Summary
Cells often respond to diverse environmental stresses by inducing stress granules (SGs) as an adaptive mechanism. SGs are generally assembled as a result of aggregation of mRNAs stalled in a translational pre-initiation complex, mediated by a set of RNA-binding proteins such as G3BP and TIA-1. SGs may serve as triage centres for storage, translation re-initiation or degradation of specific mRNAs. However, the mechanism involved in the modulation of their assembly/disassembly is unclear. Here we report that Wnt signalling negatively regulates SG assembly through Dishevelled (Dvl), a cytoplasmic Wnt effector. Overexpression of Dvl2, an isoform of Dvl, leads to impairment of SG assembly through a DEP domain dependent mechanism. Intriguingly, the Dvl2 mutant K446M, which corresponds to an analogous mutation in Drosophila Dishevelled DEP domain (dsh1) that results in defective PCP pathway, fails to antagonize SG assembly. Furthermore, we show that Dvl2 exerts the antagonistic effect on SG assembly through a mechanism involving Rac1-mediated inhibition of RhoA. Dvl2 interacts with G3BP, a downstream component of Ras signalling involved in SG assembly, and functional analysis suggests a model wherein the Dvl-Rac1-RhoA axis regulates G3BP's SG-nucleating activity. Collectively, these results define an antagonistic effect of Wnt signalling on SG assembly, and reveal a novel role for Wnt/Dvl pathway in the modulation of mRNA functions.
Keywords: Wnt, Dvl, RNA, Stress granule, G3BP
Introduction
Stress granules (SGs) and Processing bodies (P bodies) represent mRNA-containing, non-membranous, dynamic cytoplasmic foci defined by the presence of specific and common protein components (Anderson and Kedersha, 2006; Anderson and Kedersha, 2009; Balagopal and Parker, 2009; Parker and Sheth, 2007). P bodies are present at the stationary phase, whereas SGs are assembled during diverse stress conditions, as a result of aggregation of mRNAs stalled in a translational pre-initiation complex, a process mediated by RNA-binding proteins such as G3BP and TIA-1 (Gilks et al., 2004; Kedersha et al., 1999; Tourriere et al., 2003). The actively translating mRNAs (polysomes) are present mostly diffused in the cytoplasm. Although polysomes, P bodies and SGs represent mRNAs associated with different sets of cellular proteins present at distinct cytoplasmic locations, recent data suggests that the mRNAs and protein components may shuttle between these three pools (Balagopal and Parker, 2009). Thus, SGs are thought to be sites for mRNA triage, wherein the mRNAs could be further sorted into P bodies for degradation, returned to cytoplasm for translation re-initiation or stored in the SGs (Anderson and Kedersha, 2008). Although these studies highlight the importance of post transcriptional regulation of mRNAs in achieving cell homeostasis, the mechanism by which cells modulate the assembly/disassembly of these mRNA containing structures and/or determine the fate of individual mRNA is far from clear. It is possible that diverse cell signalling pathways could operate through post transcriptional regulation of specific mRNAs.
Wnt signalling is a conserved pathway that regulates cell fate determination, cell proliferation and cell polarization during growth and development of multi-cellular organisms (Boutros and Mlodzik, 1999; Clevers, 2006; Gao and Chen, 2010; Logan and Nusse, 2004; van Amerongen and Nusse, 2009; Wallingford and Habas, 2005). Wnt pathway is broadly categorized into canonical or non-canonical, based on the dependence on β-catenin for the signalling process. In canonical Wnt signalling, binding of Wnt to the cognate receptors on the cell membrane leads to β-catenin stabilization in the cytoplasm, through a mechanism involving Dishevelled (Dvl)-dependent inhibition of the β-catenin degradation complex. The accumulated β-catenin then enters the nucleus and activates transcription of Wnt responsive genes. However, the non-canonical signalling operates independent of β-catenin, but involves regulation of cytoskeleton dynamics by Dvl-dependent activation of Rac and Rho GTPases. Alternatively, Wnt could activate Ca2+ signalling, which also requires Dvl. Despite its demonstrated role in diverse cellular processes including Wnt signalling, the molecular function of Dvl is largely unknown. Mammalian cells express three isoforms of this protein, namely Dvl1, Dvl2 and Dvl3 (Klingensmith et al., 1996; Lee et al., 2008). An interesting feature of overexpressed Dvl is its ability to form cytoplasmic puncta, the nature of which remains mysterious (Axelrod et al., 1998; Kishida et al., 1999; Smalley et al., 1999). Endogenous Dvl also localizes to cytoplasmic granules (Chang et al., 2005; Miller et al., 1999; Yanagawa et al., 1995). Overexpression and live imaging studies have shown that these cytoplasmic puncta are Dvl protein assemblages, which are in dynamic equilibrium with the cytoplasmic Dvl pool (Schwarz-Romond et al., 2005; Smalley et al., 2005). Importantly, these structures are non-membranous and do not represent any known cytoplasmic vesicle compartments (Schwarz-Romond et al., 2005; Smalley et al., 2005).
Owing to the structural similarities between Dvl and SGs/P bodies, we investigated the possible connection between these cytoplasmic structures. In this study, we demonstrate that Wnt/Dvl signalling negatively regulates SG assembly, mediated through a mechanism involving Rac1-dependent inhibition of RhoA. Moreover, Dvl2 physically interacts with G3BP and inhibits its SG-nucleating activity. Our findings functionally connect Dvl to stress response, and implicate a role for Wnt/Dvl signalling in the modulation of mRNA translation and degradation.
Results
Dishevelled negatively regulates SG assembly in a DEP-dependent manner
To investigate the possible connection between Dvl and SGs or P bodies, NIH3T3 cells were transfected with full length or various deletion mutants of mouse Dvl2 (Fig. 1C), and were subjected to oxidative stress by treating with 0.5 mM sodium arsenite for 30 min. Ectopically expressed full length Dvl2 did not localize to SGs (Fig. 1A) or P bodies (Fig. 1B), but was often present juxtaposed to them. Interestingly, we found that the C-terminal region, but not N-terminal or middle region, could be independently targeted to SGs (supplementary material Fig. S1A,B,C). Further deletion analysis demonstrated that the DEP domain was sufficient for localization to SGs (supplementary material Fig. S1D). Indeed, it is known that the N-terminal DIX domain of Dvl has a polymerization activity, leading to the formation of cytoplasmic puncta when overexpressed in mammalian cells (Schwarz-Romond et al., 2007). Dvl2 protein devoid of the DIX domain (Dvl-ΔDIX), localized to SGs (supplementary material Fig. S1E), indicating that DIX-mediated oligomerization of Dvl2 may inhibit localization of full length Dvl2 to SGs. Removal of the PDZ domain (Dvl-ΔPDZ) also resulted in localization to SGs (supplementary material Fig. S1F), albeit to a lesser extent as compared to Dvl-ΔDIX.
Fig. 1. Dvl2 disassembles SGs in a DEP-dependent manner.
(A) NIH3T3 cells expressing indicated proteins were treated with sodium arsenite and immunostained for overexpressed proteins with HA antibodies (green) and for SGs with FXR1 antibodies (red). DNA was stained with Hoechst-33342 dye (blue). HA-firefly luciferase (Fluc) was used as the control. Scale bars: 10 µm. (B) NIH 3T3 cells were transfected with HA-Fluc or HA-Dvl2 and were subjected to arsenite treatment before immunostaining with HA antibodies (green) for detecting overexpressed proteins (green) and with Dcp1a antibodies (red) for identifying the P bodies. (C) Schematic representation of mouse Dvl2 constructs used in this study, and the ability of them to localize to and/or disassemble SGs upon overexpression. Amino acid numbers are as indicated. Deleted regions are shown as doted lines. ND denotes, not determined. (D) Cells were transfected with indicated mutant Dvl2 constructs and were immunostained with indicated antibodies after sodium arsenite treatment. (E) The graph depicts number of NIH3T3 cells transfected with indicated constructs displaying SG assembly after sodium arsenite treatment. Data represents average of three independent experiments. Error bars indicate ±SD. Data was analyzed by Student's t test (*P<0.05, **P<0.005).
We noticed that upon arsenite treatment, significantly less number of Dvl2 transfected cells displayed SGs, suggesting a negative role for Dvl in SG assembly (Fig. 1D,E). Studies using deletion mutants of Dvl2 indicated that the DEP domain is crucial for this function (Fig. 1D). Removal of DIX domain did not affect the ability of Dvl2 to impair SG assembly, whereas deletion of PDZ domain moderately affected Dvl's function, indicating that the PDZ might also play a role in negatively regulating SG assembly (Fig. 1E). As the DEP domain was found to be critical for Dvl's function in negatively regulating SG assembly, we wished to investigate the effect of changing lysine 446 to methionine (K446M), which corresponds to an analogous mutation in the DEP domain of Drosophila Dishevelled (dsh1) that results in defective non-canonical Wnt signalling/planar cell polarity (PCP) pathway (Axelrod et al., 1998; Axelrod, 2001), on SG assembly. Intriguingly, K446M mutation significantly affected the ability of Dvl2 to interfere with SG assembly (Fig. 1D,E), indicating that Dvl2 might act through the non-canonical pathway. However, wild type or mutants of Dvl2 neither localized to P bodies, nor did significantly affect the P body assembly during oxidative stress (P.K.S. and J.J., unpublished observations).
Dvl impairs SG assembly through Rac-mediated inhibition of Rho activity
The non-canonical Wnt/PCP pathway involves Dvl-mediated activation of Rac and Rho (Habas et al., 2001; Habas et al., 2003; Klein and Mlodzik, 2005), and overexpression of Dvl is sufficient to activate both the GTPases (Habas et al., 2003). Initially, we examined if Rac1 and/or RhoA activity is essential for arsenite-induced SG assembly by expressing a constitutively-active or dominant-negative form of Rac1 (V12Rac1 or N17Rac1, respectively) or RhoA (V14RhoA or N19RhoA, respectively), and analyzing SG assembly after arsenite treatment. Both constitutively-active Rac1 and dominant-negative RhoA interfered with arsenite-induced SG assembly (Fig. 2A upper panel, B) as compared to GFP-expressing control cells. However, expression of dominant-negative Rac1 or constitutively-active RhoA did not have significant effect on SG assembly. These data indicate that the activities of Rac1 and RhoA are modulated during SG assembly, and that both GTPases might have opposite effects. Consistent with this notion, knockdown of RhoA resulted in impairment of SG assembly (Fig. 2E,F). This finding is also in support of a previous report suggesting a positive role for RhoA in SG assembly during oxidative stress (Tsai and Wei, 2010). Interestingly, Rac1-mediated perturbation of SG assembly could be reversed by constitutively-active RhoA (V14RhoA), whereas, impairment of SG assembly caused by expression of dominant-negative RhoA (N19RhoA) could not be reverted by dominant-negative Rac1 (Fig. 2A lower panel, B). Collectively, these results suggest that Rac1-mediated inhibition of RhoA leads to inhibition of SG assembly.
Fig. 2. Dvl2 promotes SG disassembly via Rac1-mediated inhibition of RhoA.
(A, upper panel) NIH3T3 cells were transfected with GFP-control vector or GFP-tagged version of constitutively-active Rac1 (V12Rac1), dominant-negative Rac1 (N17Rac1), constitutively-active RhoA (V14RhoA) or dominant-negative RhoA (N19RhoA) construct (green) and analyzed for SG assembly using HuR as the SG marker (red). DNA was stained in blue. (A, lower panel) NIH3T3 cells were transfected with GFP-V12Rac1 or GFP-N17Rac1 (green) with myc-V14RhoA or myc-N19RhoA (blue) as indicated, and analyzed for SG assembly using HuR (red). (B) The graph depicts number of NIH3T3 cells transfected with indicated constructs displaying SG assembly after sodium arsenite treatment. (C) NIH3T3 cells were co-transfected with HA-Dvl2 (blue) and GFP-tagged version of Rac1 or RhoA constructs (green), as indicated. Cells were analyzed for the SG assembly using HuR (red) as the marker. Scale bars: 10 µm. (D) Quantitative representation of SG assembly from experiments described in C. (E) NIH3T3 cells were treated with control (siControl), Rac1 (siRac1)- or RhoA (siRhoA)-specific siRNA and the cell lysate was analyzed for indicated proteins by Western blotting. α-tubulin was used as a loading control. (F) Quantitative depiction of SG assembly in control (siControl) or RhoA-specific siRNA (siRhoA) transfected cells. (G) NIH3T3 cells were transfected with control (siControl), Rac1-specific siRNA (siRac1) alone or with HA-Dvl2 as indicated, and analyzed for the number of transfected cells showing SG assembly after arsenite treatment. Data (in B, D, F and G) represents average of three independent experiments. Error bars indicate ±SD. Data was analyzed by Student's t test (**P<0.001, N.S.; non significant).
To investigate if RhoA and Rac1 acted downstream to Dvl2, constitutively active or dominant-negative Rac1 or RhoA construct was co-transfected with Dvl2, and assessed for their ability to revert the Dvl2-mediated impairment of SG assembly. We found that dominant-negative Rac1 and constitutively-active RhoA reversed the effect of Dvl2 on SG assembly, suggesting that Rac1 and RhoA play a downstream role (Fig. 2C,D). Moreover, Dvl2 mediated inhibition of SG assembly could be significantly reversed by knockdown of endogenous Rac1 (Fig. 2E,G). Taken together, these findings indicate that Dvl2 negatively regulates SG assembly through a mechanism involving Rac1-mediated inhibition of RhoA.
Dvl2 interacts with G3BP and negatively regulates its ability to induce SGs
Previous studies have shown that phosphorylation of eIF2α is a critical event during early stages of SG assembly, which is mediated by stress activated kinases such as PKR, PERK, HRI, and GCN2, leading to inhibition of translation initiation (Anderson and Kedersha, 2006). We examined the phosphorylation status of eIF2α in HeLa S3 cells overexpressing GFP or GFP-Dvl2, after arsenite treatment (0.2 mM, 30 min). Enhanced Dvl2 level did not significantly interfere with stress mediated phosphorylation of eIF2α (Fig. 3A), suggesting a downstream role for Dvl2 for impairment of SG assembly.
Fig. 3. Dvl2 interacts with G3BP.

(A) HeLa S3 cells were transfected with GFP control or GFP-Dvl2 constructs and were subjected to arsenite treatment (0.2 mM, 30 min), and analyzed by western blotting (WB) with indicated antibodies. (B) HEK293T cells were lysed in the presence of RNasin or RNase A, and were subjected to immunoprecipitation (IP) using rabbit-IgG (control IP) or Dvl2-specific antibodies (Dvl2 IP). The immunoprecipitates were probed with indicated antibodies. (C) HEK293T cells were co-transfected with GFP or GFP-tagged Drosophila G3BP (GFP-G3BP) and HA-Dvl2 fragments as indicated. IP with GFP-specific antibody was performed and analyzed for the presence of Dvl2 fragments by Western blotting (WB). *indicates non-specific band. (D) Cells expressing GFP (control) or GFP-G3BP and indicated HA-Dvl2 deletion constructs were subjected to immunoprecipitation with GFP antibody and assayed for the presence of Dvl2 proteins by Western blotting with HA antibody. (E) Schematic representation of human G3BP (hG3BP) constructs used in this study. Different domains and phosphorylation-defective (S149A) and phospho-mimetic (S149E) mutants are indicated (for details, see (Tourriere et al., 2003). (F) HEK293T cells were co-transfected with HA-Dvl2 and indicated hG3BP constructs. Immunoprecipitation (IP) was performed using GFP-specific antibody and analyzed by Western blotting (WB) using indicated antibodies. (G & H) GFP-G3BP (green) was co-transfected with full length HA-Dvl2 (G, red) or indicated deletion mutants (H, red), and the cells were stained with HA-specific antibodies. (I) Cells were co-transfected with HA-Dvl2 (red) construct and indicated GFP-G3BP deletion mutants (green). DNA is stained in blue. Scale bars: 10 µm.
It is possible that Dvl could act at the level of SG nucleation, a process mediated by RNA-binding proteins such as G3BP1 and TIA-1 (Gilks et al., 2004; Kedersha et al., 1999; Tourriere et al., 2003). Immunoprecipitation assays revealed that endogenous Dvl2 associated with G3BP (Fig. 3B). Interestingly, removal of intact RNAs by RNase treatment of the lysate enhanced the interaction between Dvl2 and G3BP (Fig. 3B). This indicates that the binding of G3BP to RNAs inhibits its ability to interact with Dvl. The C-terminal region of Dvl2 was sufficient for the interaction with G3BP (Fig. 3C). Moreover, G3BP failed to interact with Dvl2 when the DEP region was deleted (Fig. 3D), suggesting that Dvl2 interacts with G3BP in a DEP-dependent manner. However, K446M mutation in the DEP domain did not considerably affect the interaction of Dvl2 with G3BP (Fig. 3D).
Deletion studies also suggested that the N-terminal NTF2-like domain of G3BP is involved in the interaction with Dvl2 (Fig. 3E,F). It has been reported that G3BP gets phosphorylated at Ser149 and Ser232, and the phosphorylation of Ser149 is negatively regulated by Ras signalling through RasGAP (Tourriere et al., 2001; Tourriere et al., 2002). Interestingly, we found that HA-Dvl2 interacted specifically with the phospho-mimetic (S149E), but not with the phospho-defective (S149A), mutant of G3BP (Fig. 3F). Indeed, previous studies have shown that phosphorylation at Ser149 plays a critical role in SG assembly; the dephosphorylated form (S149A) strongly induces SGs, whereas the phosphorylated G3BP (S149E) dominantly inhibits SG formation (Tourriere et al., 2003). These observations further strengthen the role for Dvl2-G3BP interaction in the modulation of SG assembly and also suggest a potential interconnection between Ras/RasGAP and Wnt/Dvl signalling pathways.
Consistent with the physical interaction data, we also observed that full length Dvl2 (HA-Dvl2) co-localized with full length G3BP (GFP-G3BP) and a fragment containing the NTF2-like domain of G3BP (GFP-G3BP-A), but not with the acidic domain (GFP-G3BP-B) (Fig. 3G,I). Moreover, the C-terminal region of Dvl2 (HA-Dvl-C) and GFP-G3BP were targeted to the same cytoplasmic structures (Fig. 3H). Also, in agreement with the interaction data, the NTF2-like domain of G3BP and HA-Dvl2 were also co-localized with each other (Fig. 3I).
As overexpression of G3BP is sufficient to induce SG assembly (Tourriere et al., 2003), we analyzed the ability of G3BP to assemble SGs in the presence or absence of ectopically expressed Dvl2. G3BP failed to assemble SGs (as assessed by HuR staining) upon co-expression with Dvl2, (Fig. 4A, right panel), indicating that Dvl2 negatively regulates G3BP's ability to induce SG assembly. Furthermore, expression of Dvl-C alone did not significantly affect the arsenite-induced SG assembly (Fig. 4C), however, its co-expression with Dvl2 substantially reversed the negative effect of Dvl2 on SG assembly (Fig. 4C), which is consistent with the notion that Dvl2-mediated perturbation of SG assembly requires functional interaction with G3BP. Dvl2-mediated inhibition of SG assembly could also be significantly overcome by overexpression of G3BP (Fig. 4D), indicating that G3BP functions downstream of Dvl2. Interestingly, upon arsenite treatment, NIH3T3 cells co-expressing GFP-G3BP and HA-Dvl2 displayed two categories of cytoplasmic puncta, which were mutually exclusive; one containing Dvl2 and G3BP (Fig. 4B, right panel, arrow heads), and the other one having G3BP and the endogenous SG marker, HuR (Fig. 4B, right panel, arrows), and none showed all three localizing together. These observations further strengthen the idea that Dvl2-mediated impairment of SG assembly would involve inhibition of the SG-nucleating activity of G3BP. Interestingly, G3BP expression also reversed the antagonistic effect of V12Rac1 or N19RhoA on SG assembly (Fig. 4E), suggesting that G3BP could operate downstream of these GTPases as well. Collectively, the results demonstrate that the Dvl-Rac-Rho axis functions through regulating G3BP activity.
Fig. 4. Dvl2 inhibits G3BP's ability to induce SGs.
(A) NIH3T3 cells were transfected with GFP-G3BP (green) alone or with HA-Dvl2 (blue) and stained for the SG marker HuR (red) in the absence (A) or presence (B) of sodium arsenite. Arrows indicate cytoplasmic puncta positive for GFP-G3BP and HuR. Arrowheads indicate puncta positive for GFP-G3BP and HA-Dvl2. Scale bars: 10 µm. (C) NIH3T3 cells were transfected with indicated constructs and subjected to arsenite treatment (0.5 mM, 30 min). Percentage of transfected cells displaying SGs, as identified by G3BP antibodies, was scored. (D) Quantitative data for experiments described in B. (E) Quantitative representation of cells showing SG assembly as assessed by HuR staining after transfection with indicated constructs. All the quantitative data represent average of three independent experiments. Error bars indicate ±SD. Data was analyzed by Student's t test (**P<0.001).
Wnt signalling antagonizes SG assembly
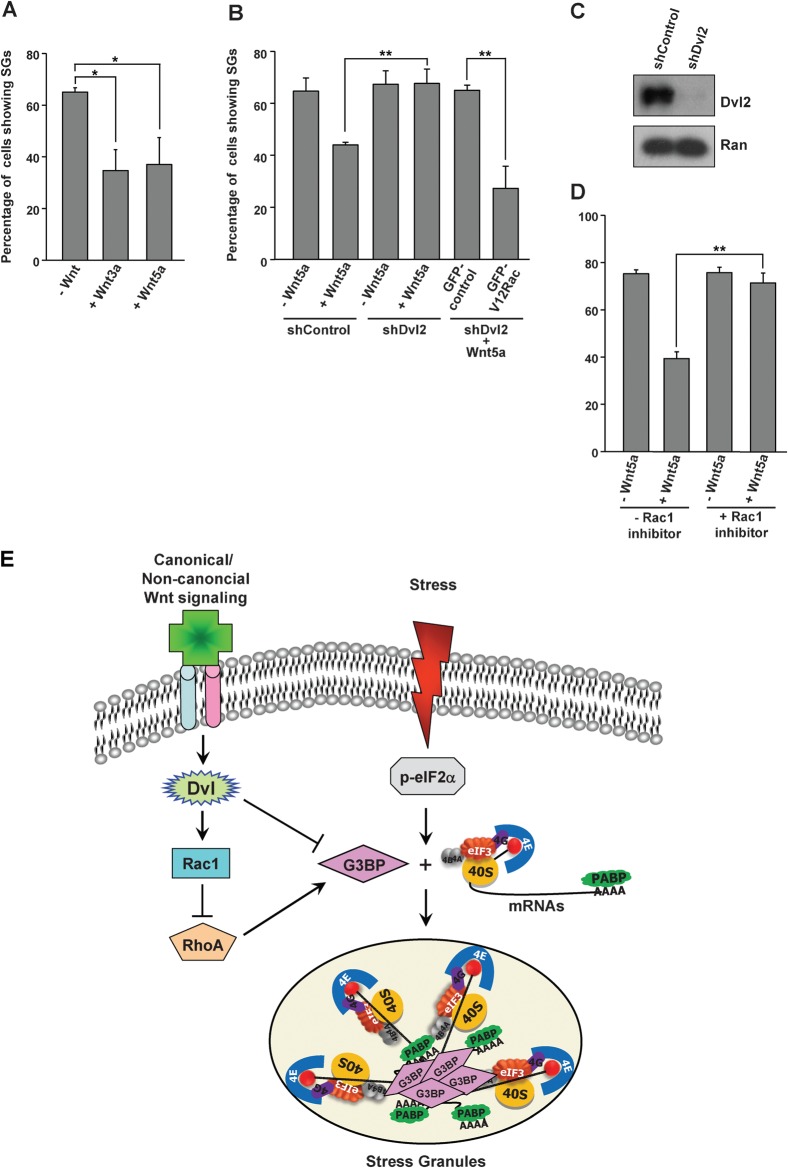
As our studies clearly indicated a role for Dvl in SG dynamics, we wished to explore whether Wnt signalling regulates SG assembly. We treated NIH3T3 cells with 100 ng/ml of Wnt3a or Wnt5a and later subjected to arsenite treatment. Both Wnt3a and Wnt5a treatment significantly reduced the number of cells exhibiting SG formation (Fig. 5A). Moreover, depletion of Dvl2 abrogated the ability of Wnt to exert its negative effect on SG assembly and expression of V12Rac1 could reverse this effect (Fig. 5B,C). Furthermore, experiments using Rac1 inhibitor (50 µM, 4h) indicated that Wnt-mediated impairment of SG assembly requires Rac1 activation (Fig. 5D). Taken together, these data imply that the antagonistic effect of Wnt on SG assembly is mediated through Dvl2 and Rac1.
Fig. 5. Wnt signalling antagonizes SG assembly.
(A) NIH3T3 cells were treated with Wnt3a or Wnt5a at a final concentration of 100 ng/ml for 13.5 h. Later, sodium arsenite (0.2 mM final concentration) was added to the medium for 30 min, and cells were stained for an SG marker, HuR. (B) NIH3T3 cells transfected with control (shControl) or Dvl2-shRNA (shDvl2) and GFP control or GFP-V12Rac1 were untreated (−) or treated (+) with Wnt5a as indicated and subjected to arsenite stress. SG assembly was analyzed by immunostaining with an SG specific marker (HuR). (C) Control or Dvl2-specific shRNA construct was transfected into NIH3T3 cells and analyzed for depletion of Dvl2 through Western blotting using affinity purified rabbit anti-Dvl2 antibodies. Ran was used as the loading control. (D) NIH3T3 cells were untreated (−) or treated (+) with Wnt5a and incubated with (+) or without (−) Rac1 inhibitor before subjecting to oxidative stress. Percentage of cells displaying SGs, as identified by HuR immunostaining, was scored. Graphical data (in A, B and D) represent averages of three independent experiments. Error bars indicate ±SD. Data was analyzed by Student's t test (*P≤0.01, **P≤0.001). (E) A working model suggesting the role of Wnt in negatively regulating SG assembly. Wnt signalling inhibits SG assembly by interfering with the ability of G3BP to aggregate translationally repressed mRNAs and to nucleate SGs, by physically interacting with Dvl2 and by a mechanism involving Rac1-mediated inhibition of Rho.
The data presented here support a working model depicted in Fig. 5E. When the Wnt signalling is off or at the basal level and cells encounter environmental stress, translationally suppressed RNA molecules could be aggregated into SGs by the action of SG nucleators such as G3BP. Upon binding of Wnt to the cognate receptors, Dvl gets activated and could negatively regulate SG assembly through inhibition of G3BP activity, possibly involving physical interaction between Dvl and G3BP. Interestingly, our results indicate that Rac1-mediated suppression of RhoA activity could also lead to interference with G3BP's SG-nucleation activity. It remains possible that Rac1 and RhoA could modulate the interaction between G3BP and Dvl to regulate the SG assembly.
Discussion
The paper describes a novel finding that mammalian Dishevelled (Dvl), a key effector of Wnt signalling, negatively regulates the SG assembly pathway. The fact that Dvl2 regulates these cytoplasmic mRNA containing foci suggests a novel role for this Wnt player in mRNA regulation. Although full length Dvl2 was not targeted to SGs, some deletion mutants showed localization to SGs. Particularly, DEP, a domain critical for non-canonical Wnt/PCP pathway, could independently localize to SGs. This indicates that full length Dvl2 might transiently localize to SGs through its DEP domain, however, other domains such as DIX and PDZ domain negatively regulate this function. More importantly we found that Dvl2 strongly interfered with SG assembly in a DEP-dependent manner, through a mechanism involving Rac1-mediated inhibition of RhoA. Furthermore, Wnt signalling antagonizes SG assembly through Dvl2/Rac1/RhoA-mediated regulation of G3BP, a protein involved in nucleating SGs.
The interaction between Dvl2 and G3BP is mediated through the NTF2 domain (A) of G3BP (Fig. 3). Interestingly, the interaction between these two proteins is mostly favoured when G3BP is phosphorylated at S149, which resides in the acidic domain (B). However, the acidic domain (B) alone or the acidic domain containing the phosphomimetic mutation did not show any specific interaction with Dvl2 (P.K.S. and J.J., unpublished data). How does the phosphorylation in acidic domain influence the interaction of G3BP with Dvl2, which is mediated through its NTF2 domain is far from clear. One possibility is that the phosphorylation of S149 could change the conformation of G3BP so as to allow the N-terminal NTF2 domain to have a better interaction with Dvl2.
Our studies have revealed a crucial role for the DEP domain in Dvl2-mediated abrogation of SG assembly. How does the DEP domain contribute to this Dvl function? It appears that DEP may be involved in multiple ways; on the one hand, this domain is essential for the physical interaction with G3BP, and thereby impairing the ability of G3BP to nucleate SGs, and on the other hand, DEP is required for activating Rac1 (Habas et al., 2003), which in turn inhibits RhoA to antagonize SG assembly. Consistent with this notion, deletion of DEP domain results in impairment of Dvl's ability to perturb SG assembly (Fig. 1D,E). However, when lysine 446 in the DEP domain is mutated to methionine (K446M), Dvl2 fails to interfere with SG assembly (Fig. 1D,E). This mutation has been shown to impair with Dvl's ability to activate Rac1 (Habas et al., 2003), but retains the ability to interact with G3BP (Fig. 3D), thus implying a crucial role for Dvl mediated Rac1 activation in interfering with SG formation. On the contrary, Dvl-C expression, which would lead to activation of Rac1, does not interfere with SG assembly (Fig. 4C). This fragment, however, when expressed along with full length Dvl2, impairs with Dvl2's ability to antagonize SG assembly (Fig. 4C), possibly by interfering with the interaction between overexpressed Dvl2 and endogenous G3BP. Taken together, these data show that, while Dvl requires DEP-mediated activation of Rac1 and interaction with G3BP are important for inhibiting SG assembly, activities imparted by other regions of Dvl2 could also contribute to this function as discussed below.
It is interesting to note that ectopic expression of Dvl2 induces activation of both Rac1 and RhoA (Habas et al., 2003). However, our studies suggest that Dvl-mediated inhibition of SG assembly requires Rac1-dependent inhibition of RhoA activity (Fig. 2). This would predict that Dvl's ability to activate Rac and Rho would be differentially modulated under stress conditions. How could this be achieved? The PDZ domain, which is required for RhoA activation through Daam1 (Habas et al., 2001), also contributes to perturbation of SG assembly (Fig. 1E). Under stress conditions, Dvl may function through negatively regulating the Daam1 activity. Detailed studies are required to test this possibility and to delineate the function of individual domain of Dvl in modulating SG dynamics.
The cross-talk between Wnt and SG assembly pathways could play decisive roles under physiological conditions as well as in cases of developmental defects and diseases caused by mis-regulation of Wnt signalling. Intriguingly, the mutant K446M, which corresponds to an analogous mutation in Drosophila Dishevelled DEP domain (dsh1) that results in defective PCP pathway, failed to antagonize SG assembly (Fig. 1D,E). It has been reported that a mutation in the Drosophila homolog of mammalian G3BP, Rasputin, leads to ommatidial polarity defects similar to dsh1 mutant. Our finding that Dvl2 K446M mutant is functionally defective in regulating SG dynamics (Fig. 1D,E) raises the exciting possibility that the PCP defect in dsh1 mutant (Boutros and Mlodzik, 1999) could, at least in part, be due to defective mRNA functions. Two recent reports further support a function for Dvl in mRNA regulation (Bikkavilli and Malbon, 2010; Maisonneuve et al., 2009).
Previously, Dvl has been shown to interact with nucleoredoxin (NRX), a protein regulated by the redox conditions of the cell (Funato et al., 2006). Under oxidative stress, the interaction between Dvl and NRX has been shown to be decreased. Moreover, NRX has been reported to modulate the Wnt/β-catenin and Wnt/PCP signaling, primarily through regulating Dvl function (Funato et al., 2006; Funato et al., 2008; Funato et al., 2010). Interestingly, impairment of SG assembly mediated by Dvl could also involve regulation by NRX. However, further studies are required to test this intriguing possibility.
Earlier studies have demonstrated that oxidative stress antagonizes Wnt signalling by diverting β-catenin from TCF-complex to FOXO-complex, and enhancing FOXO-mediated transcription (Almeida et al., 2007; Essers et al., 2005; Hoogeboom et al., 2008). Our findings suggest that Wnt can antagonize SG assembly in a Dvl-dependent manner. β-catenin may not be required for this response, as both canonical (β-catenin-dependent) and non-canonical (β-catenin-independent) Wnt signalling pathways mediated by Wnt3a and Wnt5a, respectively, interfered with SG assembly (Fig. 5A). Moreover, we find depletion or overexpression of β-catenin does not affect SG assembly (P.K.S. and J.J., unpublished data), indicating that β-catenin may be dispensable for SG assembly. As both Wnt3a and Wnt5a are shown to activate Rac (Kurayoshi et al., 2006; Schlessinger et al., 2009; Wu et al., 2008; Yamamoto et al., 2008), based on our results, we propose that canonical and non-canonical Wnts mediate disassembly of SGs through a common mechanism involving Rac-mediated inhibition of Rho.
It is interesting to note that in many cancers including colorectal cancers, where Wnt signalling is misregulated, G3BP is also overexpressed (Pazman et al., 2000). Whether increased amounts of G3BP provide any growth or survival advantage for tumour cells, is an interesting question. The findings reported here open up avenues to address the physiological relevance of the interplay between SG assembly and Wnt signalling pathways during development and in disease. Moreover, as the SGs represent dynamic structures regulating mRNA fates, their modulation by Wnt/Dvl points toward a broader regulation of mRNA functions by this important signalling pathway.
Material and Methods
Cell lines, treatments and transfection
NIH3T3, HEK293T and HeLa S3 cells were maintained in DMEM with 10% FBS and antibiotics. For generating oxidative stress, cells were grown to 60–80% confluency and were treated with 0.5 mM sodium arsenite (S.D. Fine Chem. Ltd., Mumbai, India) for 30 min. Cells were transfected with indicated constructs using polyethylene imine (Polysciences, Inc.) or Lipofectamine 2000 as per manufacturer's instructions.
For analyzing the effect of Wnt on SG assembly, NIH3T3 cells were treated with 100 ng/ml of recombinant Wnt3a and Wnt5a (R&D systems) in DMEM containing 10% FBS for 13.5 h and later sodium arsenite was added to the medium (0.2 mM final concentration) and incubated for 30 min.
For Dvl2 depletion, NIH3T3 cells were transfected with pSUPER-control or pSUPER-Dvl2 shRNA construct [kind gifts from Dr Y. Minami, Kobe University, Japan] (Nishita et al., 2006).
For RhoA depletion, NIH3T3 cells were transfected with a previously described RhoA siRNA (Noritake et al., 2004) (Dharmacon). Forty eight hours post transfection, cells were treated with 0.5 mM sodium arsenite (final concentration) for 30 min before immunostaining with indicated SG marker antibody.
For Rac1 depletion, NIH3T3 cells were transfected with previously described Rac1 siRNA (Wang et al., 2003) (Dharmacon). Forty eight hours after transfection, cells were re-seeded on glass coverslips, and 12h later, were transfected with pcDNA3.1 HA control or HA-Dvl2 construct using Lipofectamine. Thirty six hours later, cells were treated with 0.5 mM Arsenite (final concentration) for 30 min and immunostained for indicated SG marker. All the siRNA transfections were performed with Oligofectamine (Invitrogen) as per manufacturer's instructions.
In experiments conducted to study the role of Rac1 in Wnt-mediated disassembly of SGs, NIH3T3 cells were initially treated with or without Wnt5a for 12 h, followed by addition of Rac1 inhibitor (50 µM final concentration, Calbiochem) to the same medium for 3.5 h. Further, 0.2 mM sodium arsenite (final concentration) was added to the same medium and incubated for 30 min before immunostaining with indicated antibodies.
Antibodies
Rabbit polyclonal antibodies were raised against the C-terminal region (amino acids 594 to 736) of mouse Dvl2 expressed as His-tagged recombinant protein in bacteria. Antibodies were affinity purified using the antigen covalently linked to the affigel-10 matrix (Bio-Rad). Rabbit polyclonal antibodies were raised against recombinant full length GFP and were affinity purified, and was used for immunoprecipitation assays. The immunoprecipitates were probed with mouse anti-GFP antibodies (Santa Cruz Biotechnology, Inc.) for analyzing the GFP-tagged proteins. Other antibodies used in Western analysis; mouse anti-eIF2α and rabbit anti-phospho-eIF2α (Cell Signalling Technology), mouse anti-HA (Sigma-Aldrich), mouse anti-Rac1 (BD Biosciences), mouse anti-RhoA (Santa Cruz Biotechnology, Inc.), mouse anti-Tubulin (Sigma-Aldrich) and mouse anti-Ran (BD Biosciences). Commercially available antibodies and their dilutions used for immunofluorescence are: mouse anti-Dcp1a (Sigma-Aldrich, 1:200); rabbit anti-FXR1 (Santa Cruz Biotechnology, Inc., 1:50); mouse anti-HuR (Santa Cruz Biotechnology, Inc., 1:200); rat anti-HA (Roche, 1:100); mouse anti-G3BP (BD Biosciences, Inc., 1:500); rabbit anti-myc (Sigma-Aldrich, 1:50) and rabbit anti-HA (Santa Cruz Biotechnology, Inc., 1:50), mouse anti-HA (Covance, 1:1000).
DNA constructs
Mouse Dvl2 construct (pCS2-Flag-mDvl2) was a kind gift from Dr Daniel Capelluto (Virginia Tech, USA). The full length mDvl2 was amplified by PCR and cloned into pcDNA-HA-C1 vector. Later, deletion clones were generated using sub-cloning strategies involving PCR and restriction digestions (Fig. 1C). Dvl2-K446M mutant was generated by a PCR based mutagenesis approach. All the Dvl2 constructs express respective proteins fused with a HA tag at their N-termini. Detailed cloning information will be available upon request. GFP-tagged versions of constitutively active and dominant negative Rac1 (V12Rac1 and N17V12Rac1) and RhoA (V14RhoA and N19RhoA) constructs were kind gifts from Dr Francisco Sanchez-Madrid (Universidad Autonoma de Madrid, Spain). For generation myc-tagged V14RhoA and N19RhoA constructs, the corresponding cDNAs were released from GFP-tagged versions and were subcloned into pcDNA-myc vectors using appropriate restriction enzymes. GFP-G3BP wild type and mutant constructs were generously provided by Dr Jamal Tazi (Institut de Génétique Moléculaire de Montpellier, France). pEGFP-C1 (GFP, Clontech) and pcDNA-HA-firefly luciferase (Fluc) were used as controls in different experiments as indicated.
Immunofluorescence and microscopy
Cells were grown on glass coverslips, fixed and processed for immunofluorescence studies as described earlier (Joseph and Dasso, 2008). Immunofluorescence analyses were performed using an inverted microscope (Axiovert 200M; Carl Zeiss, Inc.) as described previously (Murawala et al., 2009). Images were processed using Adobe Photoshop CS2.
For quantitation of SGs, cells were fixed and stained with HuR antibodies (or others as indicated). A cell was counted SG positive, if it displayed >5 distinguishable HuR positive puncta that could be directly visualized through 63× Plan-Apochromat objective (oil, NA 1.4). Detailed analysis of the SGs from the acquired images, using Zeiss Axiovision Rel. 4.6 software, indicated that the average size of the puncta was 0.6 µm2 (with the minimum size being 0.3 µm2).
Immunoprecipitation assay
HEK293T cells expressing the indicated proteins were lysed in RIPA buffer [50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, supplemented with protease inhibitor cocktail (Roche), 10 mM NaF, 2 mM PMSF]. For immunoprecipitation of GFP-tagged proteins, cell lysates were incubated with affinity purified GFP antibody (generated in our lab) or control antibody (rabbit IgG, Banaglore Genei) pre-bound to Protein-G dynabeads (Invitrogen) for 2 hours at 40C. The immunocomplexes were washed twice with the lysis buffer and once with TBS before eluting in SDS loading dye. The immunoprecipitates were separated on SDS-PAGE and analyzed for the presence of indicated proteins with specific antibodies by Western blotting.
Endogenous Dvl2 immunoprecipitation was performed in HEK293T cells using affinity purified Dvl2 antibody generated in our lab. To study the dependability of endogenous interaction between Dvl2 and G3BP on RNA, HEK293T cells were lysed in RIPA buffer containing 200 µg/ml RNaseA (Invitrogen), or 100 U/ml RNAsin Plus (Promega), and incubated for 45 min on ice before immunoprecipitation.
Statistical analysis
All values are expressed as mean ± SD of three independent experiments. In each experiment, 100 cells were counted. P values were calculated using Student's t-test. P value ≤0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We are grateful to Drs. D. Capelluto, F. Sanchez-Madrid and J. Tazi for providing plasmids. We thank members of the Joseph and Seshadri laboratories for critical discussions. Financial support from Council of Scientific and Industrial Research, Government of India, to P.K. Sahoo, P. Murawala, M.R. Sahoo and S. Gaikwad and from Department of Biotechnology (DBT), Government of India, to P.T. Sawale and M.M. Tripathi is greatly appreciated. The work was supported by funding from DBT.
References
- Almeida M., Han L., Martin-Millan M., O'Brien C. A., Manolagas S. C. (2007). Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting beta-catenin from T cell factor- to forkhead box O-mediated transcription. J. Biol. Chem. 282, 27, 298–27, 305 10.1074/jbc.M70281120 [DOI] [PubMed] [Google Scholar]
- Anderson P., Kedersha N. (2006). RNA granules. J. Cell Biol. 172, 803–808 10.1083/jcb.200512082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P., Kedersha N. (2008). Stress granules: the Tao of RNA triage. Trends Biochem. Sci. 33, 141–150 10.1016/j.tibs.2007.12.003 [DOI] [PubMed] [Google Scholar]
- Anderson P., Kedersha N. (2009). RNA granules: post-transcriptional and epigenetic modulators of gene expression. Nat. Rev. Mol. Cell. Biol. 10, 430–436 10.1038/nrm2694 [DOI] [PubMed] [Google Scholar]
- Axelrod J. D. (2001). Unipolar membrane association of Dishevelled mediates Frizzled planar cell polarity signaling. Genes Dev. 15, 1182–1187 10.1101/gad.890501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod J. D., Miller J. R., Shulman J. M., Moon R. T., Perrimon N. (1998). Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes Dev. 12, 2610–2622 10.1101/gad.12.16.2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balagopal V., Parker R. (2009). Polysomes, P bodies and stress granules: states and fates of eukaryotic mRNAs. Curr. Opin. Cell Biol. 21, 403–408 10.1016/j.ceb.2009.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikkavilli R. K., Malbon C. C. (2010). Dishevelled-KSRP complex regulates Wnt signaling through post-transcriptional stabilization of beta-catenin mRNA. J. Cell Sci. 123, 1352–1362 10.1242/jcs.056176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros M., Mlodzik M. (1999). Dishevelled: at the crossroads of divergent intracellular signaling pathways. Mech. Dev. 83, 27–37 10.1016/S0925-4773(99)00046-5 [DOI] [PubMed] [Google Scholar]
- Chang W., Lloyd C. E., Zarkower D. (2005). DSH-2 regulates asymmetric cell division in the early C. elegans somatic gonad. Mech. Dev. 122, 781–789 10.1016/j.mod.2005.03.005 [DOI] [PubMed] [Google Scholar]
- Clevers H. (2006). Wnt/beta-catenin signaling in development and disease. Cell 127, 469–480 10.1016/j.cell.2006.10.018 [DOI] [PubMed] [Google Scholar]
- Essers M. A., Vries-Smits L. M., Barker N., Polderman P. E., Burgering B. M., Korswagen H. C. (2005). Functional interaction between beta-catenin and FOXO in oxidative stress signaling. Science 308, 1181–1184 10.1126/science.1109083 [DOI] [PubMed] [Google Scholar]
- Funato Y., Michiue T., Asashima M., Miki H. (2006). The thioredoxin-related redox-regulating protein nucleoredoxin inhibits Wnt-beta-catenin signalling through Dishevelled. Nat. Cell Biol. 8, 501–508 10.1038/ncb1405 [DOI] [PubMed] [Google Scholar]
- Funato Y., Michiue T., Terabayashi T., Yukita A., Danno H., Asashima M., Miki H. (2008). Nucleoredoxin regulates the Wnt/planar cell polarity pathway in Xenopus. Genes Cells 13, 965–975 10.1111/j.1365-2443.2008.01220.x [DOI] [PubMed] [Google Scholar]
- Funato Y., Terabayashi T., Sakamoto R., Okuzaki D., Ichise H., Nojima H., Yoshida N., Miki H. (2010). Nucleoredoxin sustains Wnt-beta-catenin signaling by retaining a pool of inactive Dishevelled protein. Curr. Biol. 20, 1945–1952 10.1016/j.cub.2010.09.065 [DOI] [PubMed] [Google Scholar]
- Gao C., Chen Y. G. (2010). Dishevelled: the hub of Wnt signaling. Cell. Signal. 22, 717–727 10.1016/j.cellsig.2009.11.021 [DOI] [PubMed] [Google Scholar]
- Gilks N., Kedersha N., Ayodele M., Shen L., Stoecklin G., Dember L. M., Anderson P. (2004). Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol. Biol. Cell 15, 5383–5398 10.1091/mbc.E04-08-0715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas R., Kato Y., He X. (2001). Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell 107, 843–854 10.1016/S0092-8674(01)00614-6 [DOI] [PubMed] [Google Scholar]
- Habas R., Dawid I. B., He X. (2003). Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 17, 295–309 10.1101/gad.1022203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogeboom D., Essers M. A., Polderman P. E., Voets E., Smits L. M., Burgering B. M. (2008). Interaction of FOXO with beta-catenin inhibits beta-catenin/T cell factor activity. J. Biol. Chem. 283, 9224–9230 10.1074/jbc.M706638200 [DOI] [PubMed] [Google Scholar]
- Joseph J., Dasso M. (2008). The nucleoporin Nup358 associates with and regulates interphase microtubules. FEBS Lett. 582, 190–196 10.1016/j.febslet.2007.11.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N. L., Gupta M., Li W., Miller I., Anderson P. (1999). RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J. Cell Biol. 147, 1431–1442 10.1083/jcb.147.7.1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida S., Yamamoto H., Hino S., Ikeda S., Kishida M., Kikuchi A. (1999). DIX domains of Dvl and axin are necessary for protein interactions and their ability to regulate beta-catenin stability. Mol. Cell. Biol. 19, 4414–4422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein T. J., Mlodzik M. (2005). Planar cell polarization: an emerging model points in the right direction. Annu. Rev. Cell Dev. Biol. 21, 155–176 10.1146/annurev.cellbio.21.012704.132806 [DOI] [PubMed] [Google Scholar]
- Klingensmith J., Yang Y., Axelrod J. D., Beier D. R., Perrimon N., Sussman D. J. (1996). Conservation of Dishevelled structure and function between flies and mice: isolation and characterization of Dvl2. Mech. Dev. 58, 15–26 10.1016/S0925-4773(96)00549-7 [DOI] [PubMed] [Google Scholar]
- Kurayoshi M., Oue N., Yamamoto H., Kishida M., Inoue A., Asahara T., Yasui W., Kikuchi A. (2006). Expression of Wnt-5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res. 66, 10,439–10,448 10.1158/0008-5472.CAN-06-2359 [DOI] [PubMed] [Google Scholar]
- Lee Y. N., Gao Y., Wang H. Y. (2008). Differential mediation of the Wnt canonical pathway by mammalian Dishevelleds-1, -2, and -3. Cell. Signal. 20, 443–452 10.1016/j.cellsig.2007.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan C. Y., Nusse R. (2004). The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20, 781–810 10.1146/annurev.cellbio.20.010403.113126 [DOI] [PubMed] [Google Scholar]
- Maisonneuve C., Guilleret I., Vick P., Weber T., Andre P., Beyer T., Blum M., Constam D. B. (2009). Bicaudal C, a novel regulator of Dvl signaling abutting RNA-processing bodies, controls cilia orientation and leftward flow. Development 136, 3019–3030 10.1242/dev.038174 [DOI] [PubMed] [Google Scholar]
- Miller J. R., Rowning B. A., Larabell C. A., Yang-Snyder J. A., Bates R. L., Moon R. T. (1999). Establishment of the dorsal-ventral axis in Xenopus embryos coincides with the dorsal enrichment of Dishevelled that is dependent on cortical rotation. J. Cell Biol. 146, 427–437 10.1083/jcb.146.2.427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murawala P., Tripathi M. M., Vyas P., Salunke A., Joseph J. (2009). Nup358 interacts with APC and plays a role in cell polarization. J. Cell Sci. 122, 3113–3122 10.1242/jcs.037523 [DOI] [PubMed] [Google Scholar]
- Nishita M., Yoo S. K., Nomachi A., Kani S., Sougawa N., Ohta Y., Takada S., Kikuchi A., Minami Y. (2006). Filopodia formation mediated by receptor tyrosine kinase Ror2 is required for Wnt5a-induced cell migration. J. Cell Biol. 175, 555–562 10.1083/jcb.200607127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noritake J., Fukata M., Sato K., Nakagawa M., Watanabe T., Izumi N., Wang S., Fukata Y., Kaibuchi K. (2004). Positive role of IQGAP1, an effector of Rac1, in actin-meshwork formation at sites of cell-cell contact. Mol. Biol. Cell 15, 1065–1076 10.1091/mbc.E03-08-0582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R., Sheth U. (2007). P bodies and the control of mRNA translation and degradation. Mol. Cell 25, 635–646 10.1016/j.molcel.2007.02.011 [DOI] [PubMed] [Google Scholar]
- Pazman C., Mayes C. A., Fanto M., Haynes S. R., Mlodzik M. (2000). Rasputin, the Drosophila homologue of the RasGAP SH3 binding protein, functions in ras- and Rho-mediated signaling. Development 127, 1715–1725 [DOI] [PubMed] [Google Scholar]
- Schlessinger K., Hall A., Tolwinski N. (2009). Wnt signaling pathways meet Rho GTPases. Genes Dev. 23, 265–277 10.1101/gad.1760809 [DOI] [PubMed] [Google Scholar]
- Schwarz-Romond T., Merrifield C., Nichols B. J., Bienz M. (2005). The Wnt signalling effector Dishevelled forms dynamic protein assemblies rather than stable associations with cytoplasmic vesicles. J. Cell Sci. 118, 5269–5277 10.1242/jcs.02646 [DOI] [PubMed] [Google Scholar]
- Schwarz-Romond T., Fiedler M., Shibata N., Butler P. J., Kikuchi A., Higuchi Y., Bienz M. (2007). The DIX domain of Dishevelled confers Wnt signaling by dynamic polymerization. Nat. Struct. Mol. Biol. 14, 484–492 10.1038/nsmb1247 [DOI] [PubMed] [Google Scholar]
- Smalley M. J., Sara E., Paterson H., Naylor S., Cook D., Jayatilake H., Fryer L. G., Hutchinson L., Fry M. J., Dale T. C. (1999). Interaction of axin and Dvl-2 proteins regulates Dvl-2-stimulated TCF-dependent transcription. EMBO J. 18, 2823–2835 10.1093/emboj/18.10.2823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley M. J., Signoret N., Robertson D., Tilley A., Hann A., Ewan K., Ding Y., Paterson H., Dale T. C. (2005). Dishevelled (Dvl-2) activates canonical Wnt signalling in the absence of cytoplasmic puncta. J. Cell Sci. 118, 5279–5289 10.1242/jcs.02647 [DOI] [PubMed] [Google Scholar]
- Tourriere H., Gallouzi I. E., Chebli K., Capony J. P., Mouaikel J., van der G. P., Tazi J. (2001). RasGAP-associated endoribonuclease G3Bp: selective RNA degradation and phosphorylation-dependent localization. Mol. Cell. Biol. 21, 7747–7760 10.1128/MCB.21.22.7747-7760.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourriere H., Chebli K., Tazi J. (2002). mRNA degradation machines in eukaryotic cells. Biochimie 84, 821–837 10.1016/S0300-9084(02)01445-1 [DOI] [PubMed] [Google Scholar]
- Tourriere H., Chebli K., Zekri L., Courselaud B., Blanchard J. M., Bertrand E., Tazi J. (2003). The RasGAP-associated endoribonuclease G3BP assembles stress granules. J. Cell Biol. 160, 823–831 10.1083/jcb.200212128 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Tsai N. P., Wei L. N. (2010). RhoA/ROCK1 signaling regulates stress granule formation and apoptosis. Cell. Signal. 22, 668–675 10.1016/j.cellsig.2009.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amerongen R., Nusse R. (2009). Towards an integrated view of Wnt signaling in development. Development 136, 3205–3214 10.1242/dev.033910 [DOI] [PubMed] [Google Scholar]
- Wallingford J. B., Habas R. (2005). The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development 132, 4421–4436 10.1242/dev.02068 [DOI] [PubMed] [Google Scholar]
- Wang H. R., Zhang Y., Ozdamar B., Ogunjimi A. A., Alexandrova E., Thomsen G. H., Wrana J. L. (2003). Regulation of cell polarity and protrusion formation by targeting RhoA for degradation. Science 302, 1775–1779 10.1126/science.1090772 [DOI] [PubMed] [Google Scholar]
- Wu X., Tu X., Joeng K. S., Hilton M. J., Williams D. A., Long F. (2008). Rac1 activation controls nuclear localization of beta-catenin during canonical Wnt signaling. Cell 133, 340–353 10.1016/j.cell.2008.01.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S., Nishimura O., Misaki K., Nishita M., Minami Y., Yonemura S., Tarui H., Sasaki H. (2008). Cthrc1 selectively activates the planar cell polarity pathway of Wnt signaling by stabilizing the Wnt-receptor complex. Dev. Cell 15, 23–36 10.1016/j.devcel.2008.05.007 [DOI] [PubMed] [Google Scholar]
- Yanagawa S., van Leeuwen F., Wodarz A., Klingensmith J., Nusse R. (1995). The Dishevelled protein is modified by wingless signaling in Drosophila. Genes Dev. 9, 1087–1097 10.1101/gad.9.9.1087 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.