Summary
When a sperm and oocyte unite into one cell upon fertilization, membranous fusion between the sperm and oocyte occurs. In mice, Izumo1 and a tetraspanin molecule CD9 are required for sperm-oocyte fusion as one of the oocyte factors, and another tetraspanin molecule CD81 is also thought to involve in this process. Since these two tetraspanins often form a complex upon cell-cell interaction, it is probable that such a complex is also formed in sperm-oocyte interaction; however, this possibility is still under debate among researchers. Here we assessed this problem using mouse oocytes. Immunocytochemical analysis demonstrated that both CD9 and CD81 were widely distributed outside the oocyte cell membrane, but these molecules were separate, forming bilayers, confirmed by immunobiochemical analysis. Electron-microscopic analysis revealed the presence of CD9- or CD81-incorporated extracellular structures in those bilayers. Finally, microinjection of in vitro-synthesized RNA showed that CD9 reversed a fusion defect in CD81-deficient oocytes in addition to CD9-deficient oocytes, but CD81 failed in both oocytes. These results suggest that both CD9 and CD81 independently work upon sperm-oocyte fusion as extracellular components.
Key words: CD81, CD9, Membrane fusion, Exosome
Introduction
In fertilization, a sperm first interacts with cumulus cells, somatic cells surrounding an oocyte (Ikawa et al., 2010). It causes detachment of cumulus cells from an oocyte by its enzymatic activities, and then adheres to the zona pellucida (ZP), the extracellular matrix fully covering the oocyte. After completion of the acrosome reaction, a specific modification of the outer membrane of a sperm, the sperm penetrates the ZP and adheres to the oocyte cell membrane (Jin et al., 2011). At this time, membrane fusion occurs between the sperm and oocyte.
CD9 and Izumo1 belong to the tetraspan membrane protein family (tetraspanin) and immunoglobulin superfamily, respectively, and play a crucial role in sperm-oocyte fusion in mice (Inoue et al., 2005; Kaji et al., 2000; Le Naour et al., 2000; Miyado et al., 2000). In other words, both CD9-deficient oocytes and Izumo1-deficient sperm are unable to fuse to their wild-type partner's cells (Inoue et al., 2005; Kaji et al., 2000; Le Naour et al., 2000; Miyado et al., 2000). Moreover, CD9-containing exosome-like vesicles are released from oocytes, transferred to the sperm head, and facilitate sperm-oocyte fusion (Ikawa et al., 2010; Miyado et al., 2008; Toshimori, 2011).
Exosomes, nano-sized microvesicles of 50–250 nm in diameter, are known to be released from various types of cells, and play a role in transferring cellular materials from cell to cell (Bobrie et al., 2011). Notably, they contain heat shock proteins (Hsp70 and Hsp90) (Lancaster and Febbraio, 2005), express tetraspanins (CD9, CD81, and CD63) (Zöller, 2009) and gangliosides (GM1 and GM3) (de Gassart et al., 2003) on their outer membrane, and often carry mRNA and microRNA (Valadi et al., 2007). Interestingly, the possible clinical application of exosomes as siRNA carriers is now being explored (Lakhal and Wood, 2011). In dendritic cells, exosomes are generated from intraluminal endosomal vesicles, and released from their cell surface in a form of multivesicular bodies (Pelchen-Matthews et al., 2004).
CD81 is expressed on the surface of oocytes, and deletion of CD81 gene in mice results in a 40% reduction of female fertility, indicating that this infertility is due to the inability of oocytes to fuse with sperm (Rubinstein et al., 2006). Furthermore, when CD81-deficient oocytes are incubated with sperm, some of the sperm penetrating into the perivitelline space of CD81-deficient oocytes fail to undergo acrosome reaction, indicating that the impaired fusibility of CD81-deficient oocytes may be in part caused by impaired acrosome reaction of sperm (Tanigawa et al., 2008). In addition, CD81 is abundantly expressed in granulosa cells, somatic cells that surround oocytes (Tanigawa et al., 2008).
Since CD9 shares homologous sequences with CD81 throughout the four transmembrane regions, and both often form a complex upon cell-cell interaction (Horváth et al., 1998), these two proteins are thought to play similar roles in regulating cellular function (Hemler, 2003). It is therefore likely that such a complex is also formed in sperm-oocyte interaction; however, this possibility is still under debate among researchers (Glazar and Evans, 2009). Here we focused on the subcellular localization of CD9 and CD81 on mouse oocytes to infer the possible roles of these two proteins in the fusion between a sperm and oocyte.
Results
Synergistic effects of anti-CD9 and anti-CD81 on sperm-oocyte fusion
Two mAbs, anti-CD9 and anti-CD81, were raised against extracellular loops of mouse CD9 (Oritani et al., 1996) and mouse CD81 (Maecker et al., 2000), respectively. The former is known to inhibit sperm-oocyte fusion (Chen et al., 1999; Miller et al., 2000; Miyado et al., 2000), to reduce the fertilization rate as well as the two-cell formation rate (Miyado et al., 2000), and to cause excess sperm penetration (>10 sperm/oocyte) (Miyado et al., 2000). The latter is known to induce homotypic adhesion of B cells (Maecker et al., 2000), and also inhibits IL-4 production in the process of antigen-specific T-B cell interaction (Deng et al., 2002). However, anti-CD81 appears to behave like anti-CD9 upon sperm-oocyte fusion (Rubinstein et al., 2006). Thus, we here focused our attention on whether sperm-oocyte fusion or the IVF rate is affected by the addition of anti-CD81 alone or by both anti-CD81 and anti-CD9.
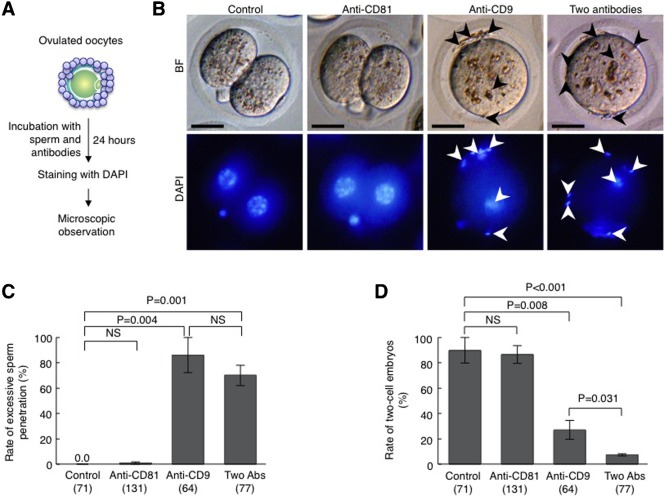
Oocytes surrounded by cumulus cells (herein referred to as ‘cumulus-intact’ oocytes) were isolated from oviducts and directly subjected to IVF in TYH medium containing anti-CD81 and/or anti-CD9, as depicted in Fig. 1A. Concomitantly, ‘cumulus-intact’ oocytes were inseminated in the medium containing pre-immune IgG, and are herein referred to as ‘control oocytes’. In addition, we used rabbit pre-immune IgG whose total concentration had been adjusted to 100 µg/ml in each condition. On observation 24 hours after insemination, oocytes fertilized in the presence of anti-CD81 had developed normally to two-cell embryos, similar to the control oocytes (Fig. 1B). Quantitative analysis showed no significant difference in the rate of two-cell embryos between these two groups (86.5±7.1% for oocytes incubated with anti-CD81 vs. 89.9±10.1% for control oocytes) (Fig. 1D). Furthermore, excess sperm penetration did not occur in either group (0.9±0.9% and 0.0±0.0%) (Fig. 1C). In contrast, in the oocytes inseminated in the presence of both mAbs or anti-CD9 alone, excess sperm penetration was observed in the perivitelline space (PVS) (Fig. 1B). This was confirmed by quantitative analysis (70.2±8.0% for oocytes incubated with both mAbs vs. 86.1±13.9% for oocytes incubated with anti-CD9 alone) (Fig. 1C). Interestingly, the rate of two-cell embryos was significantly reduced in oocytes incubated with both mAbs, comparing to that in oocytes incubated with anti-CD9 alone (7.3±0.8 vs. 27.0±7.5; P = 0.031) (Fig. 1D). This is probably due to the weak, but apparent inhibitory effect of anti-CD81 on IVF. These results suggested that CD9 and CD81 work cooperatively in sperm-oocyte fusion.
Fig. 1. Inhibitory effects of anti-CD9 and anti-CD81 on fertilization.
(A) Experimental flow for testing the rate of excessive sperm penetration in perivitelline space (PVS) of an oocyte and the rate of two-cell embryos. Oocytes collected from oviducts of superovulated female mice were subjected to IVF in the presence of anti-CD9 (50 µg/ml) and/or anti-CD81 (50 µg/ml) or a preimmune Ab (50 µg/ml) for 24 hours. They were then stained with DAPI. BF, bright field. (B) Embryos 24 hours after IVF in the presence of Abs. Arrowheads marked sperm accumulated at the PVS. Scale bars: 20 µm. (C) The rate of embryos exhibiting excess sperm penetration. Parentheses = number of oocytes examined. NS, not significant. Values are the mean±s.e.m. (D) The rate of two-cell embryos 24 hours after IVF in the presence of Abs. Parentheses = number of oocytes examined. NS, not significant. Values are the mean±s.e.m.
Extracellular localization of CD81 and CD9 in oocytes
To assess the roles of CD81 and CD9 in sperm-oocyte fusion in more detail, their subcellular localization on the oocyte cell membrane was examined using ‘zona-intact’ oocytes (Fig. 2). Since fixatives are known to affect the subcellular protein localization pattern in oocytes (Sato et al., 2011), we performed double immunocytochemical staining for CD81 and CD9 using living oocytes, according to the procedure (Miyado et al., 2000), as depicted in Fig. 2A. The confocal microscopic observation demonstrated that the CD81-rich area was confined to the ZP near PVS, while the CD9-rich area was observed in the PVS (Fig. 2B, lower set of images). This indicates the presence of two different types of layers outside the ‘zona-intact’ oocytes, as suggested previously (Miyado et al., 2008).
Fig. 2. Subcellular localization of CD9 and CD81 in oocytes.

(A) Experimental flow. Oocytes were isolated from oviducts, and cumulus cells were removed from oocytes by treatment with hyaluronidase. Oocytes were then reacted with anti-CD9 and anti-CD81 without fixation and observed under a confocal microscope. (B) Oocytes immunostained with anti-CD9 and anti-CD81. In each panel, boxes in the middle set of panels were enlarged and shown on the lower. BF, bright field; PVS, perivitelline space; ZP, zona pellucida. In each panel, scale bars: 20 µm.
To confirm this further, we employed a biochemical approach to know the presence of these two proteins in the extracellular region of ‘zona-intact’ oocytes, as depicted in Fig. 3A. The ‘zona-intact’ oocytes isolated from ovulated oocytes were subjected to treatment with collagenase (Yamatoya et al., 2011) to remove extracellular components such as ZP and PVS (hereafter referred to as ‘ZP + PVS’). The resulting two fractions, namely ‘ZP + PVS’ and ‘denuded oocytes’, were next subjected to immunoblotting (Fig. 3B). As expected, the ‘ZP + PVS’ fraction was successfully immunoreacted with anti-CD9 and anti-CD81. The ‘denuded oocytes’ fraction was only reactive with anti-CD9. In addition, the capacitated sperm lysate immunoreacted with both antibodies. These data indicated that both CD81 and CD9 are present as extracellular components in ‘zona-intact’ oocytes.
Fig. 3. Biochemical analysis for identification of CD9 and CD81 in the extracellular region of an oocyte.

(A) Experimental flow. A total of 40 oocytes were collected from oviducts of superovulated female mice, and cumulus cells were removed from oocytes by treatment with hyaluronidase. The ZP was removed from oocytes by treatment with collagenase. Extracellular components containing PVS and ZP and denuded oocytes were then subjected to immunoblotting together with epididymal sperm (1.5×103). (B) Immunoblotting using anti-CD9 and anti-CD81.
Immunoprecipitation of CD81- or CD9-containing complex from oocytes
Since CD9 shares homologous sequences with CD81, especially with respect to each of their four transmembrane domains, both proteins are considered to exhibit similar biological functions, and in some cases would form a complex, as pointed out by Horváth et al. (Horváth et al., 1998); however, it remains unknown whether complex formation occurs in mouse oocytes. To assess such a possibility, we performed an immunoprecipitation assay using lysates of ‘zona-intact’ oocytes. First, the ‘zona-intact’ oocytes were biotinylated to compare the patterns of components immunoprecipitated with anti-CD9 or anti-CD81, and then subjected to immunoprecipitation (Fig. 4A). When the lysates, corresponding to 10 oocytes per lane, were electrophoresed in SDS-PAGE, the patterns of biotinylated components were completely different between the precipitates reacting with anti-CD81 and with anti-CD9. The precipitate reacting with anti-CD9 was classified into three components, including CD9 molecule, whereas the precipitate reacting with anti-CD81 was classified into seven or more components, the molecular sizes of which were inconsistent with those in components precipitated with anti-CD9 (Fig. 4A). Second, when 500 oocytes were reacted with anti-CD81 without biotinylation and subjected to immunoblotting, the precipitate was reacted with anti-CD9 (Fig. 4B).
Fig. 4. Immunoprecipitation patterns of oocytes.
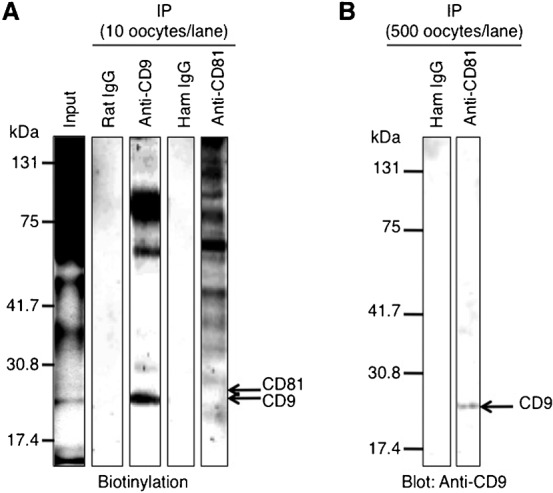
(A) Immunoprecipitation (IP) of oocyte lysates using anti-CD9 and anti-CD81. A total of 200 oocytes were collected from oviducts of superovulated female mice, and cumulus cells were removed from oocytes. Oocytes were biotinylated for 1 hour at 4°C and then lysed in 1% Brij 97-containing buffer for 3 hours at 4°C. This input lysate was next reacted with each anti-CD9 or anti-CD81 for 2 hours at 4°C, and precipitated with Sepharose beads conjugated with secondary Abs. After immunoprecipitation, the lysates corresponding to 10 oocytes were electrophoresed per lane. The preimmune rat IgG and hamster IgG (ham IgG) were concomitantly reacted with the oocyte lysates as negative controls. (B) Immunoblotting of the precipitate after reaction with anti-CD81. 500 oocytes/lane were collected from oviducts and lysed in Brij 97-containing buffer for 3 hours at 4°C. The lysates were reacted with anti-CD81 for 2 hours at 4°C and precipitated with Sepharose beads conjugated with secondary Ab. As a negative control, the oocyte lysates were precipitated with the preimmune hamster IgG (ham IgG). The precipitates corresponding to 500 oocytes were then electrophoresed per lane and immunoblotted with anti-CD9.
CD81-rich and CD9-rich layers are present in extracellular structures outside an oocyte- evidence from immunoelectron-microscopic analysis
We previously showed the presence of CD9-containing exosome-like vesicles in PVS of ‘zona-intact’ oocytes using immunoelectron microscopy (Miyado et al., 2008). In this study, we demonstrated that CD81 is localized in the inner area of the ZP of ‘zona-intact’ oocytes (Figs 2, 3). Moreover, CD81 was successfully immunoprecipitated with anti-CD81, but not with anti-CD9 (Fig. 4). Based on these results, we predicted that CD81-containing structures should exist in ZP. To prove this hypothesis, we next performed immunoelectron-microscopic analysis of extracellular components in ‘zona-intact’ oocytes (Fig. 5). As depicted in Fig. 5A, 200 ‘zona-intact’ oocytes were collected, and extracellular components including ZP and PVS were separated from ‘zona-intact’ oocytes by treatment with collagenase, as mentioned previously. After being separated from denuded oocytes, the extracellular components were subjected to immunoelectron-microscopic analysis. Gold particles bound to anti-CD81 were found in the extracellular structures corresponding to ZP, while those bound to anti-CD9 were confined to the region corresponding to the PVS (Fig. 5B). This observation confirmed the previous results that extracellular structures outside an oocyte consist of two types of layers, the so-called CD81-enriched layer and CD9-enriched layer.
Fig. 5. Electron-microscopic analysis of extracellular components containing CD9 and CD81.
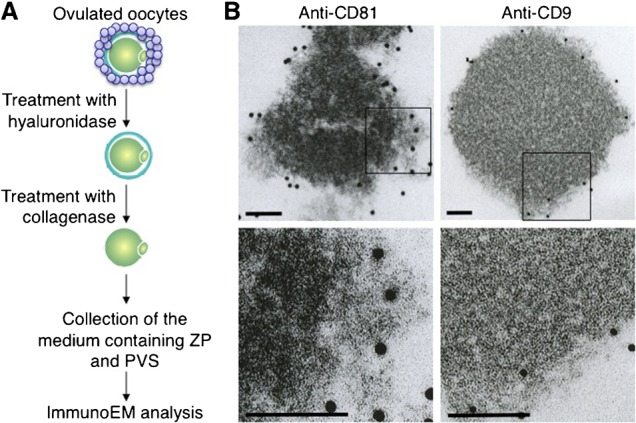
(A) Experimental flow for observing CD9-containing or CD81-containing extracellular structures. Oocytes were collected from oviducts of superovulated female mice, and cumulus cells were removed from oocytes by treatment with hyaluronidase. After ZP removal by collagenase, extracellular components containing ZP and PVS were collected, reacted with anti-CD9 or anti-CD81, and then incubated with 10 nm colloidal gold particles coupled to the secondary Abs for 1 hour at room temperature. The materials conjugated with the gold particles were spun down at 3,000 rpm for 10 min at room temperature, and the precipitates were washed with TYH medium three times. The final precipitates were fixed and subjected to electron-microscopic analysis. (B) Electron-microscopic analysis of materials bound to the gold particles. In each panel, boxes in the middle set of panels were enlarged and shown below. In each panel, scale bars: 100 nm.
Functional sharing between CD81 and CD9 in sperm-oocyte fusion - evidence from exogenous RNA expression assay
To explore possible functional sharing between CD81 and CD9 in sperm-oocyte fusion, we microinjected mRNA for CD9 into CD81-deficient oocytes or mRNA for CD81 into CD9-deficient oocytes (see Materials and Methods). For this purpose, mRNA for CD9 or CD81 was synthesized in vitro, as depicted in Fig. 6A. After microinjection of capped mRNA into immature GV-stage oocytes isolated from ovaries, RNA-injected oocytes were cultured for 24 hours for in vitro maturation and then reacted with each mAb (Fig. 6B). As expected, the CD9-deficient oocyte injected with CD9 mRNA was clearly stained with anti-CD9, and the CD81-deficient oocyte injected with CD81 mRNA was stained with anti-CD81.
Fig. 6. In vitro synthesis of RNAs encoding CD81 and CD9 and subsequent forced expression of mRNA in oocytes.
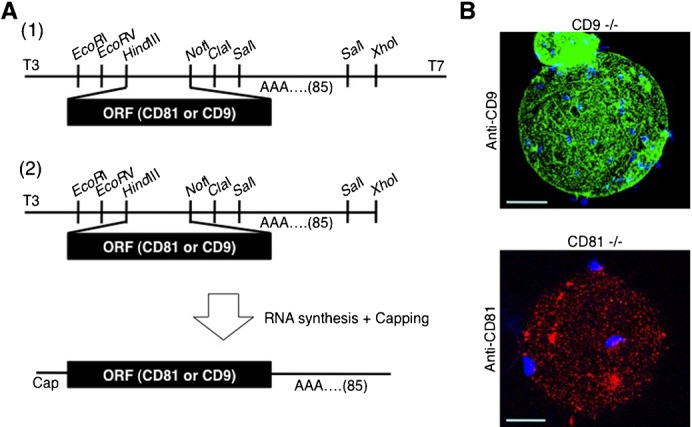
(A) Experimental flow for in vitro synthesis of RNAs encoding mouse CD81 and CD9. (1) Subcloning of CD9 and CD81 cDNAs into plasmid vectors. The ORF corresponding to each cDNA was PCR-amplified, and the amplified DNA fragments were subcloned into the Hin dIII and Not I sites in pBluescript SKII-A85, a vector containing poly(A) repeats (comprising 85 adenines) instead of polyadenylation signal. (2) RNA synthesis. The cDNA-inserted vectors were linearized by digestion with Xho I and used as templates for RNA synthesis using the mCAP RNA Capping Kit. (B) Forced expression of mRNA encoding CD9 or CD81 in oocytes. GV-stage oocytes were collected from ovaries of CD9−/− and CD81−/− female mice and subjected to RNA injection. CD9 RNA was microinjected into CD9−/− oocytes, while CD81 RNA was injected into CD81−/− oocytes. After maturing in vitro for 24 hours, these oocytes were subjected to IVF, after which they were stained with DAPI, immunostained with anti-CD9 or anti-CD81, and observed with a confocal microscope. In each panel, scale bars: 20 µm.
To count the number of sperm fused per oocyte, ‘zona-free’ oocytes prepared by immersion in acidic Tyrode's solution were preincubated with DAPI and then subjected to IVF, as shown in Fig. 7A. This procedure enabled the staining of only fused sperm nuclei by dye transfer into sperm after membrane fusion. Concomitantly, non-injected oocytes were inseminated along with RNA-injected oocytes. When the transcript encoding CD9 was injected, the fusion rate was completely reversed in both CD9-deficient oocytes (1.0±0.1 vs. 0.0±0.0 for non-injected oocytes; P<0.0001) (Fig. 7B) and CD81-deficient oocytes (2.3±0.7 vs. 0.6±0.1 for non-injected oocytes; P = 0.0002) (Fig. 7C). On the other hand, when the transcript encoding CD81 was injected, the fusion rate was unaltered in CD9-deficient oocytes (0.0±0.0 vs. 0.0±0.0 for non-injected oocytes) (Fig. 7B) as well as in CD81-deficient oocytes (0.2±0.1 vs. 0.6±0.1 for non-injected oocytes) (Fig. 7C). These results indicate that the function of CD81 is replaceable by that of CD9, whereas CD81 cannot support the task of CD9, which plays a critical role in sperm-oocyte fusion.
Fig. 7. Increased rate of sperm-oocyte fusion after forced expression of CD9 and CD81 mRNAs in CD9-deficient and CD81-deficient oocytes.
(A) Experimental flow for evaluating the rate of sperm-oocyte fusion. ZP-free oocytes were preincubated with DAPI for 1 hour prior to IVF. The number of fused sperm per oocyte was then counted as shown in the right panel, in which a wild-type ZP-free oocyte fused with several sperm. Arrowheads, sperm fused to an oocyte; arrow, oocyte chromosomes; BF, bright field. Scale bars: 20 µm. (B) Number of sperm fused per RNA-injected CD9-deficient oocyte. Poly(A)+ RNA was in vitro synthesized as depicted in Fig. 6A, and microinjected into GV-stage oocytes. After maturing in vitro for 24 h, these oocytes were subjected to IVF. Parentheses indicate the number of oocytes examined. Values are the mean±s.e.m. (C) Number of sperm fused per RNA-injected CD81-deficient oocyte. Preparation of poly(A)+RNAs, microinjection and subsequent IVF are as described in (B). Parentheses indicate the number of oocytes examined. Values are the mean±s.e.m.
Discussion
Membrane fusion occurs between two cell membranes of the sperm and oocyte. Such a process takes place widely not only in sperm-oocyte interaction in animals, but also in plants. Our present results using immunocytochemical and immunobiochemical analyses indicate that two tetraspanins, CD9 and CD81, play a role as ‘extracellular components’ in sperm-oocyte membrane fusion.
CD81 is expressed in various types of cells, whereas expression of CD9 is more restricted (Caplan et al., 2007). In contrast, CD9 and CD81 interact via at least two tetraspanin partners, CD9P-1 and EWI-2 (Stipp et al., 2003). Consistent with this, the pattern of proteins coprecipitated with CD81 or CD9 in the presence of 3-[(3-cholamidopropyl)-dimethylammonio]propanesulfonate (CHAPS) or Brij 97 is complex, but is quite similar in the CD9-transfected lymphoblast-like Raji cell line (Horváth et al., 1998). When CD9 and CD81 are co-expressed in one type of somatic cell, the majority of CD9 forms a complex with CD81 (Stipp et al., 2001). However, in mouse oocytes, the spatial distribution of these two proteins was different, and the possibility of complex formation between CD9 and CD81 appeared to be very low, as demonstrated in Fig. 2B and Fig. 4. The unique localization pattern of CD9 and CD81 in oocytes in turn suggested that CD9 is predominantly produced in oocytes, while CD81 is predominantly produced in cumulus cells, from which CD81 may become localized to the ZP. Indeed, it has been reported that the expression of CD81 is higher in cumulus cells than in oocytes (Tanigawa et al., 2008). In addition, we here demonstrated that forced expression of CD81 does not affect the fusion rate of both CD9-deficient and CD81-deficient oocytes (Fig. 7B,C), implying that CD81-containing components would not have been released from an oocyte. Taking these collective data into consideration, we here propose a model regarding the roles of CD81 and CD9 in mammalian fertilization (Fig. 8). Before membrane adhesion and subsequent fusion between sperm and oocyte, the differential distribution pattern of CD81 and CD9 may be prerequisite for subsequent fusion events.
Fig. 8. Schematic representation of the distribution of CD81 and CD9 in oocytes upon fertilization.
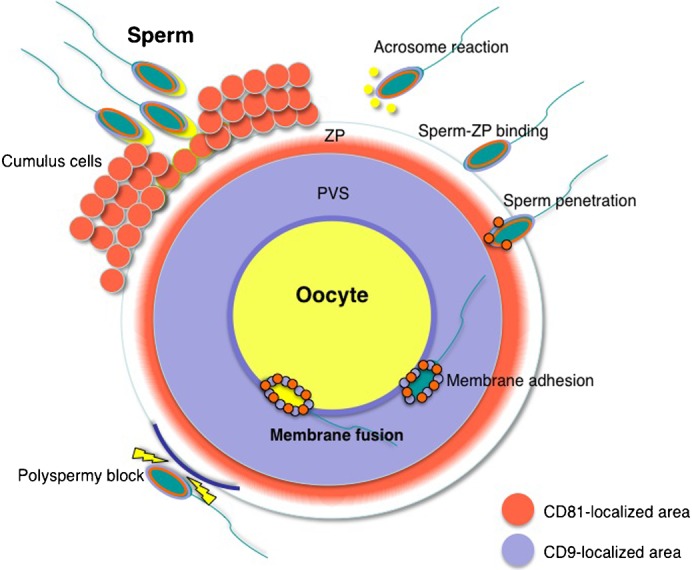
In fertilization, a sperm first interacts with cumulus cells. After the sperm has separated the cumulus cells from an oocyte by its own enzymatic activities, it commences an acrosomal reaction and then adheres to ZP (‘sperm-ZP binding’) (Jin et al., 2011). After sperm-ZP binding, the sperm penetrates the ZP and adheres to the oocyte cell membrane. At this time, membrane fusion occurs between the sperm and oocyte. Once a sperm has fused to the oocyte cell membrane, cortical granule exudates cause ZP modification (‘zona hardening’) to block polyspermic penetration. Upon fertilization, CD81 localizes in the inner region of the ZP, whereas CD9 localizes at the PVS. When the sperm penetrates the PVS, CD81 and CD9 molecules appear to adhere to the sperm surface via exosomes (Miyado et al., 2008; Ito et al., 2010; Kawano et al., 2011). Orange area, CD81-localized area; light blue area, CD9-localized area. ZP, zona pellucida; PVS, perivitelline space.
A sperm fails to fuse with the partner oocyte when a genetically defective oocyte (CD9-deficient oocyte) (Miyado et al., 2000; Le Naour et al., 2000; Kaji et al., 2000) or sperm (Izumo1-deficient sperm) (Inoue et al., 2005) is employed for IVF. Similarly, the fusion rate is reduced when CD81-deficient oocytes are subjected to IVF with normal sperm (Tanigawa et al., 2008; Rubinstein et al., 2006). Moreover, we have demonstrated that 1) CD9 is released from oocytes and becomes localized at the PVS, and 2) CD9 present in the extracellular space plays an important role in sperm-oocyte fusion (Ito et al., 2010). We have also shown that these CD9 molecules in the extracellular space are included in vesicles, termed ‘oocyte exosome-like vesicles’, which are released from an oocyte before fertilization (Miyado et al., 2008). Notably, unlike CD9-deficient oocytes, CD9-deficient sperm are able to fertilize a normal oocyte, although CD9 is strictly required at the site where sperm-oocyte fusion occurs. Furthermore, we here demonstrated that CD81 proteins are expressed in sperm (Fig. 3B), and CD81-deficient sperm are fertile, as described previously (Tanigawa et al., 2008; Rubinstein et al., 2006). Based on these accumulated data, it seems likely that extracellular components (containing these two tetraspanins) outside an oocyte are essential for sperm-oocyte fusion.
CD9 and CD81 are now recognized as components included in the exosomes (Stoorvogel, 2012). These structures often contain small RNAs that are transferred via cell to cell connection (Valadi et al., 2007); therefore, exosomes were recently considered as siRNA carriers for the clinical application of siRNA (Lakhal and Wood, 2011). It was recently reported that human sperm have small RNA (Krawetz et al., 2011), but their nuclei have neither transcriptional nor translational activities (Krawetz et al., 2011). Furthermore, sperm nuclei are not required for sperm-oocyte fusion (Barroso et al., 2009). These data suggest that small RNAs may not mediate sperm-oocyte fusion in mammals if they are included in oocyte exosome-like vesicles.
As previously reported, the expression level of CD9 and its distribution remained unaltered in CD81-deficient oocytes, and either CD81 or CD9 deficiency is expected to have no effect on membrane organization (Tanigawa et al., 2008). Our present results indicated that oocyte CD81 was mainly localized in the ZP, and was involved in sperm-oocyte fusion. Moreover, microinjection experiments demonstrated that additional expression of CD9 could improve the reduced fusion rate caused by CD81 deficiency in oocytes, while additional expression of CD81 failed to reverse a fusion defect caused by CD9 deficiency. On the other hand, since sperm-oocyte fusion occurs between their two cell membranes, CD81 localized at the inner portion of ZP may be involved in any step of fusion-related events prior to membrane fusion, supporting the function of oocyte CD9 in the fusion. Moreover, just before the sperm-oocyte fusion, CD9 inside the PVS is transferred to sperm membrane (Miyado et al., 2008). Thus, we suppose that CD81 may help to transfer of oocyte CD9 into the sperm membrane. Since CD81 can form a complex with CD9 (Horváth et al., 1998), CD81 transferred to the sperm membrane may support the subsequent transfer of CD9 on the sperm membrane through complex formation between them.
In conclusion, we have shown that 1) CD81 and CD9 are present in the extracellular regions of oocytes before sperm-oocyte fusion; 2) CD81 and CD9 partly share activities required for sperm-oocyte fusion.
Materials and Methods
Antibodies
Two monoclonal antibodies (mAbs) against mouse CD9 (clone No. KMC8; hereafter referred to as anti-CD9) and CD81 (clone No. Eat1; hereafter referred to as anti-CD81) for immunostaining, immunoblotting and immunoprecipitation were purchased from BD Biosciences (San Jose, CA) and used as the first Abs. The second antibodies used were Alexa488- or Alexa546-conjugated IgG (Molecular Probes, Eugene, OR). Anti-rat IgG and anti-hamster IgG Abs were obtained from Sigma-Aldrich Co. (St. Louis, MO).
Immunostaining
Female C57BL/6N mice (8 to 12 weeks old; purchased from Japan SLC Inc., Shizuoka, Japan) received intraperitoneal injections of 5 IU of pregnant mare's serum gonadotropin (PMSG; Merck4Biosciences, Darmstadt, Germany) followed by 5 IU of human chorionic gonadotropin (hCG; Merck4Biosciences) 48 hours apart. Oocytes at metaphase II stage were collected from oviducts of females 14–16 hours after administration of hCG. Cumulus cells were dispersed from oocytes by incubating them for 10 min at 37°C in TYH medium (Naito et al., 1988) containing hyaluronidase (300 µg/ml; Merck4Biosciences). The living oocytes were then incubated with TYH medium containing the first mAbs (0.5 µg/ml) for 1 hour at 4°C. After washing with TYH medium, these oocytes were next treated with the second Abs (0.25 µg/ml; Alexa488- or Alexa546-conjugated IgG) for 1 hour at 4°C and then washed 3 times in TYH medium, prior to the acquisition of serially sectioned fluorescent images captured by a laser scanning confocal microscope (LSM 510 model; Carl Zeiss Microimaging Inc., Thornwood, NY).
All animal experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee at the National Center for Child Health and Development.
Immunoblotting, biotinylation and immunoprecipitation
A total of 200 metaphase II oocytes were collected, lysed in Laemmli's SDS sample buffer containing 2% SDS, 62.5 mM Tris-HCl (pH 6.8), 0.005% bromophenol blue and 7% glycerol, boiled for 10 min at 95°C, and resolved in SDS-PAGE on an 8% acrylamide gel, prior to immunoblotting. Detection of the immune complex between proteins and the first antibodies (0.1 µg/ml) of interest was made by enzyme-linked color development with horseradish peroxidase (HRP)-conjugated secondary antibodies (0.01 µg/ml) (Sigma-Aldrich Co.).
Immunoprecipitation was performed according to the procedure described by Sakakibara et al. (Sakakibara et al., 2005). The ‘zona-intact’ oocytes were collected and washed three times in 500 µl HEPES-buffered solution (HBS).
For biotinylation, the oocytes were incubated in 500 µl HBS containing 0.4 mg/ml Sulfo-NHS-LC-Biotin (Pierce Biotechnology, Inc., Rockford, IL) for 1 hour on ice, and washed three times in 500 µl HBS. After biotinylation, the oocytes were incubated with anti-CD9 (1 µg/ml) or anti-CD81 (1 µg/ml) for 2 hours at 4°C, and lysed with 500 µl HBS containing 1% Brij 97 (Sigma-Aldrich Co.) for 4 hours at 4°C. After the lysates were centrifuged at 13,500 rpm for 30 min at 4°C, the supernatants were mixed with 5 µl bed volume of Sepharose beads (Merck4Biosciences) coated with anti-rat IgG or anti-hamster IgG, and rotated for 2 hours at 4°C. After washing three times with 1% Brij 97 in HBS, the Sepharose beads were lysed with Laemmli's SDS sample buffer and heated for 10 min at 95°C. The supernatants were separated by SDS-PAGE and immunoblotted, as described above and mentioned in the figure legends.
On the other hand, non-biotinylated oocytes were lysed in 1 ml of 1% Brij 97 in HBS as described above, immunoprecipitated with anti-CD81 (1 µg/ml) or pre-immune hamster IgG (Sigma-Aldrich Co.) for 2 hours at 4°C, and immunoblotted with anti-CD9 (0.1 µg/ml).
CD9- and CD81-deficient mice
We had produced CD9-deficient mice previously (Miyado et al., 2000), and CD81-deficient mice were kindly provided by Dr. Miyazaki (The University of Tokyo, Tokyo, Japan) (Miyazaki et al., 1997).
In vitro fertilization (IVF)
For IVF, oocytes were collected from the oviductal ampulla region of superovulated C57BL/6N females (8 to 12 weeks old) 14 to 16 hours after hCG injection, and placed in a 30 µl drop of TYH medium covered with paraffin oil (Nacalai Tesque, Inc., Kyoto, Japan) equilibrated with 5% CO2 in air at 37°C. Sperm collected from the epididymides of 8- to 12-week-old B6C3F1 males were induced to capacitate by incubating in TYH medium for 90 min in an atmosphere of 5% CO2 in air at 37°C before insemination. The oocytes were then inseminated with sperm in the presence of anti-CD9 (50 µg/ml) and/or anti-CD81 (50 µg/ml) or a preimmune rat IgG (50 µg/ml) as a control. In addition, we used rabbit pre-immune IgG whose total IgG concentration had been adjusted to 100 µg/ml in each condition. The final concentration of sperm added to the oocytes was 1.5×105 sperm/ml. To measure the rate of excessive sperm penetration (>10 sperm/oocyte) and two-cell embryos, the oocytes were observed under an LSM 510 confocal microscope 24 hours after incubation with Abs.
To count the number of sperm fused per oocyte, zona-free oocytes were prepared according to the procedure described by Yamatoya et al. (Yamatoya et al., 2011). They were then preincubated with 4′,6-diamidino-2-phenylindole (DAPI; Wako Pure Chemical Industries, Ltd., Osaka, Japan) at the final concentration of 10 µg/ml in TYH medium for 20 min at 37°C, and washed 3 times in separate drops of TYH medium before insemination. DAPI is a fluorescent dye that can slowly permeate the living cell membrane (semi-permeable) and will not leak out of cells after washing, relative to Hoechst33342 (permeable), according to the instructions from Invitrogen Co. (Carlsbad, CA). It enables the staining of only fused sperm nuclei as a result of the transfer of DAPI into sperm after membrane fusion. One hour after incubation in a 30 µl drop of TYH medium, the oocytes were fixed with HBS containing 2% paraformaldehyde, 0.1% glutaraldehyde and 0.1% polyvinylpyrolidone (PVP) for 30 min at room temperature. Then, the number of sperm fused per oocyte was determined by counting DAPI-transferred sperm.
Electron-microscopic analysis
The oocytes were isolated from oviducts of superovulated mice, and cumulus cells were removed from the oocytes by treatment with hyaluronidase (300 µg/ml) in TYH medium, as described previously (Takezawa et al., 2011). After zona-intact oocytes were treated with collagenase (Wako Pure Chemical Industries, Ltd.) at the final concentration of 1 mg/ml in a 30 µl drop of medium (Yamatoya et al., 2011) for 20 min at 37°C, denuded oocytes were removed from the drop with a microcapillary attached to a mouth piece and the remaining solution (<30 µl) was collected as extracellular components of oocytes. The collected components were incubated with anti-CD9 (0.5 µg/ml) or anti-CD81 (0.5 µg/ml) for 2 hours at room temperature. The collected components were then exposed to 1 µl bed volume of 10 nm colloidal gold coupled to the secondary Abs (Sigma-Aldrich Co.) for 1 hour at room temperature. The materials conjugated with the gold particles were spun down at 12,000 rpm for 30 min at room temperature, and then the precipitates were washed with TYH medium. This step was repeated 3 times, and then the precipitates were fixed with HBS solution containing 2% glutaraldehyde for 24 hours at 4°C and 2% osmic acid for 1 hour at 4°C. Ultra-thin sections were prepared as described (Toshimori et al., 1998).
In vitro synthesis of poly (A)+RNAs
The open reading frames (ORFs) encoding mouse CD9 and CD81 cDNAs were amplified by polymerase chain reaction (PCR) using two primer sets as described below: 5′-GGAAGCTTGTACCATGCCGGTC-3′ (mCD9-START-HIII) and 5′-GGGCGCCGCTCTAGACCATTTC-3′ (mCD9-STOP-NotI); 5′-GGAAGCTTGTACCATGGGGGTG-3′ (mCD81-STRAT-HIII) and 5′-GGGCGGCCGCTTCAGTACACGG-3′ (mCD81-STOP-NotI), and subcloned into the Hin dIII and Not I sites in pRc/CMV vector (Invitrogen Co.). These ORFs were cut out with two restriction enzymes, Hin dIII and Xho I, and then inserted into the Hin dIII and Xho I sites in pBluescript SKII-A85 vector [kindly provided by Dr. Sakurai (Shinshu University, Matsumoto, Japan)], which contained poly(A) repeats comprising 85 adenines to extend poly(A) tail with a definite length, as depicted in Fig. 6A. The cDNA-containing vectors were linearized with Xho I and used as templates for RNA synthesis using the mCAP RNA Capping Kit (Stratagene Cloning Systems, La Jolla, CA). After the synthesized RNAs had been qualified by agarose gel electrophoresis and quantified by UV absorbance using a spectrophotometer, they were purified by treatment with chloroform and finally precipitated with ethanol. Just before being injected into oocytes, the RNA precipitates were dried and resuspended in a solution containing 1 mM Tris-HCl (pH 8.0) and 0.1 mM EDTA by vortexing. For injection, the final concentration of RNAs was adjusted to 100 ng/µl.
Microinjection of in vitro synthesized RNAs into oocytes
For RNA injection, immature oocytes were isolated from the ovaries of C57BL/6N female mice (8 to 12 weeks old) 36 hours after injection of PMSG (5 units), as described by Vanderhyden and Armstrong (Vanderhyden and Armstrong, 1989). After the ovaries had been chopped with forceps in TYH medium, tissue fragments were dissociated using a 21-gauge needle attached to a disposable 2.5 ml syringe. The released germinal vesicle (GV) stage oocytes were collected with glass capillaries attached to a short piece of rubber tubing, and placed in a 5 µl drop of TYH medium covered with paraffin oil (Nacalai Tesque, Inc.) equilibrated with 5% CO2 in air at 37°C. Then, the synthesized RNAs were injected into the nucleus of the oocytes with glass capillaries attached to a micromanipulator (Narishige Scientific Instruments Lab., Tokyo, Japan). The injection volume was around 50 nl per oocyte, which corresponded to 5 ng RNA. After RNA injection, the oocytes were co-incubated with granulosa cells without addition of any additional reagents in TYH medium for 24 hours at 37°C up to maturation at metaphase II. The oocytes were then subjected to ZP removal by brief incubation in acid Tyrode's solution (Sigma-Aldrich Co.). The zona-free oocytes were preincubated with DAPI at a final concentration of 10 µg/ml in TYH medium for 20 min at 37°C, and washed 3 times by transferring to separate drops of TYH medium. C57BL/6N sperm (ca. 1.5×105 sperm/ml) were added to a 30 µl drop of TYH medium containing DAPI-treated zona-free oocytes and then the dish was incubated for 1 hour at 37°C. After insemination, the number of fused sperm per oocyte was counted according to the method described in the section ‘In vitro fertilization (IVF)’.
Acknowledgments
We thank M. Okabe, N. Inoue and K. Toshimori for critical discussions. This work was supported by a grant from The Ministry of Health, Labor and Welfare, and a grant-in-aid for Scientific Research, The Ministry of Education, Culture, Sports, and Technology of Japan.
Footnotes
Competing interests: The authors declare that there are no competing financial interests.
References
- Barroso G., Valdespin C., Vega E., Kershenovich R., Avila R., Avendaño C., Oehninger S. (2009). Developmental sperm contributions: fertilization and beyond. Fertil. Steril. 92, 835–848 10.1016/j.fertnstert.2009.06.030 [DOI] [PubMed] [Google Scholar]
- Bobrie A., Colombo M., Raposo G., Théry C. (2011). Exosome secretion: molecular mechanisms and roles in immune responses. Traffic 12, 1659–1668 10.1111/j.1600-0854.2011.01225.x [DOI] [PubMed] [Google Scholar]
- Caplan M. J., Kamsteeg E. J., Duffield A. (2007). Tetraspan proteins: regulators of renal structure and function. Curr. Opin. Nephrol. Hypertens. 16, 353–358 10.1097/MNH.0b013e328177b1fa [DOI] [PubMed] [Google Scholar]
- Chen M. S., Tung K. S., Coonrod S. A., Takahashi Y., Bigler D., Chang A., Yamashita Y., Kincade P. W., Herr J. C., White J. M. (1999). Role of the integrin-associated protein CD9 in binding between sperm ADAM 2 and the egg integrin alpha6beta1: implications for murine fertilization. Proc. Natl. Acad. Sci. USA 96, 11830–11835 10.1073/pnas.96.21.11830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gassart A., Geminard C., Fevrier B., Raposo G., Vidal M. (2003). Lipid raft-associated protein sorting in exosomes. Blood 102, 4336–4344 10.1182/blood-2003-03-0871 [DOI] [PubMed] [Google Scholar]
- Deng J., Dekruyff R. H., Freeman G. J., Umetsu D. T., Levy S. (2002). Critical role of CD81 in cognate T-B cell interactions leading to Th2 responses. Int. Immunol. 14, 513–523 10.1093/intimm/14.5.513 [DOI] [PubMed] [Google Scholar]
- Glazar A. I., Evans J. P. (2009). Immunoglobulin superfamily member IgSF8 (EWI-2) and CD9 in fertilisation: evidence of distinct functions for CD9 and a CD9-associated protein in mammalian sperm-egg interaction. Reprod. Fertil. Dev. 21, 293–303 10.1071/RD08158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler M. E. (2003). Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annu. Rev. Cell Dev. Biol. 19, 397–422 10.1146/annurev.cellbio.19.111301.153609 [DOI] [PubMed] [Google Scholar]
- Horváth G., Serru V., Clay D., Billard M., Boucheix C., Rubinstein E. (1998). CD19 is linked to the integrin-associated tetraspans CD9, CD81, and CD82. J. Biol. Chem. 273, 30537–30543 10.1074/jbc.273.46.30537 [DOI] [PubMed] [Google Scholar]
- Ikawa M., Inoue N., Benham A. M., Okabe M. (2010). Fertilization: a sperm's journey to and interaction with the oocyte. J. Clin. Invest. 120, 984–994 10.1172/JCI41585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue N., Ikawa M., Isotani A., Okabe M. (2005). The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature 434, 234–238 10.1038/nature03362 [DOI] [PubMed] [Google Scholar]
- Ito C., Yamatoya K., Yoshida K., Maekawa M., Miyado K., Toshimori K. (2010). Tetraspanin family protein CD9 in the mouse sperm: unique localization, appearance, behavior and fate during fertilization. Cell Tissue Res. 340, 583–594 10.1007/s00441-010-0967-7 [DOI] [PubMed] [Google Scholar]
- Jin M., Fujiwara E., Kakiuchi Y., Okabe M., Satouh Y., Baba S. A., Chiba K., Hirohashi N. (2011). Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc. Natl. Acad. Sci. USA 108, 4892–4896 10.1073/pnas.1018202108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaji K., Oda S., Shikano T., Ohnuki T., Uematsu Y., Sakagami J., Tada N., Miyazaki S., Kudo A. (2000). The gamete fusion process is defective in eggs of Cd9-deficient mice. Nat. Genet. 24, 279–282 10.1038/73502 [DOI] [PubMed] [Google Scholar]
- Kawano N., Yoshida K., Miyado K., Yoshida M. (2011). Lipid rafts: keys to sperm maturation, fertilization, and early embryogenesis. J. Lipids 2011, e264706 10.1155/2011/264706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawetz S. A., Kruger A., Lalancette C., Tagett R., Anton E., Draghici S., Diamond M. P. (2011). A survey of small RNAs in human sperm. Hum. Reprod. 26, 3401–3412 10.1093/humrep/der329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhal S., Wood M. J. (2011). Exosome nanotechnology: an emerging paradigm shift in drug delivery: exploitation of exosome nanovesicles for systemic in vivo delivery of RNAi heralds new horizons for drug delivery across biological barriers. Bioessays 33, 737–741 10.1002/bies.201100076 [DOI] [PubMed] [Google Scholar]
- Lancaster G. I., Febbraio M. A. (2005). Exosome-dependent trafficking of HSP70: a novel secretory pathway for cellular stress proteins. J. Biol. Chem. 280, 23349–23355 10.1074/jbc.M502017200 [DOI] [PubMed] [Google Scholar]
- Le Naour F., Rubinstein E., Jasmin C., Prenant M., Boucheix C. (2000). Severely reduced female fertility in CD9-deficient mice. Science 287, 319–321 10.1126/science.287.5451.319 [DOI] [PubMed] [Google Scholar]
- Maecker H. T., Todd S. C., Kim E. C., Levy S. (2000). Differential expression of murine CD81 highlighted by new anti-mouse CD81 monoclonal antibodies. Hybridoma 19, 15–22 10.1089/027245700315752 [DOI] [PubMed] [Google Scholar]
- Miller B. J., Georges-Labouesse E., Primakoff P., Myles D. G. (2000). Normal fertilization occurs with eggs lacking the integrin alpha6beta1 and is CD9-dependent. J. Cell Biol. 149, 1289–1296 10.1083/jcb.149.6.1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyado K., Yamada G., Yamada S., Hasuwa H., Nakamura Y., Ryu F., Suzuki K., Kosai K., Inoue K., Ogura A. et al. (2000). Requirement of CD9 on the egg plasma membrane for fertilization. Science 287, 321–324 10.1126/science.287.5451.321 [DOI] [PubMed] [Google Scholar]
- Miyado K., Yoshida K., Yamagata K., Sakakibara K., Okabe M., Wang X., Miyamoto K., Akutsu H., Kondo T., Takahashi Y. et al. (2008). The fusing ability of sperm is bestowed by CD9-containing vesicles released from eggs in mice. Proc. Natl. Acad. Sci. USA 105, 12921–12926 10.1073/pnas.0710608105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T., Müller U., Campbell K. S. (1997). Normal development but differentially altered proliferative responses of lymphocytes in mice lacking CD81. EMBO J. 16, 4217–4225 10.1093/emboj/16.14.4217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito K., Fukuda Y., Toyoda Y. (1988). Effects of porcine follicular fluid on male pronucleus formation in porcine oocytes matured in vitro. Gamete Res. 21, 289–295 10.1002/mrd.1120210310 [DOI] [PubMed] [Google Scholar]
- Oritani K., Wu X., Medina K., Hudson J., Miyake K., Gimble J. M., Burstein S. A., Kincade P. W. (1996). Antibody ligation of CD9 modifies production of myeloid cells in long-term cultures. Blood 87, 2252–2261. [PubMed] [Google Scholar]
- Pelchen-Matthews A., Raposo G., Marsh M. (2004). Endosomes, exosomes and Trojan viruses. Trends Microbiol. 12, 310–316 10.1016/j.tim.2004.05.004 [DOI] [PubMed] [Google Scholar]
- Rubinstein E., Ziyyat A., Prenant M., Wrobel E., Wolf J. P., Levy S., Le Naour F., Boucheix C. (2006). Reduced fertility of female mice lacking CD81. Dev. Biol. 290, 351–358 10.1016/j.ydbio.2005.11.031 [DOI] [PubMed] [Google Scholar]
- Sakakibara K., Sato K., Yoshino K., Oshiro N., Hirahara S., Mahbub Hasan A. K., Iwasaki T., Ueda Y., Iwao Y., Yonezawa K. et al. (2005). Molecular identification and characterization of Xenopus egg uroplakin III, an egg raft-associated transmembrane protein that is tyrosine-phosphorylated upon fertilization. J. Biol. Chem. 280, 15029–15037 10.1074/jbc.M410538200 [DOI] [PubMed] [Google Scholar]
- Sato B., Katagiri Y. U., Miyado K., Okino N., Ito M., Akutsu H., Okita H., Umezawa A., Fujimoto J., Toshimori K. et al. (2011). Lipid rafts enriched in monosialylGb5Cer carrying the stage-specific embryonic antigen-4 epitope are involved in development of mouse preimplantation embryos at cleavage stage. BMC Dev. Biol. 11, 22 10.1186/1471-213X-11-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stipp C. S., Orlicky D., Hemler M. E. (2001). FPRP, a major, highly stoichiometric, highly specific CD81- and CD9-associated protein. J. Biol. Chem. 276, 4853–4862 10.1074/jbc.M009859200 [DOI] [PubMed] [Google Scholar]
- Stipp C. S., Kolesnikova T. V., Hemler M. E. (2003). Functional domains in tetraspanin proteins. Trends Biochem. Sci. 28, 106–112 10.1016/S0968-0004(02)00014-2 [DOI] [PubMed] [Google Scholar]
- Stoorvogel W. (2012). Functional transfer of microRNA by exosomes. Blood 119, 646–648 10.1182/blood-2011-11-389478 [DOI] [PubMed] [Google Scholar]
- Takezawa Y., Yoshida K., Miyado K., Sato M., Nakamura A., Kawano N., Sakakibara K., Kondo T., Harada Y., Ohnami N. et al. (2011). β-catenin is a molecular switch that regulates transition of cell-cell adhesion to fusion. Sci. Rep. 1, 68 10.1038/srep00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanigawa M., Miyamoto K., Kobayashi S., Sato M., Akutsu H., Okabe M., Mekada E., Sakakibara K., Miyado M., Umezawa A. et al. (2008). Possible involvement of CD81 in acrosome reaction of sperm in mice. Mol. Reprod. Dev. 75, 150–155 10.1002/mrd.20709 [DOI] [PubMed] [Google Scholar]
- Toshimori K. (2011). Dynamics of the mammalian sperm membrane modification leading to fertilization: a cytological study. J. Electron Microsc. (Tokyo) 60 Suppl 1, S31–S42 10.1093/jmicro/dfr036 [DOI] [PubMed] [Google Scholar]
- Toshimori K., Saxena D. K., Tanii I., Yoshinaga K. (1998). An MN9 antigenic molecule, equatorin, is required for successful sperm-oocyte fusion in mice. Biol. Reprod. 59, 22–29 10.1095/biolreprod59.1.22 [DOI] [PubMed] [Google Scholar]
- Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J. J., Lötvall J. O. (2007). Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659 10.1038/ncb1596 [DOI] [PubMed] [Google Scholar]
- Vanderhyden B. C., Armstrong D. T. (1989). Role of cumulus cells and serum on the in vitro maturation, fertilization, and subsequent development of rat oocytes. Biol. Reprod. 40, 720–728 10.1095/biolreprod40.4.720 [DOI] [PubMed] [Google Scholar]
- Yamatoya K., Ito C., Araki M., Furuse R., Toshimori K. (2011). One-step collagenase method for zona pellucida removal in unfertilized eggs: easy and gentle method for large-scale preparation. Reprod. Med. Biol. 10, 97–103 10.1007/s12522-011-0075-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zöller M. (2009). Tetraspanins: push and pull in suppressing and promoting metastasis. Nat. Rev. Cancer 9, 40–55 10.1038/nrc2543 [DOI] [PubMed] [Google Scholar]