Summary
A myosphere cell is a unique type of muscle stem cell that is able to maintain its pre-myogenic state in culture over time. These cells are propagated in culture as free-floating, non-adherent spheres. We believe that the 3-dimensional adhesive cell-cell interactions involved in maintaining the sphere-like myosphere structures are also involved in maintaining their longevity in culture. We found that Sca-1, which is highly expressed by myosphere cells, plays a role in the growth and the formation of the myospheres. In comparing adhesion molecules expressed by 3-dimensionally grown myosphere cells to those expressed by 2-dimensionally grown primary myoblasts, we found that there was a distinct difference in the expression of β3 integrin. Upon further investigation we discovered that there is an adhesive interaction between Sca-1+ cells and αVβ3 integrin. Here we show that Sca-1+ cells (myosphere cells and NIH3T3 cells) adhere to αVβ3 integrin and that Sca-1− cells (primary myoblasts) do not adhere. The interaction between Sca-1 and αVβ3 integrin was confirmed using antibody blocking, shRNA knockdown of Sca-1 in Sca-1+ cells, and by expressing Sca-1 cDNA in Sca-1− cells, which demonstrated that the level of adhesion of these cells to αVβ3 integrin was dependent on the presence of Sca-1. Additionally, we found that the co-expression of Sca-1 and β3 resulted in significantly greater adhesion of Sca-1+ cells to αVβ3 integrin. In conclusion, our data indicate that Sca-1 is involved in maintaining the 3-dimensional myosphere cell-cell contacts and that Sca-1 is involved in the binding of cells to αVβ3 integrin.
Key words: αVβ3 integrin, Sca-1, Myosphere, Muscle stem cells
Introduction
Stem cell antigen 1 (Sca-1), a member of the Ly6 family, is an 18 kD glycosyl phosphatidylinositol-anchored cell surface protein (GPI-AP) composed of a globular core, where the GPI anchor attaches, and three protruding finger loops (Gumley et al., 1995). Although the purpose of Sca-1 remains unknown it is believed to be involved in balancing stem cell self-renewal and differentiation (Epting et al., 2008b; Ito et al., 2003), stem cell homing (Bradfute et al., 2005; Oh et al., 2003), cell-cell adhesion (English et al., 2000; Pflugh et al., 2002), and concentrating other membrane proteins in lipid rafts to influence signaling (Epting et al., 2008a; Holmes and Stanford, 2007; Pflugh et al., 2002). As it relates to muscle, Sca-1 is expressed by many muscle stem cells including: muscle-derived stem cells (Jankowski et al., 2002; Qu-Petersen et al., 2002), mesoangioblasts (Cossu and Bianco, 2003), side population cells (Gussoni et al., 1999; Jackson et al., 1999), and myosphere stem cells (Sarig et al., 2006; Westerman et al., 2010). In addition, satellite cells, which typically do not express Sca-1, up-regulate Sca-1 expression upon muscle injury (Kafadar et al., 2009) indicating that there is a role for Sca-1 during muscle injury. This is interesting in that it has been proposed that the expression of Sca-1 is not static but is influenced by the microenvironment (Mitchell et al., 2005). In support of this it has been shown that the presence of cytokines such as interferon α/β (Khan et al., 1993; Ma et al., 2001), TNF-α (Long et al., 2011; Sagi-Assif et al., 1996), and TGF-β1 (Long et al., 2011) can influence the expression of Sca-1 in cells, and that even the presence of crushed muscle extract resulted in an increase of Sca-1 expression in primary myoblasts (Kafadar et al., 2009).
Integrins are heterodimeric adhesion molecules composed of alpha and beta subunits. Beta 3 integrin (CD61) interacts with one of two alpha subunits, αIIb (CD41), which is expressed primarily in platelets, and αV (CD51), which is expressed by a variety of tissues including skeletal muscle (Blaschuk et al., 1997; Hynes, 2002). Because our interest in β3 integrin lies in its expression in muscle our focus is on the αVβ3 heterodimer, also known as the vitronectin receptor. αVβ3 integrin primarily uses a RGD sequence to interact with components of the extracellular matrix (Barczyk et al., 2010); however, it has also been shown to bind some of its ligands using non-RGD sequences (Pedchenko et al., 2004; Wu et al., 2006; Ylipaasto et al., 2010). This integrin interacts with a wide variety of ligands, including: ECM components, growth factors, snake venoms, viruses, and CD31 (Stupack and Cheresh, 2004). αVβ3 integrin is most well-known for its involvement in tissue remodeling and repair and is up-regulated in blood vessels after injury (Sajid and Stouffer, 2002). With regard to skeletal muscle, the expression of the αVβ3 integrin has been reported to be involved in myoblast differentiation, its expression peaking during proliferation just prior to differentiation, and then dropping off during myotube formation (Blaschuk et al., 1997; Liu et al., 2011). Most recently, it has been shown that the αVβ3 integrin, which is not normally expressed in adult muscle, is expressed by muscle after injury and by activated satellite cells (Liu et al., 2011). The importance of this expression during injury was further supported by the impaired ability of β3 null mice to repair injured muscle (Liu et al., 2011).
Previously we characterized a unique type of muscle stem cell that can be cultured non-adherently as free-floating spheres (referred to as myospheres). Myosphere cells express Sca-1, β1 integrin, CD34 and CD90, and do not express CD31 or CD45 (Westerman et al., 2010). These cells are easily isolated and can be maintained in a primitive “pre-myogenic” state in culture. Myosphere cells do not express myogenic markers Pax7, Myf5, or MyoD; however, upon differentiation, which is induced by the addition of serum, myosphere cells grow adherently, express Pax7, Myf5, and MyoD, and are able to form multinucleated myotubes (Westerman et al., 2010). In addition, our previous in vivo studies showed myosphere cells are able to contribute to regenerating myofibers as well as to mononuclear cells that reside immediately adjacent to, but within the basal lamina of the myofibers (Westerman et al., 2010). Currently the exact source of myosphere cells is unknown; however, because myosphere cells express Sca-1, which is associated with interstitial cells (Asakura et al., 2002), and because myosphere cells have characteristics similar to other interstitial muscle stem cells such as: muscle SP cells (Asakura et al., 2002; Frank et al., 2006) mesoangioblasts (De Angelis et al., 1999), myoendothelial cells (Zheng et al., 2007), pericytes (Dellavalle et al., 2007), perivascular cells (Crisan et al., 2008), PW1+/Pax7− interstitial cells (PICs) (Mitchell et al., 2010), and B4 integrin+ cells (Liadaki et al., 2012), we expect that, like these muscle stem cells, myosphere cells reside within the interstitial space. In this study we show that the expression of Sca-1 by myosphere stem cells plays a role in maintaining the cell-cell adhesive nature necessary for the formation and maintenance of the sphere-like structures in culture. In further examining the adhesive nature of myosphere cells, we discovered that there is an adhesive interaction that occurs between these muscle stem cells and αVβ3 integrin, and more importantly that this interaction involves Sca-1, indicating that a possible purpose behind the expression of Sca-1 by stem cells is to aid in the repair of injured tissue by interacting with αVβ3 integrin.
Results
Sca-1 is involved in maintaining sphere-like structures formed by myosphere cells
To determine if Sca-1 was involved in the formation and growth of myospheres in culture we isolated myosphere cells from the hind limbs of 4–8 week old C57BL6 mice, FACS sorted these cells for the Sca-1+ and Sca-1− populations (both CD31−/CD45−), and then monitored their growth overtime. After sorting the Sca-1+ and Sca-1− cells were plated at equal densities into 12 well dishes and then monitored daily for the formation and growth of myospheres. In observing 10 independent sorts we found that the cells in the Sca-1+ population were able to form and maintain myospheres more readily than their Sca-1− counterparts. Myospheres generated by the Sca-1+ cell population were found to be visually tighter and more compact than those generated by the Sca-1− cell population, Fig. 1A,B. This adhesiveness was further indicated during the passaging of the myosphere cultures; we found that when we dissociated myospheres into their single cell components using dispase/collagenase it took approximately 30 minutes to dissociate the Sca-1+ myospheres whereas the Sca-1− myospheres took less than 10 minutes. We also monitored the population doublings of the Sca-1+ and Sca-1− sorted cells over the course of 5 weeks. We found that the Sca-1+ cells went through two fold more population doublings and could be maintained in culture more readily for longer periods of time than the Sca-1− cells. This difference is demonstrated in Fig. 1C, which shows that Sca-1+ cell population doubles at twice the rate of the Sca-1− cells.
Fig. 1. Myosphere formation and Population doublings.
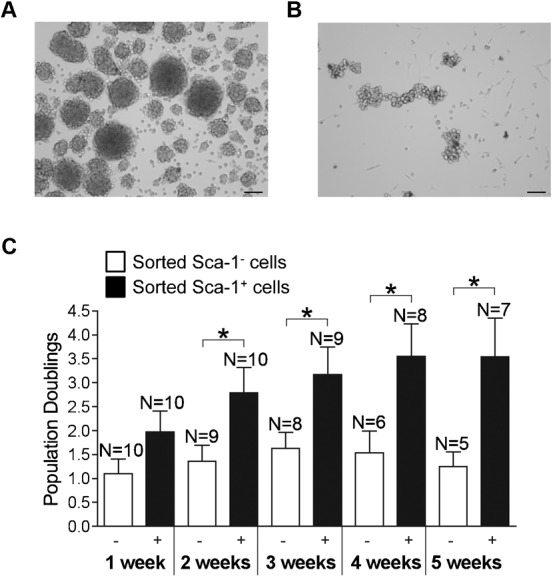
Myospheres generated by sorted (A) Sca-1+ and (B) Sca-1− cells. (C) Population doublings for Sca-1− and Sca-1+ sorted cell populations are shown over the course of 5 weeks. Sca-1− sorted cells are represented in white and Sca-1+ in black. These results were pooled from ten independent sorts. Error bars display ± s.e.m. *P<0.04 by unpaired Student's t test. Measure bars shown in A,B represent 100 µm.
To show that the differences observed between the sorted Sca-1+ and the Sca-1− cells were not simply due to characteristics of two independent cell populations, we knocked down the expression of Sca-1 in actively growing myospheres (at 15, 22 and 48 days after isolation). This was done by first dissociating actively growing myospheres into a single cell suspension using dispase/collagenase and then dividing this suspension evenly into two culture plates, one plate was transduced with a biscistronic lentiviral vector expressing Sca-1 shRNA and GFP (Fig. 2A) and the other plate was transduced with a control vector composed of the same backbone but containing a scrambled shRNA sequence. To ensure that Sca-1 was adequately knocked down by the shRNA, cells were sorted after transduction for either the GFP+/Sca-1− population in the knockdown cells or for the GFP+ population (Sca-1+ control cells); FACS profiles for these cell populations are shown in Fig. 2B–E. After sorting, cells were plated at equal densities and monitored for growth over time. In agreement with the data presented in the previous paragraph, we found the Sca-1+ cells formed tight compact spheres whereas the Sca-1− cells remained mostly independent, occasionally forming loosely attached chains of cells (Fig. 2F,G). We also found that nine days after transduction the cell number was significantly lower in the Sca-1− group than in their Sca-1+ counterparts (0.9±0.3×106 versus 2.6±0.4×106, respectively, n = 3) (Fig. 2H).
Fig. 2. shRNA knockdown of Sca-1.
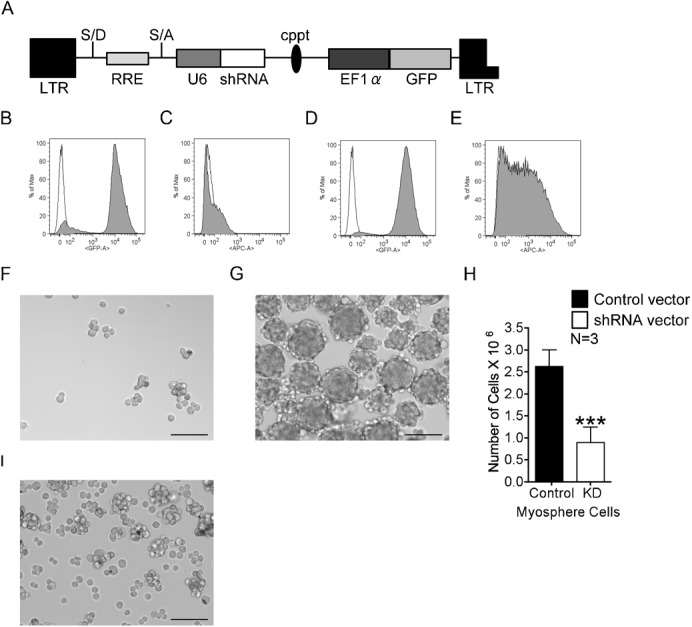
(A) Bicistronic shRNA lentiviral vector used to transduce myosphere cells. FACS profiles showing the expression of GFP and Sca-1, respectively, in myosphere cells transduced by the (B,C) shRNA Sca-1 knockdown vector and (D,E) control shRNA vector. Empty peaks represent the unstained control; filled peaks represent GFP expression and cells incubated with APC conjugated Sca-1 antibody, respectively. Myospheres generated from cells transduced with the (F) Sca-1 shRNA knockdown vector and (G) shRNA control vector. (H) Graph showing the number of myosphere cells nine days after transduction with either the control shRNA vector (black) or the shRNA Sca-1 knockdown vector (white). Error bars display ± s.e.m. ***P<0.0001 by unpaired Student's t test. (I) Myospheres isolated from Sca-1 null mice. Measure bars shown in F,G,I represent 100 µm.
To further confirm the involvement of Sca-1 in the formation and growth of myospheres, we isolated myosphere cells from Sca-1 null mice (Stanford et al., 1997). We found that like the Sca-1− sorted and Sca-1 knockdown cells, these cultures initially remained as independent free-floating cells; however, after a significant delay (approximately 28 days after isolation) the Sca-1 null cells were able to form some small myospheres, approximately 50 µm in size, with some reaching as large as 175 µm. These results indicated that Sca-1 is not essential for the formation of mysopheres but its presence significantly enhances the ability of these cells to form and maintain myospheres in culture. Additionally, we found that the myosphere cells isolated from the Sca-1 null mice did not propagate well in culture, similar to the Sca-1− sorted and knockdown cells we observed a gradual reduction in the number of cells present and the cells that remained did not maintain a uniform cell size (Fig. 2I).
Myosphere cells adhere to αVβ3 integrin
It was expected that because myosphere cells maintain 3-dimensional cell-cell contacts in culture, they would express a greater variety of adhesion molecules than conventionally cultured adherent primary myoblasts. In using reverse transcriptase PCR to examine adhesion molecules expressed by myosphere cells versus those expressed by 2-dimensionally grown primary myoblasts, we found a distinct difference in the expression of β3 integrin between the two types of cells (Fig. 3A). As a control the expression of β1 integrin is shown for these same samples (Fig. 3B). β3 integrin expression was also confirmed by FACS, in which we saw that there was not only a greater percentage of myosphere cells expressing β3 integrin (89.8±2.4%, n = 10) than primary myoblasts (31.4±5.7%, n = 8), but there was also a greater shift in the fluorescence peak, indicating the myosphere cells express a higher level of β3 integrin per cell (Fig. 3C,D).
Fig. 3. Myosphere cells adhere to αVβ3 integrin.
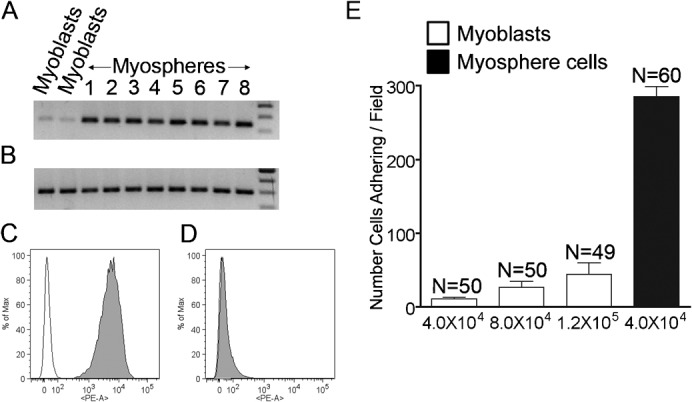
Reverse transcription PCR showing the expression of (A) β3 integrin and (B) β1 integrin in myoblasts and myosphere cells. Representative FACS profiles showing β3 integrin expression by (C) myosphere cells and (D) myoblasts. Empty peaks represent the unstained controls, filled peaks cells incubated with PE conjugated β3 integrin antibody. (E) Graph showing the number of cells adhering/field to αVβ3 integrin versus cells tested, myoblasts are shown in white and myosphere cells in black. These results were pooled from two independent experiments. Error bars display ± s.e.m.
To determine if αVβ3 integrin could be involved in the adhesive contacts that maintain myospheres in culture, we compared the adhesion of myosphere cells and primary myoblasts to αVβ3 integrin protein using a static adhesion assay. Maxisorb 96 well plates were coated with 3 µg/ml of functionally active αVβ3 integrin protein after which 4×104 primary myoblasts or myosphere cells were placed into the coated wells for 1 hr at 37°C. We found significantly more myosphere cells (285.6±13.6) adhered to the αVβ3 integrin coated wells compared to primary myoblasts (11.1±2.4) (Fig. 3E). As a control some wells were coated using an equal amount of BSA; no cells adhered to these wells. To ensure that the difference in adhesion between myosphere cells and primary myoblasts was not due to cell number, we also examined the adhesion of myoblasts to αVβ3 integrin when an excess of cells was added to the wells (8×104 and 1×105). We found that there was an increase in the number of myoblasts adhering; however, the adhesion of myoblasts to the αVβ3 integrin protein remained significantly lower than the adhesion of the myosphere cells, even when an excess of cells were used in this assay (Fig. 3E). To assure that the adhesion of the myosphere cells to the αVβ3 integrin coated wells was not due to non-specific binding but rather due to a specific interaction of these cells with the αVβ3 integrin protein, we blocked some of the αVβ3 integrin coated wells with a functionally blocking antibody (αVβ3 integrin clone LM609) and myosphere cells did not adhere to these wells.
Adhesion of myosphere cells to αVβ3 integrin is reduced by functionally blocking Sca-1 antibodies
Since the results presented in Fig. 3 suggest that Sca-1 plays a role in myosphere cell-cell contact, as well as their maintenance in culture, and because myosphere cells express considerably more Sca-1 than myoblasts (79.6±3.1%, n = 18 versus 32.3±7.9%, n = 10) (Fig. 4A,B), we examined if Sca-1 could be involved in the adhesion of myosphere cells to αVβ3 integrin. To do this we repeated the adhesion assay in the presence of two different functional blocking Sca-1 antibodies (clones E13-161.7 and D7) (Fig. 4C). In these assays we incubated myosphere cells for 30 minutes on ice with varying concentrations of the Sca-1 antibodies (18, 24, and 30 µg/ml) and samples were washed to remove excess antibody prior to placing the mAb treated cells in the αVβ3 integrin protein coated wells. To assure uniformity, those samples that were not incubated with an antibody were handled in an identical fashion as those samples incubated with antibodies. We found that there was a significant dose dependent decrease in the adhesion of myosphere cells to the αVβ3 integrin coated wells when myosphere cells were incubated with either E13 or D7 Sca-1 antibodies. The percent of myosphere cells adhering dropped from 104.5±3.1% (myosphere cells incubated without antibody) to 36.4±2.6% (for the E13-161.7 Sca-1 antibody) and 31.2±2.1% (for the D7 Sca-1 antibody). The percent of primary myoblasts adhering was 4.0±0.6%. As an additional control and to show that the loss of adhesion by the cells incubated with Sca-1 antibody was not due to structural interference caused by steric hindrance (the binding of the antibody to the cells), we incubated cells with an isotype-matched antibody directed to β1 integrin, which is another cell surface marker highly expressed by myosphere cells (Westerman et al., 2010). We found that there was a slight reduction in myosphere cell adhesion after incubation with 30 µg of β1 integrin antibody (95.9±2.4%), indicating that the loss of myosphere cell adhesion to αVβ3 integrin after incubation with a Sca-1 antibody was not simply due to structural interference caused by the antibody.
Fig. 4. Sca-1 antibodies block adhesion to αVβ3 integrin.
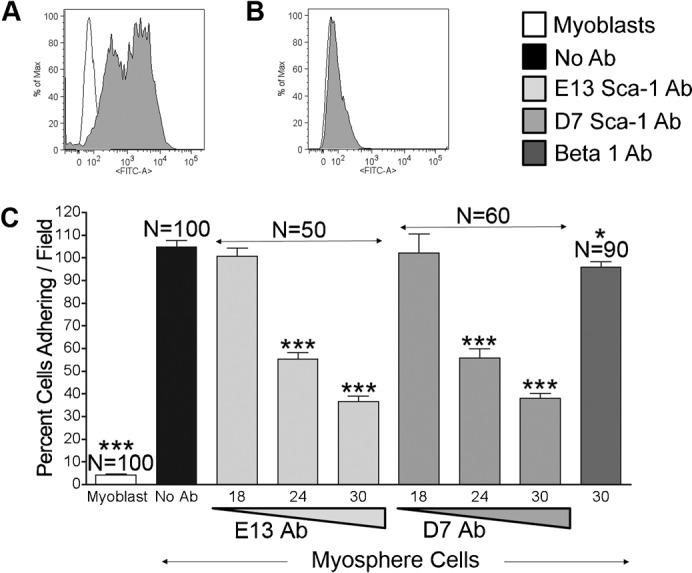
Representative FACS profiles showing Sca-1 expression by (A) myosphere cells and (B) myoblasts. Empty peaks represent unstained controls, filled peaks cells incubated with FITC conjugated Sca-1 antibody. (C) Graph showing percent of cells adhering/field to αVβ3 integrin versus cells incubated with antibody, represented are myoblasts (white), myosphere cells incubated with: no antibody (black), Sca-1 E13 antibody (light gray), Sca-1 D7 antibody (medium gray), and β1 integrin antibody (dark gray). Each of the Sca-1 antibody results was pooled from two independent experiments; the β1 antibody results were pooled from three independent experiments. Error bars display ± s.e.m. *Indicates P = 0.029, ***Indicates P<0.0001 both by unpaired Student's t test.
Knockdown of Sca-1 expression by shRNA reduces adhesion to αVβ3 integrin
In addition to myosphere cells we also examined the adhesion of a second cell type to αVβ3 integrin. The cells chosen were NIH3T3 because these cells express Sca-1 (86.5±1.0%, n = 7) (Fig. 5A) and are easily maintained and passed in culture. To determine if the NIH3T3 cells adhered to αVβ3 integrin we repeated the adhesion assay. We found that NIH3T3 cells adhere significantly more to αVβ3 integrin (53.3±1.9%) than do primary myoblasts (4.0±0.6%); however, to a lesser extent than the myosphere cells (104.5±3.1%). Additionally, when NIH3T3 cells were incubated with Sca-1 blocking antibody (E13 – 30 µg) prior to the adhesion assay we detected a significant reduction in the adhesion of these cells to αVβ3 integrin (20.3±1.1%) (Fig. 5B), which was similar to myosphere cells. In order to further demonstrate that Sca-1 is involved in the adhesion of the NIH3T3 cells to αVβ3 integrin, we knocked down the expression of Sca-1 in these cells using the bicistronic lentiviral vector shown in Fig. 2A. After verifying the loss of Sca-1 expression by FACS and sorting for the Sca-1−/GFP+ cells (Fig. 5C), we then repeated the adhesion assay. As a control we also transduced NIH3T3 cells with same vector, containing a random shRNA sequence (Fig. 5D). We found that there was a significant reduction in the adhesion of the NIH3T3 cells expressing the Sca-1 shRNA (8.8±0.5%), and that the loss of adhesion was significantly greater than that of NIH3T3 cells incubated with the Sca-1 antibody, indicating that Sca-1 is not only involved in the adhesion of these cells but may also be influencing the adhesion of other adhesion molecules expressed by these cells. The adhesion of the control shRNA cells (54.5±2.8%) was the same as the adhesion of the untransduced NIH3T3 cells.
Fig. 5. NIH3T3 cells adhere to αVβ3 integrin.
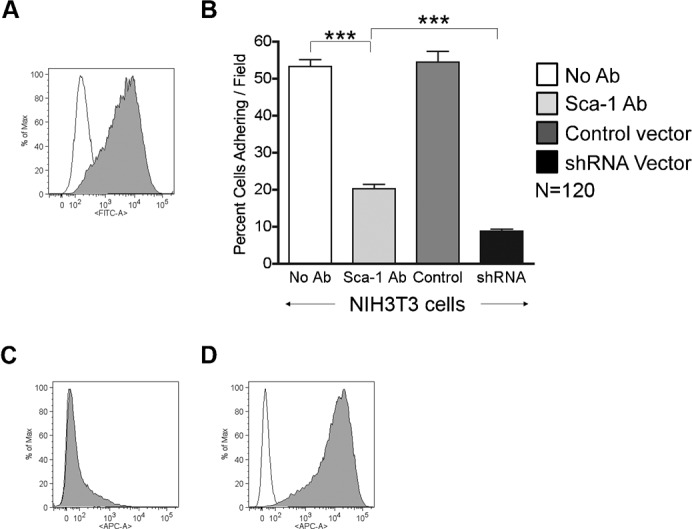
(A) Representative FACS profile showing Sca-1 expression in NIH3T3 cells. Empty peak represents the unstained control; filled peak represents cells incubated with FITC conjugated Sca-1 antibody. (B) Graph showing the percent of cells adhering/field to αVβ3 integrin versus cells tested, represented are NIH3T3 cells with no antibody (white), Sca-1 antibody (light gray), transduced with control shRNA vector (dark gray), and transduced with shRNA Sca-1 knockdown vector (black). FACS profiles show Sca-1 expression in NIH3T3 cells transduced by the (C) shRNA Sca-1 knockdown vector and (D) control shRNA vector. Empty peaks represent the unstained control; filled peaks represent cells incubated with APC conjugated Sca-1 antibody. The results in this figure are pooled from 3 independent experiments. Error bars display ± s.e.m. ***P<0.0001 by unpaired Student's t test.
Primary myoblasts expressing Sca-1 adhere to αVβ3 integrin
In addition to examining what would happen to cell adhesion to αVβ3 integrin upon the loss of Sca-1 expression, we also examined what would happen to cell adhesion in cells with a gain in Sca-1 expression. To do this we transduced cells with a bicistronic lentiviral vector that constitutively expresses Sca-1 and neomycin resistance (Fig. 6A), and as a control we also transduced cells with a vector containing the same backbone but without the Sca-1 cDNA cassette. For these studies we chose to use primary myoblasts because these cells do not adhere well to αVβ3 integrin. After selection with neomycin the expression of Sca-1 was confirmed both by FACS (Fig. 6B) and by western blot (Fig. 6C). We found that myoblasts constitutively expressing Sca-1 were able to bind significantly more to αVβ3 integrin (33.5±1.3%) than myoblasts transduced by the control vector (2.8±0.2%) or untransduced myoblasts (2.8±0.2%) (Fig. 6D). We also found that even after using concentrated virus to transduce the primary myoblasts we were not able to match the extent of adhesion by the myosphere stem cells interacting with αVβ3 integrin (96.7±2.5%), indicating that other factors that are cell type specific may also be playing a role in the adhesion (such as differences in the expression or activation of other adhesion molecules).
Fig. 6. Sca-1 expression in myoblasts increases adhesion to αVβ3 integrin.
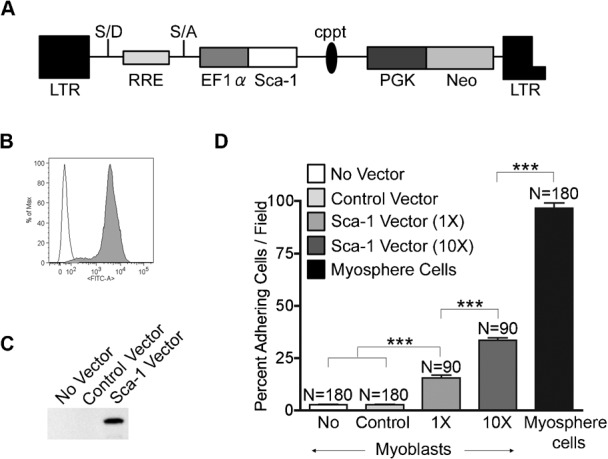
(A) Bicistronic lentiviral vector used to transduce primary myoblasts. (B) FACS profile showing Sca-1 expression by transduced myoblasts. Empty peak represents the unstained control, filled peak myoblasts incubated with FITC conjugated Sca-1 antibody. (C) Western showing the expression of Sca-1 in untransduced, control transduced, and Sca-1 transduced myoblasts. (D) Graph showing the percent of cells adhering/field to αVβ3 integrin versus cells tested, represented are myoblasts (white), myoblasts transduced by the control vector (light gray), myoblasts transduced by the Sca-1 vector (medium gray, MOI = 5) and (dark gray, MOI = 50), and myosphere cells (black). Results were pooled from 6 independent adhesion assays (3 for cell transduced at an MOI = 5, and 3 for those transduced at a MOI = 50). Error bars display ± s.e.m. ***P<0.0001 by unpaired Student's t test.
Adhesion to αVβ3 integrin is enhanced by the co-expression of Sca-1 and β3 integrin
Because myosphere cells express both Sca-1 and β3 integrin we examined if the co-expression of β3 integrin and Sca-1 within the same cell would enhance the adhesion of those cells to αVβ3 integrin. For this experiment we used NIH3T3 cells because these cells express αV integrin (88.8%, n = 13) (Fig. 7A), but do not express β3 integrin (0%, n = 15) (Fig. 7B). We transduced NIH3T3 (Sca-1+) cells with a bicistronic lentiviral vector constitutively expressing mouse β3 integrin (Cluzel et al., 2005) and neomycin resistance. As a control we transduced cells with a vector containing the same backbone but without the β3 integrin cDNA cassette. After selection with neomycin, FACS was used to confirm the expression β3 integrin (Fig. 7C). We repeated the adhesion assay, this time using trypsin to lift the NIH3T3 cells because NIH3T3 cells expressing β3 integrin remained adherent to the plates when dispase/collagenase was used. We found there was a significant increase in the adhesion of the cells that co-expressed Sca-1 and β3 integrin (67.5±2.5%) to the αVβ3 integrin protein versus those expressing Sca-1 alone (27.9±1.3%) (Fig. 7D). In addition, NIH3T3 cells that expressed neither Sca-1 nor β3 integrin had significantly lower adhesion than all of the other NIH3T3 cells tested (10.8±0.4%). The adhesion of cells transduced with the control vectors was the same as the adhesion of untransduced NIH3T3 cells (32.2±1.6%). To further confirm these results, prior to the adhesion assay we incubated the NIH3T3 cells expressing both Sca-1 and β3 integrin with antibodies that functionally block Sca-1, β3 integrin, or both Sca-1 and β3 integrin. We found that there was a significant reduction in the adhesion the cells incubated with the Sca-1 (22.6±0.7%) and β3 integrin (21.3±0.6%) antibodies and that the reduction in adhesion was greater still when cells were incubated with both antibodies (7.5±0.3%) (Fig. 7E). In addition, to show that the adhesion of the NIH3T3 cells was specific for the αVβ3 integrin protein we blocked some of the wells that were coated with the αVβ3 integrin protein with a functionally blocking antibody (αVβ3 integrin clone LM609); there was very little binding of both NIH3T3 cells (1.9±0.2%, n = 60, β3− cells) and β3 expressing NIH3T3 cells (4.5±0.2%, n = 60) to these wells. Additionally, we noted that the adhesion of the NIH3T3 cells in this study (27.9±1.3%) was lower than in the previous adhesion study (53.3±1.9%) (Fig. 5), we suspect the change in the adhesiveness may possibly be due to cleavage of adhesion molecules by trypsin which was used in place of dispase/collagenase.
Fig. 7. β3 integrin enhances the adhesion of Sca-1+ NIH3T3 cells.
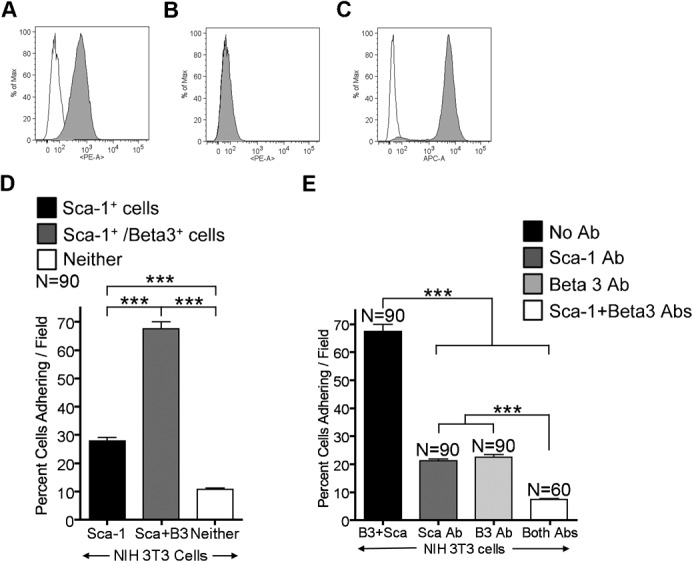
Representative FACS profiles showing the expression of (A) αV integrin and (B) β3 integrin in NIH3T3 cells. Empty peaks represent the unstained control; filled peaks represent cells incubated with PE conjugated αV and β3 integrin antibodies. (C) FACS profile of NIH3T3 cells after transduction with the β3 integrin vector. Empty peak represents the unstained control; filled peak represents cells incubated with APC conjugated β3 integrin antibody. (D) Graph showing the percent of cells adhering/field to αVβ3 integrin versus cells tested, represented are NIH3T3 cells (black), NIH3T3 cells expressing β3 integrin (dark gray), NIH3T3 cells expressing neither Sca-1 or β3 integrin (white). (E) Graph showing the percent of cells adhering/field to αVβ3 integrin versus cells tested, represented are NIH3T3 cells expressing both Sca-1 and β3 integrin incubated with: no antibodies (black), Sca-1 antibody (dark gray), β3 integrin antibody (light gray), both Sca-1 and β3 integrin antibodies (white). The results in D,E were pooled from 3 independent experiments. Error bars display ± s.e.m. ***P<0.0001 by unpaired Student's t test.
To determine if αVβ3 integrin could also be involved in the cell-cell adhesion of myospheres we isolated myosphere stem cells from β3 integrin null mice. We monitored the growth of the β3 integrin null cells and found that these cells could easily form and be propagated as myospheres in culture forming very large myospheres that were greater than 200 µm in size (Fig. 8A), indicating that β3 integrin is not essential for myosphere cell-cell adhesion. These results also suggest that the formation of myospheres is not simply due to a receptor-ligand interaction of αVβ3 integrin to Sca-1 but that perhaps there is one or more factors involved in the adhesion of Sca-1+ cells to the αVβ3 integrin protein. Additionally, we confirmed the lack of β3 integrin expression (Fig. 8B), as well as the presence of Sca-1 (Fig. 8C), in the myospheres that were isolated from the β3 integrin null mice.
Fig. 8. Growth and adhesion of β3 null myospheres.
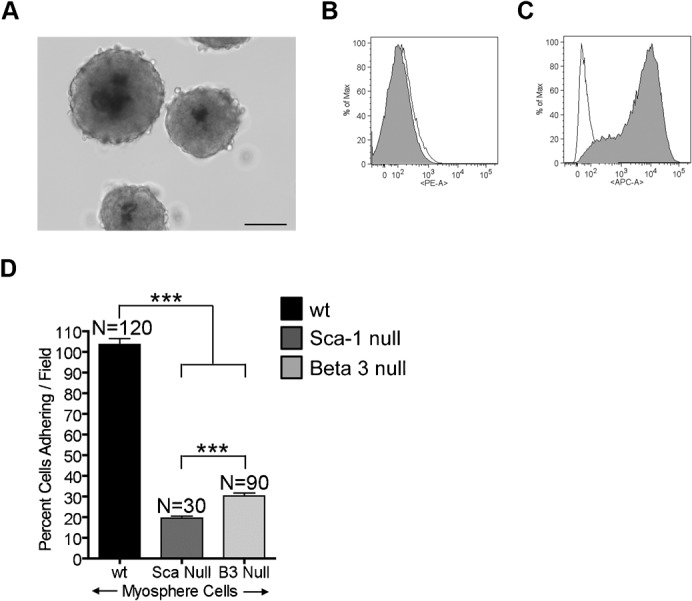
(A) Myospheres generated from β3 integrin null mice. FACS profiles showing the expression of (B) β3 integrin and (C) Sca-1 by β3 null myospheres. Empty peaks represent the unstained controls; filled peaks represents cells incubated with PE conjugated to β3 integrin antibody and APC conjugated to Sca-1 antibody, respectively. (D) Graph showing the percent of cells adhering/field to αVβ3 integrin versus cells tested; represented are myosphere cells isolated from wild type (black), Sca-1 null (dark gray), and β3 integrin null (light gray) mice. These results were pooled from 2 independent experiments. Error bars display ± s.e.m. ***P<0.0001 by unpaired Student's t test. Measure bar shown in A represents 100 µm.
To further demonstrate that the dual expression of Sca-1 and β3 integrin enhances the adhesion of cells to αVβ3 integrin protein, we isolated myosphere stem cells from Sca-1 null and β3 integrin null mice and then repeated the adhesion assay. We found that there was a significant decrease in the adhesion by myosphere cells isolated from either the Sca-1 null mice (19.5±0.9%) or β3 integrin null mice (30.3±1.4%) to αVβ3 integrin protein compared to myosphere cells isolated from wt mice (103.5±2.7%) (Fig. 8D).
Discussion
Myosphere cells are a unique type of muscle stem cell that is capable of maintaining a pre-myogenic state in culture over time (Westerman et al., 2010). We believe that the 3-dimensional cell-cell contacts resulting from the formation of the myospheres aids in their longevity in culture. To determine if Sca-1 was playing a role in these cell-cell interactions, we monitored the formation and growth of myospheres generated from Sca-1+ and Sca-1− cell populations. We found that the expression of Sca-1 is important for maintaining these cells in culture, in that we were not able to generate myospheres from the Sca-1− sorted cell populations or in myosphere cell populations in which Sca-1 was knocked down. We were able to generate some myospheres from Sca-1 null mice, but only after a significant delay and the spheres generated were smaller than myospheres generated from wt mice. The correlation between the expression of Sca-1 and the ability to form myospheres in culture supports our theory that Sca-1 is involved in the cell-cell contacts that maintain these sphere-like structures in culture. However, the ability of the Sca-1 null cells to generate myospheres after a significant delay indicates that there are other factors involved in this adhesion. Additionally, because Sca-1+ myosphere cells could be propagated and maintained more readily in culture than the Sca-1− cells, we believe that Sca-1 is also involved in the proliferation of myosphere cells further indicating the importance of Sca-1 in maintaining these cells in culture.
To determine what other factors were involved in maintaining the 3-dimensional cell-cell contacts of myospheres, we explored the differences in the expression of adhesion molecules in myospheres compared to 2-dimesionally grown primary myoblasts. We found there was a very significant difference in the level of expression of β3 integrin and that myosphere cells were able to adhesively interact with αVβ3 integrin protein whereas primary myoblasts did not. Knowing that Sca-1 has some involvement in myosphere cell-cell contacts we began to examine Sca-1 as a possible ligand for αVβ3 integrin. Using four independent approaches we were able to demonstrate that Sca-1 is involved in the adhesion of myosphere cells to αVβ3 integrin. These methods include: (1) incubating myosphere cells with Sca-1 blocking antibodies, which resulted in a significant reduction in their adhesion to αVβ3 integrin protein, (2) using a second Sca-1 expressing cell type (NIH3T3 cells) to confirm the loss of adhesion to αVβ3 integrin protein when the expression of Sca-1 is knocked down by shRNA, (3) constitutively expressing Sca-1 in primary myoblasts, which normally do not adhere to αVβ3 integrin protein, and demonstrating a significant increase in their adhesion to αVβ3 integrin protein and finally (4) testing the adhesion of myosphere cells isolated from Sca-1 null mice in which we found there was a significant reduction in the adhesion of these cells to the αVβ3 integrin protein compared to myosphere cells isolated from wt mice.
We also noted that depending on the cell type used (myosphere stem cells, myoblasts, or NIH3T3 cells) there are different degrees of adhesion to αVβ3 integrin. An example of this is the over expression of Sca-1 in myoblasts. Even though their adhesion to αVβ3 integrin significantly increased, these cells did not adhere at the same frequency as myosphere cells. This indicated to us that Sca-1 is not acting alone and that there are additional adhesion molecules involved in maintaining the adhesive nature of the myosphere cells. This hypothesis correlates with our blocking antibody studies in which we could significantly reduce the adhesion to αVβ3 integrin by incubating myosphere cells with the Sca-1 antibody but could not completely eliminate the adhesion.
Because Sca-1 and β3 integrin are both highly expressed by myosphere cells we decided to examine if the dual expression of these molecules was responsible for the enhanced adhesion of these cells to αVβ3 integrin. We found that there was a significant increase in the adhesion of the Sca-1+ NIH3T3 cells to αVβ3 integrin when these cells also expressed β3 integrin, and that myosphere cells expressing both Sca-1 and β3 integrin had much greater adhesion to αVβ3 integrin than myosphere cells that were generated from either Sca-1 null or β3 integrin null mice. Interestingly, even though the addition of β3 integrin enhances the adhesion of the Sca-1+ cells to αVβ3 integrin protein we found that β3 integrin itself is not needed to form or maintain myospheres in culture. Taken together these data indicate that β3 integrin is not interacting with Sca-1 by a trans mechanism but may be influencing the adhesion of these cells through a cis mechanism. Additionally, these data indicate that it is likely there are other unknown component(s) involved in the adhesion of the Sca-1+ cells to αVβ3 integrin, and that this/these component(s) interact with both Sca-1 and αVβ3 integrin.
It is intriguing that even though Sca-1 has been used for years as a marker for stem cells its function remains unknown (Holmes and Stanford, 2007). The possibility that Sca-1 acts as an adhesion molecule has been reported (English et al., 2000; Pflugh et al., 2002); however, a ligand for Sca-1 has never been identified. Here we report that Sca-1 is involved in cell adhesion, possibly acting as an adhesion molecule itself. These data demonstrate that the expression of Sca-1 by cells is involved in the adhesion of those cells to αVβ3 integrin. Interestingly, this is not the first suggestion that a Ly6 family member interacts with αVβ3 integrin. Previous studies by Wu et al. showed Ly6 protein cobra cardiotoxin A5 binds to αVβ3 integrin using a non-RGD binding site (Wu et al., 2006). In addition, urokinase-type plasminogen activator receptor (uPAR), another Ly6 protein, has been reported to co-immunoprecipitate with αVβ3 integrin (Maupas-Schwalm et al., 2009) and is also believed to influence the activation and adhesion of αVβ3 integrin resulting in the migration of cells by promoting the degradation of the ECM (Madsen and Sidenius, 2008; Smith and Marshall, 2010). We propose that like these other Ly6 proteins Sca-1 is interacting with αVβ3 integrin and that perhaps the purpose of Sca-1 expression is to enhance the adhesion and migration of cells needed to repair injured tissues by interacting with αVβ3 integrin. In a recent study, Sca-1 null mice were shown to exhibit impaired muscle regeneration due to an inability to remodel the extracellular matrix (Kafadar et al., 2009). This research supports our hypothesis, in that αVβ3 integrin is involved in the activation of matrix metalloproteinases (MMPs), which are needed to remodel the matrix (Brooks et al., 1996; Deryugina et al., 2001; Maupas-Schwalm et al., 2009; Rupp et al., 2008).
In summary, here we present data demonstrating for the first time that Sca-1+ cells adhere to αVβ3 integrin and that this adhesion is enhanced by the presence of β3 integrin. We believe that the interaction of Sca-1 with αVβ3 integrin is important in that it gives a view into possible mechanisms of how stem cells are involved in the repair of injured tissue. Further studies will be needed in order to determine if the relationship between Sca-1 and αVβ3 integrin is a direct ligand to receptor interaction and/or if the expression of Sca-1 is influencing the binding of other adhesion molecules that are direct ligands of αVβ3 integrin.
Materials and Methods
Cells and cell culture
Hind limb muscles from 4–12 week old wild type C57BL/6 mice (Charles River Laboratories; Wilmington, MA, USA), and β3 null mice (B6;129S2-Itgb3tm1Hyn/J, Jackson Laboratories; Bar Harbor, ME, USA) were used to isolate primary myoblasts and myospheres. Sca-1 null mice were also used to isolate myospheres, these mice were backcrossed for >10 generations on the C57BL/6J background and were provided by Dr. William Stanford (Stanford et al., 1997). All experimental procedures were conducted in accordance with the Harvard Medical School Standing Committee on Animals. Primary myoblasts were isolated using the protocol described by Richler and Yaffe (Richler and Yaffe, 1970; Yaffe, 1968) in combination with the pre-plating technique described by Qu-Peterson et al. (Qu-Petersen et al., 2002). Primary myoblasts were cultured in F10 medium (Invitrogen; Grand Island, NY, USA) containing 20% FCS (Hyclone; Waltham, MA, USA), 100 U/ml Pen/Strep (Invitrogen), and 5 ng/ml bFGF (PeproTech; Rocky Hill, NJ, USA) on collagen coated dishes. Myospheres were isolated and maintained as we previously described in (Westerman et al., 2010). Briefly, hind limb muscles were minced and then enzymatically dissociated by a mixture of 2.4 U/ml dispase and 10 mg/ml collagenase A (both from Roche; Indianapolis, IN, USA) for 1 hour at 37°C. After digestion the slurry was dissociated further using a scalpel and then F10 medium containing 20% FCS was added to inactivate the dispase/collagenase. The slurry was passed through a 70 µm cell strainer (BD Falcon; Franklin Lakes, NJ, USA) and centrifuged for 15 minutes at 156 × g. Cell pellets were resuspended in 1 ml of red blood cell lysis buffer (0.15 M ammonium chloride/0.01 M potassium bicarbonate solution, pH 7.4) for 2½ minutes on ice. After lysis 20 ml of DMEM:F12 (1:1) was added and the cells were pelleted and then resuspended in 5 ml of B27 medium (1:1 DMEM:F12 medium containing B27 supplement and 100 U/ml Pen/Strep (all from Invitrogen)), triturated, filtered through 40 µm cell strainer (BD Falcon), and then brought up and cultured in B27 medium supplemented with 20 ng/ml bFGF, 20 ng/ml hEGF (both from PeproTech), and 2 µg/ml heparin (Stem Cell Technologies; Vancouver, BC, Canada). NIH3T3 cells were cultured in DMEM containing 10% CS and 100 U/ml Pen/Strep (all from Invitrogen).
Lentiviral constructs
The shRNA vector was subcloned into a modified version of the lentiviral vector described earlier in (Westerman et al., 2007). The modified vector contained a U6 promoter, a multiple cloning site for inserting the shRNA (BamH1, EcoR1, Sal1, and Mlu1), a central polypurine tract, and an Ef1α promoter driving GFP. We used the BamH1 and Mlu1 sites to insert an oligo (from Invitrogen) containing the shRNA sequence (GAA CAA TCT TTG CTT ACC CAT), which had been reported previously to knock down Sca-1 (Upadhyay et al., 2011). The final shRNA lentiviral vector is shown in Fig. 2A. As a control we inserted a scramble shRNA sequence into the same vector (GAA CAA TCT GTG CTT ACC CAT). The Sca-1 cDNA lentiviral vector was also made by subcloning into a modified version of the lentiviral vector described in (Westerman et al., 2007). The Sca-1 cDNA was obtained from Origene (pCMV6-Kan/Neo; Rockville, MD, USA). This modified vector contained an EF1α promoter, a multiple cloning site for inserting the Sca-1 cDNA (EcoR1, Hpa1, Sal1, and Mlu1), a central polypurine tract, and a PGK promoter driving neomycin. The Origene Sca-1 plasmid was digested with EcoR1, blunted, and ligated to a blunted Xho1 site in a modified pUC19 plasmid containing cloning sites for Mlu1, Xho1, and EcoR1. The Sca-1 cDNA was then removed from the pUC19 plasmid and placed into the lentiviral vector using the EcoR1 and Mlu1 sites. The final Sca-1 lentiviral vector is shown in Fig. 6A. The β3 integrin lentiviral vector was made in a similar fashion as the Sca-1 vector. Dr. Bernhard Wehrle-Haller (Cluzel et al., 2005) provided us with a β3 integrin-GFP fusion plasmid. This plasmid was digested with BamH1 and EcoR1 and then β3 integrin-GFP cassette was ligated into a pUC19 plasmid. The β3 integrin-GFP fusion cassette was then removed from the pUC19 plasmid and placed into the lentiviral vector using the EcoR1 and Sal1 restriction sites. The backbone lentiviral vector without the cDNA of Sca-1 or β3 integrin was used as a negative control (referred to as “empty vector”).
Lentiviral infection
Virus was produced by transient transfection of 293T cells with 3.2 µg of lentiviral vector, 4.0 µg Gag-Pol-Vif plasmid, and 0.4 µg of each Rev, Tat, and VSV-G using Fugene (Roche: Indianapolis, IN, USA) as described earlier in (Westerman et al., 2007). Supernatants were collected 48 hours after transfection and cells (primary myoblasts or NIH3T3) were transduced using 1 ml virus/10 cm dish in the presence of 6 µg/ml protamine sulfate (Sigma; Saint Louis, MO, USA) for 3 hours. For cells transduced with neomycin resistant vectors, two days after transduction cells were split in medium containing 800 µg/ml G418 (Invitrogen).
Flow cytometry
Myosphere cells were sorted into Sca-1+ and Sca-1− cell populations using a BD FACSAria (BD Biosciences; Franklin Lakes, NJ, USA), cells were labeled with PE conjugated to CD31 (e-Biosciences; San Diego, CA, USA) and CD45 (BD Pharmingen; San Diego, CA USA) antibodies to eliminate any possible endothelial or hematopoietic cells, and FITC conjugated to Sca-1 (BD Pharmingen) to mark the cell populations of interest. During the sort, propidium iodide (Invitrogen) was added to gate out dead cells. For the shRNA sort myosphere cells and NIH3T3 cells transduced with the shRNA vector were labeled with APC conjugated to Sca-1 (e-Bioscience). Cells were sorted for the APC negative (Sca-1−) GFP positive population. For FACS analysis PE and FITC conjugated Sca-1 (BD Pharmingen) and PE conjugated CD61 (β3 integrin, e-Biosciences) antibodies were used. Myospheres were dissociated to single cells using a mixture of 2.4 U/ml dispase and 10 mg/ml collagenase A, and primary myoblasts were lifted from the plate using PBS containing 2% FCS and 2 mM EDTA. Myosphere cells were washed 1× with DMEM:F12 and 1× with HANKS containing 0.1% BSA. NIH3T3 cells were washed once with HANKS containing 2% FCS (HF). Cells were incubated with conjugated antibodies for 1 hr on ice. After antibody incubation, myosphere cells were washed in HANKS containing 0.1% BSA and NIH 3T3 cells were washed twice in HF. Myosphere cells were resuspended in 200 µl HANKS containing 0.5% BSA and NIH3T3 cells were resuspended in HF for analysis. For each sample 10,000 cells were analyzed. Unstained cells were used as a negative control. Data analysis was done using FlowJo (TreeStar, Inc; Ashland, OR, USA).
RNA Isolation, RT-PCR
Total RNA was extracted from myoblasts and myosphere cells using the RNeasy Plus RNA isolation kit (Qiagen; Germantown, MD, USA). GeneAMP RNA PCR kit (Applied Biosystems; Carlsbad, CA, USA) was used for reverse transcription of RNA and amplification of cDNA. Primers used for β3 Integrin were GGT ACC AAG TTG GCC TCT CA and GAT TAC GGG ACA CGC TCT GT (244 bp). β1 Integrin was used as a control; the primers used were TGG GAC ATT TGA GTG TGG AG and AGC ATT CAC AAA CAC GAC ACC (302 bp). Samples were denatured at 95°C for 1 minute, followed by amplification: 94°C for 30 seconds (denature), 60°C for 30 seconds (anneal), and 72°C for 1 minute (extension) for 35 cycles, followed by 72°C 10 minutes.
Immunoblotting
Protein was extracted using MEM-PER Eukaryotic Membrane Protein Extraction Reagent Kit (Pierce; Rockford, IL, USA) according to the manufactures protocol. Membrane and cytosolic fractions were collected separately and Slide-A-Lyzer Dialysis Cassettes (10,000 MWCO Pierce) were used to dialyze the samples overnight against 3 changes of 0.5% CHAPS buffer (200 ml). Protein concentrations were measured using the BCA Protein Assay Kit (Pierce). Sca-1 samples were run under non-reducing conditions; reducing conditions were used for control samples (GAPDH). Equal amounts of protein (30 µg) were separated on a 15% SDS-polyacrylamide gel and transferred to 0.2 µm PVDF membranes. Membranes were blocked with 5% non-fat dry milk diluted in PBST (0.05% TWEEN20 in PBS) for 1 hour and then incubated with primary antibody for 2 hours at room temperature. Primary antibodies were diluted in blocking solution, these antibodies include Sca-1 rabbit monoclonal (Epitomics; Burlingame, CA, USA) used at 1 µg/ml and GAPDH (Santa Cruz Biotechnologies; Santa Cruz, CA, USA) used at 1:500. Membranes were washed 3× for 10 min in PBST and then incubated for 1 hour with HRP-conjugated secondary antibody that was diluted at 1:5,000 (goat anti-rabbit, Pierce). The immunoreaction was detected using Supersignal West Dura Chemiluminescent Substrate (Pierce). Images and densitometric analysis were performed using Kodak Imagestation 4000 mm Pro (Carestream Health; Woodbridge, CT, USA).
Static adhesion assay
NUNC Maxisorb 96 well plates were coated with 3 µg/ml of recombinant human αVβ3 integrin protein (R&D Systems; Minneapolis, MN, USA). Control wells were coated with an equal concentration of BSA. Twenty hours after coating, wells were washed with HANKS buffer, and then blocked with HANKS containing 1 mM MgCl2 and 1 mM CaCl2 (HANKS+) and 1% BSA for 1 hr at room temperature. As an additional control some of the αVβ3 integrin coated wells were incubated with 10 µg/ml of an antibody that functionally blocks αVβ3 integrin (clone LM609, Millipore; Billerica, MA, USA). Primary myoblasts, myosphere cells, and NIH3T3 cells were collected, washed, and then resuspended at a concentration of 4×104 cells/150 µl in HANKS+ containing 0.5% BSA. Cells were placed in the wells for 1 hr at 37°C, and then washed 3× with HANKS to remove the unbound cells. To determine if Sca-1 was adhering to αVβ3 integrin, we blocked interactions with Sca-1 by incubating cells with 30 µg/ml of Sca-1 antibodies (clone E13-161.7, BD Pharmingen or D7, e-Bioscience), on ice for 30 minutes. As a control cells were incubated with 30 µg/ml β1 integrin antibody (clone 265917, R&D Systems). Samples were washed to remove excess antibody, and then applied to the appropriate wells for 1 hr at 37°C. Cell samples for each well were prepared independently, those samples that were not incubated with an antibody were handled the same as those incubated with antibodies to maintain uniformity from one sample to the next. Adhesion was quantified by counting the number of cells that adhere in ten 20× fields for each sample tested. The percent of cells adhering was determined relative to the adhesion of myosphere cells incubated with the matching isotype control antibody (number of test cells adhering/field divided by the mean number of isotype control cells adhering/field ×100).
Statistical analysis
In all experiments, results are expressed as means ± s.e.m. Statistical differences between two sets of data were determined using the unpaired Student's t test; P<0.05 was considered statistically different. All statistical calculations were performed using GraphPad Prism 5 software (GraphPad Software; La Jolla, CA, USA).
Acknowledgments
We thank Charles A. Vacanti for his encouragement and support of this research. We thank William Stanford for generously providing the Sca-1 null mice and Bernhard Wehrle-Haller for kindly providing the mouse β3 integrin cDNA. We also thank Emanuela Gussoni, Paul D. Allen, and Francis W. Luscinskas for their critical reading of the manuscript. This work was supported by a grant from NIH-NIAMS (K01AR52372) to K.A.W. and by the Brigham and Women's Hospital Department of Anesthesia, Perioperative and Pain Medicine research fund.
Footnotes
Competing interests: The authors have no competing interests to declare.
References
- Asakura A., Seale P., Girgis-Gabardo A., Rudnicki M. A. (2002). Myogenic specification of side population cells in skeletal muscle. J. Cell Biol. 159, 123–134 10.1083/jcb.200202092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barczyk M., Carracedo S., Gullberg D. (2010). Integrins. Cell Tissue Res. 339, 269–280 10.1007/s00441-009-0834-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschuk K. L., Guérin C., Holland P. C. (1997). Myoblast αvβ3 integrin levels are controlled by transcriptional regulation of expression of the β3 subunit and down-regulation of β3 subunit expression is required for skeletal muscle cell differentiation. Dev. Biol. 184, 266–277 10.1006/dbio.1997.8527 [DOI] [PubMed] [Google Scholar]
- Bradfute S. B., Graubert T. A., Goodell M. A. (2005). Roles of Sca-1 in hematopoietic stem/progenitor cell function. Exp. Hematol. 33, 836–843 10.1016/j.exphem.2005.04.001 [DOI] [PubMed] [Google Scholar]
- Brooks P. C., Strömblad S., Sanders L. C., von Schalscha T. L., Aimes R. T., Stetler-Stevenson W. G., Quigley J. P., Cheresh D. A. (1996). Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin αvβ3. Cell 85, 683–693 10.1016/S0092-8674(00)81235-0 [DOI] [PubMed] [Google Scholar]
- Cluzel C., Saltel F., Lussi J., Paulhe F., Imhof B. A., Wehrle-Haller B. (2005). The mechanisms and dynamics of αvβ3 integrin clustering in living cells. J. Cell Biol. 171, 383–392 10.1083/jcb.200503017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossu G., Bianco P. (2003). Mesoangioblasts–vascular progenitors for extravascular mesodermal tissues. Curr. Opin. Genet. Dev. 13, 537–542 10.1016/j.gde.2003.08.001 [DOI] [PubMed] [Google Scholar]
- Crisan M., Yap S., Casteilla L., Chen C. W., Corselli M., Park T. S., Andriolo G., Sun B., Zheng B., Zhang L. et al. (2008). A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3, 301–313 10.1016/j.stem.2008.07.003 [DOI] [PubMed] [Google Scholar]
- De Angelis L., Berghella L., Coletta M., Lattanzi L., Zanchi M., Cusella-De Angelis M. G., Ponzetto C., Cossu G. (1999). Skeletal myogenic progenitors originating from embryonic dorsal aorta coexpress endothelial and myogenic markers and contribute to postnatal muscle growth and regeneration. J. Cell Biol. 147, 869–878 10.1083/jcb.147.4.869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellavalle A., Sampaolesi M., Tonlorenzi R., 1, Tagliafico E., Sacchetti B., Perani L., Innocenzi A., Galvez B. G., Messina G., Morosetti R. et al. (2007). Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat. Cell Biol. 9, 255–267 10.1038/ncb1542 [DOI] [PubMed] [Google Scholar]
- Deryugina E. I., Ratnikov B., Monosov E., Postnova T. I., DiScipio R., Smith J. W., Strongin A. Y. (2001). MT1-MMP initiates activation of pro-MMP-2 and integrin αvβ3 promotes maturation of MMP-2 in breast carcinoma cells. Exp. Cell Res. 263, 209–223 10.1006/excr.2000.5118 [DOI] [PubMed] [Google Scholar]
- English A., Kosoy R., Pawlinski R., Bamezai A. (2000). A monoclonal antibody against the 66-kDa protein expressed in mouse spleen and thymus inhibits Ly-6A.2-dependent cell-cell adhesion. J. Immunol. 165, 3763–3771. [DOI] [PubMed] [Google Scholar]
- Epting C. L., King F. W., Pedersen A., Zaman J., Ritner C., Bernstein H. S. (2008a). Stem cell antigen-1 localizes to lipid microdomains and associates with insulin degrading enzyme in skeletal myoblasts. J. Cell. Physiol. 217, 250–260 10.1002/jcp.21500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epting C. L., López J. E., Pedersen A., Brown C., Spitz P., Ursell P. C., Bernstein H. S. (2008b). Stem cell antigen-1 regulates the tempo of muscle repair through effects on proliferation of α7 integrin-expressing myoblasts. Exp. Cell Res. 314, 1125–1135 10.1016/j.yexcr.2007.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank N. Y., Kho A. T., Schatton T., Murphy G. F., Molloy M. J., Zhan Q., Ramoni M. F., Frank M. H., Kohane I. S., Gussoni E. (2006). Regulation of myogenic progenitor proliferation in human fetal skeletal muscle by BMP4 and its antagonist Gremlin. J. Cell Biol. 175, 99–110 10.1083/jcb.200511036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumley T. P., McKenzie I. F., Sandrin M. S. (1995). Tissue expression, structure and function of the murine Ly-6 family of molecules. Immunol. Cell Biol. 73, 277–296 10.1038/icb.1995.45 [DOI] [PubMed] [Google Scholar]
- Gussoni E., Soneoka Y., Strickland C. D., Buzney E. A., Khan M. K., Flint A. F., Kunkel L. M., Mulligan R. C. (1999). Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature 401, 390–394 10.1038/43919 [DOI] [PubMed] [Google Scholar]
- Holmes C., Stanford W. L. (2007). Concise review: stem cell antigen-1: expression, function, and enigma. Stem Cells 25, 1339–1347 10.1634/stemcells.2006-0644 [DOI] [PubMed] [Google Scholar]
- Hynes R. O. (2002). Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 10.1016/S0092-8674(02)00971-6 [DOI] [PubMed] [Google Scholar]
- Ito C. Y., Li C. Y., Bernstein A., Dick J. E., Stanford W. L. (2003). Hematopoietic stem cell and progenitor defects in Sca-1/Ly-6A-null mice. Blood 101, 517–523 10.1182/blood-2002-06-1918 [DOI] [PubMed] [Google Scholar]
- Jackson K. A., Mi T., Goodell M. A. (1999). Hematopoietic potential of stem cells isolated from murine skeletal muscle. Proc. Natl. Acad. Sci. USA 96, 14482–14486 10.1073/pnas.96.25.14482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski R. J., Deasy B. M., Cao B., Gates C., Huard J. (2002). The role of CD34 expression and cellular fusion in the regeneration capacity of myogenic progenitor cells. J. Cell Sci. 115, 4361–4374 10.1242/jcs.00110 [DOI] [PubMed] [Google Scholar]
- Kafadar K. A., Yi L., Ahmad Y., So L., Rossi F., Pavlath G. K. (2009). Sca-1 expression is required for efficient remodeling of the extracellular matrix during skeletal muscle regeneration. Dev. Biol. 326, 47–59 10.1016/j.ydbio.2008.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan K. D., Shuai K., Lindwall G., Maher S. E., Darnell J. E., Jr and Bothwell A. L. (1993). Induction of the Ly-6A/E gene by interferon α/β and γ requires a DNA element to which a tyrosine-phosphorylated 91-kDa protein binds. Proc. Natl. Acad. Sci. USA 90, 6806–6810 10.1073/pnas.90.14.6806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liadaki K., Casar J. C., Wessen M., Luth E. S., Jun S., Gussoni E., Kunkel L. M. (2012). β4 integrin marks interstitial myogenic progenitor cells in adult murine skeletal muscle. J. Histochem. Cytochem. 60, 31–44 10.1369/0022155411428991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Niu A., Chen S. E., Li Y. P. (2011). β3-integrin mediates satellite cell differentiation in regenerating mouse muscle. FASEB J. 25, 1914–1921 10.1096/fj.10-170449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long K. K., Montano M., Pavlath G. K. (2011). Sca-1 is negatively regulated by TGF-β1 in myogenic cells. FASEB J. 25, 1156–1165 10.1096/fj.10-170308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Ling K. W., Dzierzak E. (2001). Cloning of the Ly-6A (Sca-1) gene locus and identification of a 3′ distal fragment responsible for high-level γ-interferon-induced expression in vitro. Br. J. Haematol. 114, 724–730 10.1046/j.1365-2141.2001.02986.x [DOI] [PubMed] [Google Scholar]
- Madsen C. D., Sidenius N. (2008). The interaction between urokinase receptor and vitronectin in cell adhesion and signalling. Eur. J. Cell Biol. 87, 617–629 10.1016/j.ejcb.2008.02.003 [DOI] [PubMed] [Google Scholar]
- Maupas-Schwalm F., Bedel A., Augé N., Grazide M. H., Mucher E., Thiers J. C., Salvayre R., Nègre-Salvayre A. (2009). Integrin αvβ3, metalloproteinases, and sphingomyelinase-2 mediate urokinase mitogenic effect. Cell. Signal. 21, 1925–1934 10.1016/j.cellsig.2009.08.010 [DOI] [PubMed] [Google Scholar]
- Mitchell K. J., Pannérec A., Cadot B., Parlakian A., Besson V., Gomes E. R., Marazzi G., Sassoon D. A. (2010). Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nat. Cell Biol. 12, 257–266 10.1038/ncb2025 [DOI] [PubMed] [Google Scholar]
- Mitchell P. O., Mills T., O'Connor R. S., Kline E. R., Graubert T., Dzierzak E., Pavlath G. K. (2005). Sca-1 negatively regulates proliferation and differentiation of muscle cells. Dev. Biol. 283, 240–252 10.1016/j.ydbio.2005.04.016 [DOI] [PubMed] [Google Scholar]
- Oh H., Bradfute S. B., Gallardo T. D., Nakamura T., Gaussin V., Mishina Y., Pocius J., Michael L. H., Behringer R. R., Garry D. J. et al. (2003). Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc. Natl. Acad. Sci. USA 100, 12313–12318 10.1073/pnas.2132126100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedchenko V., Zent R., Hudson B. G. (2004). αvβ3 and αvβ5 integrins bind both the proximal RGD site and non-RGD motifs within noncollagenous (NC1) domain of the α3 chain of type IV collagen. Implication for the mechanism of endothelia cell adhesion. J. Biol. Chem. 279, 2772–2780 10.1074/jbc.M311901200 [DOI] [PubMed] [Google Scholar]
- Pflugh D. L., Maher S. E., Bothwell A. L. (2002). Ly-6 superfamily members Ly-6A/E, Ly-6C, and Ly-6I recognize two potential ligands expressed by B lymphocytes. J. Immunol. 169, 5130–5136. [DOI] [PubMed] [Google Scholar]
- Qu-Petersen Z., Deasy B., Jankowski R., Ikezawa M., Cummins J., Pruchnic R., Mytinger J., Cao B., Gates C., Wernig A. et al. (2002). Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J. Cell Biol. 157, 851–864 10.1083/jcb.200108150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richler C., Yaffe D. (1970). The in vitro cultivation and differentiation capacities of myogenic cell lines. Dev. Biol. 23, 1–22 10.1016/S0012-1606(70)80004-5 [DOI] [PubMed] [Google Scholar]
- Rupp P. A., Visconti R. P., Czirók A., Cheresh D. A., Little C. D. (2008). Matrix metalloproteinase 2-integrin αvβ3 binding is required for mesenchymal cell invasive activity but not epithelial locomotion: a computational time-lapse study. Mol. Biol. Cell 19, 5529–5540 10.1091/mbc.E07-05-0480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi-Assif O., Traister A., Katz B. Z., Anavi R., Eskenasy M., Witz I. P. (1996). TNFα and anti-Fas antibodies regulate Ly-6E.1 expression by tumor cells: a possible link between angiogenesis and Ly-6E.1. Immunol. Lett. 54, 207–213 10.1016/S0165-2478(96)02675-2 [DOI] [PubMed] [Google Scholar]
- Sajid M., Stouffer G. A. (2002). The role of αvβ3 integrins in vascular healing. Thromb. Haemost. 87, 187–193. [PubMed] [Google Scholar]
- Sarig R., Baruchi Z., Fuchs O., Nudel U., Yaffe D. (2006). Regeneration and transdifferentiation potential of muscle-derived stem cells propagated as myospheres. Stem Cells 24, 1769–1778 10.1634/stemcells.2005-0547 [DOI] [PubMed] [Google Scholar]
- Smith H. W., Marshall C. J. (2010). Regulation of cell signalling by uPAR. Nat. Rev. Mol. Cell Biol. 11, 23–36 10.1038/nrm2821 [DOI] [PubMed] [Google Scholar]
- Stanford W. L., Haque S., Alexander R., Liu X., Latour A. M., Snodgrass H. R., Koller B. H., Flood P. M. (1997). Altered proliferative response by T lymphocytes of Ly-6A (Sca-1) null mice. J. Exp. Med. 186, 705–717 10.1084/jem.186.5.705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupack D. G., Cheresh D. A. (2004). Integrins and angiogenesis. Curr. Top. Dev. Biol. 64, 207–238 10.1016/S0070-2153(04)64009-9 [DOI] [PubMed] [Google Scholar]
- Upadhyay G., Yin Y., Yuan H., Li X., Derynck R., Glazer R. I. (2011). Stem cell antigen-1 enhances tumorigenicity by disruption of growth differentiation factor-10 (GDF10)-dependent TGF-β signaling. Proc. Natl. Acad. Sci. USA 108, 7820–7825 10.1073/pnas.1103441108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerman K. A., Ao Z., Cohen E. A., Leboulch P. (2007). Design of a trans protease lentiviral packaging system that produces high titer virus. Retrovirology 4, 96 10.1186/1742-4690-4-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerman K. A., Penvose A., Yang Z., Allen P. D., Vacanti C. A. (2010). Adult muscle ‘stem’ cells can be sustained in culture as free-floating myospheres. Exp. Cell Res. 316, 1966–1976 10.1016/j.yexcr.2010.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P. L., Lee S. C., Chuang C. C., Mori S., Akakura N., Wu W. G., Takada Y. (2006). Non-cytotoxic cobra cardiotoxin A5 binds to αvβ3 integrin and inhibits bone resorption. Identification of cardiotoxins as non-RGD integrin-binding proteins of the Ly-6 family. J. Biol. Chem. 281, 7937–7945 10.1074/jbc.M513035200 [DOI] [PubMed] [Google Scholar]
- Yaffe D. (1968). Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc. Natl. Acad. Sci. USA 61, 477–483 10.1073/pnas.61.2.477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylipaasto P., Eskelinen M., Salmela K., Hovi T., Roivainen M. (2010). Vitronectin receptors, αv integrins, are recognized by several non-RGD-containing echoviruses in a continuous laboratory cell line and also in primary human Langerhans' islets and endothelial cells. J. Gen. Virol. 91, 155–165 10.1099/vir.0.012450-0 [DOI] [PubMed] [Google Scholar]
- Zheng B., Cao B., Crisan M., Sun B., Li G., Logar A., Yap S., Pollett J. B., Drowley L., Cassino T. et al. (2007). Prospective identification of myogenic endothelial cells in human skeletal muscle. Nat. Biotechnol. 25, 1025–1034 10.1038/nbt1334 [DOI] [PubMed] [Google Scholar]


