Summary
Tubulobulbar complexes are actin-related endocytic structures that form at sites of intercellular attachment in the seminiferous epithelium and are proposed to internalize intact junctions. In this study, we test the prediction that altering the structure/function of tubulobulbar complexes results in failure to release mature spermatids from Sertoli cells. We used an in vivo knockdown strategy to target cortactin, a component of tubulobulbar complexes, in Sprague Dawley rats. In each animal, one testis was surgically injected with cortactin siRNA reagents and the other testis was injected with non-targeting siRNA. After three days, experimental and control testes were processed for immunoblotting, electron microscopy or immunofluorescence microscopy. In testis sections immunostained for cortactin or labeled for filamentous actin, fluorescence microscopy revealed that tubulobulbar complexes were shorter in siRNA-treated testes relative to controls. Significantly, in the knockdown testes, spermiation was delayed in some tubules and had failed in others. When evaluated by electron microscopy, adhesion complexes (ectoplasmic specializations) remained associated with mature spermatids that failed to be released from Sertoli cells. Immunoblots both of whole testis lysates and of isolated seminiferous epithelial lysates confirmed that cortactin expression was knocked-down in experimental testes and in the seminiferous epithelium respectively, relative to controls. Moreover, in testes injected with siRNA reagents with a dye modification on one of the four targeting siRNA sequences, dye clusters were detected at the base of the epithelium confirming that the reagents entered Sertoli cells. Our results are consistent with the hypothesis that tubulobulbar complexes internalize intercellular junctions and that they are a significant component of the sperm release mechanism.
Key words: Tubulobulbar complexes, Cortactin, Spermatogenesis, Testis, Spermiation, Actin
Introduction
The disassembly of massive intercellular adhesion junctions between Sertoli cells and mature spermatids is fundamental to the process of sperm release (spermiation) from the seminiferous epithelium. During this disassembly, unique structures termed “tubulobulbar complexes” form at the intercellular attachment sites (Russell and Clermont, 1976), and have been proposed to be responsible for internalizing ‘intact’ junctions into Sertoli cells (Guttman et al., 2004b; Young et al., 2009b; Young et al., 2012).
We previously have shown that tubulobulbar complexes contain adhesion proteins (α6β1 integrin, nectin2 [Sertoli cell] and nectin3 [spermatid]) known to be present at ectoplasmic specializations (Guttman et al., 2004b; Young et al., 2009b). Moreover, regions of the complexes enriched in junction proteins also are associated with the endosomal markers Rab5 (Young et al., 2012) and EEA1 (Young et al., 2009b), as well as with LAMP1, SGP1 and PKCα (Guttman et al., 2004b). Significantly, the absence of tubulobulbar complexes in estradiol treated rats (D'Souza et al., 2009) and in amphiphysin knockout animals (Kusumi et al., 2007) is correlated with a failure to release mature spermatids from the seminiferous epithelium. In addition, spermiation failure is one of the first phenotypes observed in men undergoing hormonal methods of contraception (McLachlan et al., 2002). Together, these observations are consistent with our working hypothesis that tubulobulbar complexes that form between Sertoli cells and spermatids are subcellular machines that internalize intact intercellular adhesion junctions and that the complexes are a key element of the sperm release mechanism.
Although tubulobulbar complexes are unique to the seminiferous epithelium they bear a striking resemblance to podosomes, particularly those in osteoclasts (Ochoa et al., 2000), to membrane tubules reconstituted in cell-free systems (Wu et al., 2010), and to models of clathrin-based endocytosis generally in cells (Taylor et al., 2011). Podosomes in osteoclasts, reconstituted membrane tubules, the necks of mature clathrin coated pits, and tubulobulbar complexes all consist of elongate cores of tubular membrane surrounded by networks of actin filaments and related proteins. However, unlike in the other systems where the tubular central core consists of a single membrane, the core of tubulobulbar complexes consists of a ‘double-membrane’ tube formed by the attached plasma membranes of two adjacent cells. Among key components present in many of these systems are Arp2/3, N-WASp, amphiphysin, dynamin, and cortactin (Kusumi et al., 2007; Ochoa et al., 2000; Taylor et al., 2011; Vaid et al., 2007; Wu et al., 2010; Young et al., 2009a). Knockdown of cortactin in osteoclasts prevents podosome formation even though actin-related structures in other parts of cells are unaffected (Tehrani et al., 2006).
If tubulobulbar complexes are a significant part of the sperm release mechanism, then perturbation of tubulobulbar complex structure/function should lead to spermiation failure. Because there are no culture models of sperm release, the approach we currently are taking to verify this prediction is to deplete cortactin, a known component of tubulobulbar complexes in rats, by intratesticular injection of siRNA reagents, determine if the knockdown has an effect on tubulobulbar complexes, and then determine whether there is a spermiation defect.
In this study we report the results of cortactin knockdown experiments. Although tubulobulbar complexes were present in siRNA treated rat testes, they were shorter three days after injection than those in contralateral control testes injected with a scrambled sequence. Moreover, spermiation delay and failure were detected in the knockdown testes, and ectoplasmic specializations (testis specific adhesion junctions) remained associated with those spermatids retained by the epithelium. We conclude that apical tubulobulbar complexes are a significant part of the normal process of sperm release and that interfering with their structure/function results in spermiation failure.
Results
Normal arrangement and structure of tubulobulbar complexes in rat
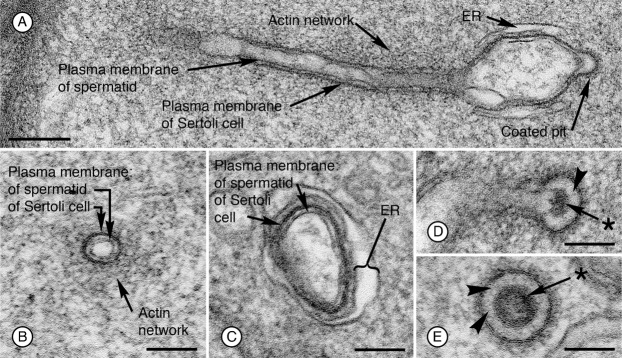
Tubulobulbar complexes that form in apical regions of the seminiferous epithelium are elongate tubular extensions of the attached plasma membranes of the spermatid and Sertoli cell that project into the Sertoli cell in regions previously occupied by unique adhesion junctions known as ectoplasmic specializations. In rats, as many as twenty-four complexes, each ranging from 2–3 µm in length develop in two parallel rows associated with the concave face of the “hook shaped” spermatid heads (Russell and Clermont, 1976; Russell, 1979c) (Fig. 1). The “double-membrane” core of each complex is cuffed by a dendritic actin network (Young et al., 2009a) (Fig. 2A,B). In addition to actin, other molecular components of tubulobulbar complexes include Arp2/3 (Vaid et al., 2007), N-WASp (Young et al., 2009a), cortactin (Young et al., 2009a), dynamin (Vaid et al., 2007), amphiphysin (Kusumi et al., 2007), and cofilin (Guttman et al., 2004a). Formation of each complex is initiated in the Sertoli cell by a clathrin-coated pit (Young et al., 2009a), which retains an attachment to the neighboring germ cell plasma membrane as the complex elongates and matures (Russell and Clermont, 1976) (Fig. 2A). In regions of the coated pit where the germ cell plasma membrane remains attached to the Sertoli cell, fine filamentous connections occur between the two cells, and the germ cell membrane contains a distinct sub-membrane density (Russell and Clermont, 1976) (Fig. 2D,E). As the complex matures a swelling develops in the distal third of the structure just proximal to the coated pit (Fig. 2C). This bulbar region is not associated with actin. Rather, it is closely associated with a cistern of endoplasmic reticulum. The bulbar region eventually buds from the complex and is internalized by the Sertoli cell (Russell and Clermont, 1976; Russell, 1979c).
Fig. 1. Position and arrangement of tubulobulbar complexes associated with Sertoli/spermatid attachment sites in rat seminiferous epithelium.
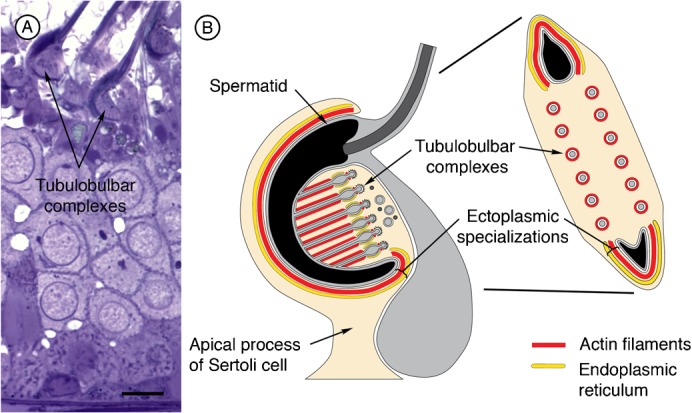
(A) 1 µm thick section at stage VII of spermatogenesis showing clusters of tubulobulbar complexes adjacent to the concave surfaces of hook-shaped spermatid heads at the apex of the epithelium. Bar = 10 µm. (B) Schematic illustration of tubulobulbar arrangement adjacent to mature spermatids.
Fig. 2. Ultrastructural features of tubulobulbar complexes associated with Sertoli/spermatid attachment sites at the apex of rat seminiferous epithelium.
(A) Longitudinal section of a tubulobulbar complex. Bar = 200 nm. (B) Cross section through proximal tubular region. Bar = 100 nm. (C) Cross section through bulbar region. Bar = 100 nm. (D) Longitudinal section through distal tubular region and coated-pit. Notice the density (asterisk) associated with the spermatid plasma membrane and the connections (arrowhead) with the Sertoli cell plasma membrane of the coated pit. Bar = 100 nm. (E) Cross section through a coated pit illustrating fine filamentous attachments between the plasma membranes of the Sertoli cell and the spermatid (arrowheads), and the density associated with the spermatid plasma membrane (asterisk). Bar = 100 nm.
Cortactin knockdown by intratesticular siRNA
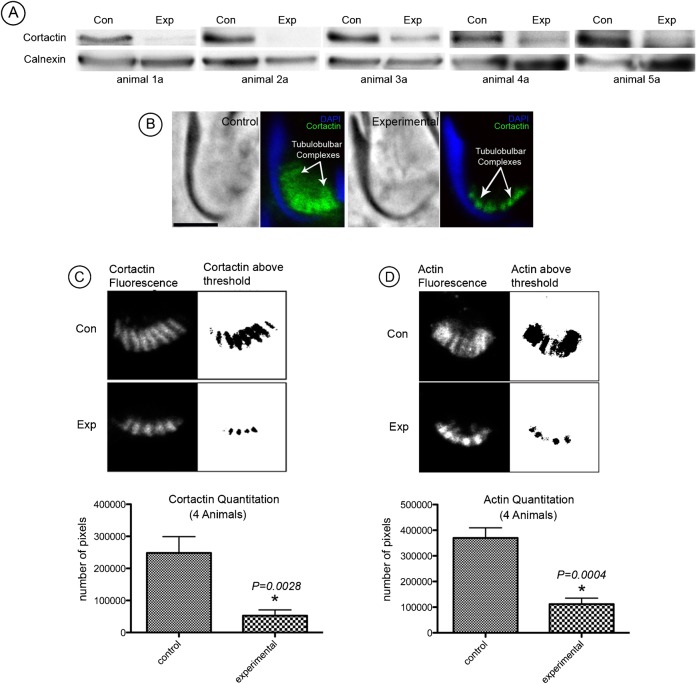
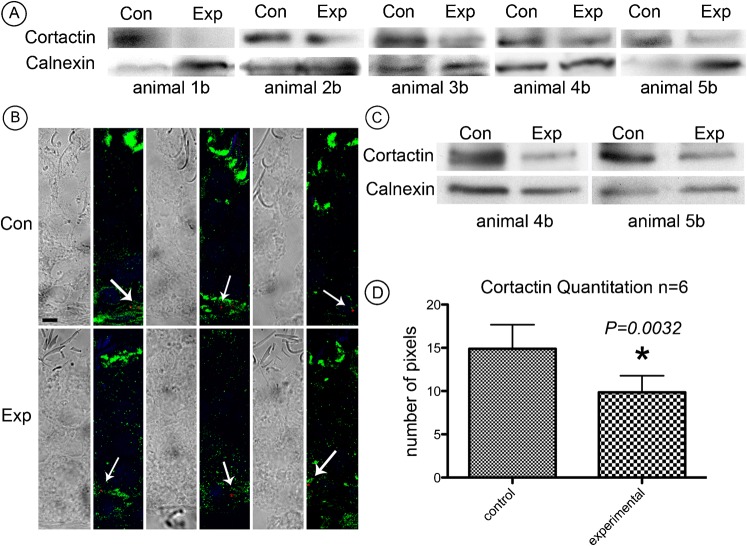
In the first set of experiments on five animals, cortactin bands on Western blots of whole testis lysates from testes injected with cortactin (Cttn) targeted siRNAs were clearly weaker relative to those of similar lysates from contralateral control testes injected with non-targeting siRNAs (Fig. 3A). We concluded that cortactin in the testis was successfully knocked down by intratesticular injection of siRNA.
Fig. 3. Evidence of cortactin knockdown.
(A) Western blots of seminiferous epithelial lysate from five animals indicate weaker cortactin bands from experimental testes in comparison to control. Calnexin was used as a loading control. (B) Immunofluorescence of stage VII spermatids labeled for cortactin (green) and DAPI (blue). Cortactin staining outlines individual tubulobulbar complexes from experimental and control testes and reveals a difference in length. Bar = 5 µm. (C) Quantitation of knockdown was determined by probing cryosections from control and experimental tissue labeled for cortactin. Mature spermatids were randomly selected and set to a predetermined threshold using ImageJ software. The number of positive pixels was determined using the “analyze particles” function. The number of pixels was considered to be an index of tubulobulbar complex length in each cluster. Experimental tubulobulbar complexes were shown to be significantly different from control tubulobulbar complexes (P = 0.0028) (D) Quantitation of knockdown determined by probing cryosections from control and experimental tissue labeled for filamentous actin. Experimental tubulobulbar complexes were shown to be significantly different from those in controls (P = 0.0004).
Tubulobulbar complexes are shorter in cortactin knockdown testes
Tubulobulbar complexes in sections of stage VII seminiferous epithelium appeared qualitatively shorter in cortactin knockdown testes relative to control testes when double labeled for cortactin (Fig. 3B,C) and actin (Fig. 3D).
As an index of tubulobulbar complex length we quantified the numbers of pixels above a set threshold occupied by the total cluster of tubulobulbar complexes adjacent to the concave face of mature spermatids at Stage VII of spermatogenesis in experimental and control testes labeled for either cortactin or for actin (Fig. 3C,D). There was a significant difference between experimental and control values (P<0.0028 for cortactin and P<0.0004 for actin) (Fig. 3C,D).
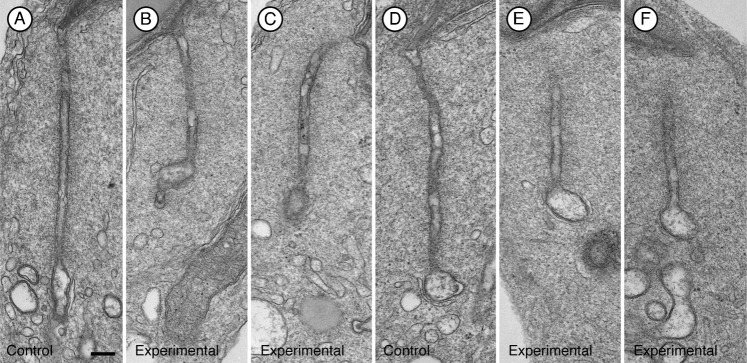
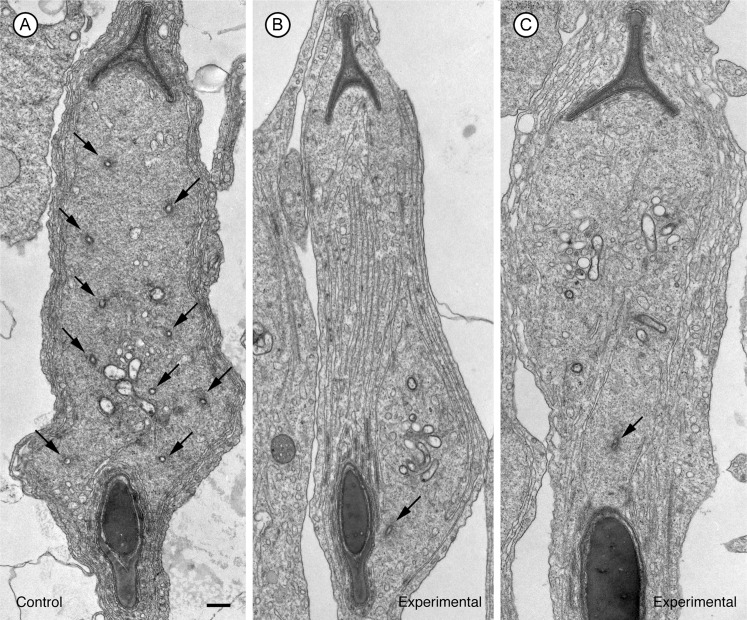
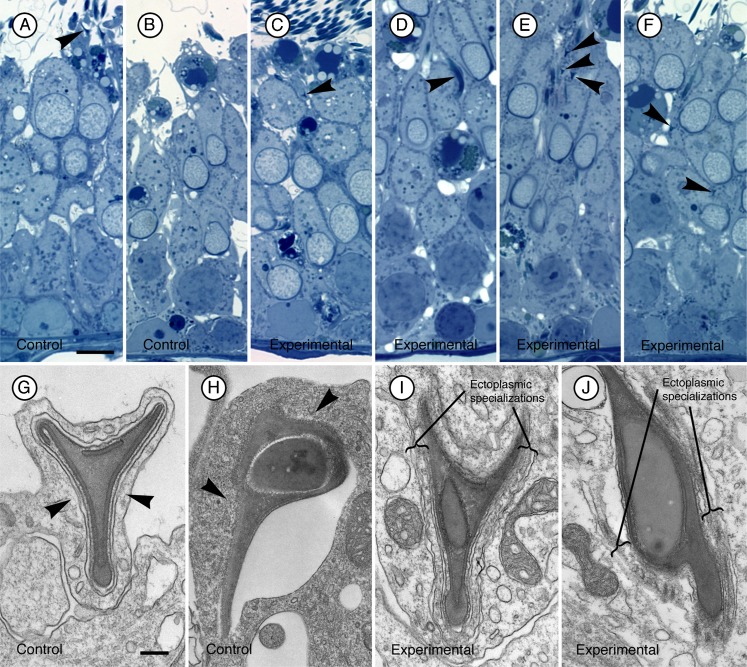
To gain a better understanding of the morphological effects of the siRNA, we examined the structures at the ultrastructural level. In electron micrographs of spermatids and adjacent Sertoli cell regions in favorable sections where tubulobulbar complexes could be seen along their entire length, the structures were clearly shorter in experimental (Fig. 4B,C,E,F) than in control tissue (Fig. 4A,D). Moreover, when apical Sertoli processes containing mature spermatids at Stage VII of spermatogenesis were sectioned so that the total cluster of tubulobulbar complexes was cut in cross section in control testes (Fig. 5A), many fewer profiles of tubulobulbar complexes were visible in experimental material when cut at the same level (Fig. 5B,C). This is a result consistent with that predicted if tubulobulbar complexes were shorter in experimental than in control testes. We conclude that cortactin knockdown resulted in shorter tubulobulbar complexes.
Fig. 4. Representative longitudinal sections of tubulobulbar complexes from two different rats.
(A–C) first rat, (D–F) second rat. Relative to tubulobulbar complexes in the control testis of each animal (A,D) similar complexes are noticeably shorter in the cortactin siRNA treated contralateral testes (B,C,E,F). Bar = 200 nm.
Fig. 5. Cross sections through apical Sertoli cell lobules containing mature spermatids at Stage VII of spermatogenesis in control (A) and cortactin siRNA treated (B,C) testes.
The plane of section for each panel is similar to that shown in the right image on Fig. 1B. Notice that there are many fewer profiles of proximal tubular regions of tubulobulbar complexes (arrowheads) in the siRNA treated material relative to control tissue. This is because the complexes are shorter in cortactin knockdown tissue relative to controls. Bar = 200 nm.
Spermatids retained in the epithelium
Mature spermatids are released as spermatozoa into the lumen of seminiferous tubules in Stage VIII of spermatogenesis (Leblond and Clermont, 1952). During this spermiation process adhesion junctions (ectoplasmic specializations) between Sertoli cells and spermatids disappear, Sertoli cell cytoplasm is gradually withdrawn from spermatid heads, spermatids detach from the apex of the epithelium, and residual lobes from the released spermatids are retained by Sertoli cells. In light micrographs of the seminiferous epithelium from control testes at Stage VIII, spermatids were being released in the normal fashion (Fig. 6A,B). In the epithelium at stage VIII of experimental animals retraction of the Sertoli cell cytoplasm away from spermatids appeared delayed in some tubules (arrows in Fig. 6C). Significantly, after most spermatids in controls had been released (Fig. 7A,B) some spermatids in experimental testes appeared not to be released and were retained by the epithelium (arrow marked by asterisk in Fig. 6C, arrowheads in Fig. 7C–F). When evaluated ultrastructurally, ectoplasmic specializations were absent from Sertoli cell regions adjacent to spermatids being released from control epithelia (arrowheads in Fig. 7G,H), but were still present in association with spermatids retained at the apex of experimental testes (Fig. 7I,J). We conclude that there is evidence of a delay and a failure of spermiation in experimental testes, and that adhesion junctions (ectoplasmic specializations) persisted at intercellular attachment sites where spermatids were retained by the epithelium.
Fig. 6. Evidence of delayed spermiation.
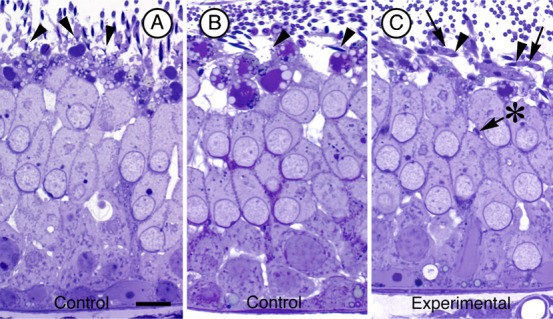
Light micrographs of Stage VIII seminiferous tubules from control (A,B) and cortactin targeted siRNA (C) injected testes. Notice that in panel (C) Sertoli cell cytoplasm (arrows) still engulfs the spermatid heads (arrowheads) at the apex of the epithelium whereas this cytoplasm has been withdrawn in controls. Also notice that there is one spermatid head (arrow marked with asterisk) that is positioned deep in the epithelium in (C). Bar = 10 µm.
Fig. 7. Evidence of spermiation failure and persistence of adhesion junctions in cortactin siRNA testes.
(A–F) 1 µm thick plastic sections stained with toluidine blue of stages VIII (late)/IX of spermatogenesis. In sections from control testes (A,B), spermatids are in the final stages of release (arrowhead in (A)) or have been released as spermatozoa from the epithelium (B). In comparable sections from cortactin siRNA testes (C–F), numerous spermatids (arrowheads) are observed at the apex of the epithelium, some still surrounded by the apical process of the Sertoli cell (arrowhead in (C)). Others are positioned deeper in the epithelium (arrowheads in D,E,F). Bar = 10 µm. When viewed at the ultrastructural level, spermatids being released from the epithelium at late stage VIII in sections of control testes lack ectoplasmic specializations in Sertoli cell regions adjacent to spermatid heads (arrowheads in (G,H) and apical regions of the Sertoli cell are being withdrawn from the heads. In sections at stages VIII (late)/IX of cortactin siRNA testes (I,J), ectoplasmic specializations are present adjacent to spermatid heads that are positioned in the epithelium similar to those shown in panels D,E,F. Bar = 200 nm.
siRNA reagents enter Sertoli cells and result in cortactin knockdown in isolated seminiferous epithelia
To verify that the siRNA administered by intratesticular injection actually entered Sertoli cells and resulted in cortactin knockdown in the seminiferous epithelium, we ran additional experiments using the same pool of cortactin targeting and non-targeting duplexes but with one nucleotide from each pool modified with DY547 on the 5′ end of the sense strand. This nucleic acid tag can be visualized by confocal microscopy using an appropriate laser. The dye was detected in the seminiferous epithelium of all animals that received the modified siRNA and non-targeting reagents (Fig. 8B).
Fig. 8. Cortactin knockdown occurs in the seminiferous epithelium and siRNA reagents enter Sertoli cells.
(A) Western blots of whole testis lysate from five animals used in the dye studies. Cortactin bands are weaker from experimental testes than from control testes even when the protein concentration is greater as indicated by the calnexin staining. (B) Dye appears as clusters in immunofluorescence micrographs of seminiferous epithelium from testes injected with the modified siRNA and the non-targeting control. Bar = 10 µm. (C) To confirm that cortactin knockdown was in the seminiferous epithelium, two animals used to prepare whole testes lysate were also used to prepare isolated seminiferous epithelium lysate. In both animals, bands from experimental lysates were weaker than from control lysates. (D) The quantitation of cortactin determined by the positive pixels above threshold. The number of pixels was significantly less (P = 0.0032) from experimental testes than from control testes.
To confirm that cortactin was indeed knocked-down in experimental testes, Western blots were run on whole testis lysates from animals using the same reagents as those used for the dye morphological studies. Cortactin bands were weaker in experimental lysates than in contralateral control testis lysates (Fig. 8A). To confirm that cortactin knockdown occurred in the seminiferous epithelium, seminiferous epithelium was isolated from two of the above animals and then analysed by immunoblotting. In both animals, successful depletion of cortactin was detected (Fig. 8C). As in our previous experiments, the number of positive pixels above threshold was significantly less adjacent to mature spermatids in experimental animals than in controls when sections were labeled for cortactin (Fig. 8D). We conclude that siRNA reagents entered Sertoli cells and that the reagents targeting cortactin resulted in lower overall levels of the protein in the seminiferous epithelium than in controls. These results confirm the successful delivery of the siRNA into the seminiferous epithelium.
Discussion
There is a growing body of evidence that tubulobulbar complexes that develop adjacent to mature spermatids are subcellular machines that internalize intact intercellular junctions (Guttman et al., 2004b; Young et al., 2009b; Young et al., 2012). In this study we use intratesticular injection of siRNA reagents to target a known component of tubulobulbar complexes, alter tubulobulbar complex structure and correlate this altered structure with a fundamental biological event during spermatogenesis – sperm release.
Apical tubulobulbar complexes each consist of a tubular process of the spermatid plasma membrane that extends into a corresponding invagination of the neighboring Sertoli cell (Russell and Clermont, 1976). A clathrin-coated pit in the Sertoli cell initiates the formation of the structure and remains attached to the tip of the complex as it lengthens. A network of actin filaments cuffs the double-membrane tubular core and as the complex matures, a large swelling or bulb forms in distal regions (Russell, 1979a). The bulb buds from the complex and enters endosomal compartments of the Sertoli cell (Guttman et al., 2004b; Young et al., 2012).
The location, timing and unique structure of tubulobulbar complexes have led to a number of suggested functions. Among these are that they eliminate cytoplasm from spermatids (Russell, 1979c), anchor elongate spermatids to the epithelium (Russell and Clermont, 1976), facilitate shaping of spermatid heads (Kierszenbaum and Tres, 2004), and remove excess acrosomal contents from spermatids (Tanii et al., 1999). The observation that tubulobulbar complexes also occur at the base of the seminiferous epithelium in association with junction complexes between neighboring Sertoli cells indicates that the primary function of tubulobulbar complexes is probably not related specifically to spermatids per se, but is more related to a cellular process or event common both to apical and to basal sites. At the apex of the epithelium, ectoplasmic specializations located between Sertoli cells and spermatids must ‘disassemble’ to enable sperm release. At the base of the epithelium, similar junctions located between Sertoli cells need to remodel as the next generation of spermatogenic cells translocate through the junctions to complete meiosis and then to develop into sperm cells. A unifying function for tubulobulbar complexes in the seminiferous epithelium is that they internalize intercellular junctions (Guttman et al., 2004b; Russell, 1979b).
There now is substantial evidence that tubulobulbar complexes do internalize adhesion junctions that occur between Sertoli cells and spermatids. First, tubulobulbar complexes develop in areas previously occupied by testis specific adhesion junctions known as ectoplasmic specializations, and the structures develop prior to release of spermatids from the epithelium. Second, integral membrane junction proteins have been localized in tubulobulbar complexes and are concentrated at their ends (Guttman et al., 2004b; Young et al., 2012). Third, these proteins colocalize with early endocytic markers Rab5 and EEA1 (Young et al., 2009b; Young et al., 2012). Significantly, nectin 3, one of the adhesion molecules present in the spermatid plasma membrane, is concentrated at the ends of tubulobulbar complexes and is absent from spermatozoa after release from the epithelium. These data, together with the observation that the double membrane bulbs bud from the structures, are consistent with the conclusion that apical tubulobulbar complexes are subcellular endocytic machines that internalize ‘intact’ intercellular adhesion junctions into Sertoli cells.
If tubulobulbar complexes associated with spermatids do in fact internalize junctions, then perturbing their structure/function should prevent junction turnover ultimately delaying or preventing sperm release from the epithelium. In this study we used an in vivo siRNA approach to knockdown cortactin, a protein concentrated at tubulobulbar complexes in the seminiferous epithelium (Young et al., 2009a), and a component of the actin cuff surrounding the central double-membrane core of each structure.
Cortactin is known to coordinate membrane dynamics and cytoskeletal remodeling in other systems (Schafer, 2002) – two features that also make it a key player during the process of clathrin-mediated endocytosis (Merrifield et al., 2005). Two of cortactin's five functional domains allow it to simultaneously bind F-actin and Arp2/3. Therefore, cortactin not only promotes nucleation and branching but also acts to stabilize newly formed branches (Weaver et al., 2001). These features, paired with cortactin's affinity to bind newly incorporated actin molecules (Bryce et al., 2005) make it a key component in the formation of an actin network.
Temporarily silencing the expression of cortactin leads to instable actin-based structures in a number of systems. In the absence of cortactin, lamellipodial protrusions retract due to instability (Bryce et al., 2005). Cortactin knockdown in osteoclasts causes a loss of podosome formation (Tehrani et al., 2006). Significantly, podosomes somewhat resemble tubulobulbar complexes (Young et al., 2009b) in that they are tubular structures with a central membrane core cuffed by a dendritic actin network.
Here we show that when administered to rats by intratesticuar injection, siRNA reagents enter Sertoli cells and caused a knockdown of cortactin in the seminiferous epithelium. As observed in other systems (Tehrani et al., 2006) where knockdown of cortactin prevented the formation of some structures without altering other cortactin containing features, reduced cortactin levels in the seminiferous epithelium altered the phenotype of tubulobulbar complexes without appearing to effect actin bundles in ectoplasmic specializations where cortactin also has been localized (Kai et al., 2004; Young et al., 2009b).
When labeled for cortactin and/or actin, tubulobulbar complexes were clearly shorter in epithelia from testes injected with targeting siRNA reagents relative to similar epithelia from contralateral testes injected with non-targeting siRNA reagents. This result was confirmed quantitatively using a threshold method to evaluate the numbers of positive pixels generated by labeling tubulobulbar clusters adjacent to spermatid heads. Using this method, the pixels above threshold remaining in each image were counted and considered an index of tubulobulbar complex length. Numbers of pixels present in tubulobulbar complexes from knockdown animals were significantly different from controls, both for actin and cortactin stained material, supporting our conclusion that cortactin knockdown reduces tubulobulbar complex length.
Significantly, in knockdown epithelium we detected a delay in retraction of Sertoli cell away from mature spermatids and spermiation failure. Moreover, in cases where spermatids remained attached to the epithelium, adhesion junctions (ectoplasmic specializations) were also present, an observation consistent with a failure of the normal junction removal mechanism.
We propose that short tubulobulbar complexes in the knockdown epithelia cause a failure of the structures to completely internalize intercellular adhesion junctions between Sertoli cells and spermatids and that this results in a delay in and possibly eventual failure of sperm release. Because we only evaluated one time point (3 days) after siRNA injections, we can not distinguish between (1) an initial complete disassembly of tubulobulbar complexes followed by partial recovery over the 3-day time period, and (2) a continuous prevention of tubulobulbar complexes to develop to their full length. Collins and coworkers (Collins et al., 2011), on the basis of a high resolution ultrastructural study, conclude that one of the actin filament network's primary roles during clathrin-mediated endocytosis is to elongate the bud neck in order to drive the endocytosed vesicles away from the plasma membrane. This is consistent with our observation of short tubulobulbar complexes after cortactin knockdown. Due to a lack of stability in the actin network caused by a depletion of cortactin, tubulobulbar complexes may not be able to elongate and acquire or maintain normal length and thereby fail to internalize the same amount of junctional membrane.
The time period (3 days or roughly 72 hrs) from injection of the siRNA reagents until evaluation of phenotype in our study was sufficient for us to observe effects on tubulobulbar complexes and on sperm release. In the rat, stage VII of spermatogenesis, the stage when tubulobulbar complexes are most apparent, lasts roughly 56 hours (Russell, 1984). Sperm release generally occurs in late stage VIII and this stage lasts 29 hours. In our study, siRNA reagents effecting stage VII tubules soon after injection would progress through at least to stage VIII when the study was terminated. Also, because tubulobulbar complexes begin development prior to stage VII and because of the length of stage VII, we would anticipate also seeing effects on tubulobulbar complexes in stage VII when the experiment was terminated.
Interestingly, cortactin homozygous knockout mice appear phenotypically normal and live a normal life span (Schnoor et al., 2011); however, their fertility and testicular morphology have not been reported. Similarly, the anti-androgen flutamide is reported to reduce testicular cortactin levels and to cause phenotypic changes in ectoplasmic specializations (Anahara et al., 2006), but any effects on tubulobulbar complex morphology or on spermiation have not been evaluated.
Our data presented here are consistent with the hypothesis that tubulobulbar complexes are subcellular machines that internalize intact intercellular adhesion junctions and that their function is essential for normal sperm release. Significantly, these findings introduce a new paradigm for junction internalization generally in cells and link the mechanism to a biologically significant event – sperm release.
Materials and Methods
Reagents
The paraformaldehyde was obtained from Fisher Scientific (Ottawa, ON, CAN). The secondary antibodies and phallotoxins conjugated to Alexa fluorochromes were obtained from Invitrogen (Burlington, ON, CAN) and those conjugated to horseradish peroxidase were purchased from Jackson ImmunoResearch Laboratories, Inc. (Westgrove, USA). Unless otherwise indicated, all other reagents used in the studies were obtained from Sigma–Aldrich (Oakville, ON, CAN).
siRNAs
An ON-TARGETplus pool of four siRNAs synthesized by Dharmacon (Lafayette, CO) was used to target rat cortactin (Cttn). An ON-TARGETplus non-targeting pool – a pool of four non-targeting siRNAs – was used as a negative control.
For dye modification studies, the same pool of four oligonucleotides was used with one of the four duplexes having a DY547 modification on the 5′ end of the sense strand.
PEI-mediated siRNA delivery
siRNAs were mixed with in vivo-jetPEI (Polyplus, Illkirch, France) at an N/P ratio of 1/8 at room temperature. For intratesticular injection administration, 50 µl of the Cttn targeting siRNA mixture was injected into right testis and 50 µl of the non-targeting control was injected into the left testis.
Animals
Animals used in this study were reproductively active male Sprague Dawley rats. They were obtained from Charles River Animal Research Laboratories. The rats were maintained according to guidelines established by the Canadian Council on Animal Care and using protocols approved by the UBC and SFU animal care committees. All experiments were done at least in duplicate using tissue from different animals.
Animal surgeries
Handling
All animals were handled daily for a minimum of one week before surgical treatment to decrease animal anxiety around investigators and staff. Handling included weighing and a brief physical inspection. A food ‘treat’ was given after each handling session.
Surgical preparation
Animals were weighed and anesthesia was induced with 5% isoflurane. Anesthesia was maintained with 2–3% isoflurane for the remainder of the surgical protocol.
Surgical sites were shaved and then washed 3 times by surgical soap followed by 70% ethanol. Lacrilube was applied to protect the eyes, and a lactate ringer's solution (LRS) was injected subcutaneously to maintain hydration.
Pre-emptive pain management consisted of a single dose each of buprenorphine at 0.05 mg/kg and of ketaprophen at 5.0 mg/kg injected subcutaneously. In addition, a local analgesia, bupivicaine, was injected subcutaneously along each incision site.
Surgical procedure
After surgical preparation, animals were transferred to a surgical table equipped with a heat source. Heart rate and oxygen saturation were monitored with a pulse oximeter equipped with a foot clip sensor. Deep anesthesia was ensured by a lack of withdraw reflex upon toe pinch. Using sterile technique, an incision of approximately 1–1.5 cm was made through the right side of the scrotum and skin was opened to expose the spermatic fascia enclosing the testis. An additional incision was made through the fascia to expose the surface of the testis. The testis was positioned to avoid major blood vessels and 32-gauge needle attached to a LASI (Hamilton, Reno, NV, USA) syringe system was inserted through the capsule and into the testis. A total volume of 50 µL of Cttn targeting siRNA and PEI mixture was injected into the testis. The needle was withdrawn and the spermatic fascia sutured closed with two or three discontinuous sutures. Subcuticular sutures were used to close the skin incision. Using the same surgical procedure, 50 µL of a non-targeting siRNA and PEI mixture were injected into the left testis as a control.
Post-surgical care and processing
After surgery, rats were placed in a cage under an infrared heater and allowed to recover. The cage contained clean, dry bedding, hydrogel and bacon softies. In most cases, animals were up and eating within 30 minutes. Animals were then transferred into their home cages with their original cage mates that also had the same surgery on the same day. Animals were monitored closely for the remainder of the experiment.
After three days, and in each experiment, animals were deeply anesthetized and the testes removed for processing. The testes of two animals were fixed for electron microscopy, those of another two animals were fixed and cryo-sectioned for immunofluorescence analysis and those of a third pair were processed for immunoblotting.
Light and electron microscopy
Testes were removed from anesthetized animals and then perfused, through the spermatic artery, for 2 min with warm (33°C) PBS (150 mM NaCl, 5 mM KCl, 0.8 mM KH2PO4, 3.2 mM Na2HPO4 pH 7.3) to clear the organ of blood. This was immediately followed by perfusion for 30 min with fixative (0.1 M sodium cacodylate, 1.5% paraformaldehyde, and 1.5% glutaraldehyde, pH 7.3). After perfusion, each testis was cut into small pieces and immersion fixed for an additional 2 hrs. The testis material was then washed with 0.1 M sodium cacodylate for three 10 min washes, then further fixed on ice for 60 min in 1% OsO4 in 0.1 M sodium cacodylate buffer. Following the incubation, the material was washed three times with ddH2O, 10 min each wash, then stained en bloc for one hour with 0.1% uranyl acetate. The material was then washed another three times in ddH2O, then dehydrated in an ascending alcohol series (30%, 50%, 70%, 95%, 100%) for ten minutes at each concentration. This was followed by two incubations of 15 min each in propylene oxide. The blocks then were left in a 1:1 solution of propylene oxide:EM-bed 812 (EM Sciences) overnight. The material was embedded in 100% EM-bed 812 and then incubated at 60°C for 24 hours. Thick (1 µm) sections were stained with toluidine blue and photographed on a Zeiss Imager. A1 microscope using bright-field optics. Thin sections were viewed and images acquired on either a Philips 300 electron microscope operated at 60 kV or a FEI Tecnai G2 Spirit Transmission electron microscope operated at 120 kV.
Immunofluorescence
Tissue preparation
Isolated testes were removed from anesthetized animals and perfused briefly with warm PBS to clear blood and then perfused with warm fixative (PBS, 3% paraformaldehyde, pH 7.3) for 30 min.
Frozen sections
Fixed testes were frozen onto aluminum stubs with OCT compound (Sakura Finetek USA, Torrence, CA) by immersion into liquid nitrogen. Sections of 5 µm were cut using a cryo-microtome, attached to poly-L-lysine coated glass slides, immediately plunged into −20°C acetone for 5 min, air dried, and then processed for immunofluorescence.
Immunostaining
Slides with attached cryosections were rehydrated and blocked with 5% normal goat serum (NGS) in TPBS-BSA (PBS containing 0.05% Tween-20 and 0.1% bovine serum albumin) for 20 min at room temperature. The primary antibody used to probe cryosections was rabbit anti-cortactin (KE-20) (Sigma–Aldrich, St. Louis, MO, USA). The primary antibody added to the slides was made up in TPBS-BSA with 1% NGS, and incubated overnight at 4°C in a humidity chamber. The material was washed extensively with the TPBS-BSA (wash buffer), then incubated for 60 minutes at 37°C with secondary antibody conjugated to a fluorochrome (goat anti-rabbit AlexaFluor 568 (Invitrogen)). The slides were again washed and then coverslips were mounted using Vectashield (Vector Labs, Burlington, ON). The tissue was visualized using a Zeiss Axiophot microscope fitted with appropriate filter sets for detecting fluorescence and with appropriate optics for phase microscopy and an inverted Zeiss Axiovert LSM 5 confocal microscope equipped with epifluorescence and LSM 5 Pascal software. Image acquisition was done with the LSM 5 Pascal software.
Filamentous actin was labeled by making up AlexaFluor 568 phalloidin in TPBS-BSA and staining slides for 20 minutes at room temperature. Slides were then extensively washed in TPBS-BSA.
Immunoblotting
Western blots were performed on either whole testis alone, or on whole testis and seminiferous epithelium lysates both from experimental and control (non-targeting siRNA) testes. Blots were probed first with cortactin antibody, and then the blots were stripped and stained with an antibody to calnexin as a loading control.
For whole testis lysates, testes were removed from rats under deep anesthesia and decapsulated. The whole testis tissue was extensively homogenized in RIPA lysis buffer (150 mM NaCl, 50 mM Tris, pH 7.4, 5 mM EDTA, 1% NonIdet P-40, 1% deoxycholic acid [sodium salt], 10% SDS) with complete, mini-EDTA-free protease inhibitor (Roche, Mississauga, ON, CAN).
For blots run on both whole testis and seminiferous epithelium, half of each testis was prepared as described above and the other half was cut into small pieces in ice-cold PEM/250 buffer (0.2 M Pipes, 0.1M EGTA, 0.1M MgCl2, Sucrose). The small pieces of seminiferous tubules were collected, placed in fresh PEM/250 buffer and epithelium was separated from tubule walls as described elsewhere (Guttman et al., 2007). The isolated sheets of epithelium were collected and pelletted by centrifugation. Pellets were resuspended and extensively homogenized in RIPA buffer.
Lysates were loaded into wells of 1-mm-thick 10% SDS-PAGE gels at equal concentrations as determined by protein assay and run according to standard protocols (Laemmli, 1970). Proteins were transferred onto Immobilon-P transfer membrane (Millipore, Billerica, MA) and then washed for 5 minutes at room temperature with TBST (500 mM Tris, pH 7.5, 150 mM NaCl, 0.1% Tween-20). The blots were blocked for 12 hours at 4°C using 4–5% non-fat milk (Blotto, Santa Cruz Biotechnology, Santa Cruz, CA). Membranes were washed 3 times, 5 minutes each wash, and then incubated for 1 hour at room temperature with rabbit-anti-cortactin (Sigma–Aldrich, Oakville, ON, CAN) at a concentration of 1:10,000. Blots were washed 3 times; 5 minutes each wash with TBST and then incubated for 1 hour at room temperature with goat anti-rabbit horseradish peroxidase-conjugated antibody. Blots were washed 3 times; 5 minutes each with TBST and then reacted with ECL (GE Lifesciences, Pittsburgh, PA, USA) and bands were visualized with Bioflex EC film (Interscience, Markham, ON, CAN).
Controls for loading were performed by stripping membranes with methanol for 10 minutes at room temperature. After stripping, blots were washed in TBST 3 times for 5 minutes each wash. The blots were blocked for 12 hours at 4°C using 4–5% non-fat milk. Membranes were washed 3 times, 5 minutes each wash, and then incubated for 1 hour at room temperature with rabbit-anti-Calnexin (Sigma–Aldrich, Oakville, ON, CAN) at a concentration of 1:40,000. Blots were then washed 3 times; 5 minutes each wash with TBST and then incubated for 1 hour at room temperature with goat anti-rabbit horseradish peroxidase-conjugated antibody. Blots were washed 3 times, 5 minutes each with TBST and then reacted with ECL (GE Lifesciences, Pittsburgh, PA, USA) and bands were visualized with Bioflex EC film (Interscience, Markham, ON, CAN).
Relative quantitation and statistical analysis of actin and cortactin fluorescence as an indication of tubulobulbar complex length
Quantitation of knockdown was determined by probing cryosections of tissue from control and experimental tissue labeled for cortactin or for actin. Ten mature spermatids with associated labeled clusters of tubulobulbar complexes at stage VII of spermatogenesis from each control testis and ten cells from each experimental testis were randomly selected and each cell was set to a predetermined threshold using ImageJ software (Abramoff et al., 2004). When set to a common threshold, the number of positive pixels was determined using the “analyze particles” function. The number of pixels was considered to be an index of tubulobulbar length in each cluster.
Statistics
To determine if there were any significant differences between the mean values from experimental and then control animals, a one-way analysis of variance was used (ANOVA). No significant differences were found (P>0.05) and mean values were combined. Mean values plus SD are indicated in figure legends (Fig. 3C,D, Fig. 8D); one-tailed, unpaired t test (with Welch's correction) for statistical significance.
Acknowledgments
We would like to thank Mary Dearden at the SFU animal care facility for assistance with the surgeries. This work was supported by Natural Sciences and Engineering Research Council of Canada grant RGPIN 155397-08 to A.W.V.
Footnotes
Competing interests: The authors have no competing interests to declare.
References
- Abramoff M. D., Magalhães P. J., Ram S. J. (2004). Image processing with ImageJ. Biophotonics International 11, 36–42. [Google Scholar]
- Anahara R., Toyama Y., Maekawa M., Kai M., Ishino F., Toshimori K., Mori C. (2006). Flutamide depresses expression of cortactin in the ectoplasmic specialization between the Sertoli cells and spermatids in the mouse testis. Food Chem. Toxicol. 44, 1050–1056 10.1016/j.fct.2005.12.010 [DOI] [PubMed] [Google Scholar]
- Bryce N. S., Clark E. S., Leysath J. L., Currie J. D., Webb D. J., Weaver A. M. (2005). Cortactin promotes cell motility by enhancing lamellipodial persistence. Curr. Biol. 15, 1276–1285 10.1016/j.cub.2005.06.043 [DOI] [PubMed] [Google Scholar]
- Collins A., Warrington A., Taylor K. A., Svitkina T. (2011). Structural organization of the actin cytoskeleton at sites of clathrin-mediated endocytosis. Curr. Biol. 21, 1167–1175 10.1016/j.cub.2011.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza R., Pathak S., Upadhyay R., Gaonkar R., D'Souza S., Sonawane S., Gill-Sharma M., Balasinor N. H. (2009). Disruption of tubulobulbar complex by high intratesticular estrogens leading to failed spermiation. Endocrinology 150, 1861–1869 10.1210/en.2008-1232 [DOI] [PubMed] [Google Scholar]
- Guttman J. A., Obinata T., Shima J., Griswold M., Vogl A. W. (2004a). Non-muscle cofilin is a component of tubulobulbar complexes in the testis. Biol. Reprod. 70, 805–812 10.1095/biolreprod.103.022723 [DOI] [PubMed] [Google Scholar]
- Guttman J. A., Takai Y., Vogl A. W. (2004b). Evidence that tubulobulbar complexes in the seminiferous epithelium are involved with internalization of adhesion junctions. Biol. Reprod. 71, 548–559 10.1095/biolreprod.104.028803 [DOI] [PubMed] [Google Scholar]
- Guttman J., Vaid K., Vogl A. W. (2007). Enrichment and disassembly of ectoplasmic specializations in the rat testis. Molecular Motors: Methods And Protocols, Vol. 392 (ed. Sperr A O.), pp. 159–170 Totowa, NJ: Humana Press. [DOI] [PubMed] [Google Scholar]
- Kai M., Irie M., Okutsu T., Inoue K., Ogonuki N., Miki H., Yokoyama M., Migishima R., Muguruma K., Fujimura H.et al. (2004). The novel dominant mutation Dspd leads to a severe spermiogenesis defect in mice. Biol. Reprod. 70, 1213–1221 10.1095/biolreprod.103.024802 [DOI] [PubMed] [Google Scholar]
- Kierszenbaum A. L., Tres L. L. (2004). The acrosome-acroplaxome-manchette complex and the shaping of the spermatid head. Arch. Histol. Cytol. 67, 271–284 10.1679/aohc.67.271 [DOI] [PubMed] [Google Scholar]
- Kusumi N., Watanabe M., Yamada H., Li S. A., Kashiwakura Y., Matsukawa T., Nagai A., Nasu Y., Kumon H., Takei K. (2007). Implication of amphiphysin 1 and dynamin 2 in tubulobulbar complex formation and spermatid release. Cell Struct. Funct. 32, 101–113 10.1247/csf.07024 [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 10.1038/227680a0 [DOI] [PubMed] [Google Scholar]
- Leblond C. P., Clermont Y. (1952). Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann. N. Y. Acad. Sci. 55, 548–573 10.1111/j.1749-6632.1952.tb26576.x [DOI] [PubMed] [Google Scholar]
- McLachlan R. I., O'Donnell L., Stanton P. G., Balourdos G., Frydenberg M., de Kretser D. M., Robertson D. M. (2002). Effects of testosterone plus medroxyprogesterone acetate on semen quality, reproductive hormones, and germ cell populations in normal young men. J. Clin. Endocrinol. Metab. 87, 546–556 10.1210/jc.87.2.546 [DOI] [PubMed] [Google Scholar]
- Merrifield C. J., Perrais D., Zenisek D. (2005). Coupling between clathrin-coated-pit invagination, cortactin recruitment, and membrane scission observed in live cells. Cell 121, 593–606 10.1016/j.cell.2005.03.015 [DOI] [PubMed] [Google Scholar]
- Ochoa G. C., Slepnev V. I., Neff L., Ringstad N., Takei K., Daniell L., Kim W., Cao H., McNiven M., Baron R.et al. (2000). A functional link between dynamin and the actin cytoskeleton at podosomes. J. Cell Biol. 150, 377–389 10.1083/jcb.150.2.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell L. D. (1979a). Further observations on tubulobulbar complexes formed by late spermatids and Sertoli cells in the rat testis. Anat. Rec. 194, 213–232 10.1002/ar.1091940204 [DOI] [PubMed] [Google Scholar]
- Russell L. D. (1979b). Observations on the inter-relationships of Sertoli cells at the level of the blood- testis barrier: evidence for formation and resorption of Sertoli-Sertoli tubulobulbar complexes during the spermatogenic cycle of the rat. Am. J. Anat. 155, 259–279 10.1002/aja.1001550208 [DOI] [PubMed] [Google Scholar]
- Russell L. D. (1979c). Spermatid-Sertoli tubulobulbar complexes as devices for elimination of cytoplasm from the head region late spermatids of the rat. Anat. Rec. 194, 233–246 10.1002/ar.1091940205 [DOI] [PubMed] [Google Scholar]
- Russell L. D. (1984). Spermiation - the sperm release process: ultrastructural observations and unresolved problems. Ultrastructure Of Reproduction: Gametogenesis, Fertilization, And Embryogenesis (ed. Van Blerkom J, Motta P M.), pp. 46–66 Boston, MA: Martinus Nijhoff. [Google Scholar]
- Russell L. D., Clermont Y. (1976). Anchoring device between Sertoli cells and late spermatids in rat seminiferous tubules. Anat. Rec. 185, 259–277 10.1002/ar.1091850302 [DOI] [PubMed] [Google Scholar]
- Schafer D. A. (2002). Coupling actin dynamics and membrane dynamics during endocytosis. Curr. Opin. Cell Biol. 14, 76–81 10.1016/S0955-0674(01)00297-6 [DOI] [PubMed] [Google Scholar]
- Schnoor M., Lai F. P., Zarbock A., Kläver R., Polaschegg C., Schulte D., Weich H. A., Oelkers J. M., Rottner K., Vestweber D. (2011). Cortactin deficiency is associated with reduced neutrophil recruitment but increased vascular permeability in vivo. J. Exp. Med. 208, 1721–1735 10.1084/jem.20101920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanii I., Yoshinaga K., Toshimori K. (1999). Morphogenesis of the acrosome during the final steps of rat spermiogenesis with special reference to tubulobulbar complexes. Anat. Rec. 256, 195–201 [DOI] [PubMed] [Google Scholar]
- Taylor M. J., Perrais D., Merrifield C. J. (2011). A high precision survey of the molecular dynamics of mammalian clathrin-mediated endocytosis. PLoS Biol. 9, e1000604 10.1371/journal.pbio.1000604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehrani S., Faccio R., Chandrasekar I., Ross F. P., Cooper J. A. (2006). Cortactin has an essential and specific role in osteoclast actin assembly. Mol. Biol. Cell 17, 2882–2895 10.1091/mbc.E06-03-0187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaid K. S., Guttman J. A., Babyak N., Deng W., McNiven M. A., Mochizuki N., Finlay B. B., Vogl A. W. (2007). The role of dynamin 3 in the testis. J. Cell. Physiol. 210, 644–654 10.1002/jcp.20855 [DOI] [PubMed] [Google Scholar]
- Weaver A. M., Karginov A. V., Kinley A. W., Weed S. A., Li Y., Parsons J. T., Cooper J. A. (2001). Cortactin promotes and stabilizes Arp2/3-induced actin filament network formation. Curr. Biol. 11, 370–374 10.1016/S0960-9822(01)00098-7 [DOI] [PubMed] [Google Scholar]
- Wu M., Huang B., Graham M., Raimondi A., Heuser J. E., Zhuang X., De Camilli P. (2010). Coupling between clathrin-dependent endocytic budding and F-BAR-dependent tubulation in a cell-free system. Nat. Cell Biol. 12, 902–908 10.1038/ncb2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. S., Guttman J. A., Vaid K. S., Shahinian H., Vogl A. W. (2009a). Cortactin (CTTN), N-WASP (WASL), and clathrin (CLTC) are present at podosome-like tubulobulbar complexes in the rat testis. Biol. Reprod. 80, 153–161 10.1095/biolreprod.108.070615 [DOI] [PubMed] [Google Scholar]
- Young J. S., Guttman J. A., Vaid K. S., Vogl A. W. (2009b). Tubulobulbar complexes are intercellular podosome-like structures that internalize intact intercellular junctions during epithelial remodeling events in the rat testis. Biol. Reprod. 80, 162–174 10.1095/biolreprod.108.070623 [DOI] [PubMed] [Google Scholar]
- Young J. S., Takai Y., Kojic K. L., Vogl A. W. (2012). Internalization of adhesion junction proteins and their association with recycling endosome marker proteins in rat seminiferous epithelium. Reproduction 143, 347–357 10.1530/REP-11-0317 [DOI] [PubMed] [Google Scholar]