood, but it has been shown that vasa is indispensable for germ cell development and it is present in the germline of many animal species, suggesting a conserved role throughout evolution (reviewed by de Sousa Lopes and Roelen, 2010). Immunohistochemical analyses, using specific antibodies against CVH protein, demonstrated that CVH-expressing cells were detectable during early embryogenesis of chicken embryos, starting from the first cleavage of fertilized eggs, (Tsunekawa et al., 2000), suggesting that a preformation mode of germline specification was adopted in chicken.
At stage X [the roman numerals refer to the staging system used by Eyal-Giladi and Kochav (Eyal-Giladi and Kochav, 1976)], the PGCs are localized in the central zone of the area pellucida, on the ventral surface of the epiblast (Ginsburg, 1994). At this stage, the PGCs are gradually translocated from the epiblast to an extra-embryonic structure, the hypoblast and carried anteriorly by the hypoblast to the so-called germinal crescent region, away from the primitive streak that starts to move forward from the posterior area of the blastodisc (Ginsburg, 1994). At HH4–5 [referring to the staging system used by Hamburger and Hamilton in 1951, but reprinted in 1992 (Hamburger and Hamilton, 1992)], the germinal crescent containing the PGCs is localized at the border region between the area pellucida and area opaca, anterior to the developing embryonic disk (Nakamura et al., 2007; Swift, 1914). The PGCs move from the hypoblast layer to accumulate in the extraembryonic mesoderm localized between the ectoderm and hypoblast. Subsequently, the PGCs become lodged in the vascular system as the blood islands are formed in the yolk sac around HH10 and by HH12 use those extraembryonic blood vessels as a vehicle to reach the embryo (Fujimoto et al., 1976; Nakamura et al., 2007; Swift, 1914). By HH15, the PGCs start leaving the vascular system close to the genital ridges, just caudally from the vitelline arteries and by HH17 the majority of the PGCs have settled in the genital ridges (Fujimoto et al., 1976; Meyer, 1964; Nakamura et al., 2007; Swift, 1914; Ukeshima et al., 1987). The mechanism by which the PGCs enter the vascular system is less well understood than the mechanism by which the PGCs exit the vascular system (SDF1–CXCR4) to colonize the gonads (Stebler et al., 2004) that has clear similarities with the process of homing of lymphocytes during inflammation and tumor metastasis (Alsayed et al., 2007; Ueda et al., 2004).
Here, we have investigated the vasculatory route used by the PGCs from the extraembryonic germinal crescent to the intraembryonic vascular system as this has also not been well described to date. We observed that PGCs concentrate and make effective use of the two large calibre blood vessels that flow into the embryo from left and right: the anterior part of the sinus terminalis and the anterior vitelline veins. A defective development of the anterior vitelline veins or the direct blocking of the blood flow through the vitelline veins resulted in an accumulation of PGCs anteriorly and a concomitant decrease in the number of PGCs that reached the genital ridges. We propose a novel model of PGC migration in chicken embryos.
Materials and Methods
Embryo collection and manipulation
Fertilized White Leghorn chicken (Gallus gallus) eggs were incubated in a humidified atmosphere at 37.0°C until the desired HH stage (Hamburger and Hamilton, 1992). Embryos were washed and manipulated on 2% agar-coated petri dishes containing phosphate buffer solution (PBS). The vitelline membrane was removed, the embryos were isolated with intact area opaca and pellucida and fixed overnight (o/n) at 4°C either in 4% paraformaldehyde (PFA) for whole mount immunofluorescence or in Bouin's solution (Sigma) for immunohistochemistry and stored in PBS at 4°C until further use.
Whole mount immunofluorescence
Fixed embryos were permeabilized with 0.5% Triton (Sigma) in PBS (PBT) o/n at 4°C with rotation. Thereafter, they were washed in PBS and incubated 24 hours at 4°C with the first antibodies diluted in 1% bovine serum albumin (BSA, Fraction V) (Gibco) in PBS. The first antibodies used were rabbit anti-CVH IgG at 1:500 and mouse anti-SSEA1 IgM (TG1) at 1:10. Next, the embryos were washed in PBS for 1 hour and incubated with the respective secondary antibodies diluted in 1% BSA/PBS for 24 hours at 4°C. The secondary antibodies used were Alexa Fluor 488 donkey anti-rabbit IgG (Molecular Probes) and Alexa Fluor 568 goat anti-mouse IgM (Molecular Probes), both used at 1:1000. The embryos had a final rinse in PBS and were enclosed with Vectashield with Dapi (Vector), covered with a cover glass and sealed with nail polish. For this analysis, we considered only embryos that showed normal morphology, including the presence of PGCs. The total number of PGCs per embryo was counted and plotted.
Immunohistochemistry
For paraffin inclusion, embryos from HH13 were dehydrated following a graded series of ethanol (70%, 80%, 90% and 100%) and cleared in xylene. The embryos were individually embedded in paraffin (2× 30 minutes) at 70°C and stored at 4°C. The embryos were sectioned (transverse sections, 5 µm) using a rotatory microtome RM2255 (Leica, Nussloch, Germany). The sections were rehydrated starting with xylene and followed by a decreasing series of ethanol (100%, 90%, 80%, 70%) followed by milli-Q water and PBS. The inhibition of endogenous peroxidase activity was performed by treatment with a freshly prepared 0.3% H2O2 in PBS for 20 minutes at room temperature (RT). Next, the sections were blocked for 1 hour at RT in fresh 1% BSA/PBS. The slides were incubated with rabbit anti-CVH IgG at 1:500 diluted in blocking o/n at 4°C, washed in PBS and incubated with BrightVision Poly-HRP anti-rabbit (Immunologic) for 30 minutes at RT. Thereafter, the slides were washed first with PBS, than with 0.05M Tris-maleate buffer (pH 7.6), revealed with a solution of 0.4 mg/ml 3,3′-diaminobenzidine (DAB) and finally counterstained with Mayer's Hematoxylin. The sections were washed in water, dehydrated in an increasing series of ethanol and finally xylene. Thereafter, the samples were mounted in Entellan (Merck). For this analysis, we considered only embryos that showed normal morphology, including the presence of PGCs. The total number of PGCs per embryo was counted and plotted.
In ovo clamp experiments
At HH14, an opening was made in the shell of eggs and part of the vitelline membrane was removed to expose the embryo and some drops of PBS were added to avoid embryo drought. To block blood flow though the anterior vitelline veins a knot was tied using a small semicircular multipass needle attached to a prolene monofilament (Ethicon). The opening made in the eggs was closed and the eggs incubated for 6 hours (until HH15). The control embryos were treated similarly, but the vitelline veins were not clamped. After the incubation time, the embryos were isolated, fixed in 4% PFA o/n and processed for whole mount immunofluorescence. The total number of PGCs per embryo was counted and statistical analysis to compare the distribution of PGCs in the two groups of embryos was performed using the non-parametric Mann–Whitney test.
Ex ovo culture of chicken embryos
Preparation of the embryos for ex ovo culture was performed as described (Nagai et al., 2011). This culture system allows the growth of chicken embryos without the vitelline membrane in a fish embryo-like topology on top of a “mini yolk sac-like”. HH5 embryos were removed from the egg, cleared of excessive yolk with PBS and folded by the anterior–posterior axis into a half circle. Forceps were used to gently press the edges of the area opaca together to create a “sealed” half circle. Outside the sealed area, the rest of the area opaca was cut off with micro scissors and the embryo was left to heal undisturbed for 30 minutes in Pannett–Compton solution (Streit and Stern, 2008) at RT. Thereafter, the embryos were cultured for 30 hours (HH13) or 48 hours (HH17) on a petri dish in suspension in medium consisting of a mix 2:1 of thin albumen and Pannett–Compton solution containing 1:300 Penicillin/Streptomycin (Gibco) at 37°C with humidity on air. After the incubation time, the embryos were isolated, fixed in 4% PFA o/n and processed for whole mount immunofluorescence.
Image acquisition and analysis
Whole mount embryos were imaged on a Leica M420 stereoscope (Leica, Rijswijk, the Netherlands) equipped with a Nikon E4500 coolpix camera (Nikon, Tokyo, Japan), fluorescence images were made on a Leica MZFIII stereoscope (Leica, Rijswijk, the Netherlands) equipped with a Leica DFC90 camera (Leica, Heerbrugg, Switzerland) and confocal images were made on a Leica TCS SP5 confocal inverted microscope (Leica, Mannheim, Germany) operating under the Leica Application Suite Advanced Fluorescence software (Leica, Mannheim, Germany).
Sections were imaged on an Olympus AX70 microscope (Olympus, Zoeterwoude, Netherlands) equipped with either an Olympus XC50 camera (Olympus, Tokyo, Japan) or a Spot RT3 camera (Diagnostic Instruments, Sterling Heights, MI, USA). For 3D reconstruction, serial paraffin sections immunostained for CVH followed by Hematoxylin staining were digitalized using a Pannoramic MIDI scanner (3D Histech, Budapest, Hungary) and reconstructed with Amira 4.1 software (Visage Imaging, Carlsbad, CA, USA).
Results
The number of chicken PGCs remained constant, but increasing numbers of PGCs expressed SSEA1 between HH8–HH19
We analysed the number of PGCs in White Leghorn chicken embryos between HH5–HH19 (n = 42) by whole mount double immunofluorescence for CVH and SSEA1, counting the total number of PGCs present in the embryo, area pellucida and area opaca. We observed a high variation in the total number of PGCs between embryos of the same developmental stage; however, the average number of PGCs present in the germinal crescent at HH5 was similar to the average number of PGCs present in the genital ridges between HH16–HH19. The majority of the embryos exhibited between 200 and 450 PGCs (Fig. 1A).
Fig. 1. Chicken PGCs between HH5–HH19.
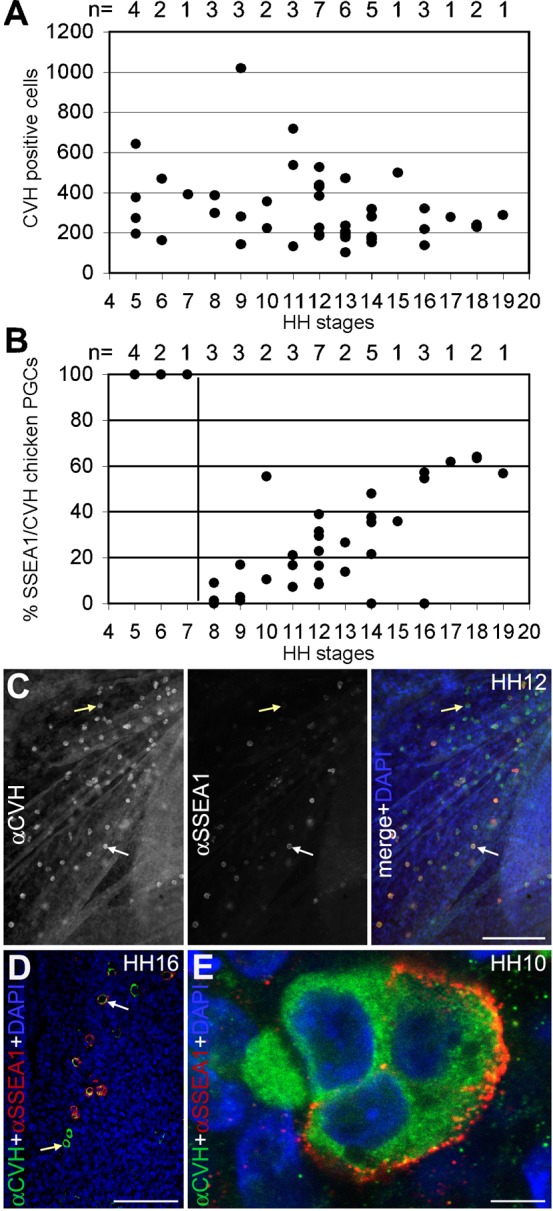
(A) Total number of CVH positive cells present between HH5–HH19. n is the total number of embryos analyzed. (B) Percentage of SSEA1-positive cells in the CVH-positive population of PGCs between HH5–HH19. n is the total number of embryos analyzed. (C) Expression of CVH and SSEA1 in area pellucida, lateral to the head region at HH12. The PGCs (CVH positive) were SSEA1-positive (white arrows) or SSEA1-negative (yellow arrows). (D) In the genital ridges at HH16 the PGCs (CVH-positive) were SSEA1-positive (white arrows) or SSEA1-negative (yellow arrows). (E) PGCs from the same cluster showed different expression of SSEA1. CVH (green) is expressed in the cytoplasm while SSEA1 (red) expression is restricted to the cell surface. Scale bars: 100 µm in C,D and 5 µm in E.
Between HH5–HH7, all the CVH-positive cells were positive for SSEA1 (Fig. 1B), but SSEA1 was observed in many other cells and tissues in the embryo, making SSEA1 an inadequate marker of the germline at those stages. At HH8, SSEA1 was drastically downregulated in the CVH-positive PGCs and was then slowly upregulated in a fraction of the germ cells (Fig. 1B,C) until it stabilized at 60% of the cells by the time the PGCs colonized the genital ridges at HH16–HH19 (Fig. 1B,D). Interestingly, in the typically 3–4 cell clusters of germ cells, already described in 1914 by Swift (Swift, 1914), we often observed both SSEA1-positive and SSEA1-negative cells (Fig. 1E). In agreement, Swift noticed pronounced differences in the yolk content and yolk coloration among PGCs (Swift, 1914) and this may be directly linked to the heterogeneity observed in SSEA1 staining.
At HH13, the PGCs localized to the sinus terminalis and anterior vitelline veins
We analysed the distribution of the CVH-positive PGCs in detail between HH5–HH19 in whole mount chicken embryos and observed PGCs in three different structures: the area opaca, the area pellucida and the genital ridges (Fig. 2A,B). Between HH5–HH8, the great majority of the PGCs were located at the anterior region of area pellucida, bordering with the area opaca, the germinal crescent (Fig. 2B). However, at HH8–HH10, the PGCs were displaced to the area opaca adjacent to the germinal crescent, where they were predominantly found between HH11–HH12 (Fig. 2B). From there, the PGCs migrated transiently through the anterior area pellucida, towards the embryo, during a period of 12 hours between HH13–HH15. By HH16, the majority of the PGCs had reached the genital ridges (Fig. 2B). The number of PGCs present in the posterior part of the embryo, both in the area opaca and area pellucida, was consistently low during the period of development analysed (Fig. 2B).
Fig. 2. Tracking of the migration of PGCs using CVH as a marker.
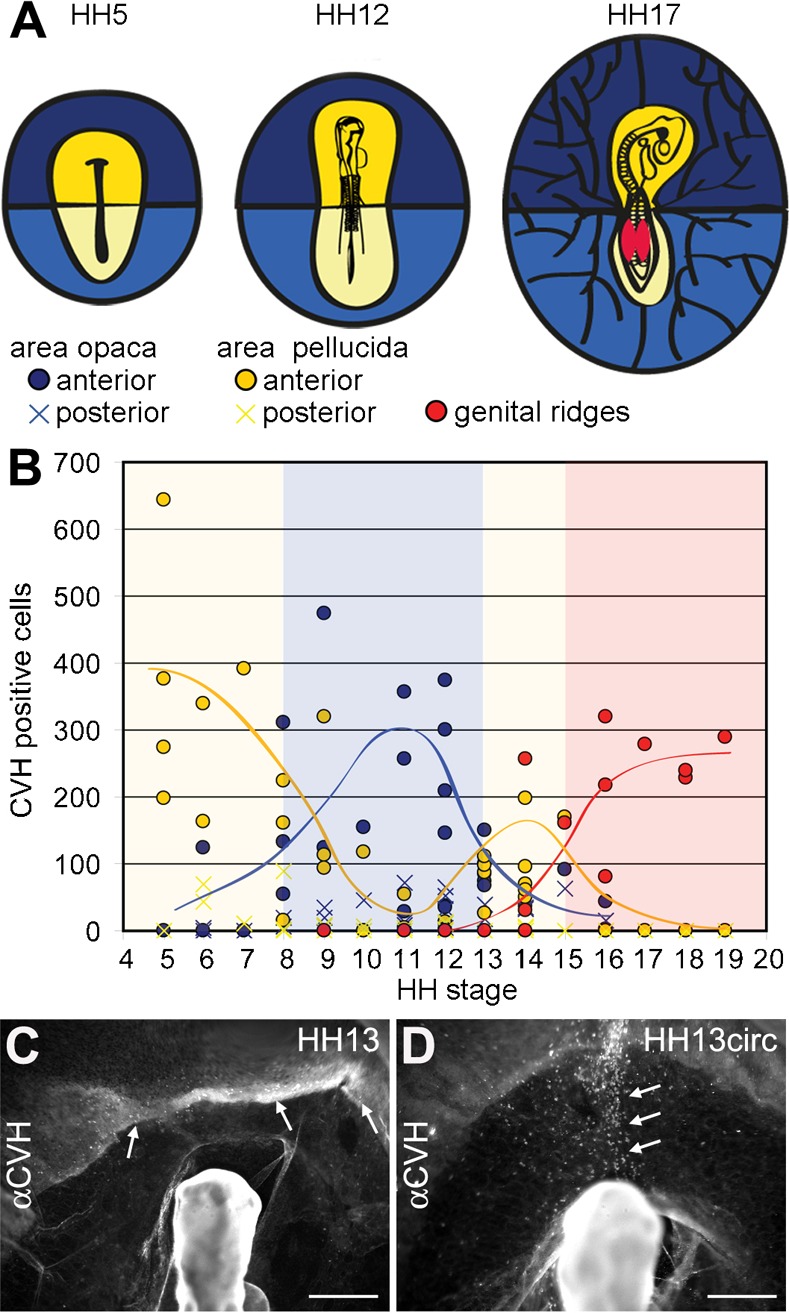
(A) Cartoon defining the different regions analysed during chicken development in several stages (HH5, HH12, HH17): anterior (dark blue/circle) and posterior (light blue/cross) regions of the area opaca; anterior (dark yellow/circle) and posterior (light yellow/cross) regions of area pellucida; and genital ridges (red/circle). (B) Total number of PGCs in the areas defined (A) showed predominant localization in 4 different structures during migration: at HH5–8 PGCs localized in the anterior region of area pellucida (dark yellow circle), at HH8–12 there was a displacement of the PGCs to the anterior region of area opaca (dark blue circle), from there the PGCs are migrating back to the anterior region of area pellucida (dark yellow circle) at HH13–15, and from HH14 on, the PGCs started to settle on the genital ridges (red circle). (C,D) At HH13, PGCs are either sparsely localized between area opaca and area pellucida (white arrows) (C) or they have aligned axially in the area pellucida (white arrows). We define this novel stage as HH13circ. Scale bars: 500 µm in C,D.
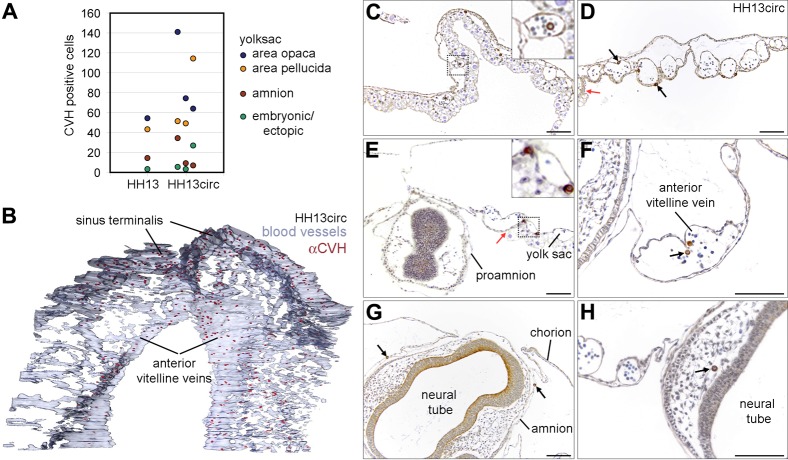
At HH13, the PGCs start to transit between the anterior region of area opaca, area pellucida and the genital ridges and this coincides with the period of initiation of the (vitelline) blood circulation. Therefore, we zoomed in at HH13 and defined two developmental sub-stages, HH13 and HH13circ. At HH13, the PGCs were relatively dispersed in the anterior central part of the area opaca and anterior central area pellucida (Fig. 2C). However, in some HH13 embryos, the PGCs concentrated in a narrower continuous axial region between the area opaca and the area pellucida (Fig. 2D). Both HH13 and HH13circ embryos contained about 17–19 somites and approximately the same number of PGCs (with similar medians) in the yolk sac in the area opaca and in the area pellucida, the amnion and ectopically, in particular in the head vasculature (Fig. 3A).
Fig. 3. At HH13circ the majority of PGCs is localized in the sinus terminalis and anterior vitelline veins.
(A) Analysis of position of PGCs in sectioned embryos at stage HH13circ. PGCs at HH13 and HH13circ are present in similar numbers in the yolk sac in the anterior area opaca and pellucida; the amnion and ectopically in the embryo head. (B) 3D reconstruction of the extraembryonic vasculature of embryos at HH13circ has shown that the PGCs were mainly localized in the anterior vitelline veins and the sinus terminalis. (C–E) Transverse sections of HH13circ embryos immunostained for CVH. PGCs were dispersed in the area opaca (C) and area pellucida (D) anterior from the head and at the level of the head (E). PGCs were observed inside and outside the blood vessels (black arrows). The junction between the area opaca and pellucida is marked by a red arrow. Note in E, that the head at the level of the prosencephalon is completely surrounded by proamnion. (F) PGCs (black arrow) in the anterior anterior vitelline veins. (G,H) Ectopic PGCs (black arrows) were found in the amnion (G) and in the capillary network of the head (H). Scale bars: 100 µm in C–H.
The histological analysis and 3D reconstruction of the vasculature and the position of the PGCs confirmed that at HH13circ the majority of PGCs are concentrated in specific blood vessels in the yolk sac, namely in the sinus terminalis (in the area opaca) and continuous to the developing anterior vitelline veins (in the area pelucida) (Fig. 3B). Our observations contrast with the current model where the PGCs were thought to be scattered broadly throughout the yolk sac vasculature and enter the embryo through the omphalomesenteric veins.
Also of note was the fact that, at HH13circ the PGCs were clearly present both inside (in the lumen of the vessel) and outside the blood vessels in the yolk sac (Fig. 3C–F), suggesting that they are not simply engulfed by the blood vessels as they form. In addition, PGCs were also observed frequently in the amnion (somatopleura) (Fig. 3G) and ectopically in the vasculature of the embryo head (Fig. 3H). The PGCs in the amnion probably mislocated when the somatopleura (amnion/chorion) and splanchnopleura (yolk sac) separated.
Finally, we also report that the head (at the level of the prosencephalon) at stage HH13circ, while extending anteriorly, becomes transiently enveloped in the proamnion, a bilaminar tissue consisting of hypoblast and epiblast (and no mesoderm); and, as a result, both the somatopleura (amnion/chorion) and splanchnopleura (yolk sac) are transiently localized above the developing head (Fig. 3E).
The PGCs migrated towards the embryo primarily using the anterior vitelline veins
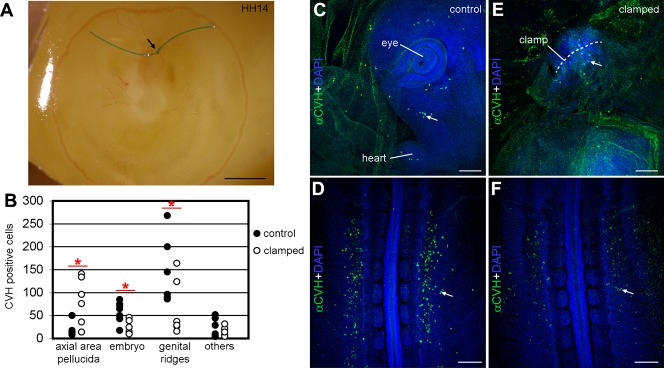
To experimentally test whether the anterior vitelline veins play a key role in the migration of PGCs from the extraembryonic to the intraembryonic vasculature, we blocked the anterior vitelline veins (by clamping the veins) in embryos at stage HH14 (Fig. 4A) and allowed the embryos to develop in ovo for 6 hours (from HH14 to HH15) to check whether that impacted on PGC migration. After 6 hours of culture, in control embryos (n = 7) the majority of PGCs found their way to the embryo and colonized the genital ridges (Fig. 4B–D). However, in experimental embryos (n = 6), the PGCs remained clustered in the region of the clamped anterior vitelline veins in the axial anterior area pellucida (Fig. 4B,E) and showed a reduced number of PGCs transiting through the embryo and in the genital ridges when compared to the controls (Fig. 4B,F). Our results indicate that blocking the blood flow from the anterior vitelline veins at HH14 has a significant effect in the correct migration of PGCs towards the genital ridges. We concluded that the anterior vitelline veins are the main vehicle used by the PGCs during their migration from the extraembryonic vasculature into the intraembryonic vasculature.
Fig. 4. Blocking the anterior vitelline veins prevented the correct migration of PGCs towards the genital ridges.
(A) The anterior vitelline veins were clamped in HH14 embryos growing in ovo and the embryos were allowed to develop for 6 hours. (B) Analysis of the total number of PGCs in control (n = 7, black dots) and experimental embryos (n = 6, white dots) in different regions. The differences in distribution of the PGCs in the axial area pellucida, the embryo and genital ridges were statistically significant (P<0.05) using the non-parametric Mann–Whitney test [(*) P<0.05]. (C–F) The number of PGCs (white arrows) present ectopically in the embryo head (C) and genital ridges (D) was consistently higher in control embryos than in experimental embryos, where the PGCs concentrated surrounding the clamped vitelline veins (E) and the number of PGCs settled in the genital ridges was reduced (F). Scale bars: 500 µm in A, 100 µm in C,E and 200 µm in D,F.
To further confirm the role of the anterior vitelline veins in the migration of PGCs from the extraembryonic to the intraembryonic circulation, we analyzed embryos cultured ex ovo using a modified Cornish pasty method that results in primary defects in the morphogenesis of the anterior extraembryonic structures (Nagai et al., 2011). Using this method, the somatopleura (amnion/chorion) and the splanchnopleura (yolk sac) separate, but the amniotic folds from the head, lateral and tail do not form leaving the embryo exposed. Moreover, the anterior axial conversion of the sinus terminalis and the anterior vitteline veins does not occur and therefore we investigated whether the PGCs were able to find their way into the genital ridges from the germinal crescent. For the modified Cornish pasty method, embryos at HH5 were removed from the egg, folded in two by their anterior–posterior axis and the edges of the semi-circle were pressed together to create a “mini yolk sac-like” (Fig. 5A) and cultured in suspension.
Fig. 5. A new model for PGCs migration in chicken embryos.
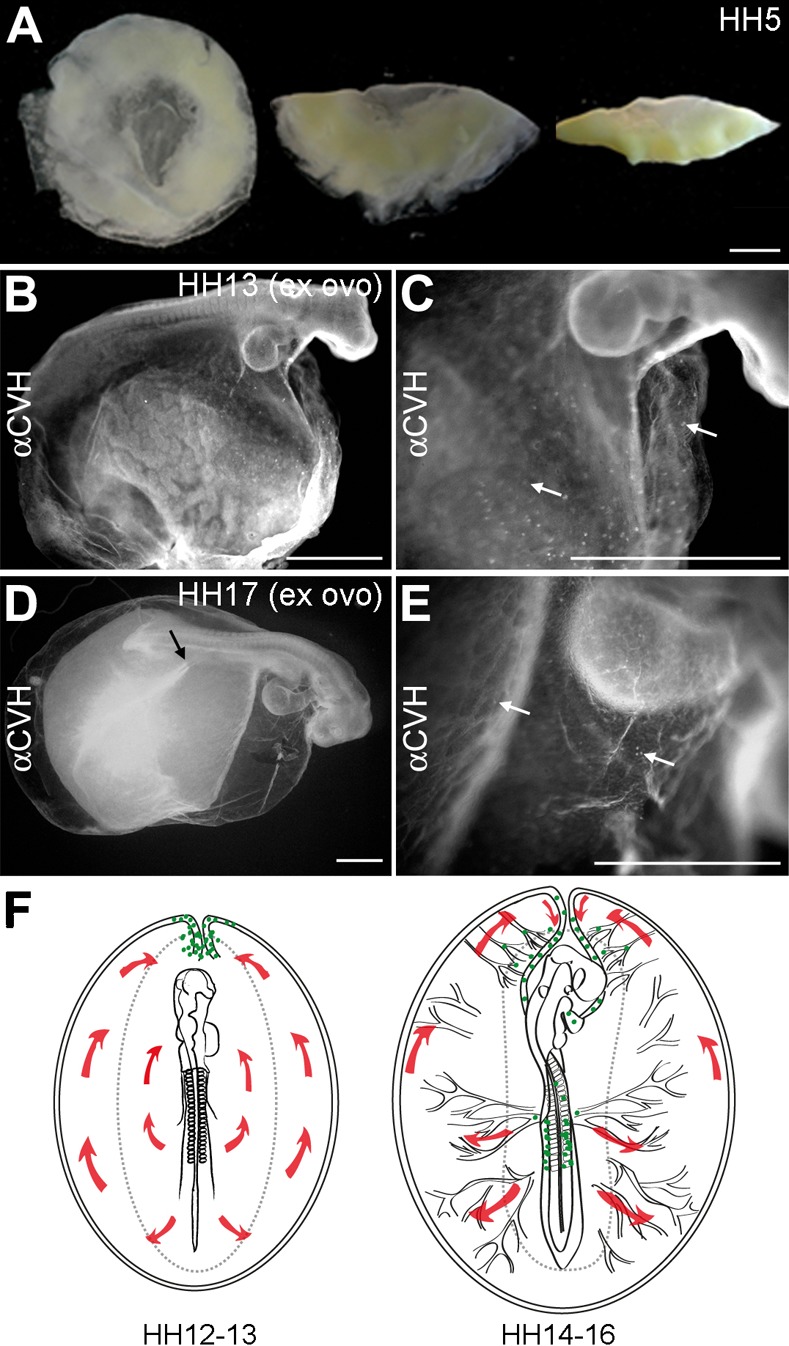
(A) Embryos at HH5 prepared to be cultured using the Cornish pasty method. (B,C) Ex ovo embryos at HH13 showed a relatively normal embryonic morphology (B) and the PGCs were observed in the germinal crescent area in both the somatopleura and splanchnopleura (white arrows). (D,E) Ex ovo embryos at HH17 showed a relatively normal embryonic morphology and the formation of well-developed posterior vitelline arteries (black arrow) (D) and the PGCs were still observed in the germinal crescent area in both the somatopleura and splanchnopleura (white arrows) (E). (F) A new model for PGCs migration in chicken embryos. At HH12–13, the yolk sac circulation courses in loop (red arrows) to enter the embryo via the heart. At this stage, the majority of PGCs (green dots) localized axially at the border between the area opaca and pellucida, where the sinus terminalis converged in the anterior vitelline veins. At HH14–16, the PGCs (green dots) circulated effectively towards the embryo via the sinus terminalis and the anterior vitelline veins towards the heart. Thereafter, the PGCs traffic via the aorta to the caudal part of the embryo and become lodged in the genital ridges. Scale bars: 100 µm in A–E.
After 30 hours ex ovo, embryos at stage HH13 were collected and immunostained for CVH (Fig. 5B,C). In general, the head showed a normal development, the embryos had 17–19 somites (n = 10), the heart was beating and the extraembryonic circulation well-established in the posterior region of the embryo. As expected, the headfold of the amnion did not form leaving the head exposed and tilting upwards. We observed many PGCs (± 200) dispersed in the splanchnopleura, anterior to the head at the border between the area opaca and pellucida and some PGCs mislocated in the somatopleura (Fig. 5C).
After 48 hours ex ovo, we could collect embryos corresponding to stage HH17 (Fig. 5D,E) showing a beating heart with visible blood flow and about 29–32 defined somites (n = 10), which corresponds to stage HH17 in ovo. HH17 ex ovo embryos showed a well-established circulation in the “mini yolk sac” and well-developed posterior vitelline arteries (Fig. 5D). However, there were clear defects in the morphogenesis and positioning of the large calibre anterior vitelline veins. We observed a general defect in the axial movement and fusion of the left and right side of the sinus terminalis and the two anterior vitelline veins. We counted the total number of (CVH-positive) PGCs in several HH17 ex ovo embryos (n = 5) and the majority of the PGCs (± 150) were present in the region of the splanchnopleura between the left and right vitelline veins and anterior to the heart. In those embryos, only a very low number of PGCs (< 10) was observed in the region of the genital ridges. We concluded that due to the developmental defects in the position of the sinus terminalis and the anterior vitelline veins in the HH13 and HH17 ex ovo embryos, the PGCs fail to find and ingress these blood vessels and therefore remained ectopically in the “germinal crescent” region. We propose a novel model for the migration of PGCs in the chicken with a key role for the sinus terminalis and the anterior vitelline veins (Fig. 5E) as part of a defined or preferred vascular pathway used by the PGCs to travel from their position in the germinal crescent into the heart via the omphalomesenteric veins.
Discussion
SSEA1 has been used as an appropriate marker to identify and isolate PGCs from chicken embryos (Lee et al., 2011; Minematsu et al., 2008; Motono et al., 2008; Motono et al., 2010; Mozdziak et al., 2005; Petitte et al., 1997). However, by performing double immunofluorescence analyses, using antibodies against CVH and SSEA1, we have now demonstrated that although SSEA1 marked PGCs, it is only expressed by a fraction, albeit increasing, of CVH-positive PGCs between HH8–HH19, but not in the entire population of PGCs. This perhaps explains why the fraction of circulating PGCs at HH13–HH15 isolated by fluorescence-activated cell sorting (FACS) on the basis of SSEA1 expression by Mozdziak and colleagues was smaller than the fraction of circulating PGCs isolated using a Nycodenz density gradient (Zhao and Kuwana, 2003) or found in chicken blood by PAS staining (Tajima et al., 1999). Interesting, pluripotent mouse embryonic stem cells, which are closely related to PGCs, also show pronounced heterogeneity for SSEA1 staining (Hayashi et al., 2008).
It will be important to identify additional lineage-specific markers, like Nanog and Dead end (Aramaki et al., 2007; Cañón et al., 2006; Fernandez-Tresguerres et al., 2010) and in particular novel cell surface markers to study chicken PGCs. This will be important to investigate both embryonic pluripotency and PGC development at very early developmental stages, where the CVH antibody is the only available to identify PGCs.
We and others (Tajima et al., 1999) have observed a large individual variation in the number of PGCs between HH5 and HH19. This results from genetic variation as demonstrated by Tajima and colleagues that showed that specific hens layed eggs with embryos containing consistently high or low number or circulating PGCs (Tajima et al., 1999). Maybe due to this high variability in PGC numbers, we were unable to observe an increasing number of PGCs between HH5 and HH19 as reported (Nakamura et al., 2007). In our hands, the number of PGCs remained approximately constant between HH5 and HH19. In agreement, Fujimoto and colleagues described 312 PGCs at HH10 (Fujimoto et al., 1976), Motono and colleagues referred to about 300 PGCs at HH13–HH16 (Motono et al., 2008) and Nakamura described embryos containing on average 300 PGCs from HH14 to HH20 (Nakamura et al., 1991). Together, we concluded that from HH5, when the PGCs are present in the germinal crescent, until HH19, when the PGCs have reached the genital ridges, the number of PGCs remained constant and range from 200–400.
Two studies have mapped the position of PGCs during their migration from the germinal crescent (HH4) to the genital ridges (HH17) by analysis of whole amount embryos (Fujimoto et al., 1976; Nakamura et al., 2007). None of them mentioned the vitelline veins, even though there is a clear concentration of PGCs visible at the junction between the sinus terminalis and the vitelline veins at HH10–11. Curiously, neither Fujimoto nor Nakamura analysed embryos at stage HH13 and this could be the reason why the concentration of PGCs in the anterior vitelline veins has remained unnoticed.
Different groups have collected circulating PGCs at HH13–HH14 using blood from the sinus terminalis, the vitelline vessels, the heart and the dorsal aorta (Motono et al., 2010; Mozdziak et al., 2005; Nakamura et al., 2010; Nakamura et al., 2007; Tajima et al., 1999; Yamamoto et al., 2007; Yasuda et al., 1992; Zhao and Kuwana, 2003). Although none referred to a specific vascular route used by the PGCs, their methods to collect circulating PGCs support the idea that the majority of the PGCs indeed concentrate and use the sinus terminalis and anterior vitelline veins as an effective way to reach the embryo via the omphalomesenteric veins that enter the heart. Circumstantial evidence was also provided by Nakamura and colleagues by injecting quail PGCs in chick vitelline vessels at HH15 and later observing those quail PGCs in the recipient chicks' genital ridges (Nakamura et al., 1992). We now provide functional data indicating a key role for the anterior vitelline veins transporting PGCs towards the embryo.
The PGCs leave the heart between HH13–HH15 and use the aorta to transit towards the genital ridges. At these stages, the ventral aorta develops first cranially before it turns caudally, via the first aortic arch, into the dorsal aorta. It is therefore not surprising that we and others (Nakamura et al., 1988; Nakamura et al., 1991) observed that HH13–HH15 PGCs had the tendency to become trapped in the cephalic capillary network when being pumped out of the heart, instead of performing the U-turn towards the dorsal aorta. In embryos in which the posterior part, including the gonads, have been excised, the PGCs still accumulate in the head capillaries (Nakamura et al., 1991). Interestingly, stromal cell-derived factor-1 (SDF1/Cxcl12), a chemokine involved in the extravasation of the PGCs from the vascular system to the mesenchyme of the genital ridges (Stebler et al., 2004), is expressed at HH12–HH15 specifically both in the area of the genital ridges and the head region (Rehimi et al., 2008) and could promote migration of PGCs into both areas.
The signaling pathway involved in attracting the PGCs into the vascular system is less understood. Having a better understanding of the vascular route taken by the PGCs and the markers that can be used to follow the population of PGCs will greatly facilitate the investigation of the mechanisms used by PGCs to enter the vascular system. In turn this may prove an important model to understand how metastatic cells behave on their way to form secondary tumors and how leukocytes behave during processes like infection and inflammation.
Acknowledgments
We thank the following: P. Beverly for mouse anti-SSEA1; S. Maas, C. van Munsteren and L. van Iperen for help with the egg-logistics; B. Wisse for help with the Amira software; and R. Poelman for discussions. This research was supported by the European Commission's Livelong Learning Programme (Leonardo da Vinci 2011-1-PT1-LEO02-08199) to A.M.B. and by the Netherlands Organization for Scientific Research (NWO Aspasia 015.007.037) to S.M.C.S.L.
Footnotes
Competing interests: The authors have no competing interests to declare.
References
- Alsayed Y., Ngo H., Runnels J., Leleu X., Singha U. K., Pitsillides C. M., Spencer J. A., Kimlinger T., Ghobrial J. M., Jia X.et al. (2007). Mechanisms of regulation of CXCR4/SDF-1 (CXCL12)-dependent migration and homing in multiple myeloma. Blood 109, 2708–2717 10.1182/blood-2006-07-035857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aramaki S., Sato F., Kato T., Soh T., Kato Y., Hattori M. A. (2007). Molecular cloning and expression of dead end homologue in chicken primordial germ cells. Cell Tissue Res. 330, 45–52 10.1007/s00441-007-0435-1 [DOI] [PubMed] [Google Scholar]
- Cañón S., Herranz C., Manzanares M. (2006). Germ cell restricted expression of chick Nanog. Dev. Dyn. 235, 2889–2894 10.1002/dvdy.20927 [DOI] [PubMed] [Google Scholar]
- Chuva de Sousa Lopes S. M., Roelen B. A. (2010). On the formation of germ cells: The good, the bad and the ugly. Differentiation 79, 131–140 10.1016/j.diff.2009.11.003 [DOI] [PubMed] [Google Scholar]
- de Sousa Lopes S. M., Roelen B. A. (2010). An overview on the diversity of cellular organelles during the germ cell cycle. Histol. Histopathol. 25, 267–276. [DOI] [PubMed] [Google Scholar]
- Eyal–Giladi H., Kochav S. (1976). From cleavage to primitive streak formation: a complementary normal table and a new look at the first stages of the development of the chick. I. General morphology. Dev. Biol. 49, 321–337 10.1016/0012-1606(76)90178-0 [DOI] [PubMed] [Google Scholar]
- Fernandez–Tresguerres B., Cañon S., Rayon T., Pernaute B., Crespo M., Torroja C., Manzanares M. (2010). Evolution of the mammalian embryonic pluripotency gene regulatory network. Proc. Natl. Acad. Sci. USA 107, 19955–19960 10.1073/pnas.1010708107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto T., Ukeshima A., Kiyofuji R. (1976). The origin, migration and morphology of the primordial germ cells in the chick embryo. Anat. Rec. 185, 139–153 10.1002/ar.1091850203 [DOI] [PubMed] [Google Scholar]
- Ginsburg M. (1994). Primordial germ cell formation in birds. Ciba Found. Symp. 182, 52–61; discussion 61–67. [DOI] [PubMed] [Google Scholar]
- Hamburger V., Hamilton H. L. (1992). A series of normal stages in the development of the chick embryo. 1951. Dev. Dyn. 195, 231–272 10.1002/aja.1001950404 [DOI] [PubMed] [Google Scholar]
- Hayashi K., Lopes S. M., Tang F., Surani M. A. (2008). Dynamic equilibrium and heterogeneity of mouse pluripotent stem cells with distinct functional and epigenetic states. Cell Stem Cell 3, 391–401 10.1016/j.stem.2008.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. D., Richardson E., Bachvarova R. F., Crother B. I. (2011). Evolution of the germ line-soma relationship in vertebrate embryos. Reproduction 141, 291–300 10.1530/REP-10-0474 [DOI] [PubMed] [Google Scholar]
- Lee S. I., Lee B. R., Hwang Y. S., Lee H. C., Rengaraj D., Song G., Park T. S., Han J. Y. (2011). MicroRNA-mediated posttranscriptional regulation is required for maintaining undifferentiated properties of blastoderm and primordial germ cells in chickens. Proc. Natl. Acad. Sci. USA 108, 10426–10431 10.1073/pnas.1106141108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D. B. (1964). The migration of primordial germ cells in the chick embryo. Dev. Biol. 10, 154–190 10.1016/0012-1606(64)90009-0 [DOI] [PubMed] [Google Scholar]
- Minematsu T., Harumi T., Naito M. (2008). Germ cell-specific expression of GFP gene induced by chicken vasa homologue (Cvh) promoter in early chicken embryos. Mol. Reprod. Dev. 75, 1515–1522 10.1002/mrd.20894 [DOI] [PubMed] [Google Scholar]
- Motono M., Ohashi T., Nishijima K., Iijima S. (2008). Analysis of chicken primordial germ cells. Cytotechnology 57, 199–205 10.1007/s10616-008-9156-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motono M., Yamada Y., Hattori Y., Nakagawa R., Nishijima K., Iijima S. (2010). Production of transgenic chickens from purified primordial germ cells infected with a lentiviral vector. J. Biosci. Bioeng. 109, 315–321 10.1016/j.jbiosc.2009.10.007 [DOI] [PubMed] [Google Scholar]
- Mozdziak P. E., Angerman–Stewart J., Rushton B., Pardue S. L., Petitte J. N. (2005). Isolation of chicken primordial germ cells using fluorescence-activated cell sorting. Poult. Sci. 84, 594–600. [DOI] [PubMed] [Google Scholar]
- Nagai H., Lin M. C., Sheng G. (2011). A modified cornish pasty method for ex ovo culture of the chick embryo. Genesis 49, 46–52 10.1002/dvg.20690 [DOI] [PubMed] [Google Scholar]
- Nakamura M., Kuwana T., Miyayama Y., Fujimoto T. (1988). Extragonadal distribution of primordial germ cells in the early chick embryo. Anat. Rec. 222, 90–94 10.1002/ar.1092220113 [DOI] [PubMed] [Google Scholar]
- Nakamura M., Kuwana T., Miyayama Y., Yoshinaga K., Fujimoto T. (1991). Ectopic colonization of primordial germ cells in the chick embryo lacking the gonads. Anat. Rec. 229, 109–115 10.1002/ar.1092290112 [DOI] [PubMed] [Google Scholar]
- Nakamura M., Yoshinaga K., Fujimoto T. (1992). Histochemical identification and behavior of quail primordial germ cells injected into chick embryos by the intravascular route. J. Exp. Zool. 261, 479–483 10.1002/jez.1402610415 [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Yamamoto Y., Usui F., Mushika T., Ono T., Setioko A. R., Takeda K., Nirasawa K., Kagami H., Tagami T. (2007). Migration and proliferation of primordial germ cells in the early chicken embryo. Poult. Sci. 86, 2182–2193. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Usui F., Ono T., Takeda K., Nirasawa K., Kagami H., Tagami T. (2010). Germline replacement by transfer of primordial germ cells into partially sterilized embryos in the chicken. Biol. Reprod. 83, 130–137 10.1095/biolreprod.110.083923 [DOI] [PubMed] [Google Scholar]
- Nieuwkoop P. D., Sutasurya L. A. (1979). Primordial Germ Cells In The Chordates: Embryogenesis And Phylogenesis. Cambridge: Cambridge University Press. [Google Scholar]
- Pain B., Clark M. E., Shen M., Nakazawa H., Sakurai M., Samarut J., Etches R. J. (1996). Long-term in vitro culture and characterisation of avian embryonic stem cells with multiple morphogenetic potentialities. Development 122, 2339–2348. [DOI] [PubMed] [Google Scholar]
- Petitte J. N., Karagenç L., Ginsburg M. (1997). The origin of the avian germ line and transgenesis in birds. Poult. Sci. 76, 1084–1092. [DOI] [PubMed] [Google Scholar]
- Rehimi R., Khalida N., Yusuf F., Dai F., Morosan–Puopolo G., Brand–Saberi B. (2008). Stromal-derived factor-1 (SDF-1) expression during early chick development. Int. J. Dev. Biol. 52, 87–92 10.1387/ijdb.072374rr [DOI] [PubMed] [Google Scholar]
- Solter D., Knowles B. B. (1978). Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1). Proc. Natl. Acad. Sci. USA 75, 5565–5569 10.1073/pnas.75.11.5565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebler J., Spieler D., Slanchev K., Molyneaux K. A., Richter U., Cojocaru V., Tarabykin V., Wylie C., Kessel M., Raz E. (2004). Primordial germ cell migration in the chick and mouse embryo: the role of the chemokine SDF-1/CXCL12. Dev. Biol. 272, 351–361 10.1016/j.ydbio.2004.05.009 [DOI] [PubMed] [Google Scholar]
- Streit A., Stern C. D. (2008). Operations on primitive streak stage avian embryos. Methods Cell Biol. 87, 3–17 10.1016/S0091-679X(08)00201-X [DOI] [PubMed] [Google Scholar]
- Swift C. H. (1914). Origin and early history of the primordial germ cells in the chick. Am. J. Anat. 15, 483–516 10.1002/aja.1000150404 [DOI] [Google Scholar]
- Tajima A., Hayashi H., Kamizumi A., Ogura J., Kuwana T., Chikamune T. (1999). Study on the concentration of circulating primordial germ cells (cPGCs) in early chick embryos. J. Exp. Zool. 284, 759–764 [DOI] [PubMed] [Google Scholar]
- Tsunekawa N., Naito M., Sakai Y., Nishida T., Noce T. (2000). Isolation of chicken vasa homolog gene and tracing the origin of primordial germ cells. Development 127, 2741–2750. [DOI] [PubMed] [Google Scholar]
- Ueda Y., Yang K., Foster S. J., Kondo M., Kelsoe G. (2004). Inflammation controls B lymphopoiesis by regulating chemokine CXCL12 expression. J. Exp. Med. 199, 47–58 10.1084/jem.20031104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukeshima A., Kudo M., Fujimoto T. (1987). Relationship between genital ridge formation and settlement site of primordial germ cells in chick embryos. Anat. Rec. 219, 311–314 10.1002/ar.1092190312 [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Usui F., Nakamura Y., Ito Y., Tagami T., Nirasawa K., Matsubara Y., Ono T., Kagami H. (2007). A novel method to isolate primordial germ cells and its use for the generation of germline chimeras in chicken. Biol. Reprod. 77, 115–119 10.1095/biolreprod.107.061200 [DOI] [PubMed] [Google Scholar]
- Yasuda Y., Tajima A., Fujimoto T., Kuwana T. (1992). A method to obtain avian germ-line chimaeras using isolated primordial germ cells. J. Reprod. Fertil. 96, 521–528 10.1530/jrf.0.0960521 [DOI] [PubMed] [Google Scholar]
- Zhao D. F., Kuwana T. (2003). Purification of avian circulating primordial germ cells by Nycodenz density gradient centrifugation. Br. Poult. Sci. 44, 30–35 10.1080/0007166031000085382 [DOI] [PubMed] [Google Scholar]