Abstract
Darunavir/ritonavir monotherapy maintains HIV suppression in most patients who have achieved an undetectable viral load on combination antiretroviral treatment, and is increasingly used in the clinic. However, concerns have been raised about the effectiveness of ritonavir-boosted protease inhibitor (PI/r) monotherapy in the prevention of HIV replication in the central nervous system (CNS). Here we report the cases of 2 patients on darunavir/r maintenance monotherapy with cerebrospinal fluid viral breakthrough together with increased immunoactivation and biomarker signs of neuronal injury. These 2 cases raise concerns about the effectiveness of darunavir/ritonavir monotherapy in HIV CNS infection. Thus, we recommend caution with protease inhibitor monotherapy until CNS results have been obtained from clinical studies.
Keywords: HIV, cerebrospinal fluid, darunavir, monotherapy, neuronal injury
Introduction
Infection of cells in the central nervous system (CNS) is a general aspect of systemic human immunodeficiency virus (HIV) infection. While combination antiretroviral therapy (cART) has effectively reduced the more severe neurological complications of HIV infection, milder forms of cognitive impairment are often still present in treated subjects [1,2], although the real prevalence is the subject of debate [3]. The traditionally used treatment regimens containing 2 nucleoside reverse transcriptase inhibitors (NRTI) together with either a non-nucleoside reverse transcriptase inhibitor (NNRTI) or a ritonavir-boosted protease inhibitor (PI/r) most often have good effects in the CNS, with a suppressive effect on the cerebrospinal fluid (CSF) viral load, intrathecal immunoactivation, and markers of brain damage [4–6].
Switching to PI/r monotherapy after achieving HIV RNA suppression by traditional cART has been demonstrated to have high efficacy for maintaining viral suppression in the blood [7,8]. PI/r monotherapy, mainly ritonavir-boosted darunavir (DRV/r), is increasingly used as maintenance therapy in parts of Europe. The main reasons for this are to avoid NRTI toxicity and to reduce treatment costs [9]. In the latest European AIDS Clinical Society (EACS) guidelines, PI/r monotherapy is considered an option in patients with intolerance to NRTI or for treatment simplification in patients without a history of failure on prior PI-based therapy and who have been virologically suppressed for at least 6 months (http://www.europeanaidsclinicalsociety.org/images/stories/EACS-Pdf/eacsguidelines-v6_english.pdf)). Concerns about the efficacy of PI/r monotherapy in preventing HIV replication in the CNS have, however, been raised.
Here we report 2 subjects on DRV/r monotherapy with CSF viral breakthrough together with increased CNS immunoactivation and signs of neuronal damage. Both were part of an ongoing prospective research cohort, currently containing more than 1500 CSF samples from more than 400 HIV-infected subjects, and the only subjects in the cohort on DRV/r monotherapy maintenance therapy. The study was approved by the regional ethics review board of Gothenburg and patients have provided informed consent for participation.
Case reports
Case 1
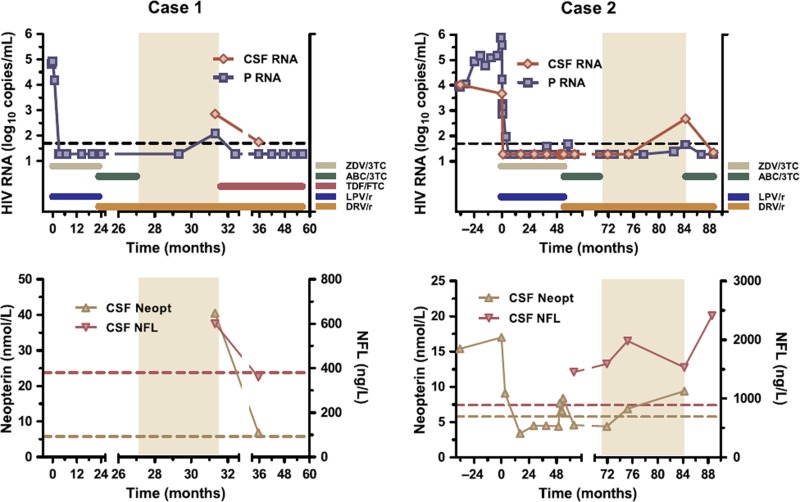
The first case is that of a black woman born in 1985 and diagnosed with HIV during pregnancy in 2007. Her CD4 count was 170 cells/μl at diagnosis and she started treatment in May 2007 with zidovudine (ZDV), lamivudine (3TC), and lopinavir/ritonavir (LPV/r). The plasma HIV RNA level (Taqman, Roche) decreased from 82,400 to < 20 copies/ml after approximately 3 months of treatment and remained undetectable thereafter (Figure 1). In April 2009 her treatment regimen was changed to abacavir (ABC), 3TC, and DRV/r 800/100 mg once daily. ABC and 3TC were stopped in August 2009 because of a rash and she continued with DRV/r as monotherapy. The rash was mild and probably not associated with ABC-induced hypersensitivity syndrome; she does not carry the HLA-B*5701 allele. A lumbar puncture was performed as part of the study protocol approximately 4 months later and showed a viral load of 119 copies/ml in blood and 709 copies/ml in CSF. At the same time she had an increased CSF lymphocyte count (30 cells/μl, normal < 3) and increased CSF beta-2 microglobulin (2.6 mg/l, normal < 1.2; nephelometry) and CSF neopterin (40.5 nmol/l, normal < 5.8; ELISA/RIA BRAHMS) as markers of macrophage/microglial activation [4]. Furthermore, we found an increased CSF concentration of the light subunit of neurofilament (NFL), a sensitive marker of axonal damage (600 ng/l, normal < 380, age- related; ELISA, UmanDiagnostics) [10]. No primary resistance mutations were found on sequencing of the virus from plasma or CSF. Subsequently, tenofovir (TDF) and emtricitabine (FTC) were added to DRV/r and her plasma viral load decreased to < 20 copies/ml again within 1 month. Another lumbar puncture was performed 4 months later and at that time her CSF viral load had decreased to 56 copies/ml. The CSF beta-2 microglobulin had decreased to 1.6 mg/l, CSF neopterin concentration to 6.9 nmol/l, and CSF NFL had normalized (360 ng/l). She did not experience any neurological or cognitive symptoms during this time period or after, but no neuropsychological testing was done.
Figure 1.
Two cases of CSF escape during darunavir/r monotherapy (period shaded). Upper panels show cerebrospinal fluid (CSF) and plasma (P) HIV RNA together with treatment history; time 0 represents first initiation of antiretroviral treatment; dotted lines correspond to 50 copies/ml. Lower panels show CSF neopterin and neurofilament light protein (NFL) concentrations; dotted lines represent upper normal limits.
Case 2
The second case is that of a Caucasian man born in 1961 who was diagnosed with HIV in 2001 when presenting with a peripheral facial palsy; he recovered fully from the palsy within 5 months. His CD4 count decreased from 390 to 160 cells/μl within the first 3 y and he started cART in 2004 with ZDV, 3TC, and LPV/r. In 2009 he participated in a treatment intensification study [11] and received in addition to his other cART ,4 weeks enfuvirtide and 4 weeks maraviroc, followed by his previous treatment regimen. Because of side effects, he changed treatment in August 2009 to ABC, 3TC, and DRV/r. In November 2010 he simplified his treatment to DRV/r monotherapy 800/100 mg once daily. His plasma viral load remained below 50 copies/ml from when he was first suppressed in May 2005 throughout his entire treatment history, and his CD4 count increased to 790 cells/μl. Lumbar punctures were performed in total 15 times in the prospective study, including 2 performed before treatment initiation (Figure 1). All 11 CSF samples taken during the period on cART showed a CSF viral load < 50 copies/ml. Thereafter, lumbar punctures were performed after 3 and 12 months of DRV/r monotherapy; at the second time point the viral load had increased to 478 copies/ml in CSF, while it was 46 copies/ml in plasma. There were also signs of increased intrathecal immunoactivation with elevated CSF lymphocyte count (17 cells/μl), CSF beta-2 microglobulin (1.6 mg/l), and CSF neopterin (9.4 nmol/l). Furthermore, we found an increased CSF NFL concentration (1530 ng/l, normal < 890, age-related), but while the increased CSF viral load and intrathecal immunoactivation developed first after switching to DRV/r monotherapy, increased NFL had also been present before. No symptoms or complaints arose and he remained normal on neuropsychological testing by CogState (detection, identification, 1 card learning, 1 back speed, 1 back accuracy) [12] when examined before and 12 months after changing to DRV/r monotherapy. No primary resistance mutations were found in blood or CSF.
He was reintroduced to ABC, 3TC, and DRV/r, and the plasma viral load decreased to < 20 copies/ml within 1 month. A lumbar puncture was performed after 3 months and his CSF viral load had decreased to < 20 copies/ml again. CSF neopterin was not analyzed at that time, but both beta-2 microglobulin (1.4 mg/l) and the CSF lymphocyte count (< 3 cells/μl) had decreased. However, the CSF NFL concentration continued to be increased (2410 ng/l).
Discussion
PI/r monotherapy maintenance regimens, mainly with DRV/r, are increasingly being used in parts of the world, and its effect on the systemic infection has been shown to be satisfactory. However, the 2 cases presented here raise concerns about the effects of DRV/r monotherapy on HIV CNS infection. In the MONOI study, 2 patients who were virologically suppressed in plasma developed mild neurological symptoms, and when a lumbar puncture was done they were both found to have CSF escape (330 and 580 copies/ml) [8]. Together, these cases suggest that DRV/r monotherapy should be used with caution until the results of studies on CSF are available. Concerns have also been raised regarding monotherapy with other PIs, i.e. atazanavir/r and lopinavir/r [13,14].
As well as viral CSF breakthrough and intrathecal immunoactivation with pleocytosis and increased markers of macrophage/microglial activation (beta-2 microglobulin and neopterin), signs of neuronal damage with increased CSF NFL levels were found in both our subjects. As a comparison, increased CSF NFL was found in only 1 out of 38 samples drawn from 21 subjects on cART that included DRV/r in our research cohort (data not shown). It has to be noted that in one of the reported cases, NFL was already increased before switching to DRV/r monotherapy and also continued to be increased after the reintroduction of ABC/3TC. The reason for this is unknown and increased CSF NFL concentrations could not be ascribed to the DRV/r monotherapy in this subject.
NFL is a very sensitive marker of neuronal destruction and increased levels can, for example, be found in various types of dementia, cerebrovascular disease, and in a variety of neurodegenerative disorders [10]. Increased CSF NFL levels are also consistently found in HIV-associated dementia, often in very high concentrations [15]. We have also found CSF NFL to be a good predictive marker with increased concentrations in asymptomatic patients who subsequently develop HIV-associated dementia [16].
Despite ongoing brain injury, none of the subjects in this report developed any neurological symptoms. However, both were closely followed and changed their treatment to include NRTIs; we do not know what would have happened if they had continued on DRV/r monotherapy. Several cases on effective cART who have developed neurological symptoms and who have been shown to have concomitant CSF escape have recently been reported [17]; many of these patients were on atypical or incomplete ART regimens when they became neurosymptomatic and were discovered to have CSF/plasma discordance. An increased CSF viral load can also be found in approximately 10% of virologically suppressed neurologically asymptomatic patients with more conventional antiretroviral regimens [18]. However, CSF HIV RNA in such cases is only slightly increased (< 200 copies/ml) and might represent CSF viral ‘blips’.
It is intriguing that the only 2 cases on DRV/r monotherapy that we examined both had CSF viral breakthrough and increased intrathecal immunoactivation. The longitudinal progress of CSF viral breakthrough is not known, nor are the potential clinical consequences. Until better studied, we suggest caution in using PI/r monotherapy and other experimental ART combinations outside clinical studies.
Acknowledgments
Declaration of interest: This work was supported by the Swedish Research Council (K2008-58P-20930-04-1, K2008-58X-20931-01-1, K2010-63P-21562-01-4, and K2011-61X-20401-05-6) and the Sahlgrenska Academy at the University of Gothenburg (ALFGBG-141741 and ALFGBG-144341). MG has received lecture fees from and/or participated on scientific advisory boards for Abbott, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Jansen/Tibotec, Merck, and Pfizer. LH has received lecture fees from Abbott and Roche and has participated on scientific advisory boards for Pfizer and Meda. BS has received research grants from Chrontech Pharma (former Tripep AB) and GlaxoSmithKline.
DF and HZ declare no competing interests.
References
- 1.Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology. 2010;75:2087–96. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simioni S, Cavassini M, Annoni JM, Rimbault Abraham A, Bourquin I, Schiffer V, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24:1243–50. doi: 10.1097/QAD.0b013e3283354a7b. [DOI] [PubMed] [Google Scholar]
- 3.Gisslen M, Price RW, Nilsson S. The definition of HIV-associated neurocognitive disorders: are we overestimating the real prevalence? BMC Infect Dis. 2011;11:356. doi: 10.1186/1471-2334-11-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hagberg L, Cinque P, Gisslen M, Brew BJ, Spudich S, Bestetti A, et al. Cerebrospinal fluid neopterin: an informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res Ther. 2010;7:15. doi: 10.1186/1742-6405-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mellgren A, Price RW, Hagberg L, Rosengren L, Brew BJ, Gisslen M. Antiretroviral treatment reduces increased CSF neurofilament protein (NFL) in HIV-1 infection. Neurology. 2007;69:1536–41. doi: 10.1212/01.wnl.0000277635.05973.55. [DOI] [PubMed] [Google Scholar]
- 6.Mellgren A, Antinori A, Cinque P, Price RW, Eggers C, Hagberg L, et al. Cerebrospinal fluid HIV-1 infection usually responds well to antiretroviral treatment. Antivir Ther. 2005;10:701–7. [PubMed] [Google Scholar]
- 7.Clumeck N, Rieger A, Banhegyi D, Schmidt W, Hill A, Van Delft Y, et al. 96 week results from the MONET trial: a randomized comparison of darunavir/ritonavir with versus without nucleoside analogues, for patients with HIV RNA < 50 copies/ml at baseline. J Antimicrob Chemother. 2011;66:1878–85. doi: 10.1093/jac/dkr199. [DOI] [PubMed] [Google Scholar]
- 8.Katlama C, Valantin MA, Algarte-Genin M, Duvivier C, Lambert-Niclot S, Girard PM, et al. Efficacy of darunavir/ritonavir maintenance monotherapy in patients with HIV-1 viral suppression: a randomized open-label, noninferiority trial, MONOI-ANRS 136. AIDS. 2010;24:2365–74. doi: 10.1097/QAD.0b013e32833dec20. [DOI] [PubMed] [Google Scholar]
- 9.Gazzard B, Hill A, Anceau A. Cost-efficacy analysis of the MONET trial using UK antiretroviral drug prices. Appl Health Econ Health Policy. 2011;9:217–23. doi: 10.2165/11592220-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 10.Norgren N, Rosengren L, Stigbrand T. Elevated neurofilament levels in neurological diseases. Brain Res. 2003;987:25–31. doi: 10.1016/s0006-8993(03)03219-0. [DOI] [PubMed] [Google Scholar]
- 11.Yilmaz A, Verhofstede C, D’Avolio A, Watson V, Hagberg L, Fuchs D, et al. Treatment intensification has no effect on the HIV-1 central nervous system infection in patients on suppressive antiretroviral therapy. J Acquir Immune Defic Syndr. 2010;55:590–6. doi: 10.1097/QAI.0b013e3181f5b3d1. [DOI] [PubMed] [Google Scholar]
- 12.Maruff P, Thomas E, Cysique L, Brew B, Collie A, Snyder P, et al. Validity of the CogState brief battery: relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Arch Clin Neuropsychol. 2009;24:165–78. doi: 10.1093/arclin/acp010. [DOI] [PubMed] [Google Scholar]
- 13.Gutmann C, Cusini A, Gunthard HF, Fux C, Hirschel B, Decosterd LA, et al. Randomized controlled study demonstrating failure of LPV/r monotherapy in HIV: the role of compartment and CD4-nadir. AIDS. 2010;24:2347–54. doi: 10.1097/QAD.0b013e32833db9a1. [DOI] [PubMed] [Google Scholar]
- 14.Vernazza P, Daneel S, Schiffer V, Decosterd L, Fierz W, Klimkait T, et al. The role of compartment penetration in PI-monotherapy: the Atazanavir-Ritonavir Monomaintenance (ATARITMO) trial. AIDS. 2007;21:1309–15. doi: 10.1097/QAD.0b013e32814e6b1c. [DOI] [PubMed] [Google Scholar]
- 15.Abdulle S, Mellgren A, Brew BJ, Cinque P, Hagberg L, Price RW, et al. CSF neurofilament protein (NFL)—a marker of active HIV-related neurodegeneration. J Neurol. 2007;254:1026–32. doi: 10.1007/s00415-006-0481-8. [DOI] [PubMed] [Google Scholar]
- 16.Gisslen M, Hagberg L, Brew BJ, Cinque P, Price RW, Rosengren L. Elevated cerebrospinal fluid neurofilament light protein concentrations predict the development of AIDS dementia complex. J Infect Dis. 2007;195:1774–8. doi: 10.1086/518043. [DOI] [PubMed] [Google Scholar]
- 17.Canestri A, Lescure FX, Jaureguiberry S, Moulignier A, Amiel C, Marcelin AG, et al. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis. 2010;50:773–8. doi: 10.1086/650538. [DOI] [PubMed] [Google Scholar]
- 18.Eden A, Fuchs D, Hagberg L, Nilsson S, Spudich S, Svennerholm B, et al. HIV-1 viral escape in cerebrospinal fluid of subjects on suppressive antiretroviral treatment. J Infect Dis. 2010;202:1819–25. doi: 10.1086/657342. [DOI] [PMC free article] [PubMed] [Google Scholar]