Summary
How cell-intrinsic regulation of the cell cycle and the extrinsic influence of the niche converge to provide proliferative quiescence, safeguard tissue integrity, and provide avenues to stop stem cells from giving rise to tumors is a major challenge in gene therapy and tissue engineering. We explore this question in sumoylation-deficient mutants of Drosophila. In wild type third instar larval lymph glands, a group of hematopoietic stem/progenitor cells acquires quiescence; a multicellular niche supports their undifferentiated state. However, how proliferative quiescence is instilled in this population is not understood. We show that Ubc9 protein is nuclear in this population. Loss of the SUMO-activating E1 enzyme, Aos1/Uba2, the conjugating E2 enzyme, Ubc9, or the E3 SUMO ligase, PIAS, results in a failure of progenitors to quiesce; progenitors become hyperplastic, misdifferentiate, and develop into microtumors that eventually detach from the dorsal vessel. Significantly, dysplasia and lethality of Ubc9 mutants are rescued when Ubc9wt is provided specifically in the progenitor populations, but not when it is provided in the niche or in the differentiated cortex. While normal progenitors express high levels of the Drosophila cyclin-dependent kinase inhibitor p21 homolog, Dacapo, the corresponding overgrown mutant population exhibits a marked reduction in Dacapo. Forced expression of either Dacapo or human p21 in progenitors shrinks this population. The selective expression of either protein in mutant progenitor cells, but not in other hematopoietic populations, limits overgrowth, blocks tumorogenesis, and restores organ integrity. We discuss an essential and complex role for sumoylation in preserving the hematopoietic progenitor states for stress response and in the context of normal development of the fly.
Keywords: Dacapo, dysplasia, hematopoiesis, microtumor, niche, organ integrity, p21, quiescence, stem cell, sumoylation, tumor suppressor, Ubc9
Introduction
Tissue and organ regeneration in patients with lesions from disease or surgery, or due to ageing, is a primary challenge in biomedical research. Tissue engineering requires understanding how normal tissues arise, develop, renew themselves, and maintain their proliferative quiescence and homeostasis. Stem cells provide proliferative quiescence and tissue integrity over time (Morrison and Spradling, 2008). Proliferative quiescence is characteristic property of some stem cells, which, as compared to their more differentiated progenitors, undergo infrequent divisions (Moore and Lyle, 2011). Loss of proliferative quiescence in pre-malignant cells frequently accompanies the development of cancer.
Mammalian cancers are composed of heterogeneous cell populations that include few stem/stem-like cells and many more differentiated cells with limited proliferative potential (Morrison and Spradling, 2008; Wang, 2010). The growth and development of a tumor depends on the complex interplay of both, the cell-intrinsic mechanisms and the microenvironment. Tumors are further characterized by dormancy or metastasis, and the nature of these processes in relation to their origin remains largely unclear (Morrison and Spradling, 2008; Wang, 2010). The mechanism of proliferative quiescence in normal stem and related cancer cells is not well understood (Moore and Lyle, 2011).
Drosophila has served as an excellent model system for cancer research. One approach to studying cancer in flies is to screen the genome for mutations in larval cells that promote tumorogenesis and metastasis. In this approach, mutations are induced selectively in specific tissues, where genetically affected mutant cells form tumors in an otherwise wild type larval body. The effects of a known or new oncogenic or tumor-suppressive mutation can be studied in such mosaic animals (Potter et al., 2000; Vidal and Cagan, 2006). In an “inverse mosaic” approach, germline mutants that develop tumors with high spatial and temporal specificity are studied by genetically manipulating specific regions of the tumor, or its environment, by expressing either the missing protein, or another protein, suspected to play a role in tumor development (Manfruelli et al., 1996; Qiu et al., 1998; Chiu et al., 2005). In either case, mosaic animals can be created with fly or human proteins.
In this study, we examined the origin of hematopoietic microtumors in Ubc9 mutants of Drosophila (Chiu et al., 2005; Huang et al., 2005). Microtumors are structures of at least 10,000 µm2 in projection area, consisting of at least 50 cells, and aggregates are structures<10,000 µm2 in projection area (Kalamarz, 2010). Both classes of structures are found in more than 80% of the Ubc9 mutants (Kalamarz, 2010). Microtumors are composed mostly of blood cells (hemocytes), including lamellocytes, and vary in the degree of melanization (Kalamarz, 2010). Ubc9 is the E2 SUMO-conjugating enzyme. Along with the SUMO-activating E1 enzymes, Aos1 and Uba2, and the SUMO E3 ligase, PIAS, Ubc9 participates in a highly-conserved protein modification system (Mabb and Miyamoto, 2007; Talamillo et al., 2008).
Blood cells in normal Drosophila larvae circulate freely in the hemolymph. Groups of blood cells are also present within the hematopoietic organ, called lymph gland. The predominant cell type is the macrophage-like plasmatocyte (Kurucz et al., 2007b), which phagocytoses microbes and dead cells. The remaining lineages are crystal cells and lamellocytes, both of which facilitate melanization reactions (Kurucz et al., 2007a; Nam et al., 2008). Large, adhesive lamellocytes differentiate in response to parasitic wasp infection in both, circulation and the lymph gland (Rizki and Rizki, 1992; Lanot et al., 2001; Sorrentino et al., 2002).
The lymph gland originates in the embryo (Mandal et al., 2004) and develops through larval stages (Lanot et al., 2001; Holz et al., 2003). The lobes are arranged bilaterally and flank the dorsal vessel in the anterior body segments (Shrestha and Gateff, 1982; Lanot et al., 2001; Qiu et al., 1998; Jung et al., 2005) (also see Fig. 1A,B). By the first instar, anterior lobes form compact cell clusters and by third instar they develop three zones (Jung et al., 2005; Mandal et al., 2007; Minakhina and Steward, 2010). A small multicellular niche controls cell states in the other two zones (Crozatier et al., 2004; Jung et al., 2005; Krzemien et al., 2007; Mandal et al., 2007), which are located up to as many as 50 cell diameters away. Cells in medullary and cortical zone divide actively until the third instar, when cells of the medullary zone become proliferatively quiescent (Jung et al., 2005; Mandal et al., 2007). The cell cycle mechanisms responsible for quiescence of these multipotent hematopoietic stem cells and progenitors remain largely unknown.
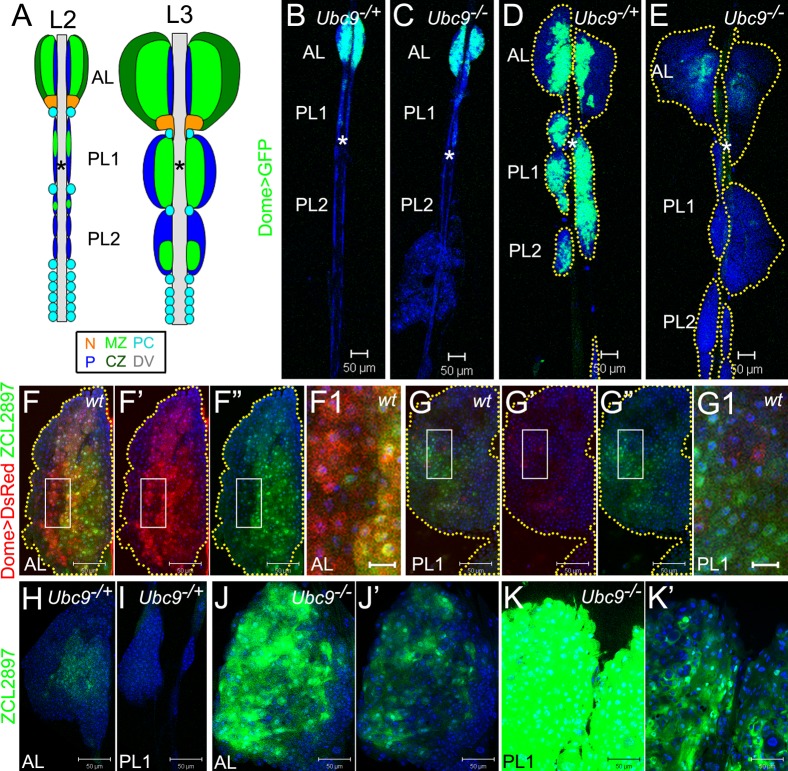
Fig. 1. Aberrant gene expression in progenitors of Ubc9 lymph glands.
Labeling: AL – anterior lobe(s), PL1 – first set of posterior lobes, PL2 – second set of posterior lobes; asterisk – dorsal vessel (DV). (A) Lymph glands in second (L2) and third (L3) larval instars. Medullary zone (MZ, light green), cortical zone (CZ, dark green); the niche (N, orange); unclassified cells (navy blue, P); pericardial cells (PC, light blue). Pairs of lobes aligned along the antero-posterior axis; PL1, PL2 consist of smaller lobes (2–3 pairs each) distinguishable at L2, but forming a continuous lobe at L3. (B–E) Dome>GFP (green) in lymph glands of 4-day L2: Ubc9−/+ (B), Ubc9−/−(C) and 6-day L3: Ubc9−/+ (D), Ubc9−/− (E). Lobes outlined in dotted marking (D,E). (F–G1) ZCL2897 (green) and Dome>DsRed (red) in wild type L3: AL (F–F”), PL (G–G”). Dome>DsRed (F',G') and ZCL2897 (F”,G”) shown separately; overlap of expressions (F,F1,G,G1). Regions of F,G (white rectangles) shown magnified in F1,G1, respectively. Yellow dotted markings outline the lobes (F–F”,G–G”). (H–K') ZCL2897 (green) expression in Ubc9−/+ (H, AL; I, PL1) and Ubc9−/− (J, AL; K, PL1) lymph glands; note: lobes in J and K are representative examples from different lymph glands. Increased ZCL2897 expression in Ubc9−/− (J,K) produced overexposure and samples were therefore re-imaged after reducing detector gain (J',K'). K–K' is a fragment of S2L. Brightness and contrast were slightly modified in panels F–G1 for clarity in merged images. Confocal sections (B–K'). Scale bars: 50 µm, except F1,G1 – 10 µm.
We show that Ubc9 microtumors derive from an initially quiescent, heterogeneous, progenitor population of the medullary zones of the anterior and posterior lobes. The largest microtumors are likely derived from the highly enlarged posterior lobes, as they abandon normal heterochronic development, and undergo dysplasia, while still attached to the dorsal vessel, but then detach from the dorsal vessel into the hemolymph as intact tumors. Dysplastic growth is niche-independent. Other sumoylation cascade enzymes, E1 subunits, and E3 ligase, PIAS, are also needed for progenitor quiescence. Our studies suggest that the cell cycle of this population is regulated, in part, by Dacapo/p21. Of dozens of hematopoietic Drosophila mutants reported to date, this is the first study where a clear cellular origin of microtumors is defined. Changes in Ubc9 expression have been linked to primary tumors in humans (Moschos et al., 2010). p21 is a known drug target in cancer therapy. Its potential regulation via sumoylation in Drosophila provides new insights into the regulation of quiescence in an in vivo model system and into the earliest steps in oncogenesis in humans.
Results
Loss of Ubc9 affects gene expression, and size and integrity of third instar lymph gland
Post-embryonic wild type lymph gland development is heterochronic (Fig. 1A,B). From the onset of the third instar, the posterior lobes of wild type lymph glands expand and coalesce so that the initially distinct four to six pairs of cell clusters form two sets of posterior lobes (Fig. 1A,B,D). The growth of posterior lobes is developmentally synchronous in that the first set expands earlier than the second set (Fig. 1B,D, supplementary material Fig. S1A,C). We call them posterior lobes, first set (PL1), and posterior lobes, second set (PL2) (Fig. 1A,B,D).
Mutant Ubc9 lymph glands are variably overgrown and exhibit aberrant differentiation of hemocytes (Chiu et al., 2005). Careful analysis of scores of mutant glands revealed differential effects on anterior versus posterior lobes (Fig. 1D,E, supplementary material Fig. S1C,D). In many glands of 6–7-day third instar larvae, the anterior lobes are completely absent or are partially dispersed where peripheral cells in the cortex are lost to the hemolymph (see below). In contrast, most posterior lobes are severely overgrown and either remain tethered to the dorsal vessel or detach (Fig. 1, supplementary material Fig. S1D, and see below). Loss of posterior lobes coincides with the appearance of large compact tumors in the hemolymph. This trend suggests that the lymph gland itself may be the direct source of the microtumors.
To examine whether Ubc9 has one primary function in normal hematopoiesis and probe if all four defects (overgrowth, misdifferentiation, lobe dispersal, and lobe detachment) are triggered from an initial disruption of this primary function, we compared the expression patterns of Dome>GFP, Hml>GFP, and 76B>GFP in developing heterozygous and Ubc9 lymph glands. We found no striking difference in late second or even early third instar (day 4 after egg lay) animals (Fig. 1B,C, supplementary material Fig. S1A,B). Most cells of the posterior lobes do not express mature hemocyte markers, but express Dome>GFP, when the Dome promoter is active (Fig. 1B,D) (Jung et al., 2005; Krzemien et al., 2007). Dome encodes the receptor for JAK-STAT signaling (Brown et al., 2001). At mid to late third instar (day 5 to 6), all heterozygous anterior lobes remain relatively small and structurally intact, while anterior lobes of the mutant glands are either larger than control, or they disperse. Mutant posterior lobes expand dramatically, but remain largely intact (Fig. 1D,E, supplementary material Fig. S1C,D, Fig. 4B,C,E,F). We found that the overgrown lobes themselves are displaced and begin to detach from the dorsal vessel (supplementary material Fig. S1D, Fig. S2J,L, Fig. 4E,F).
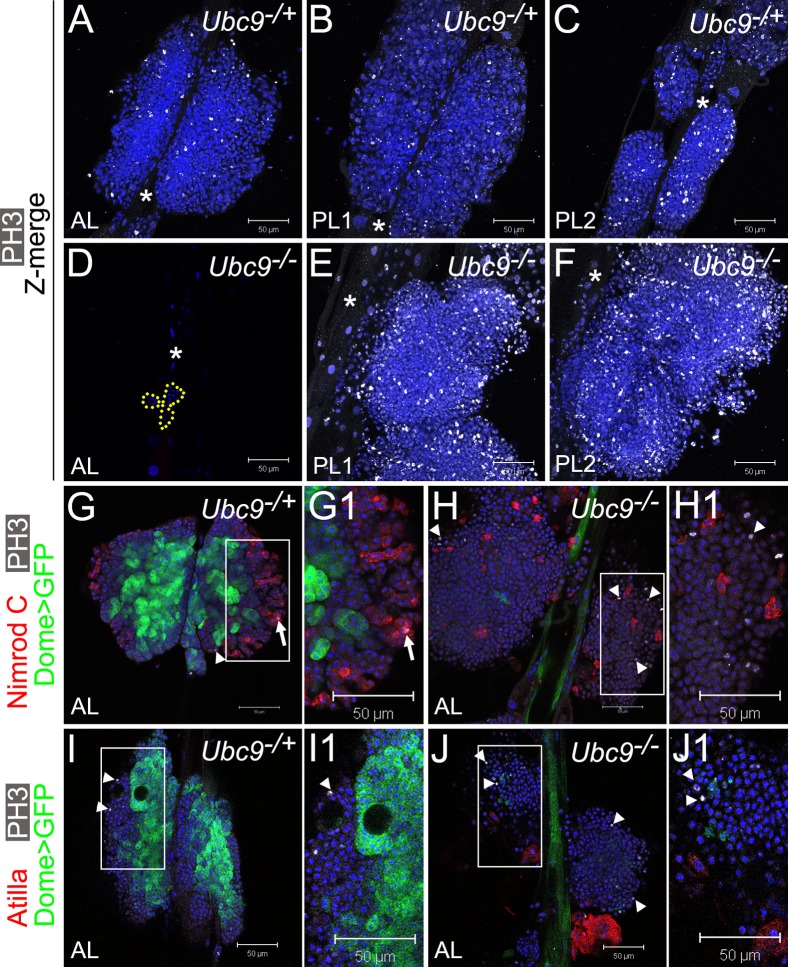
Fig. 4. Overproliferation of immature cells in Ubc9 lymph gland.
(A–F) Phosphorylated histone H3 (PH3, white) in 6.5–7-day L3 Ubc9−/+ AL, PL1, PL2 (A,B,C, respectively) and Ubc9−/− AL (D; remaining cells outlined), PL1, PL2 (E,F, respectively). Star marks DV. Optical Z-sections merged (A–C,E–F). Mutant PL1 and PL2 (E,F) are partially detached from the DV and misaligned; lobe orientation (top – anterior, bottom – posterior) is reverse of the DV. (G–J1) Dome>GFP (green), PH3 (white; arrowheads) and Nimrod C (red, G–H1), or Atilla (red, I–J1) in 6-day AL in Ubc9−/+ (G,G1,I,I1) and Ubc9−/− (H,H1,J,J1) animals. PH3/Nimrod C localization in the same cell (G1, arrow). Regions indicated in G,H,I,J magnified in G1,H1,I1,J1, respectively. Confocal sections (A–J1). Scale bars: 50 µm.
The expression of Dome>GFP in heterozygous lymph glands remains high, while in mutant glands, it gradually decreases during third instar and is virtually absent by late 6 day (Fig. 1D,E). Loss of Dome>GFP expression in mutant lobes does not result from increased apoptosis, as only less than 1% of cells in the lobes of either genetic background are positive for cleaved pro-caspase 3.
Dome>GFP expression is undetectable in circulating hemocytes of both, control and mutant animals. Single Dome>GFP cells in circulation or within microtumors are rare (supplementary material Fig. S2A–D). Surprisingly, while Dome>GFP is expressed weakly in the dorsal vessel of control animals, it is highly upregulated after the onset of anterior lobe dispersal in the mutant background (Fig. 1D,E, Fig. 5A,B; asterisk). Together, these results suggest that a primary hematopoietic effect of Ubc9 loss is on the cells of the medullary zone. Additionally, Ubc9-dependent gene regulation in the dorsal vessel coincides with loss of lobe integrity.
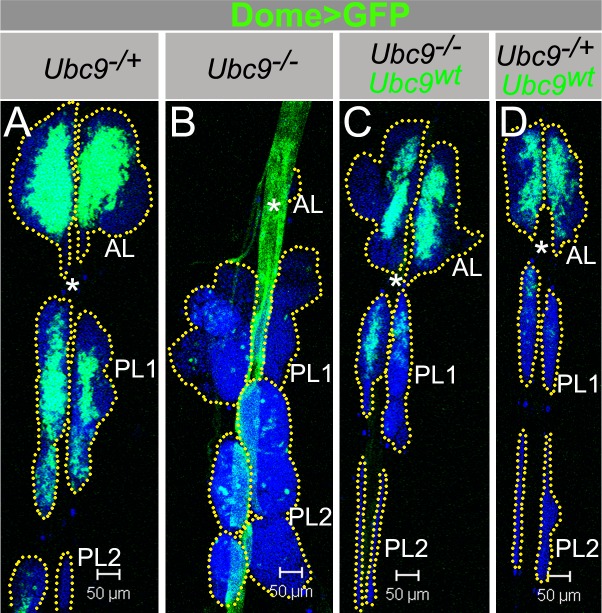
Fig. 5. Dome>Ubc9wt restores Ubc9 lymph gland size and Dome>GFP expression.
(A–D) Dome>GFP in Ubc9−/+ (A); Ubc9−/− (B); Ubc9−/−, Dome>Ubc9wt (C); Ubc9−/+, Dome>Ubc9wt (D). Asterisk (DV); dotted line outlines the lobes (A–D). Confocal sections (A–D). Scale bars: 50 µm.
The expression of Hml>GFP is limited largely to the periphery in all 6 day control anterior lobes and in approximately 10% (n = 1/8) of the first set of posterior lobes (supplementary material Fig. S1C). In all examined mutant anterior lobes and about 40% (n = 3/8) of first posterior lobes, Hml>GFP cells are scattered throughout the body of the lobe (supplementary material Fig. S1D). The expanded posterior lobes of mutant glands contain more Hml>GFP-expressing cells than the control posterior lobes (supplementary material Fig. S1D). That both, Dome>GFP and Hml>GFP expression becomes more pronounced in the first posterior lobes of control glands at third instar, supports the notion that this capacity to acquire zonation is heterochronic; it emerges only after the anterior lobes have matured. Second, at third instar Dome expression decreases in Ubc9 lymph gland and Hml expression increases slightly in posterior lobes compared to controls. These changes in the expression patterns occur simultaneously with lymph gland overgrowth.
The medullary zone exhibits heterogeneity
To understand the effects of the Ubc9 mutation on cells of the medullary zone, we simultaneously expressed Dome>DsRed with ZCL2897 (a GFP protein trap) (Morin et al., 2001) in wild type glands. ZCL2897 is expressed in cells of the medullary zone of control animals (Fig. 1F,H) (Jung et al., 2005). Despite substantial overlap in the expression of Dome>DsRed and ZCL2897, there is significant heterogeneity in gene expression (Fig. 1F–G1). At least three cell types are observed: those that express both markers (Domehi ZCL2897hi, Fig. 1F1, cells with yellow hue) and those that are strongly positive for one marker (Domehi ZCL2897lo, red cells, or Domelo ZCL2897hi, green cells, Fig. 1F1,G1). Among the doubly-positive cells, there is no apparent correlation in signal intensity of the two markers, suggesting that the medullary zone population consists of distinct cell types.
We next monitored ZCL2897 expression in heterozygous and Ubc9 third instar animals and found that, in contrast to Dome>GFP, loss of Ubc9 activates ZCL2897 expression in anterior and posterior lobes (Fig. 1H–K'). Unlike Dome>GFP, high ZCL2897 expression is also found in mutant circulating hemocytes, microtumors and overgrown lobes which are easily spotted through the cuticle (supplementary material Fig. S2E–L). Such overgrown, intact lobes, while still attached to the dorsal vessel (Fig. 1E, supplementary material Fig. S2J,L; also see Fig. 4E,F, Fig. 5B), correspond to the freely circulating microtumors in size and shape (supplementary material Fig. S2G,O; also see supplementary material Fig. S5B,H, supplementary material Fig. S6C, supplementary material Fig. S7H). This significant expansion of the ZCL2897hi cell population suggests that Ubc9 restrains division, keeps progenitors from entering an aberrant differentiation program, and maintains organ integrity.
To test if ZCL2897 expression marks lamellocytes, we examined relative expression of either MSNF9mo-mCherry (MSNF9, supplementary material Fig. S3A–D), or Atilla (supplementary material Fig. S3E,F) with ZCL2897. Both methods revealed that while a significant number of mutant ZCL2897-positive cells also express Atilla or MSNF9 (yellow signal, supplementary material Fig. S3C,D,F; white arrowheads in supplementary material Fig. S3G–R'), a number of ZCL2897hi cells do not express either lamellocyte marker (supplementary material Fig. S3G–R', white arrows). We also identified rare cells with low or absent ZCL2897 expression but positive for MSNF9 (supplementary material Fig. S3O,O', blue arrowheads) or Atilla (M.K. and S.G., unpublished data). Thus, expansion of ZCL2897 population in the mutant supports the idea that Ubc9 maintains proliferative quiescence in the progenitor population and prevents their aberrant and lamellocyte differentiation.
Ubc9 affects cells of the transition zone
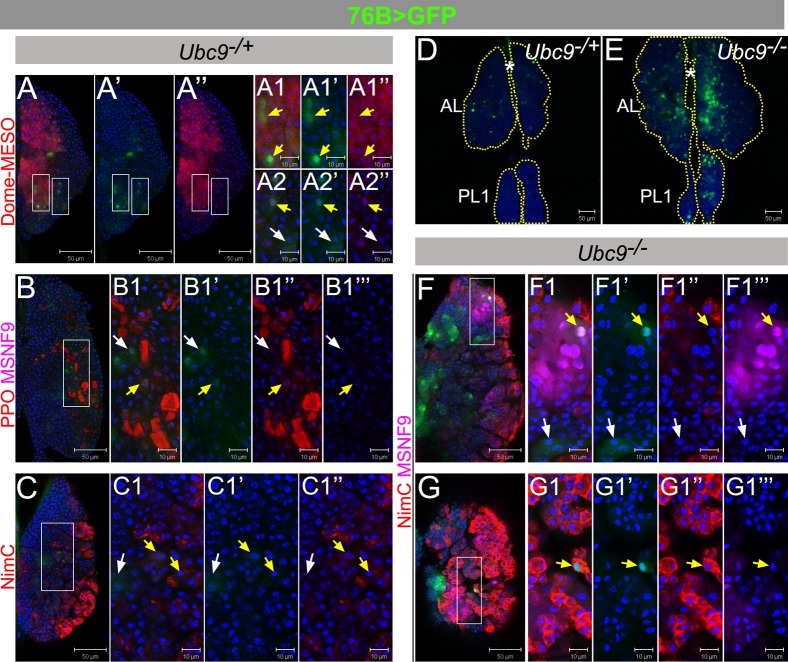
To probe the properties of the expanded population in mutant glands with a Gal4 driver, whose expression is not downregulated by the effects of the mutation, we examined the expression of the 76B>Gal4. This driver is expressed in few cells of the lymph gland (Paddibhatla et al., 2010), although the identity of these cells is not known. We found that at late third instar, many heterozygous 76B>GFP-expressing cells are located outside the Dome-MESO boundary (i.e., they are negative for Dome-MESO; Fig. 2A, white arrows) and do not express the Pro-PO (Fig. 2B–B1”), Nim C (Fig. 2C–C1”), or MSNF9 (Fig. 2B–B1‴), although rare exceptions are observed (zoomed panels in Fig. 2A–C; yellow arrows). Thus, 76B>GFP expression marks the cells that are intermediate to the Dome-MESO-positive progenitors in the medullary zone and the differentiated cells in the cortex. Because most of the cells expressing 76B>GFP reside outside the Dome-MESO boundary, interspersed in the cortex, and the double positives with either Dome-MESO (Fig. 2A) or the Pro-PO/Nim C (Fig. 2B,C) are rare, they most likely represent the transitional precursors that are derived from the medullary zone progenitors, but have not yet assumed a final differentiated identity. The existence of this transition zone has been suggested in recent studies (Krzemien et al., 2010).
Fig. 2. 76B-Gal4 characterization in control and Ubc9 lymph glands.
(A–C1″) L3 (6-day) Ubc9−/+ anterior lobes expressing 76B>GFP (green), co-labeled with Dome-MESO (red; A–A2″), or Pro-Phenol Oxidase and misshapen (PPO, red, and MSNF9, magenta, respectively; B–B1‴), or Nimrod C (NimC, red; C–C1″). (D–E) 76B>GFP (green) expression in 6-day Ubc9−/+ (D) and Ubc9−/−(E) lymph glands. (F–G1‴) L3 (6-day) Ubc9−/− anterior lobes expressing 76B>GFP (green), labeled with Nimrod C and misshapen (NimC, red and MSNF9, magenta, respectively); presented are lower (F–F1‴) and upper (G–G1‴) optical sections of two lymph glands. Split-channel images show green (′), red (″) or magenta (‴). Selected regions (white rectangles) are shown as high magnifications (panels labeled with numbers 1–2). White arrows – cells expressing singly 76B>GFP, and yellow arrows – two markers; star–DV. Confocal sections (A–G1‴). Scale bars: 50 µm (A–A″,B,C,D,E,F,G) and 10 µm (remaining panels).
Unlike Dome>GFP and Hml>GFP, 76B>GFP population is significantly expanded in Ubc9 mutant glands (Fig. 2D,E). Some mutant 76B>GFP cells are also positive for either MSNF9 or Nim C (Fig. 2F–G1‴; yellow arrows). [Ubc9 lymph glands have very few crystal cells (Chiu et al., 2005) and these were therefore not examined in mutant glands.] 76B>GFP expression is also expanded in single cells in circulation or those in microtumors in the hemolymph (supplementary material Fig. S2M–P). This expanded expression of 76B>GFP parallels the expression dynamics of ZCL2897 (supplementary material Fig. S2E–L) in the mutants.
Ubc9 is expressed throughout the lymph glands
Ubc9 protein is ubiquitously expressed in the anterior and posterior lobes of the control third instar animals, in both, medulla (nuclear and cytoplasmic) and cortex (mainly nuclear; supplementary material Fig. S4A–D'). In addition to the diffuse nuclear signal (supplementary material Fig. S4B,B',D,D'), speckles are also present (supplementary material Fig. S4B,B'; white arrows). Ubc9 is also expressed in the dorsal vessel (supplementary material Fig. S4A,C,E,G; star). Ubc94-3/5 mutants exhibit significantly lower levels of the protein in the entire organ (supplementary material Fig. S4E–H'). Both hypomorphic alleles have been previously characterized molecularly (Apionishev et al., 2001).
SUMO pathway components in hematopoiesis
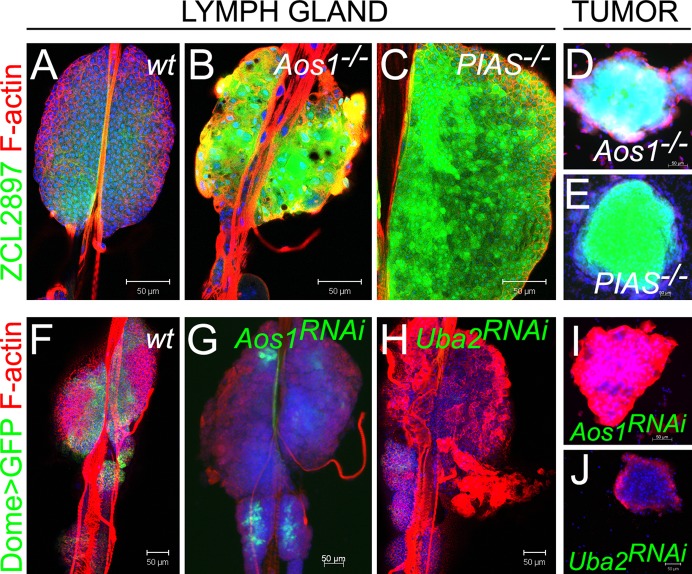
If changes observed in Ubc9 mutant hematopoietic organ are due to loss of sumoylation, then other enzymes of the sumoylation cascade should be similarly required. To test this idea, we examined larvae carrying loss-of-function mutations in E1 (Aos1c06048) and E3/PIAS (Su(var)2-101/Su(var)2-102) genes. E1 is an activating heterodimer of Aos1 and Uba2 subunits, while PIAS, encoded by Su(var)2-10, serves as the E3 ligase. Like Ubc9 glands, Aos1 and PIAS glands exhibit significant activation of ZCL2897 (Fig. 3A–C). Mutants in each background produce hematopoietic tumors (Fig. 3D,E) marked by increased expression of ZCL2897. Numerous lamellocytes appear in dispersing anterior lobes and in circulation (Fig. 3A–E and M.K. and S.G., unpublished data).
Fig. 3. Sumoylation enzymes in larval hematopoiesis.
(A–E) ZCL2897 (green) expression in wild type (A), Aos1−/− (B) and PIAS−/− (C) lymph glands and in tumors of Aos1−/− (D) and PIAS−/− (E) larvae. (F–H) Dome>GFP (green) in control lymph gland (F, without RNAi constructs). Reduction of Dome>GFP in lymph glands expressing Dome>Aos1RNAi (G) and Dome>Uba2RNAi (H). (I, J) Tumors form in animals expressing Dome>Aos1RNAi (I) and Dome>Uba2RNAi (J). Parental UAS-Aos1RNAi and UAS-Uba2RNAi classes do not produce tumors. Confocal sections (A–C,F,H,J) and fluorescent microscopy (D,E,G,I). Scale bars: 50 µm.
To test if Dome>GFP expression is compromised by loss of sumoylation enzymes, we performed knockdown of E1 subunits via RNAi. Knock-down of either Aos1 or Uba2 led to significant reduction of the Dome>GFP expression, lamellocyte differentiation, anterior lobe dispersal (Fig. 3F–H), and tumorogenesis (Fig. 3I,J). These observations parallel those for Ubc9 mutants and demonstrate that sumoylation is a fundamental mechanism through which cell division and differentiation of hematopoietic progenitors is simultaneously regulated.
Ubc9 microtumors arise from progenitor hyperplasia of anterior and posterior lobes
To more directly study the role of Ubc9 in the cell cycle, we stained lymph glands in late third instar stage (day 6.5–7) for phospho-histone H3 (Fig. 4A–F). At this stage, most control animals pupariated or are about to pupariate; their lymph gland lobes are relatively large and mitotically active (Fig. 4A–C). In mutants, the anterior lobes are dispersed with only few cells remaining (Fig. 4D, outline). The enlarged posterior lobes have numerous mitotically-active cells; these lobes show signs of detachment from the dorsal vessel (e.g., in Fig. 4E–F, only single partially detached posterior lobes are visible). Lobes of both PL1 and PL2 are severely affected (Fig. 4E–F, merged confocal Z sections) and the number of phospo-histone H3-positive cells ranges between 200–800 per posterior lobe set, compared to 30–80 phospho-histone H3-positive cells in the corresponding control lobes.
To clarify the identity of mitotic cells and examine their relation to Dome>GFP expression, we stained anterior lobes of slightly younger early 6-day lymph glands (where Dome>GFP is still detectable) and visualized differentiated plasmatocytes (anti-Nimrod C antibody) or lamellocytes (anti-Atilla antibody) with anti-phospho-histone H3 antibody. Most of the Dome>GFP cells in control glands are phospho-histone H3-negative, confirming proliferative quiescence of this cell population (Fig. 4G,G1,I,I1, arrowheads indicate mitotic cells). Not surprisingly, markers for mitosis and Nimrod C rarely colocalized in cells of either genotype (Fig. 4G–H1, arrow). None of the lamellocytes were in division (Fig. 4I–J1). Notably however, loss of Dome>GFP precedes increase in proliferation, as phospho-histone H3 staining is observed in regions of mutant lobes with low Dome>GFP signal, but only rarely among the Dome>GFP-positive cells (Fig. 4H,H1,J,J1).
Collectively, these observations strongly suggest that the solid compact large Ubc9 microtumors result primarily from the excessive mitoses in the lymph gland lobes. The expanded lobes are severed from the dorsal vessel to become free-floating microtumors. Some small tumors and aggregates are likely derived from clusters of cells dispersed from the anterior lobes. These conclusions are supported by the following: (1) Extensive mitoses and overgrowth in the anterior and posterior mutant lobes of 6 to 7 day old organs and their partial dispersal (Figs 1, 3, 4, 5, supplementary material Figs S1, S2). (2) Massive overgrowth of the remaining posterior lobes with enhanced expression of ZCL2897 (Figs 1K, supplementary material Fig. S2J,L, supplementary material Fig. S3D,F) or 76B (Fig. 2E, supplementary material Fig. S2O,P) in the lobes and microtumors. (3) The morphologies of overgrown ZCL2897hi and 76B>GFPhi lobes match those of the microtumors in the hemolymph. (4) The time of microtumor appearance in the hemolymph correlates with observed detachment of the overgrown lobes from the dorsal vessel.
Ubc9 function is essential in hematopoietic progenitors
To delineate the spatio-temporal requirement of Ubc9 in restraining division and differentiation of hematopoietic progenitors, we provided wild type Ubc9 protein to these populations via Dome-Gal4 and 76B-Gal4. The experimental rescue class (Ubc9; Dome>Ubc9wt) animals exhibit simultaneous and remarkable amelioration from the differential effects of the mutation on the anterior and posterior lobes: (1) The normal temporal and spatial regulation of the Dome promoter is restored in both anterior and posterior lobes and cells of the dorsal vessel (Fig. 5A–C). (2) The normal course of lobe development is restored, i.e., not only are the rescued posterior lobes comparable in size to control posterior lobes, they remain tethered to the dorsal vessel. (3) Even though the cortical zone of some rescue class glands shows differentiating lamellocytes, the overall proportions of the medullary and cortical zones return to normal. Overexpression of Dome>Ubc9wt reduces the number of Dome>GFP cells very slightly (Fig. 5D). (4) A stark reduction in tumorogenesis is noted as reduction in the proportion of animals carrying free microtumors (supplementary material Fig. S5D; microtumor penetrance from 75% to 11%) or aggregates (clusters of 15–50 cells; supplementary material Fig. S5E; from 82% to 23%). Other non-hematopoietic defects, i.e., delay in the onset of pupariation and adult lethality, are also rescued. These rescued adults carry no visible microtumors.
Significantly, like Dome>Ubc9wt, 76B>Ubc9wt also rescues Ubc9 defects. Since its expression is high in mutant cells (Fig. 2D,E), it is possible to visualize the remedial effects of 76B>Ubc9wt as it shrinks the GFP-positive cell population, restores coherent lymph gland lobes (I.P. and S.G., unpublished data), prevents posterior lobe detachment, and reduces the tumor burden (supplementary material Fig. S5F–I).
In contrast to the full rescue with the Dome>Ubc9wt and 76B>Ubc9wt transgenes, we found that large microtumors persisted with Collagen>Ubc9wt expression (supplementary material Fig. S6A–D; Cg-Gal4 is expressed in the lymph gland cortical zone, circulating hemocytes, and fat body) (Asha et al., 2003). All together, these observations are consistent with the interpretation that even though Ubc9 influences all hematopoietic compartments and the integrity of the lymph gland, the primary function of the protein is to maintain quiescence in hematopoietic progenitors. Sumoylation appears to serve a critical tumor-suppressive function by regulating the gene expression and the cell cycle of hematopoietic progenitors of the third instar larval lymph gland.
Ubc9 hyperplasia is niche-independent
To examine the requirement for Ubc9 in the niche, we compared niche morphology and size, and the membranous projections emanating from the niche into the medullary zone (Krzemien et al., 2007; Mandal et al., 2007) in heterozygous and mutant glands. We found no significant difference in the niche size, measured either as the number of cells expressing Antennapedia protein (supplementary material Fig. S7A–C, 5 day old animals) or Antp>GFP (supplementary material Fig. S7D–F, 6 day old animals). There was no difference in the niche projections, which were sparse in both backgrounds (supplementary material Fig. S7D,E). Cells of the dorsal vessel immediately adjacent to the niche express Antp (by both criteria), although we found no difference in its expression between heterozygous and mutant glands (supplementary material Fig. S7A,B,D,E; asterisks). An occasional population of Antp>GFP cells is found in the posterior lobes of the mutant or in microtumors (M.K. and S.G., unpublished data).
To link Ubc9 function in the niche to overproliferation, we examined Ubc9−, Antp>Ubc9wt progeny. These rescue class larvae did not experience relief from hematopoietic defects (supplementary material Fig. S7G–K) and died during pupal stages, just like their mutant siblings. Overexpression of Ubc9wt in the niche (Antp-Gal4) did not modify the niche or lobe morphology, nor did it induce lamellocytes (M.K. and S.G., unpublished data). Likewise, mutants were not rescued when wild type protein was supplied in the niche by Collier-Gal4 (M.K. and S.G, unpublished data) (Crozatier et al., 2004). These observations demonstrate that progenitor hyperplasia in mutants is niche-independent and that its function is autonomous with respect to the progenitor pool.
Loss of Ubc9 is linked to reduction of Dacapo levels
Protein interaction data suggested direct association of Ubc9 with Drosophila CDK inhibitor Dacapo (Dap) (Stanyon et al., 2004). To test if Dap levels are affected in Ubc9 cells, we stained lymph glands with anti-Dap antibody (described in de Nooij et al.) (de Nooij et al., 1996). In control glands, levels of Dap protein differ: cytoplasmic Dap is somewhat higher in the compact region of the medullary zone (dotted lines Fig. 6A,A'), than in the cytoplasm of Dome>GFP-negative cells. This correlation is maintained in Ubc9 glands, where cytoplasmic Dap signal is significantly reduced in cells with lower Dome>GFP signal and loss of the compact architecture (Fig. 6A–B'). The overall correlation between high Dome>GFP and high Dap signals suggests that sumoylation maintains quiescence by controlling cell cycle exit by sustaining high levels of Dacapo. While in both, heterozygous and mutant glands, Dacapo levels are lower in cells outside the medulla, in both backgrounds Dap protein is clearly detected (Fig. 6A–B').
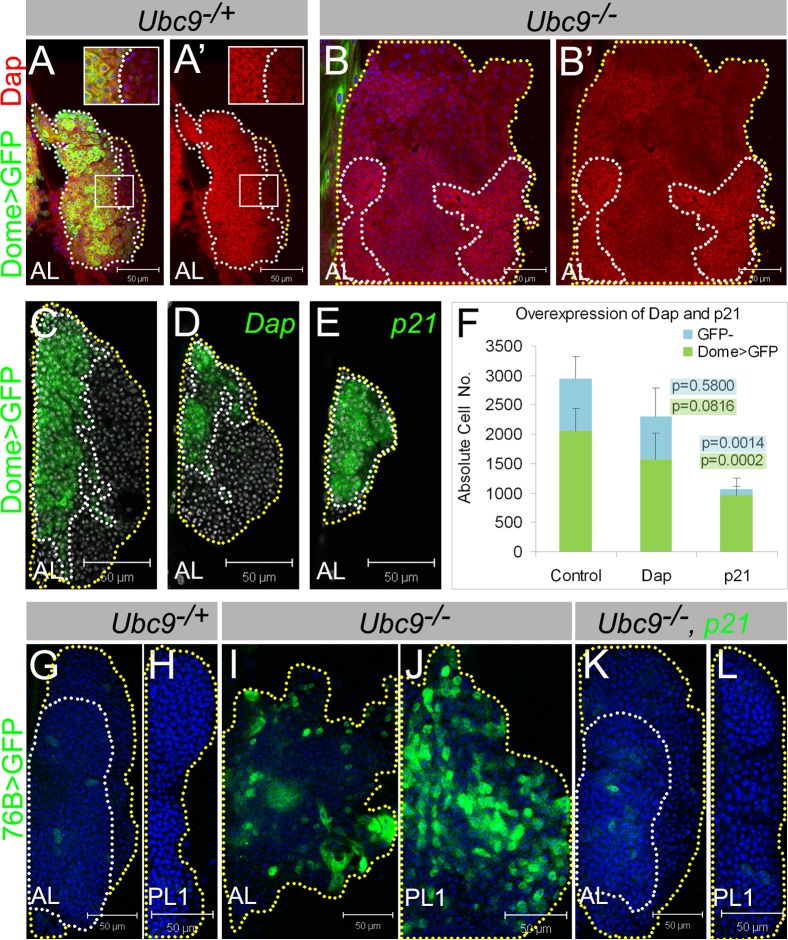
Fig. 6. Lymph gland cells respond to cell cycle inhibitors Dacapo/p21 and human p21 rescues Ubc9 tumorogenesis.
(A–B') Dacapo (red) and Dome>GFP (green) expression in L3 Ubc9−/+ (A,A') and Ubc9−/− (B,B') lymph glands. Indicated regions (A,A') are magnified in the insets. (A',B') show Dacapo staining only. (C–F) Lymph glands with Dome>GFP (C); Dome>Dap, GFP (D); Dome>p21, GFP (E) expression; nuclei labeled in white. Absolute cell numbers in Dome>GFP; Dome>Dap, GFP; Dome>p21, GFP (F; average ± SE, n≥5 animals per genotype); p values relative to control are shown on the graph (for Dome>GFP populations with green highlight, for remaining GFP− cells with blue highlight). Dotted lines outline MZ (white) and CZ (yellow). (G–L) 76B>GFP (green) in Ubc9−/+ AL (G), PL1 (H); Ubc9−/− AL (I), PL1 (J); Ubc9−/−, 76B>p21, GFP AL (K), PL1 (L). Outlines: compact tissue (white line), lobe edges (yellow). Confocal sections (A–L). Brightness of images in A–B' was slightly increased for clarity without modifying the result. Scale bars: 50 µm.
Expression of human p21 relieves Ubc9 overproliferation
Dacapo shares structural and functional similarity with vertebrate cyclin/cyclin-dependent kinase (CDK) inhibitors, p21/p27 (de Nooij and Hariharan, 1995; de Nooij et al., 1996; Lane et al., 1996). Like overexpression of Ubc9wt, both Dome>Dap and Dome>p21 lead to reduction of the progenitor population (Fig. 6C–F). The effect of Dome>p21 is stronger than that of Dome>Dap [n = 2060±384 cells in control (average ± SD, per both anterior lobes), to n = 1566±449 in Dome>Dap, and n = 952±301 in Dome>p21 (Fig. 6C–F)].
If the primary function of sumoylation is to maintain quiescence in progenitors, expression of p21 in this population may be sufficient to partially restore lymph gland homeostasis. To test this hypothesis, we created Dome>p21; Ubc9 animals. Unlike Dome>Ubc9wt, Dome>p21 resulted in only temporary and weak rescue (supplementary material Fig. S8A) presumably because in Dome>p21; Ubc9 glands, Dome>GFP levels continue to remain low (M.K. and S.G., unpublished data).
In contrast to Dome>p21, both, 76B>Dap and 76B>p21 prevent overgrowth of the progenitor population in mutant glands, restoring their normal compact morphology. There is a decline in the 76B>GFP-positive cells (Fig. 6K,L), the lobes do not disperse or dislocate, and microtumor penetrance is significantly reduced (Fig. 6K,L, supplementary material Fig. S8B). However, when p21 was provided in cells of the cortical zone and circulating hemocytes (with SrpHemo, Hemese, Hml, or Cg), we found no evidence of tumor rescue. Thus, downregulation of Dap expression in Ubc9 mutant lymph gland progenitors and Ubc9 rescue with 76B>Dap/p21 confirm the tumor-suppressive function of Ubc9 in the hematopoietic progenitors and suggest that cell cycle inhibition is likely maintained through sumoylation.
Discussion
Mammalian cancer stem cells, characterized in many cancer types, persist for a long time, and like their putative parental cells, remain proliferatively quiescent. This phenotype is thought to make them resistant to chemotherapy. Whether quiescence plays a role in cancer stem cell biology and how these cells retain proliferative quiescence, despite transitioning into a diseased state, is not clearly understood (reviewed in Moore and Lyle, 2011). Our studies here provide an important avenue to investigate the regulatory cell cycle mechanisms of normal and quiescent cancer cells at the earliest stage of cancer development.
Tumorogenesis results from failure to quiesce, dysplasia of heterogeneous progenitors, and dispersal and detachment of lobes
In a quest to identify the source of microtumors in Ubc9 mutants, we discovered that even though Ubc9 protein is ubiquitously expressed, it plays a specific and essential, niche-independent function in maintaining proliferative quiescence within progenitors of the medullary and transition zones. Reduction of sumoylation via knockdown of any of the other core enzymes of the pathway also leads to progenitor dysplasia and tumorogenesis. Once detached from the dorsal vessel, the microtumors float in the hemolymph (Fig. 1, supplementary material Figs S1, S2, Fig. 3, supplementary material Fig. S5).
The progenitor population that serves as the source of microtumors is heterogeneous with respect to Dome>GFP and ZCL2897 expression. One of the earliest detectable effects of the mutation is on the differential expression of Dome>GFP and ZCL2897 or 76B>GFP in the expanding population (Fig. 1, supplementary material Fig. S2, Figs 3, 7). The onset of the effects of Ubc9 mutation coincides with the period when the progenitors in the medulla of the anterior lobes undergoes proliferative restraint (Jung et al., 2005). At the same time, cells of the posterior lobes lag behind; they continue to divide and follow a defined heterochronic developmental pattern (Fig. 1A,B,D, supplementary material Fig. S1A,C). It is somewhat surprising that even though the Ubc9 mutation has differential effects on cells of the anterior versus posterior lobes, the overproliferation defects in both are largely rescued by ectopic expression of p21/Dap. This observation suggests a fundamental role for the enzyme in inhibiting cell cycle progression and conferring quiescence to progenitors. Since the decline in Dome>GFP expression precedes overproliferation in mutant lobes (Fig. 4) and each defect can be rescued by the expression of wild type Ubc9, it is possible that Dome>GFP expression marks the quiescent cell state. The inability of p21 or Dap to restore normal Dome>GFP expression attests to the notion that the sequential series of events, even at the earliest stages of tumorogenesis, can be genetically teased out in vivo.
Fig. 7. Sumoylation controls proliferation of progenitor cells along with Dacapo.

Hematopoietic progenitors express high level of either one or both, ZCL2897 (green) and Dome>GFP (red). Some of these cells and cells in transition zone express 76B-Gal4 (cyan). At third instar stage, progenitors enter quiescence. Heterogeneity and absence of mature marker expression suggest similarity to mammalian transit amplifying cells (Morrison and Spradling, 2008; Shaker and Rubin, 2010) or Drosophila testis progenitors (Shivdasani and Ingham, 2003). We propose that sumoylation regulates multiple events including maintenance of high levels of Dacapo protein in these cells. In the absence of sumoylation enzymes Aos1/Uba2, Ubc9, and PIAS, these cells fail to quiesce and progress into G2/M phase (cells with purple outline) and misdifferentiate (dark green cells); dysplasia and tumorogenesis follow.
While the changes in cell identities in mutant lobes are complex, the discovery of heterogeneity in the medullary zone populations of anterior and first posterior lobes is consistent with recent reports that this population has distinct fate-restricted cell populations (Krzemien et al., 2010; Minakhina and Steward, 2010). Our results suggest that lymph gland progenitors are similar to mammalian transit amplifying cells (Morrison and Spradling, 2008; Shaker and Rubin, 2010) or those in the Drosophila testis (Shivdasani and Ingham, 2003), that have limited proliferative capacity and possess a restricted differentiation potential relative to their multipotent stem cells. With an appropriate immune or developmental cue, Drosophila hematopoietic progenitors may re-enter the cell cycle to produce differentiated progeny.
What is the physiological significance of retaining some cells in quiescence at this stage in larval life? One possibility is that mitotic exit shelters progenitors from precocious development and provides a mechanism that determines the number of times they must divide before they differentiate. Additionally, a reserve of progenitors, ready to divide and differentiate rapidly guards larvae against natural enemies such as parasitic wasps that attack them at this stage of the life cycle (Sorrentino et al., 2002). This tactic parallels mitotic exit of hematopoietic stem cells (HSCs) in mice about three weeks after birth, or in humans, at about four years of age, when they become adult HSCs. The dormant adult HSCs are activated as the organism recovers from injury (Trumpp et al., 2010).
This similarity in strategies between flies and humans in normal hematopoiesis is further reinforced even when the process becomes aberrant. Like in dUbc9 mutants, uncontrolled proliferation of progenitors in human leukemias can occur independently of the signals from the niche (supplementary material Fig. S7) (Passegue et al., 2003). It is intriguing that Antp, a niche marker, is also expressed in the dorsal vessel (supplementary material Fig. S7). Furthermore, Dome>GFP expression, undetectable in normal cells, is strongly activated in mutant cells of the dorsal vessel (Figs 1, 5). Thus, it is possible that cues from the cells of the dorsal vessel influence the state of the hematopoietic progenitors and integrity of the lobes. Conversely, the status of the progenitors themselves may determine the association of the lobes to the dorsal vessel. Further analysis of Ubc9 mutants will clarify the role of the microenvironment in supporting progenitor quiescence and maintaining tissue integrity.
Dacapo/p21 contributes to progenitor quiescence
A key mechanism by which sumoylation maintains proliferative quiescence in larval hematopoiesis is cell cycle regulation through Dacapo/p21. In the embryo, Dap/p21 binds to cyclin E/Cdk2 complexes to block the G1/S transition in cell cycle (Lane et al., 1996). Furthermore, the human p21 protein can block mitosis in the Drosophila eye (Tseng and Hariharan, 2002). This function of Dap/p21 in larval hematopoiesis is similar to the roles of p27KIP1 (Fero et al., 1996) or p21CIP1/WAF1 (Cheng et al., 2000) in enforcing HSC quiescence.
We found that Dap is expressed in Dome>GFP progenitors in wild type and mutant glands, and is reduced shortly after Dome>GFP is downregulated in mutant glands (Fig. 6). Overexpression of Dap/p21 in these cells leads to decrease in progenitor number. It is noteworthy that dap mutants do not exhibit apparent tumorous overgrowth (M.K. and S.G., unpublished data) (de Nooij et al., 1996; Lane et al., 1996), a trait that is similar to young p21 null mice (Adnane et al., 2000; Martin-Caballero et al., 2001). However, with age, or in the presence of other mutations (e.g., oncogenic Ras), p21 null mice are prone to developing tumors (Adnane et al., 2000; Martin-Caballero et al., 2001; Jackson et al., 2002). It is therefore very likely that tumorogenesis in Ubc9 mutants is supported not only by loss of Dap/p21 but also by the activation of other oncogenic and pro-inflammatory proteins (Fig. 7).
The mechanism by which Ubc9 controls Dap protein levels is not known. dap transcription has been studied in embryonic development where it regulates mitotic exit (de Nooij et al., 1996; Lane et al., 1996; Liu et al., 2002). High dap transcript levels in stage 16 embryonic central and peripheral nervous system, or in differentiating postmitotic cells of a developing eye disc, correlate with exit from mitosis (de Nooij et al., 1996; Lane et al., 1996; Liu et al., 2002). These observations suggest that regulation of dap transcription is coupled with mitotic exit, and it is therefore possible that its transcription in the lymph gland progenitors is similarly synchronized. Microarray experiments of whole Ubc9 larvae compared to their heterozygous siblings indicate dap transcript downregulation (S.G., unpublished data). An intriguing possibility is that Dacapo itself, or another protein in complex with Dap, is a sumoylation target. In high throughput yeast two-hybrid assay, Dap was found to physically interact with Ubc9 (Stanyon et al., 2004). Future experiments including biochemical analyses of Dap and interacting proteins are required to test this idea.
Unscrambling Ubc9 functions in cancer and inflammation
The causal relationship between cancer and inflammation is now widely accepted, even though the mechanisms that establish and sustain this relationship remain unresolved (Karin and Greten, 2005; Mantovani et al., 2008). Drosophila Toll-Dorsal pathway not only manages immunity, but also governs hematopoietic development (Qiu et al., 1998; Govind, 2008). Ubc9 microtumor development requires Rel/NF-kappa B family transcription factors Dorsal and Dif (Chiu et al., 2005; Huang et al., 2005). Aberrant activation of NF-kappa B signaling in Ubc9 mutants resembles hematopoieitic malignancies in vertebrates that arise due to ectopic germline or somatic disruption of the pathway (Courtois and Gilmore, 2006).
We recently discovered that sumoylation provides a homeostatic mechanism to restrain systemic inflammation in the fly larva, where it keeps the Toll/Dorsal-dependent immune response in check. Ubc9 controls the “set point” by maintaining normal levels of IκB/Cactus protein in immune tissues (Paddibhatla et al., 2010). The Ubc9 cancer-inflammation model offers novel opportunities to examine the dynamics of tumor growth, its relationship to metastasis, and the links between cancer and inflammation. Ubc9 tumors are sensitive to aspirin (I.P. and S.G., unpublished results). This model is well-suited for identifying and testing drugs that target highly-conserved biochemical mechanisms, such as sumoylation, which oversee self-renewal pathways in progenitor populations.
Material and Methods
Fly strains and culture
The following lines were obtained: y w; Ubc94-3 FRT40A/CyO y+ and y w; Ubc95 FRT40A/CyO y+ (Dr S. Tanda) (Chiu et al., 2005); w1118; PBac{w+mC = PB}Aos1c06048/TM6B, Tb1 (17744) and y w; Smt3K06307/CyO, act-GFP (10419; Drosophila Bloomington Stock Center); PIAS alleles Su(var)2-101/CyO, act-GFP and Su(var)2-102/CyO, act-GFP (Dr G. Karpen) (Hari et al., 2001); MSNF9mo-mCherry from Dr R. Schultz (Tokusumi et al., 2009), Dome-MESO (Hombria et al., 2005; Krzemien et al., 2007). ZCL2897 was obtained from Yale GFP Protein Trap Collection (Morin et al., 2001).
UAS lines: UAS-Aos1RNAi (Vienna Stock Center) and UAS-Uba2RNAi (TRiP, Harvard Medical School); UAS-Ubc9wt (Dr S. Tanda) (Apionishev et al., 2001); UAS-p21 and UAS-Dap (Dr I. Hariharan) (Tseng and Hariharan, 2002); UAS-mCD8-GFP (5137), UAS-DsRed (6280) and UAS-myr-mRFP (7119) from Drosophila Bloomington Stock Center.
Gal4 lines: Domeless-Gal4 (Bourbon et al., 2002) and Collier-Gal4 (Krzemien et al., 2007) from Dr M. Crozatier, Antennapedia-Gal4 (Dr S. Minakhina) (Emerald and Cohen, 2004), HemolectinΔ-Gal4 (Sinenko and Mathey-Prevot, 2004) and Collagen-Gal4 (7011; Bloomington Stock Center) (Asha et al., 2003); Hemese-Gal4 (Dr I. Ando) (Zettervall et al., 2004) and Serpent-Gal4 (Bruckner et al., 2004) are expressed only in some lymph gland cells and circulating hemocytes; 76B-Gal4 (Harrison et al., 1995).
The ZCL2897, UAS and Gal4 transgenes were integrated into mutant backgrounds by standard crosses. ZCL2897 and Dome>myr-mRFP combination was lethal in the Ubc94-3/5 background.
Drosophila cultures were maintained on standard media. Six- or twelve-hour egglays were cultured at 23.5°C. Ubc94-3/5 and Su(var)2-101/2 were studied. Comparison of mutant and heterozygote was done on the same day, where available. Heterozygotes pupate at day 6; some of the Ubc9 mutants remain in L3 at day 8.
Rescue experiments
The Gal4/UAS system (Brand and Perrimon, 1993) was used. Dome>Ubc9wt rescue: y w UAS-Ubc9wt/Y; Ubc95, UAS-mCD8-GFP/CyO y+ and y w Dome-Gal4/FM7c; Ubc94-3/CyO y+ flies were crossed; simultaneous cross: y w/Y; Ubc95, UAS-mCD8-GFP/CyO y+ and y w Dome-Gal4/FM7c; Ubc94-3/CyO y+; F1: heterozygote Dome-Gal4/y w; Ubc95, UAS-mCD8-GFP/CyO y+, mutant Dome-Gal4/y w; Ubc95, UAS-mCD8-GFP/Ubc94-3, rescue Dome-Gal4/y w UAS-Ubc9wt; Ubc95, UAS-mCD8-GFP/Ubc94-3 and overexpression Dome-Gal4/y w UAS-Ubc9wt; Ubc95, UAS-mCD8-GFP/CyO y+ classes were scored.
Remaining rescues were similarly designed: UAS-Ubc9wt, UAS-Dap, or UAS-p21-carrying flies in Ubc94-3 or Ubc95 background were crossed to those carrying selected Gal4 (Dome, 76B, Hemolectin, Collagen, Hemese, Serpent, Antp) and Ubc95 or Ubc94-3 allele; UAS-GFP or UAS-mCD8-GFP transgenes were carried by either one, or both parents. The F1 Ubc94-3/5 mutant combination, rescue, overexpression and heterozygous control were scored.
Tumor penetrance in larvae was scored after dissection (at a magnification of 200 x), except in 76B>Ubc9wt and 76B>p21 rescue experiments where tumors were scored in intact animals. Since tumor penetrance is inherently variable, for well-controlled conditions and comparable results, all control, mutant, rescue and overexpression animals were grown and scored simultaneously under the same conditions. All experiments were performed in duplicate or triplicate.
Immunohistochemistry
Standard antibody staining protocol was used (described in Paddibhatla et al.) (Paddibhatla et al., 2010). Antibodies used: rabbit anti-phospho-histone H3 (1:200, Molecular Probes), mouse anti-P1/Nimrod C1 and mouse anti-L1/Atilla (1:10) (Vilmos et al., 2004; Kurucz et al., 2007a), mouse anti-Prophenol Oxidase (1:10, Dr T. Trenczek, University of Giessen), rabbit anti-Ubc9 (1:1500, received from Dr R. Tanguay) (Joanisse et al., 1998), anti-Antennapedia 8C11 (1:20, Developmental Studies Hybridoma Bank, The University of Iowa), mouse anti-Dacapo (1:4, received from Dr I. Hariharan (de Nooij and Hariharan, 1995), mouse anti-beta-galactosidase 40-1a (1:10, Developmental Studies Hybridoma Bank, The University of Iowa). Fluorescently-labeled secondary antibodies (Molecular Probes and Jackson Immunological), Phalloidin (Invitrogen) and nuclear dye Hoechst 33258 (Molecular Probes) were used.
Image acquisition and processing
Whole larvae were imaged in Leica stereomicroscope. Images of dissected and stained tissues were acquired in a Zeiss Laser Scanning Confocal or Zeiss Axioscope 2 Plus Fluorescence microscopes, and formatted in Zeiss LSM5 and AxioVision LE 4.5 software, respectively. Figures were assembled in Adobe Photoshop CS5. Cell counts were performed using Volocity software (Perkin Elmer). Nuclear staining is represented in the figures in blue, unless stated otherwise. Slight adjustments of brightness and contrast were applied equally to images of both, control and mutant, where applicable, and are explicitly stated in corresponding figure legend. None of these modifications affect or modify the result in a significant way.
Supplementary Material
Acknowledgments
We are grateful to our colleagues for stocks and reagents and to members of the Govind Lab for feedback. We thank J. Uribe, C. Chand, R. Rajwani, and Z. Papadopol for help with experiments and D. Fimiarz for help with confocal imaging, image processing and data retrieval. We thank the TRiP facility at Harvard Medical School for RNAi lines. This work was supported in part by funds from NSF (1121817), USDA (NRI/USDA CSREES 2006-03817 and 2009-35302-05277), NIH NIGMS S06 GM08168, G12-RR03060, P50-GM68762), and PSC-CUNY.
References
- Adnane J., Jackson R. J., Nicosia S. V., Cantor A. B., Pledger W. J., Sebti S. M. (2000). Loss of p21WAF1/CIP1 accelerates Ras oncogenesis in a transgenic/knockout mammary cancer model. Oncogene 19, 5338–5347 10.1038/sj.onc.1203956 [DOI] [PubMed] [Google Scholar]
- Apionishev S., Malhotra D., Raghavachari S., Tanda S., Rasooly R. S. (2001). The Drosophila UBC9 homologue lesswright mediates the disjunction of homologues in meiosis I. Genes Cells 6, 215–224 10.1046/j.365-2443.2001.00413.x [DOI] [PubMed] [Google Scholar]
- Asha H., Nagy I., Kovacs G., Stetson D., Ando I., Dearolf C. R. (2003). Analysis of Ras-induced overproliferation in Drosophila hemocytes. Genetics 163, 203–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourbon H. M., Gonzy-Treboul G., Peronnet F., Alin M. F., Ardourel C., Benassayag C., Cribbs D., Deutsch J., Ferrer P., Haenlin M. et al. (2002). A P-insertion screen identifying novel X-linked essential genes in Drosophila. Mech. Dev. 110, 71–83 10.1016/S0925-4773(01)00566-4 [DOI] [PubMed] [Google Scholar]
- Brand A. H., Perrimon N. (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 [DOI] [PubMed] [Google Scholar]
- Brown S., Hu N., Hombria J. C. (2001). Identification of the first invertebrate interleukin JAK/STAT receptor, the Drosophila gene domeless. Curr. Biol. 11, 1700–1705 10.1016/S0960-9822(01)00524-3 [DOI] [PubMed] [Google Scholar]
- Bruckner K., Kockel L., Duchek P., Luque C. M., Rorth P., Perrimon N. (2004). The PDGF/VEGF receptor controls blood cell survival in Drosophila. Dev. Cell 7, 73–84 10.1016/j.devcel.2004.06.007 [DOI] [PubMed] [Google Scholar]
- Cheng T., Rodrigues N., Shen H., Yang Y., Dombkowski D., Sykes M., Scadden D. T. (2000). Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science 287, 1804–1808 10.1126/science.287.5459.1804 [DOI] [PubMed] [Google Scholar]
- Chiu H., Ring B. C., Sorrentino R. P., Kalamarz M., Garza D., Govind S. (2005). dUbc9 negatively regulates the Toll-NF-kappa B pathways in larval hematopoiesis and drosomycin activation in Drosophila. Dev. Biol. 288, 60–72 10.1016/j.ydbio.2005.08.008 [DOI] [PubMed] [Google Scholar]
- Courtois G., Gilmore T. D. (2006). Mutations in the NF-kappaB signaling pathway: implications for human disease. Oncogene 25, 6831–6843 10.1038/sj.onc.1209939 [DOI] [PubMed] [Google Scholar]
- Crozatier M., Ubeda J. M., Vincent A., Meister M. (2004). Cellular immune response to parasitization in Drosophila requires the EBF orthologue collier. PLoS Biol. 2, e196 10.1371/journal.pbio.0020196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nooij J. C., Hariharan I. K. (1995). Uncoupling cell fate determination from patterned cell division in the Drosophila eye. Science 270, 983–985 10.1126/science.270.5238.983 [DOI] [PubMed] [Google Scholar]
- de Nooij J. C., Letendre M. A., Hariharan I. K. (1996). A cyclin-dependent kinase inhibitor, Dacapo, is necessary for timely exit from the cell cycle during Drosophila embryogenesis. Cell 87, 1237–1247 10.1016/S0092-8674(00)81819-X [DOI] [PubMed] [Google Scholar]
- Emerald B. S., Cohen S. M. (2004). Spatial and temporal regulation of the homeotic selector gene Antennapedia is required for the establishment of leg identity in Drosophila. Dev. Biol. 267, 462–472 10.1016/j.ydbio.2003.12.006 [DOI] [PubMed] [Google Scholar]
- Fero M. L., Rivkin M., Tasch M., Porter P., Carow C. E., Firpo E., Polyak K., Tsai L. H., Broudy V., Perlmutter R. M. et al. (1996). A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell 85, 733–744 10.1016/S0092-8674(00)81239-8 [DOI] [PubMed] [Google Scholar]
- Govind S. (2008). Innate immunity in Drosophila: pathogens and pathways. Insect Sci. 15, 29–43 10.1111/j.1744-7917.2008.00185.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari K. L., Cook K. R., Karpen G. H. (2001). The Drosophila Su(var)2-10 locus regulates chromosome structure and function and encodes a member of the PIAS protein family. Genes Dev. 15, 1334–1348 10.1101/gad.877901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D. A., Binari R., Nahreini T. S., Gilman M., Perrimon N. (1995). Activation of a Drosophila Janus kinase (JAK) causes hematopoietic neoplasia and developmental defects. EMBO J. 14, 2857–2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz A., Bossinger B., Strasser T., Janning W., Klapper R. (2003). The two origins of hemocytes in Drosophila. Development 130, 4955–4962 10.1242/dev.00702 [DOI] [PubMed] [Google Scholar]
- Hombria J. C., Brown S., Hader S., Zeidler M. P. (2005). Characterisation of Upd2, a Drosophila JAK/STAT pathway ligand. Dev. Biol. 288, 420–433 10.1016/j.ydbio.2005.09.040 [DOI] [PubMed] [Google Scholar]
- Huang L., Ohsako S., Tanda S. (2005). The lesswright mutation activates Rel-related proteins, leading to overproduction of larval hemocytes in Drosophila melanogaster. Dev. Biol. 280, 407–420 10.1016/j.ydbio.2005.02.006 [DOI] [PubMed] [Google Scholar]
- Jackson R. J., Adnane J., Coppola D., Cantor A., Sebti S. M., Pledger W. J. (2002). Loss of the cell cycle inhibitors p21(Cip1) and p27(Kip1) enhances tumorigenesis in knockout mouse models. Oncogene 21, 8486–8497 10.1038/sj.onc.1205946 [DOI] [PubMed] [Google Scholar]
- Joanisse D. R., Inaguma Y., Tanguay R. M. (1998). Cloning and developmental expression of a nuclear ubiquitin-conjugating enzyme (DmUbc9) that interacts with small heat shock proteins in Drosophila melanogaster. Biochem. Biophys. Res. Commun. 244, 102–109 10.1006/bbrc.1998.8214 [DOI] [PubMed] [Google Scholar]
- Jung S. H., Evans C. J., Uemura C., Banerjee U. (2005). The Drosophila lymph gland as a developmental model of hematopoiesis. Development 132, 2521–2533 10.1242/dev.01837 [DOI] [PubMed] [Google Scholar]
- Kalamarz M. (2010). Origin and development of hematopoietic tumors in sumoylation mutants of Drosophila melanogaster. PhD dissertation, pp. 112, Department of Biology, The Graduate Center of The City University of New York, USA [Google Scholar]
- Karin M., Greten F. R. (2005). NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 5, 749–759 10.1038/nri1703 [DOI] [PubMed] [Google Scholar]
- Krzemien J., Dubois L., Makki R., Meister M., Vincent A., Crozatier M. (2007). Control of blood cell homeostasis in Drosophila larvae by the posterior signalling centre. Nature 446, 325–328 10.1038/nature05650 [DOI] [PubMed] [Google Scholar]
- Krzemien J., Oyallon J., Crozatier M., Vincent A. (2010). Hematopoietic progenitors and hemocyte lineages in the Drosophila lymph gland. Dev. Biol. 346, 310–319 10.1016/j.ydbio.2010.08.003 [DOI] [PubMed] [Google Scholar]
- Kurucz E., Vaczi B., Markus R., Laurinyecz B., Vilmos P., Zsamboki J., Csorba K., Gateff E., Hultmark D., Ando I. (2007a). Definition of Drosophila hemocyte subsets by cell-type specific antigens. Acta Biol. Hung. 58 Suppl., 95-111 10.1556/ABiol.58.2007.Suppl.8 [DOI] [PubMed] [Google Scholar]
- Kurucz E., Markus R., Zsamboki J., Folkl-Medzihradszky K., Darula Z., Vilmos P., Udvardy A., Krausz I., Lukacsovich T., Gateff E. et al. (2007b). Nimrod, a putative phagocytosis receptor with EGF repeats in Drosophila plasmatocytes. Curr. Biol. 17, 649–654 10.1016/j.cub.2007.02.041 [DOI] [PubMed] [Google Scholar]
- Lane M. E., Sauer K., Wallace K., Jan Y. N., Lehner C. F., Vaessin H. (1996). Dacapo, a cyclin-dependent kinase inhibitor, stops cell proliferation during Drosophila development. Cell 87, 1225–1235 10.1016/S0092-8674(00)81818-8 [DOI] [PubMed] [Google Scholar]
- Lanot R., Zachary D., Holder F., Meister M. (2001). Postembryonic hematopoiesis in Drosophila. Dev. Biol. 230, 243–257 10.1006/dbio.2000.0123 [DOI] [PubMed] [Google Scholar]
- Liu T. H., Li L., Vaessin H. (2002). Transcription of the Drosophila CKI gene dacapo is regulated by a modular array of cis-regulatory sequences. Mech. Dev. 112, 25–36 10.1016/S0925-4773(01)00626-8 [DOI] [PubMed] [Google Scholar]
- Mabb A. M., Miyamoto S. (2007). SUMO and NF-kappaB ties. Cell Mol. Life Sci. 64, 1979–1996 10.1007/s00018-007-7005-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal L., Banerjee U., Hartenstein V. (2004). Evidence for a fruit fly hemangioblast and similarities between lymph-gland hematopoiesis in fruit fly and mammal aorta-gonadal-mesonephros mesoderm. Nat. Genet. 36, 1019–1023 10.1038/ng1404 [DOI] [PubMed] [Google Scholar]
- Mandal L., Martinez-Agosto J. A., Evans C. J., Hartenstein V., Banerjee U. (2007). A Hedgehog- and Antennapedia-dependent niche maintains Drosophila haematopoietic precursors. Nature 446, 320–324 10.1038/nature05585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfruelli P., Arquier N., Hanratty W. P., Semeriva M. (1996). The tumor suppressor gene, lethal(2)giant larvae (1(2)g1), is required for cell shape change of epithelial cells during Drosophila development. Development 122, 2283–2294 [DOI] [PubMed] [Google Scholar]
- Mantovani A., Allavena P., Sica A., Balkwill F. (2008). Cancer-related inflammation. Nature 454, 436–444 10.1038/nature07205 [DOI] [PubMed] [Google Scholar]
- Martin-Caballero J., Flores J. M., Garcia-Palencia P., Serrano M. (2001). Tumor susceptibility of p21(Waf1/Cip1)-deficient mice. Cancer Res. 61, 6234–6238 [PubMed] [Google Scholar]
- Minakhina S., Steward R. (2010). Hematopoietic stem cells in Drosophila. Development 137, 27–31 10.1242/dev.043943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore N., Lyle S. (2011). Quiescent, slow-cycling stem cell populations in cancer: a review of the evidence and discussion of significance. J. Oncol. 2011, pii: 396076 10.1155/2011/396076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin X., Daneman R., Zavortink M., Chia W. (2001). A protein trap strategy to detect GFP-tagged proteins expressed from their endogenous loci in Drosophila. Proc. Natl. Acad. Sci. USA 98, 15050–15055 10.1073/pnas.261408198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S. J., Spradling A. C. (2008). Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 132, 598–611 10.1016/j.cell.2008.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschos S. J., Jukic D. M., Athanassiou C., Bhargava R., Dacic S., Wang X., Kuan S. F., Fayewicz S. L., Galambos C., Acquafondata M. et al. (2010). Expression analysis of Ubc9, the single small ubiquitin-like modifier (SUMO) E2 conjugating enzyme, in normal and malignant tissues. Hum. Pathol. 41, 1286–1298 10.1016/j.humpath.2010.02.007 [DOI] [PubMed] [Google Scholar]
- Nam H. J., Jang I. H., Asano T., Lee W. J. (2008). Involvement of pro-phenoloxidase 3 in lamellocyte-mediated spontaneous melanization in Drosophila. Mol. Cells 26, 606–610 [PubMed] [Google Scholar]
- Paddibhatla I., Lee M. J., Kalamarz M. E., Ferrarese R., Govind S. (2010). Role for sumoylation in systemic inflammation and immune homeostasis in Drosophila larvae. PLoS Pathog 6, e1001234 10.1371/journal.ppat.1001234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passegue E., Jamieson C. H. M., Ailles L. E., Weissman I. L. (2003). Normal and leukemic hematopoiesis: are leukemias a stem cell disorder or a reacquisition of stem cell characteristics? Proc. Natl. Acad. Sci. USA 100, 11842–11849 10.1073/pnas.2034201100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter C. J., Turenchalk G. S., Xu T. (2000). Drosophila in cancer research. An expanding role. Trends Genet. 16, 33–39 10.1016/S0168-9525(99)01878-8 [DOI] [PubMed] [Google Scholar]
- Qiu P., Pan P. C., Govind S. (1998). A role for the Drosophila toll/cactus pathway in larval hematopoiesis. Development 125, 1909–1920 [DOI] [PubMed] [Google Scholar]
- Rizki T. M., Rizki R. M. (1992). Lamellocyte differentiation in drosophila larvae parasitized by leptopilina. Dev. Comp. Immunol. 16, 103–110 10.1016/0145-305X(92)90011-Z [DOI] [PubMed] [Google Scholar]
- Shaker A., Rubin D. C. (2010). Intestinal stem cells and epithelial-mesenchymal interactions in the crypt and stem cell niche. Transl. Res. 156, 180–187 10.1016/j.trsl.2010.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivdasani A. A., Ingham P. W. (2003). Regulation of stem cell maintenance and transit amplifying cell proliferation by TGF-beta signaling in Drosophila spermatogenesis. Curr. Biol. 13, 2065–2072 10.1016/j.cub.2003.10.063 [DOI] [PubMed] [Google Scholar]
- Shrestha R., Gateff E. (1982). Ultrastructure and cyto-chemistry of the cell-types in the larval hematopoietic organs and hemolymph of Drosophila-Melanogaster. Dev. Growth Differ. 24, 65–82 10.1111/j.1440-169X.1982.00065.x [DOI] [PubMed] [Google Scholar]
- Sinenko S. A., Mathey-Prevot B. (2004). Increased expression of Drosophila tetraspanin, Tsp68C, suppresses the abnormal proliferation of ytr-deficient and Ras/Raf-activated hemocytes. Oncogene 23, 9120–9128 10.1038/sj.onc.1208156 [DOI] [PubMed] [Google Scholar]
- Sorrentino R. P., Carton Y., Govind S. (2002). Cellular immune response to parasite infection in the Drosophila lymph gland is developmentally regulated. Dev. Biol. 243, 65–80 10.1006/dbio.2001.0542 [DOI] [PubMed] [Google Scholar]
- Stanyon C. A., Liu G., Mangiola B. A., Patel N., Giot L., Kuang B., Zhang H., Zhong J., Finley R. L., Jr (2004). A Drosophila protein-interaction map centered on cell-cycle regulators. Genome Biol. 5, R96 10.1186/gb-2004-5-12-r96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talamillo A., Sanchez J., Barrio R. (2008). Functional analysis of the SUMOylation pathway in Drosophila. Biochem. Soc. Trans. 36, 868–873 10.1042/BST0360868 [DOI] [PubMed] [Google Scholar]
- Tokusumi T., Shoue D. A., Tokusumi Y., Stoller J. R., Schulz R. A. (2009). New hemocyte-specific enhancer-reporter transgenes for the analysis of hematopoiesis in Drosophila. Genesis 47, 771–774 10.1002/dvg.20561 [DOI] [PubMed] [Google Scholar]
- Trumpp A., Essers M., Wilson A. (2010). Awakening dormant haematopoietic stem cells. Nat. Rev. Immunol. 10, 201–209 10.1038/nri2726 [DOI] [PubMed] [Google Scholar]
- Tseng A. S., Hariharan I. K. (2002). An overexpression screen in Drosophila for genes that restrict growth or cell-cycle progression in the developing eye. Genetics 162, 229–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal M., Cagan R. L. (2006). Drosophila models for cancer research. Curr. Opin. Genet. Dev. 16, 10–16 10.1016/j.gde.2005.12.004 [DOI] [PubMed] [Google Scholar]
- Vilmos P., Nagy I., Kurucz E., Hultmark D., Gateff E., Ando I. (2004). A rapid rosetting method for separation of hemocyte sub-populations of Drosophila melanogaster. Dev. Comp. Immunol. 28, 555–563 10.1016/j.dci.2003.10.001 [DOI] [PubMed] [Google Scholar]
- Wang J. C. (2010). Good cells gone bad: the cellular origins of cancer. Trends Mol. Med. 16, 145–151 10.1016/j.molmed.2010.01.001 [DOI] [PubMed] [Google Scholar]
- Zettervall C. J., Anderl I., Williams M. J., Palmer R., Kurucz E., Ando I., Hultmark D. (2004). A directed screen for genes involved in Drosophila blood cell activation. Proc. Natl. Acad. Sci. USA 101, 14,192–14,197 10.1073/pnas.0403789101 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.