Summary
The dermomyotome is a pool of progenitor cells on the surface of the myotome. In zebrafish, dermomyotome precursors (anterior border cells, ABCs) can be first identified in the anterior portion of recently formed somites. They must be prevented from undergoing terminal differentiation during segmentation, even while mesodermal cells around them respond to signaling cues and differentiate.
T-box containing transcription factors regulate many aspects of mesoderm fate including segmentation and somite patterning. The fused somites (fss) gene is the zebrafish ortholog of tbx6. We demonstrate that in addition to its requirement for segmentation, fss/tbx6 is also required for the specification of ABCs and subsequently the central dermomyotome. The absence of Tbx6-dependent central dermomyotome cells in fss/tbx6 mutants is spatially coincident with a patterning defect in the myotome.
Using transgenic fish with a heat-shock inducible tbx6 gene in the fss/tbx6 mutant background, we further demonstrate that ubiquitous fss/tbx6 expression has spatially distinct effects on recovery of the dermomyotome and segment boundaries, suggesting that the mechanism of Fss/Tbx6 action is distinct with respect to dermomyotome development and segmentation.
We propose that Fss/Tbx6 is required for preventing myogenic differentiation of central dermomyotome precursors before and after segmentation and that central dermomyotome cells represent a genetically and functionally distinct subpopulation within the zebrafish dermomyotome.
Key words: Myogenesis, Slow muscle, Fast muscle, Muscle pioneer, Somite, Anterior Border Cells, Segmentation
Introduction
In vertebrates, the paraxial mesoderm is the source of all the skeletal muscle of the trunk and limbs as well as the dermis and axial skeleton of the trunk. The specification of paraxial mesoderm into the precursors of these cell types occurs during the segmentation period, with the appearance of the myotome, the dermomyotome, and the sclerotome within the somites.
The dermomyotome contains precursors to the embryonic myotome, and to satellite cells that underlie post-embryonic muscle growth and repair (Buckingham and Vincent, 2009). In avians and mammals, the earliest myotome cells differentiate after their incorporation into somites, at about the same time as the appearance of the dermomyotome (Denetclaw et al., 1997; Venters et al., 1999; Kahane et al., 2007). In contrast, in most teleosts, primary myotome cells start to differentiate prior to somite formation and prior to the appearance of the dermomyotome (Stellabotte and Devoto, 2007). In both amniotes and teleosts, signals from tissues surrounding the somite, including the notochord, spinal cord, and surface ectoderm trigger myogenic differentiation.
Much less is known about the initial development of the dermomyotome, or about the mechanisms that maintain a dermomyotome while some of its cells differentiate into muscle fibres during early embryogenesis. In zebrafish, dermomyotome precursors can first be identified in the anterior portion of recently formed somites (Hollway et al., 2007; Stellabotte et al., 2007). These anterior border cells (ABCs) migrate to the lateral surface of the somite, forming a layer of cells on the external surface of the myotome, as in other vertebrates (Devoto et al., 2006). These cells express pax7, pax3, meox, and dacD (Groves et al., 2005; Devoto et al., 2006; Feng et al., 2006; Hammond et al., 2007; Hollway et al., 2007).
The dermomyotome can be subdivided along the dorsal-ventral axis into separate domains based on gene expression. In amniotes, the central portion of the dermomyotome has been called the intercalated region, because it is located between the dorsal and the ventral regions (Spörle, 2001). It can be distinguished from the dorsal and the ventral dermomyotome by its expression of engrailed (Davis et al., 1991; Gardner and Barald, 1992) and sim (Spörle, 2001). In zebrafish, the central-most dermomyotome cells are distinguished by their expression of the chemokine SDF1a (David et al., 2002; Svetic et al., 2007).
Five genes are known to be required for segmentation of the paraxial mesoderm: beamter, deadly seven, after eight, mind bomb, and fused somites (van Eeden et al., 1996). The first four of these genes are notch pathway genes; they are not required for the formation of the anterior three to nine somites but are required for the proper segregation of anterior and posterior half somite-markers, which in mutants are expressed in a ‘salt and pepper’ pattern. The fused somites (fss) gene is not part of the notch pathway, it encodes a transcription factor of the tbx gene family which is expressed in the anterior presomitic mesoderm (PSM) and in the anterior half of recently formed somites. fss is required for the formation of somites throughout the trunk and tail and for the development of anterior half-somite identity (Nikaido et al., 2002; van Eeden et al., 1996). tbx family members encode proteins with a conserved DNA binding motif known as the T-box and play important roles in embryonic mesoderm development. In mice, eomesodermin is required for the initial formation of the mesoderm (Showell et al., 2004), brachyury is required for notochord and posterior mesoderm development (Showell et al., 2004), tbx6 is required for paraxial mesoderm development as well as somite patterning (Chapman and Papaioannou, 1998; White et al., 2003), and tbx18 is required for the maintenance of anterior identity in newly formed somites (Bussen et al., 2004). Tbx proteins are sequence-specific DNA binding proteins which can serve as transcriptional repressors or activators (Wardle and Papaioannou, 2008).
We have investigated the role of the segmentation gene fss in the specification and differentation of paraxial mesoderm cell types. Sequence analysis demonstrates that the fss gene is the zebrafish ortholog of tbx6; we show that it is required for the specification of ABCs and for the development of the central dermomyotome and the underlying myotome in the posterior trunk and tail.
Using transgenic fish expressing heat-shock inducible tbx6 in the fss/tbx6 mutant background, we show that the ubiquitous expression of fss/tbx6 has spatially distinct effects on restoration of central dermomyotome and on segmentation in mutant embryos. We propose that the role of Fss/Tbx6 is to prevent the differentiation of central dermomyotome precursors in response to myogenic signals before and after segmentation and that in zebrafish the central dermomyotome is a genetically distinct dermomyotome subpopulation.
Results
We have examined the expression of the dermomyotome marker Pax7 in segmentation mutants to investigate a possible connection between segmentation and the specification of dermomyotome precursors (ABCs) in zebrafish. We found that formation of the dermomyotome is unaffected in segmentation mutants of the notch gene pathway (not shown), suggesting that ABC specification is not dependent on segment boundary formation. However, Pax7 expression seemed to be altered in the fused somites (fss) mutant.
Orthology between the fss gene and tbx6
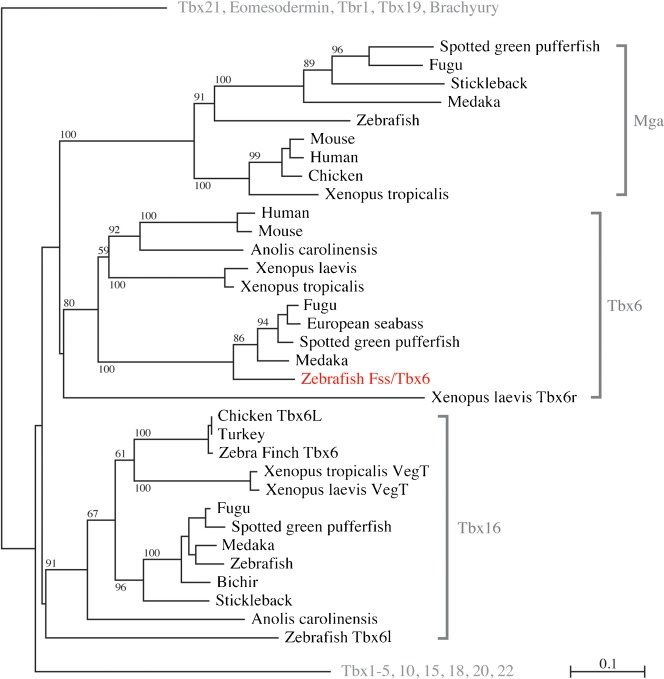
We have re-examined the homology of the fss gene to other members of the tbx gene family. fss was initially named tbx24 (Nikaido et al., 2002), when few other teleost tbx gene sequences were available for comparison and the diversity within the tbx6 subfamily was not apparent, and because there was a previously cloned zebrafish gene which had been named tbx6 (Hug et al., 1997). With further sequences available, it is clear that the Fss amino acid sequence is homologous in its T-domain to the Tbx6 subfamily, nesting alongside Tbx6 proteins of other teleosts, amphibians, lizard, and mammals (Fig. 1; supplementary material Fig. S1, cladogram of the entire tree; supplementary material Table S1, list of gene accession numbers). The two nearest subgroups, Mga and Tbx16, also contain zebrafish genes nested with those of other species. The originally named zebrafish Tbx6 falls within the Tbx16 subgroup. In support of this, the chromosomal region containing the fss gene in zebrafish is syntenic with the chromosomal region containing tbx6 in mouse (34 paralogous genes), human (69 genes), and medaka (188 genes) (Catchen et al., 2009) (supplementary material Fig. S2). Using the same parameters, the zebrafish gene previously named tbx6 has no neighboring genes in common with tbx6 in mouse or human. According to the nomenclature guidelines of the zebrafish community, fss/tbx24 has been renamed to fss/tbx6, to facilitate communication between those studying this gene in mammals and those studying it in fish. To avoid confusion, the gene previously named tbx6 has been renamed to tbx6l.
Fig. 1. Cladogram of Tbx6, Tbx16 and MGA members of the Tbx protein family.
Tree is an internal branch taken from a neighbor-joining tree of the Tbx family of proteins based solely on their T-Box domain. Numbers represent bootstrap values (100 replications, values >50 shown). Sequence alignment using ClustalX 2.0 (Larkin et al., 2007), and tree building using SeaView 4.2.12 (Gouy et al., 2010). Note that zebrafish Fss/Tbx6 (red) nests within the tbx6 clade. For outgroup branching patterns and support values, see supplementary material Fig. S1. Protein accession numbers are listed in supplementary material Table S1.
Tbx6 is required for development of the central dermomyotome
Tbx6 is required for patterning of the paraxial mesoderm in mouse (Chapman et al., 1996). We examined the expression of dermomyotome markers (Pax7, pax3 and meox) together with the expression of myogenic regulatory factors (MRFs) in fss/tbx6 mutant embryos at different stages to characterize the role of Tbx6 in the development of the major paraxial mesoderm cell types in zebrafish.
We find that fss/tbx6 is required for the development of the dermomyotome, but with regional differences: in the anterior trunk dermomyotome cells develop independently of tbx6 function (Fig. 2A,C), whereas in the posterior trunk and tail the dermomyotome is subdivided into a tbx6 independent dorsal/ventral domain and a tbx6 dependent central domain at the level of the notochord (Fig. 2B,D–F).
Fig. 2. fss/tbx6 is required for the central dermomyotome development in the posterior trunk and tail.
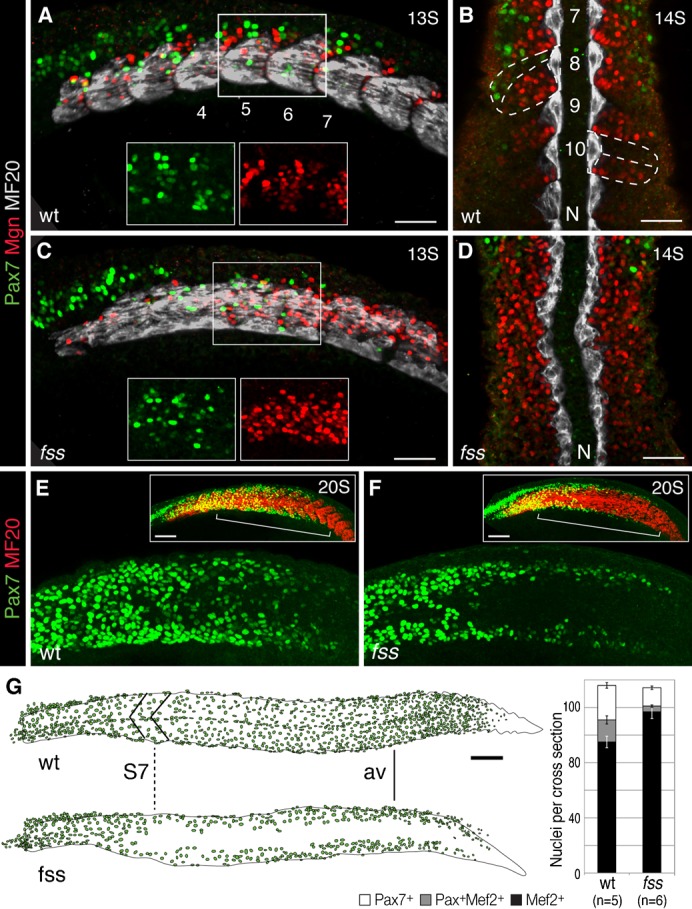
(A–D) Pax7 (green), Myogenin (Mgn, red), and MF20 (MyHC, white) expression in the anterior trunk (A,C; confocal z-stack, insets show higher magnification) and the posterior trunk (B,D) of wild type sibling (A,B) and fss/tbx6 mutant (C,D) at 13–14S stage. N, Notochordd, numbers indicate somite number. Wild type siblings express all three markers within distinct compartments of the somites (dashed lines in B). In fss/tbx6 mutants, Pax7 expressing central dermomyotome cells are restricted to the anterior trunk; the central posterior trunk shows more cells expressing Mgn. (E,F) Pax7 (green) and MF20 (MyHC, red) expression in wild type sibling (E) and fss/tbx6 mutant (F) embryo at 20S stage. Boxes show flattened confocal z-stacks of the entire trunk. In the posterior trunk Pax7 is initially expressed in cells of the dorsal and ventral dermomyotome; central Pax7 expression is delayed in wild type sibling (E), but absent in fss/tbx6 mutant (F). (G) Tracing of Pax7+ dermomyotome nuclei in representative wild type sibling and fss/tbx6 embryos at the 24 h stage. In this mutant embryo, the central dermomyotome deficit/defect in fss/tbx6 mutants starts at trunk levels correlating to somite 7 in the wild type sibling (dashed line, S7). Graph showing number of Pax7+, Mef2+ and differentiating (Pax7+/Mef2+) cells (mean±s.e.m.) on sections at anal vent (av) level in wild type siblings and fss/tbx6 mutants. Scale bars: 50 µm (A–D), 100 µm (E–G).
During the early segmentation period, at the 13S stage, dermomyotome development has reached the level of somites 7–8 in wildtype embryos. Similarly, Pax7-positive dermomyotome cells are found on the lateral surface of the myotome of fss/tbx6 mutant siblings (Fig. 2A,C). As the dermomyotome matures in the posterior trunk in wild type embryos, the dorsal/ventral domain is labeled by Pax7 antibody earlier than the central domain. During the same period, dorsal/ventral dermomyotome cells appears at the same axis levels. In fss/tbx6 mutants, however, Pax7-positive cells are completely missing in the central domain of the posterior trunk in fss/tbx6 mutants (Fig. 2E,F).
At the end of the segmentation period (24 h stage), the deficit in the central dermomyotome in fss/tbx6 mutant embryos is highly specific and quite pronounced. Beginning in a region ranging from the equivalent of somite 6 to somite 8 of wild type siblings (n = 25), Pax7-positive dermomyotome cells are absent in the central domain of all of the posterior trunk and tail (Fig. 2G, Fig. 3A,B). We found similar results with other markers of the dermomyotome, including meox, and pax3 (data not shown).
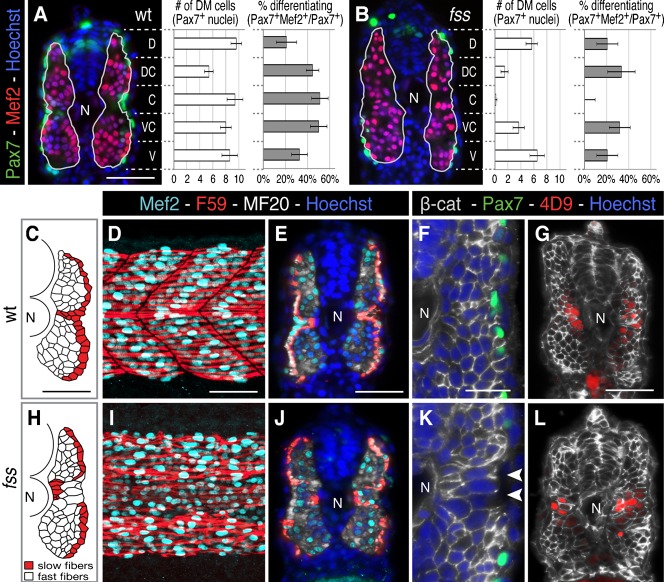
Fig. 3. Premature dermomyotome progenitor differentiation disrupts myotome patterning.
(A,B) Cross-sections and graphs showing dorsal-ventral distribution of dermomyotome (average of Pax7+ cells ± s.e.m.) and % differentiating dermomyotome (Pax7+/Mef2+) in wild type sibling and fss/tbx6 mutant (green, Pax7; red, Mef2; blue, Hoechst 33258). (C–L) Myotome morphology in wild type siblings (D–H) and fss/tbx6 (I–M) at the 24 h stage. (C,H) Tracing of fast (white) and slow (red) muscle fibres on representative cross-sections. In fss/tbx6 mutants, the layer of slow fibres is disrupted and large fast muscle fibres are found lateral to the slow fibres in the central myotome. (D,E,I,J) Whole mounts (posterior trunk) and cross-sections labeled with F59 (red, labels slow myosin heavy chain at the 24 h stage), Mef2 (aqua) and MF20 (white). (F,G,K,L) β-catenin labeled cross-sections double-labeled for either Pax7 (green, F,G) or 4D9 (red, engrailed, G,L); nuclei labeled with Hoechst. Arrowheads indicate large diameter, lateral fast fibres. N, notochord. Scale bars: 50 µm (A,C–E,G), 25 µm (F). Corresponding images of fss are to the same scale.
To facilitate quantitative comparisons between embryos with and without segments we used the anal vent as a landmark (Fig. 2G). In fss/tbx6 mutants the total number of Pax7-positive dermomyotome cells per cross section is reduced by more than 50 percent (Fig. 4A).
Fig. 4. Dermomyotome recovery and muscle growth.
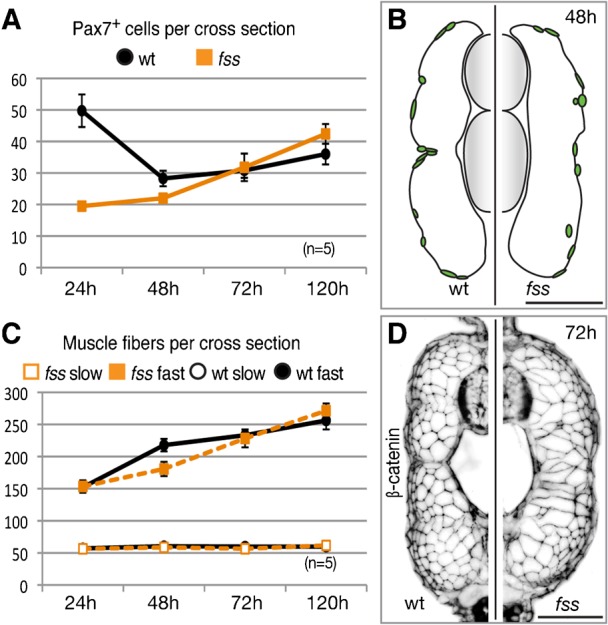
(A) Graph showing mean number of Pax7+ cells (±s.e.m.) over time. (B) Tracing of Pax7+ dermomyotome nuclei in representative transverse section of wild type sibling (left) and fss/tbx6 mutant (right) at the 48 h stage. (C) Graph showing mean number of slow and fast muscle fibres over time (± s.e.m.). (D) Cross-sections showing myotome morphology (β-catenin) at the 72 h stage. Scale bars: 50 µm.
Loss of Tbx6 leads to myogenic differentiation of central dermomyotome progenitors
Specification of the precursors of slow muscle fibres, adaxial cells, is indistinguishable between fss/tbx6 mutant and wildtype embryos (van Eeden et al., 1996) (Fig. 2B,D). However, specification of ABCs and fast muscle precursors, which develop from cells initially lateral to adaxial cells, is altered in mutants. In wild type embryos, MRF genes are expressed segmentally, in the posterior of recently formed somites, and not in the prospective dermomyotome precursors in the anterior (Weinberg et al., 1996). In fss/tbx6 mutants in contrast, MRF expression is more widespread in the paraxial mesoderm (Fig. 2B,D); we could not detect any myogenin-negative cells in the posterior trunk of fss/tbx6 mutant embryos, suggesting that ABCs differentiate prematurely into primary myotome cells. To test this, we determined the number of dermomyotome and myogenic nuclei (Mef2-positive) (Ticho et al., 1996) in the paraxial mesoderm of embryos at the end of segmentation (24 h stage). The loss of more than half of the Pax7-positive dermomyotome cells correlates with a significantly higher number of myogenic nuclei (P≤0.0002), while the total number of labeled nuclei is not affected in fss/tbx6 mutants (Fig. 2G). The simplest explanation of these results is that the precursors to the central dermomyotome have prematurely differentiated into primary myotome.
In wild type embryos at the end of segmentation, the central dermomyotome is distinguished from the dorsal and ventral dermomyotome by its higher proportion of differentiating cells, as indicated by the co-expression of Pax7 and MRFs (Fig. 3A). Approximately 50% of Pax7-positive cells in the central dermomyotome of wild type embryos co-express Mef2 at the 24 h stage, compared to about half that number in the dorsal/ventral domain. While Pax7-positive and differentiating dermomyotome cells are absent in the central domain in fss/tbx6 mutants at the end of segmentation, the most dorsal and most ventral dermomyotome cells are present in approximately normal numbers and differentiate at percentages similar to wild type (Fig. 3B).
The deficit in central dermomyotome in fss/tbx6 mutants spatially correlates with a myotome patterning defect
At the end of segmentation, the wild type zebrafish myotome consists of deep fast muscle fibres, covered laterally by a continuous monolayer of slow muscle fibres (Waterman, 1969). This slow muscle monolayer extends from the lateral surface of the myotome to the notochord, at the position of the future horizontal myoseptum, and separates the fast muscle fibres into epaxial and hypaxial regions (Fig. 3C–E). In fss/tbx6 mutant embryos, the arrangement and morphology of slow and fast fibres is normal in the anterior trunk, where the central dermomyotome is present (not shown). However, it is profoundly disrupted in the posterior trunk and tail, where dermomyotome precursors have prematurely differentiated into muscle fibres. The continuity of the slow muscle monolayer is interrupted, with numerous fast muscle fibres superficial to the slow fibres in the central region (Fig. 3H–J). These superficial fast fibres can span several segment lengths, have very large cross sectional diameters, and have an unusually large number of nuclei, with several present in transverse sections (Fig. 3F,K). The morphology and position of these fast fibres suggest that the prematurely differentiated dermomyotome precursors joined the fast primary myotome. To examine muscle cell identities, we examined expression of Engrailed, which is a marker for both slow muscle pioneers and for medial fast muscle fibres in the central myotome (Hatta et al., 1991; Wolff et al., 2003). Despite the altered position of slow and fast fibres, Engrailed expression is similar to that in wild type embryos (Fig. 3G,L).
Dorsal/ventral dermomyotome cells are sufficient to support larval muscle growth in fss/tbx6 mutants
At the 24 h stage, fss/tbx6 mutant embryos have fewer than half the number of dermomyotome cells per section through the posterior trunk, compared to wild type embryos (Fig. 2G, Fig. 4A). However, by the 48 h stage, wild type and mutant embryos have similar numbers of Pax7-positive dermomyotome cells. This is in part due to a decrease of central dermomyotome cells in wild type embryos as they differentiate into muscle fibres (Fig. 3A), and in part due to an increase in the number of Pax7-positive dermomyotome cells in mutant embryos. Furthermore, the distribution of cells along the dorsal-ventral axis of the myotome is similar (Fig. 4B). This suggests the possibility that in fss/tbx6 mutants the dorsal and ventral most dermomyotome provides a pool of precursor cells that populates the central domain. Accordingly we found Pax7-positive dermomyotome cells bridging the central domain at multiple locations throughout the posterior trunk and tail at the 36 h stage (not shown).
The premature differentiation of dermomyotome progenitors does not lead to an increased number of muscle fibres in mutant embryos at the end of segmentation, but rather to an increased number of nuclei per fibre in the ectopic fast fibres (Fig. 3K, Fig. 4A,C). The addition of muscle fibres in the fast myotome in fss/tbx6 mutants is delayed at 48 h but recovers to wild type levels by 72 h (Fig. 4C). We presume this reflects the reduced number of myogenic precursor cells, with a 24 hour delay. Unusually large fast muscle fibres remain in the central domain of the myotome of fss/tbx6 mutants at the 72 h stage, but new fibres, developing on the outside of the embryonic fast myotome during the period of stratified hyperplasia (Rowlerson and Veggetti, 2001) have diameters similar to those in wild type (Fig. 4D). These morphological differences between the wildtype and the fss/tbx6 myotome persist into later larval stages (not shown).
Induced fss/tbx6 expression rescues segmental gene expression and dermomyotome formation
To confirm that the segmentation defect and the loss of central dermomyotome in fss/tbx6 mutants both result from the loss of the fss/tbx6 gene, we created transgenic fish with tbx6 under the regulation of the inducible promoter of the hsp70 gene (Halloran et al., 2000). We assayed the early response to Tbx6 induction by examining the expression of ripply1, fgf8 and myoD shortly after heat shock. ripply1 and fgf8 are expressed in the anterior of newly formed somites in a Tbx6-dependent manner (Sawada et al., 2000; Kawamura et al., 2005), while the expression of the MRF myoD is restricted from the anterior of newly formed somites in a Tbx6-dependent manner (van Eeden et al., 1996). We found that a pulse of Tbx6 during the segmentation period restored partly segmented expression of ripply1 and fgf8, and restored gaps in the myoD expression domain (Fig. 5), indicating the restoration of anterior half-somite identity in presumptive segments.
Fig. 5. A pulse of Tbx6 expression in fss/tbx6 mutants induces segmental expression of ripply1 and fgf8, and locally represses myoD.
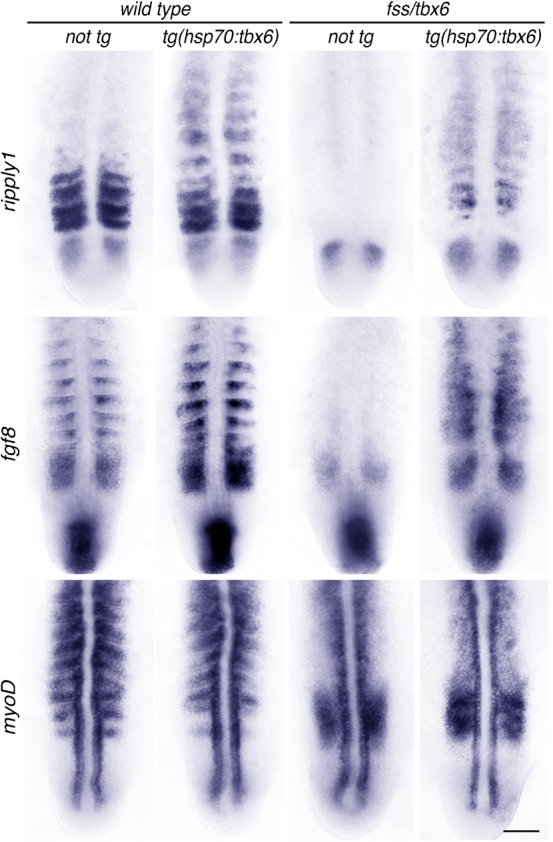
Expression of genes expressed specifically in the anterior (ripply1, fgf8) and posterior (myoD) somite compartments in non-tg and tg(hsp70:tbx6) wild type sibling and fss/tbx6 mutant embryos. Embryos were heat shocked at the 9–10S stage, fixed at the 13–14S (myoD) and 15–16S stage (ripply1, fgf8), and flat-mounted for documentation. Scale bar: 50 µm.
Heat shock induced expression of Tbx6 also restored central dermomyotome cells in mutant embryos. In wild type embryos during the segmentation period, most proliferative cells are dermomyotome cells on the surface of the somite, with the highest number at the dorsal and ventral extremes (Fig. 6A) (Barresi et al., 2001; Stellabotte et al., 2007). The loss of fss/tbx6 leads to the complete absence of proliferative cells in the central region (Fig. 6A). Pulsed expression of Tbx6 restored proliferative cells in the central region of the somite, consistent with a rescue of the central dermomyotome. In the region of the trunk with maximal numbers of induced Pax7-positive cells, fss/tbx6 mutants showed a similar number of dermomyotome cells as wild type siblings (Fig. 6B). These results suggest that segmentation and dermomyotome development are both restored after heat shock induced fss/tbx6 expression.
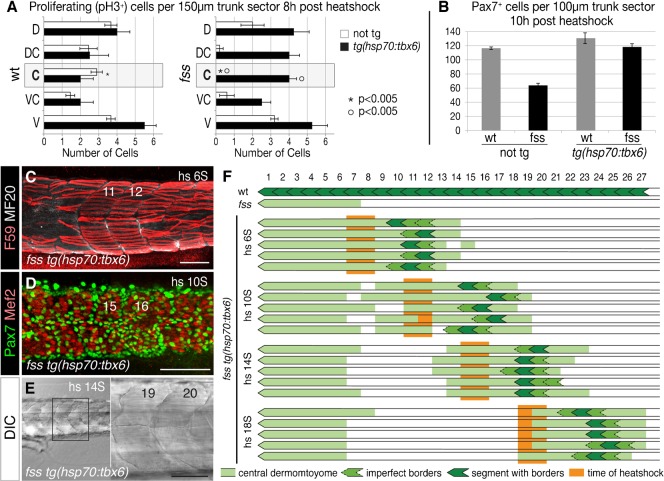
Fig. 6. Induced Tbx6 expression differentially rescues central dermomyotome and segmentation.
(A) Dorsal/ventral distribution of proliferative (phosphorylated Histone H3, pH3+) cells in non-tg and tg(hsp70:tbx6) wild type sibling and fss/tbx6 mutant embryos 8 hours after heat-shocking at the 8S stage. Data are presented as mean ± s.e.m. (B) Graph showing number of Pax7+ cells in non-tg and tg(hsp70:tbx6) wild type sibling and fss/tbx6 mutant embryos at 10 hours after heat-shocking at the 10S stage. (C–E) Rescue of dermomyotome and segmentation in fss/tbx6 mutant tg(hsp70:tbx6) visualized using F59 (red) and MF20 (white) (C) and Pax7 (green) and Mef2 (white) (D) labeling, and DIC imaging (E). Specimen stages are 5 d (C,E) and 24 h (D), rescued segments are numbered according to the corresponding region in wild-type siblings. (F) Schematic showing the location of rescued dermomyotome (light green), segments (dark green) in individual embryos after heat shocks at various times (orange). Note that a Tbx6 pulse rescues the formation of 1–3 somite boundaries and central dermomyotome over 8–10 somite lengths. Scale bars: 100 µm (C,D), 50 µm (E).
Ubiquitous fss/tbx6 expression differentially rescues dermomyotome and segmentation
To further characterize the requirement of fss/tbx6 in the formation of central dermomyotome cells and segment boundaries, we induced a pulse of Tbx6 expression at varying times during the segmentation period, and monitored segmentation and the development of the dermomyotome. We found that a Tbx6 pulse rescued the formation of 1 to 3 remarkably normal somites with continuous somite boundaries, as visualized by DIC imaging and perfectly aligned slow muscle fibres, along with an 8–10 somite length region of rescued Pax7-positive central dermomyotome (Fig. 6C–E).
The best somite was consistently formed about 2 to 4 somite-lengths posterior to the somites that would have been forming at the time of heat shock. For example, if we heat shocked a clutch of embryos at the 14S stage, somites 15 and 16 were formed during heat shock and we consistently observed that fss−/−;hsp70:tbx6 embryos formed at least one complete segment in the trunk region equivalent to somite 19 to 20 in fss heterozygous siblings (Fig. 6D,F).
The heat shock pulse leads to ubiquitous expression of Tbx6, with the highest protein levels between one and two hours (corresponding to the addition of 2 to 4 somites) after the end of heat shock and rapidly decreasing levels thereafter (not shown). Thus, the cells becoming incorporated into a segment in response to heat shock induced fss/tbx6 expression were located in the posterior presomitic mesoderm (PSM) at the beginning of heat shock and in the anterior PSM, about 2 somite-lengths posterior to the most recently formed segment in wild type siblings, when protein levels were highest. This indicates that the potential to form segment boundaries is spatially restricted to cells in the anterior PSM and that it requires high levels of fss/tbx6 expression.
Pax7-positive central dermomyotome cells were restored in a much broader region of the trunk and tail, extending anterior and posterior to the induced segment boundaries. This shows that the dermomyotome rescue is not dependent on the formation of segment boundaries, which is consistent with the observation that dermomyotome development is unaffected in segmentation mutants of the notch signaling pathway (data not shown) and suggests that lower levels of fss/tbx6 expression are sufficient for specification of central dermomyotome cells.
The restoration of central dermomyotome even anterior to the segments formed during heat shock indicates that Fss/Tbx6 can specify ABCs even after segment boundary formation. The difference in extent of dermomyotome restoration anterior to the segmentation rescue in different heat shock stages may be explained by different rates of cell maturation along anterior-posterior axis. Precursor cells remain uncommitted longer in the anterior segments than in the posterior segments of the trunk, relative to their incorporation into a somite. Thus, the dermomyotome extends more anteriorly in embryos heat shocked during maturation of the trunk (10S stage) than it does in embryos heat shocked during maturation of the tail (18S stage).
Discussion
We have investigated the role of the segmentation gene fss/tbx6 in the specification of paraxial mesoderm cells in zebrafish. We show that the loss of fss/tbx6 leads to premature differentiation of central dermomyotome precursors into primary fast fibres, causing a patterning defect within the central myotome. The requirement for fss/tbx6 subdivides the dermomyotome into distinct populations of cells along both the anterior-posterior axis and the dorsal-ventral axis.
Model for tbx6 action
Signals that promote primary myogenesis are uniformly distributed along the anterior-posterior axis, as they are derived from notochord, surface ectoderm, neural tube, and the pronephros (Cossu et al., 1996). This raises the question of how the segmentally arranged dermomyotome precursors, the ABCs, are protected from the influence of these myogenesis promoting signals. We propose that Fss/Tbx6 is a necessary component of a segmentally restricted myogenic inhibitor, and that this inhibition is required for the development of the dermomyotome.
Fss/Tbx6 is unlikely to be sufficient for the inhibition of myogenesis. It is expressed in all of the cells of the anterior PSM before it becomes restricted to the anterior of recently formed somites (Nikaido et al., 2002). Moreover, uniform expression of Tbx6 using a heat shock promoter does not prevent myogenesis in the posterior of newly formed somites. In the context of segmentation, fss/tbx6 is part of a network of genes that regulate each other and other genes, including notch pathway genes, mesp-b, and ripply (Brend and Holley, 2009; reviewed by Oates et al., 2012). The spatial restriction of the activity of one or more of these gene products may restrict Fss/Tbx6 function to the ABCs.
The temporary, uniform expression of fss/tbx6 function restores dermomyotome in a wider domain of the paraxial mesoderm than it restores segmentation. The spatial pattern of segmentation rescue is consistent with a role for Tbx6 in the wavefront region of the PSM. This region of the PSM is characterized by a gradual decrease in fgf8 and her13.2 in wildtype embryos and has been proposed to determine the position of future segment boundaries (reviewed by Holley, 2007). The spatial pattern of dermomyotome rescue after heat shock induced fss/tbx6 expression might indicate that the competence of paraxial mesoderm cells to develop into dermomyotome is more widespread in the paraxial mesoderm than the competence to form a segment boundary and/or that lower levels of gene expression are sufficient to prevent dermomyotome precursors from prematurely differentiating into primary myotome.
The dermomyotome deficit in fss/tbx6 mutant embryos correlates with altered muscle fibre morphologies and a disruption of cellular arrangements in the central trunk and tail. The presence of large muscle fibres with multiple nuclei per cross section—without any decrease in the number of muscle fibres—suggests that additional myonuclei join the fast fibre population in the central myotome. Variations in the shape of these fast fibres might result from the lack of myotome boundaries restricting their length (Henry et al., 2005) and/or the lack of central slow fibre migration promoting their elongation (Henry and Amacher, 2004).
Slow muscle fibres are specified normally in fss/tbx6 mutant embryos. We suggest that the disrupted position of the central slow muscle fibres is an indirect effect of the fss/tbx6 mutation on dermomyotome precursors. Ectopic differentiation of ABCs may cause a change in cell migration, preventing the normal displacement of the slow fibres by the fast fibres. Also, central dermomyotome cells may play a crucial role in displacing muscle fibres during cellular rearrangements in the maturing somite. These effects are likely independent of cell fate determination in the central domain of the myotome, as the muscle pioneers and the medial fast fibres express Engrailed, as in wild type (Wolff et al., 2003).
Whereas zebrafish tbx6 mutants show a loss of segmentation and of the central dermomyotome, mouse tbx6 mutants have an enlarged tailbud, transformation of paraxial mesoderm into neural tube, and loss of laterality in the node (Chapman and Papaioannou, 1998; Hadjantonakis et al., 2008). However, partial loss of tbx6 in mouse has similar consequences to the complete loss of tbx6 in zebrafish—a disruption of segmentation. In particular, partial loss of tbx6 leads to the loss of anterior somite compartment identity, as indicated by the expression of tbx18 (White et al., 2003), and the appearance of fused ribs and vertebrae. These observations are consistent with the acquisition of additional functions by the tbx6 gene in mouse. Those functions may be carried out in zebrafish by the spt/tbx16 gene, which is expressed earlier in the maturation of the PSM, and which when mutant leads to the development of an enlarged tailbud and the loss of laterality in the zebrafish equivalent of the node (Ho and Kane, 1990; Gourronc et al., 2007). Although the tbx16 gene is present in non-mammalian tetrapods, it has apparently been lost in mammals (Fig. 1). If tbx6 in mammals has acquired the combined functions of the tbx6 and tbx16 genes, then the loss of tbx6 would lead to a more severe phenotype in mouse than in zebrafish.
Regionalization of the dermomyotome along the anterior-posterior axis
fss/tbx6 is required for segmentation throughout the entire anterior-posterior extent of the trunk and tail (van Eeden et al., 1996), thus we were surprised to find that the requirement of fss/tbx6 for dermomyotome development is regionally restricted. The regional specificity for the fss/tbx6 requirement along the anterior-posterior axis is reminiscent of other developmental differences between anterior and posterior paraxial mesoderm. In the anterior-most somites, cell differentiation is delayed relative to segmentation: expression of myoD, snail1a, and engrailed arises simultaneously in roughly the first six somites, but sequentially with the formation of each segment in the posterior trunk and tail (Hatta et al., 1991; Ekker et al., 1992; Weinberg et al., 1996; Jülich et al., 2005). Similarly, slow muscle fibres are displaced to the lateral surface simultaneously in the anterior six somites, but sequentially with segmentation in the posterior somites (S.H.D., unpublished; Felsenfeld et al., 1991). The Notch pathway mutants also reveal a regional specificity, as they disrupt segmentation in the posterior trunk and tail but not in the anterior three to nine somites (reviewed by Holley, 2007). Differences between the anterior-most and the more posterior segments are also observed in amniotes. Mouse embryos mutant for either of the transcription factors mesogenin and tbx6 form only the anterior seven somites: the posterior trunk and tail lacks segmented paraxial mesoderm (Chapman and Papaioannou, 1998; Yoon and Wold, 2000).
We suggest that the dermomyotome phenotype in fss/tbx6 mutants indicates that the dermomyotome, like many other aspects of somite patterning, is distinctly regulated in the anterior somites compared to the rest of the trunk and tail.
Regionalization of the dermomyotome along the dorsal-ventral axis
In amniotes, engrailed and sim are expressed in the central portion of the dermomyotome, but not in the dorsal and the ventral dermomyotome (Davis et al., 1991; Gardner and Barald, 1992; Spörle, 2001). Also, the central portion of the dermomyotome de-epithelializes earlier than the dorsomedial and ventrolateral lips of the dermomyotome (Ben-Yair and Kalcheim, 2005; Gros et al., 2005).
In zebrafish, the central dermomyotome is adjacent to the engrailed-expressing muscle pioneer slow fibres, and specifically expresses the sdf1a gene product, which serves as a guidance cue for the migrating lateral line primordium (David et al., 2002; Svetic et al., 2007). We show that the cells of the central dermomyotome begin to express Pax7 later than the cells of the dorsal/ventral dermomyotome and are more likely to differentiate (co-express Pax7 and MRFs) before the end of the segmentation period. Dorsal/ventral dermomyotome cells, in contrast, have higher rates of proliferation after the end of segmentation and show increased MRF co-expression beginning in early larval development (data not shown). These observations suggest that in zebrafish at 24 h, the central dermomyotome is responsible for the initial growth of the primary myotome, while the dorsal/ventral dermomyotome is responsible for a later phase of myogenesis, such as stratified hyperplasia (Steinbacher et al., 2006; Steinbacher et al., 2007).
The requirement for fss/tbx6 provides the first genetic distinction between these two subdivisions of the dermomyotome, and also suggests that the dorsal/ventral portion of the dermomyotome can compensate for the central region. Thus, embryos lacking the tbx6-dependent central dermomyotome domain show only a very early and temporally restricted deficit in muscle growth. We propose that the dorsal and/or ventral dermomyotome is the source of the cells responsible for the recovery of myogenesis in the early larval period. Whether these dorsal/ventral dermomyotome cells and central dermomyotome cells have distinct embryonic origins remains to be determined.
Materials and Methods
Zebrafish, transgenesis, and heat shock
All experiments were done on zebrafish (Danio rerio) from the Wesleyan University strain of wild type fish, and the ti1 or te314a allele of fss/tbx6, which behaves as a null (Nikaido et al., 2002). We detected no differences between wild type and heterozygotes, all embryos labeled “wt” in figures are heterozygous siblings of the homozygous mutants in the same figure. Embryos were cared for using standard procedures (Westerfield, 1995).
The full open reading frame of zebrafish tbx6 was amplified by PCR from a cDNA kindly provided by Scott Holley (New Haven, Connecticut, USA), with Gateway recombination sites added at each end. The PCR product was recombined into the donor vector pDONR221 to make the plasmid pME-TBX6. pME-TBX6 was recombined with the 5′ entry clone containing the zebrafish hsp70promoter (p5E-hsp70), and with the 3′ entry clone containing 6× myc tag plus the SV40 late poly adenylation signal (p3E-MTpA), and a destination vector with tol2 sites and egfp under the control of the cardiac myosin light chain 2 promoter (pDestTol2CG2) (Villefranc et al., 2007). The resulting plasmid (RAD510) is hsp70-tbx6-myc. We also recombined pME-TBX6 with p5E-hsp70, a 3′ entry clone containing cherry (p3Emcherry), and pDestTol2CG2. This resulting plasmid (RAD521) is hsp70-tbx6-cherry.
Single cell fss/tbx6 mutant embryos were injected with hsp70-tbx6-myc or hsp70-tbx6-cherry and tol2 mRNA, and grown to adulthood. A founder was identified on the basis of cardiac eGFP expression in its offspring. These offspring were raised to adulthood and outcrossed to fss/tbx6 homozygous mutants. All crosses generated 50% transgenic embryos, suggesting the presence of only one transgene insertion. All of the shown experiments were done on the v8 allele of tg(hsp70:tbx6-myc), but we obtained qualitatively similar results using tg(hsp70:tbx6-cherry), the allele we used is v7.
Embryos were heat shocked at the indicated time by transferring them in small mesh-bottom wells to embryo medium pre-warmed to 37°C for one hour, and then returned to the standard temperature (28.5°C) for development after heat shock.
In situ hybridization and immunocytochemistry
RNA in situ hybridization and immunohistochemistry was carried out as previously described (Barresi et al., 2000). Embryos in Fig. 2B,D are shown anterior to the top. All other embryos are shown dorsal to the top, and for whole mount labeling, anterior to the left. Imaging of whole mount labeling was with a Zeiss LSM510 confocal microscope, individual optical sections were flattened for each image, except for Fig. 2B,D, which show single optical sections. Black and white were inverted in Fig. 4D for clarity of presentation. Tracings of individual labeled nuclei (Fig. 2G, Fig. 4B) and muscle fibres (Fig. 3C,H) were done on computer projections.
Supplementary Material
Acknowledgments
We thank Frank Stellabotte for initial experiments on the dermomyotome in fss/tbx6 mutant embryos. We thank John Postlethwaite for assistance with the analysis of synteny. We thank Scott Holley and Nathan Lawson for sharing fish and reagents. Our work was supported by NIH: R01 HD37509.
Footnotes
Competing Interests: The authors have no competing interests to declare.
References
- Barresi M. J., Stickney H. L., Devoto S. H. (2000). The zebrafish slow-muscle-omitted gene product is required for Hedgehog signal transduction and the development of slow muscle identity. Development 127, 2189–2199. [DOI] [PubMed] [Google Scholar]
- Barresi M. J., D'Angelo J. A., Hernández L. P., Devoto S. H. (2001). Distinct mechanisms regulate slow-muscle development. Curr. Biol. 11, 1432–1438 10.1016/S0960-9822(01)00428-6 [DOI] [PubMed] [Google Scholar]
- Ben-Yair R., Kalcheim C. (2005). Lineage analysis of the avian dermomyotome sheet reveals the existence of single cells with both dermal and muscle progenitor fates. Development 132, 689–701 10.1242/dev.01617 [DOI] [PubMed] [Google Scholar]
- Brend T., Holley S. A. (2009). Expression of the oscillating gene her1 is directly regulated by Hairy/Enhancer of Split, T-box, and Suppressor of Hairless proteins in the zebrafish segmentation clock. Dev. Dyn. 238, 2745–2759 10.1002/dvdy.22100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham M., Vincent S. D. (2009). Distinct and dynamic myogenic populations in the vertebrate embryo. Curr. Opin. Genet. Dev. 19, 444–453 10.1016/j.gde.2009.08.001 [DOI] [PubMed] [Google Scholar]
- Bussen M., Petry M., Schuster-Gossler K., Leitges M., Gossler A., Kispert A. (2004). The T-box transcription factor Tbx18 maintains the separation of anterior and posterior somite compartments. Genes Dev. 18, 1209–1221 10.1101/gad.300104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catchen J. M., Conery J. S., Postlethwait J. H. (2009). Automated identification of conserved synteny after whole-genome duplication. Genome Res. 19, 1497–1505 10.1101/gr.090480.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman D. L., Papaioannou V. E. (1998). Three neural tubes in mouse embryos with mutations in the T-box gene Tbx6. Nature 391, 695–697 10.1038/35624 [DOI] [PubMed] [Google Scholar]
- Chapman D. L., Agulnik I., Hancock S., Silver L. M., Papaioannou V. E. (1996). Tbx6, a mouse T-Box gene implicated in paraxial mesoderm formation at gastrulation. Dev. Biol. 180, 534–542 10.1006/dbio.1996.0326 [DOI] [PubMed] [Google Scholar]
- Cossu G., Tajbakhsh S., Buckingham M. (1996). How is myogenesis initiated in the embryo? Trends Genet. 12, 218–223 10.1016/0168-9525(96)10025-1 [DOI] [PubMed] [Google Scholar]
- David N. B., Sapède D., Saint-Etienne L., Thisse C., Thisse B., Dambly-Chaudière C., Rosa F. M., Ghysen A. (2002). Molecular basis of cell migration in the fish lateral line: role of the chemokine receptor CXCR4 and of its ligand, SDF1. Proc. Natl. Acad. Sci. USA 99, 16297–16302 10.1073/pnas.252339399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. A., Holmyard D. P., Millen K. J., Joyner A. L. (1991). Examining pattern formation in mouse, chicken and frog embryos with an En-specific antiserum. Development 111, 287–298. [DOI] [PubMed] [Google Scholar]
- Denetclaw W. F., Jr, Christ B., Ordahl C. P. (1997). Location and growth of epaxial myotome precursor cells. Development 124, 1601–1610. [DOI] [PubMed] [Google Scholar]
- Devoto S. H., Stoiber W., Hammond C. L., Steinbacher P., Haslett J. R., Barresi M. J., Patterson S. E., Adiarte E. G., Hughes S. M. (2006). Generality of vertebrate developmental patterns: evidence for a dermomyotome in fish. Evol. Dev. 8, 101–110 10.1111/j.1525-142X.2006.05079.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekker M., Wegner J., Akimenko M. A., Westerfield M. (1992). Coordinate embryonic expression of three zebrafish engrailed genes. Development 116, 1001–1010. [DOI] [PubMed] [Google Scholar]
- Felsenfeld A. L., Curry M., Kimmel C. B. (1991). The fub-1 mutation blocks initial myofibril formation in zebrafish muscle pioneer cells. Dev. Biol. 148, 23–30 10.1016/0012-1606(91)90314-S [DOI] [PubMed] [Google Scholar]
- Feng X., Adiarte E. G., Devoto S. H. (2006). Hedgehog acts directly on the zebrafish dermomyotome to promote myogenic differentiation. Dev. Biol. 300, 736–746 10.1016/j.ydbio.2006.08.056 [DOI] [PubMed] [Google Scholar]
- Gardner C. A., Barald K. F. (1992). Expression patterns of engrailed-like proteins in the chick embryo. Dev. Dyn. 193, 370–388 10.1002/aja.1001930410 [DOI] [PubMed] [Google Scholar]
- Gourronc F., Ahmad N., Nedza N., Eggleston T., Rebagliati M. (2007). Nodal activity around Kupffer's vesicle depends on the T-box transcription factors Notail and Spadetail and on Notch signaling. Dev. Dyn. 236, 2131–2146 10.1002/dvdy.21249 [DOI] [PubMed] [Google Scholar]
- Gouy M., Guindon S., Gascuel O. (2010). SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 27, 221–224 10.1093/molbev/msp259 [DOI] [PubMed] [Google Scholar]
- Gros J., Manceau M., Thomé V., Marcelle C. (2005). A common somitic origin for embryonic muscle progenitors and satellite cells. Nature 435, 954–958 10.1038/nature03572 [DOI] [PubMed] [Google Scholar]
- Groves J. A., Hammond C. L., Hughes S. M. (2005). Fgf8 drives myogenic progression of a novel lateral fast muscle fibre population in zebrafish. Development 132, 4211–4222 10.1242/dev.01958 [DOI] [PubMed] [Google Scholar]
- Hadjantonakis A. K., Pisano E., Papaioannou V. E. (2008). Tbx6 regulates left/right patterning in mouse embryos through effects on nodal cilia and perinodal signaling. PLoS ONE 3, e2511 10.1371/journal.pone.0002511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halloran M. C., Sato-Maeda M., Warren J. T., Su F., Lele Z., Krone P. H., Kuwada J. Y., Shoji W. (2000). Laser-induced gene expression in specific cells of transgenic zebrafish. Development 127, 1953–1960. [DOI] [PubMed] [Google Scholar]
- Hammond C. L., Hinits Y., Osborn D. P., Minchin J. E., Tettamanti G., Hughes S. M. (2007). Signals and myogenic regulatory factors restrict pax3 and pax7 expression to dermomyotome-like tissue in zebrafish. Dev. Biol. 302, 504–521 10.1016/j.ydbio.2006.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatta K., Bremiller R., Westerfield M., Kimmel C. B. (1991). Diversity of expression of engrailed-like antigens in zebrafish. Development 112, 821–832. [DOI] [PubMed] [Google Scholar]
- Henry C. A., Amacher S. L. (2004). Zebrafish slow muscle cell migration induces a wave of fast muscle morphogenesis. Dev. Cell 7, 917–923 10.1016/j.devcel.2004.09.017 [DOI] [PubMed] [Google Scholar]
- Henry C. A., McNulty I. M., Durst W. A., Munchel S. E., Amacher S. L. (2005). Interactions between muscle fibers and segment boundaries in zebrafish. Dev. Biol. 287, 346–360 10.1016/j.ydbio.2005.08.049 [DOI] [PubMed] [Google Scholar]
- Ho R. K., Kane D. A. (1990). Cell-autonomous action of zebrafish spt-1 mutation in specific mesodermal precursors. Nature 348, 728–730 10.1038/348728a0 [DOI] [PubMed] [Google Scholar]
- Holley S. A. (2007). The genetics and embryology of zebrafish metamerism. Dev. Dyn. 236, 1422–1449 10.1002/dvdy.21162 [DOI] [PubMed] [Google Scholar]
- Hollway G. E., Bryson-Richardson R. J., Berger S., Cole N. J., Hall T. E., Currie P. D. (2007). Whole-somite rotation generates muscle progenitor cell compartments in the developing zebrafish embryo. Dev. Cell 12, 207–219 10.1016/j.devcel.2007.01.001 [DOI] [PubMed] [Google Scholar]
- Hug B., Walter V., Grunwald D. J. (1997). tbx6, a Brachyury-related gene expressed by ventral mesendodermal precursors in the zebrafish embryo. Dev. Biol. 183, 61–73 10.1006/dbio.1996.8490 [DOI] [PubMed] [Google Scholar]
- Jülich D., Geisler R., Holley S. A. Tübingen 2000 Screen Consortium(2005). Integrinalpha5 and delta/notch signaling have complementary spatiotemporal requirements during zebrafish somitogenesis. Dev. Cell 8, 575–586 10.1016/j.devcel.2005.01.016 [DOI] [PubMed] [Google Scholar]
- Kahane N., Ben-Yair R., Kalcheim C. (2007). Medial pioneer fibers pattern the morphogenesis of early myoblasts derived from the lateral somite. Dev. Biol. 305, 439–450 10.1016/j.ydbio.2007.02.030 [DOI] [PubMed] [Google Scholar]
- Kawamura A., Koshida S., Hijikata H., Ohbayashi A., Kondoh H., Takada S. (2005). Groucho-associated transcriptional repressor ripply1 is required for proper transition from the presomitic mesoderm to somites. Dev. Cell 9, 735–744 10.1016/j.devcel.2005.09.021 [DOI] [PubMed] [Google Scholar]
- Larkin M. A., Blackshields G., Brown N. P., Chenna R., McGettigan P. A., McWilliam H., Valentin F., Wallace I. M., Wilm A., Lopez R. et al. (2007). Clustal W and Clustal X version 2.0. Bioinformatics 23, 2947–2948 10.1093/bioinformatics/btm404 [DOI] [PubMed] [Google Scholar]
- Nikaido M., Kawakami A., Sawada A., Furutani-Seiki M., Takeda H., Araki K. (2002). Tbx24, encoding a T-box protein, is mutated in the zebrafish somite-segmentation mutant fused somites. Nat. Genet. 31, 195–199 10.1038/ng899 [DOI] [PubMed] [Google Scholar]
- Oates A. C., Morelli L. G., Ares S. (2012). Patterning embryos with oscillations: structure, function and dynamics of the vertebrate segmentation clock. Development 139, 625–639 10.1242/dev.063735 [DOI] [PubMed] [Google Scholar]
- Rowlerson A., Veggetti A. (2001). Cellular mechanisms of post-embryonic muscle growth in aquaculture species. Muscle Development And Growth Johnston I A, ed103–140San Diego, CA, USA: Academic Press. [Google Scholar]
- Sawada A., Fritz A., Jiang Y. J., Yamamoto A., Yamasu K., Kuroiwa A., Saga Y., Takeda H. (2000). Zebrafish Mesp family genes, mesp-a and mesp-b are segmentally expressed in the presomitic mesoderm, and Mesp-b confers the anterior identity to the developing somites. Development 127, 1691–1702. [DOI] [PubMed] [Google Scholar]
- Showell C., Binder O., Conlon F. L. (2004). T-box genes in early embryogenesis. Dev. Dyn. 229, 201–218 10.1002/dvdy.10480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spörle R. (2001). Epaxial-adaxial-hypaxial regionalisation of the vertebrate somite: evidence for a somitic organiser and a mirror-image duplication. Dev. Genes Evol. 211, 198–217 10.1007/s004270100139 [DOI] [PubMed] [Google Scholar]
- Steinbacher P., Haslett J. R., Six M., Gollmann H. P., Sänger A. M., Stoiber W. (2006). Phases of myogenic cell activation and possible role of dermomyotome cells in teleost muscle formation. Dev. Dyn. 235, 3132–3143 10.1002/dvdy.20950 [DOI] [PubMed] [Google Scholar]
- Steinbacher P., Haslett J. R., Obermayer A., Marschallinger J., Bauer H. C., Sänger A. M., Stoiber W. (2007). MyoD and Myogenin expression during myogenic phases in brown trout: a precocious onset of mosaic hyperplasia is a prerequisite for fast somatic growth. Dev. Dyn. 236, 1106–1114 10.1002/dvdy.21103 [DOI] [PubMed] [Google Scholar]
- Stellabotte F., Devoto S. H. (2007). The teleost dermomyotome. Dev. Dyn. 236, 2432–2443 10.1002/dvdy.21253 [DOI] [PubMed] [Google Scholar]
- Stellabotte F., Dobbs-McAuliffe B., Fernández D. A., Feng X., Devoto S. H. (2007). Dynamic somite cell rearrangements lead to distinct waves of myotome growth. Development 134, 1253–1257 10.1242/dev.000067 [DOI] [PubMed] [Google Scholar]
- Svetic V., Hollway G. E., Elworthy S., Chipperfield T. R., Davison C., Adams R. J., Eisen J. S., Ingham P. W., Currie P. D., Kelsh R. N. (2007). Sdf1a patterns zebrafish melanophores and links the somite and melanophore pattern defects in choker mutants. Development 134, 1011–1022 10.1242/dev.02789 [DOI] [PubMed] [Google Scholar]
- Ticho B. S., Stainier D. Y., Fishman M. C., Breitbart R. E. (1996). Three zebrafish MEF2 genes delineate somitic and cardiac muscle development in wild-type and mutant embryos. Mech. Dev. 59, 205–218 10.1016/0925-4773(96)00601-6 [DOI] [PubMed] [Google Scholar]
- van Eeden F. J., Granato M., Schach U., Brand M., Furutani-Seiki M., Haffter P., Hammerschmidt M., Heisenberg C. P., Jiang Y. J., Kane D. A. et al. (1996). Mutations affecting somite formation and patterning in the zebrafish, Danio rerio. Development 123, 153–164. [DOI] [PubMed] [Google Scholar]
- Venters S. J., Thorsteinsdóttir S., Duxson M. J. (1999). Early development of the myotome in the mouse. Dev. Dyn. 216, 219–232 [DOI] [PubMed] [Google Scholar]
- Villefranc J. A., Amigo J., Lawson N. D. (2007). Gateway compatible vectors for analysis of gene function in the zebrafish. Dev. Dyn. 236, 3077–3087 10.1002/dvdy.21354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle F. C., Papaioannou V. E. (2008). Teasing out T-box targets in early mesoderm. Curr. Opin. Genet. Dev. 18, 418–425 10.1016/j.gde.2008.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman R. E. (1969). Development of the lateral musculature in the teleost, Brachydanio rerio: a fine structural study. Am. J. Anat. 125, 457–493 10.1002/aja.1001250406 [DOI] [PubMed] [Google Scholar]
- Weinberg E. S., Allende M. L., Kelly C. S., Abdelhamid A., Murakami T., Andermann P., Doerre O. G., Grunwald D. J., Riggleman B. (1996). Developmental regulation of zebrafish MyoD in wild-type, no tail and spadetail embryos. Development 122, 271–280. [DOI] [PubMed] [Google Scholar]
- Westerfield M. (1995). The Zebrafish Book: A Guide For The Laboratory Use Of Zebrafish (Danio Rerio) Eugene, OR, USA: University of Oregon Press. [Google Scholar]
- White P. H., Farkas D. R., McFadden E. E., Chapman D. L. (2003). Defective somite patterning in mouse embryos with reduced levels of Tbx6. Development 130, 1681–1690 10.1242/dev.00367 [DOI] [PubMed] [Google Scholar]
- Wolff C., Roy S., Ingham P. W. (2003). Multiple muscle cell identities induced by distinct levels and timing of hedgehog activity in the zebrafish embryo. Curr. Biol. 13, 1169–1181 10.1016/S0960-9822(03)00461-5 [DOI] [PubMed] [Google Scholar]
- Yoon J. K., Wold B. (2000). The bHLH regulator pMesogenin1 is required for maturation and segmentation of paraxial mesoderm. Genes Dev. 14, 3204–3214 10.1101/gad.850000 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.