Summary
Some photosynthetic organisms benefit from elevated levels of carbon dioxide, but studies on the effects of elevated PCO2 on the algal symbionts of animals are very few. This study investigated the impact of hypercapnia on a photosynthetic symbiosis between the anemone Anthopleura elegantissima and its zooxanthella Symbiodinium muscatinei. Anemones were maintained in the laboratory for 1 week at 37 Pa PCO2 and pH 8.1. Clonal pairs were then divided into two groups and maintained for 6 weeks under conditions naturally experienced in their intertidal environment, 45 Pa PCO2, pH 8.1 and 231 Pa PCO2, pH 7.3. Respiration and photosynthesis were measured after the 1-week acclimation period and after 6 weeks in experimental conditions. Density of zooxanthellal cells, zooxanthellal cell size, mitotic index and chlorophyll content were compared between non-clonemate anemones after the 1-week acclimation period and clonal anemones at the end of the experiment. Anemones thrived in hypercapnia. After 6 weeks, A. elegantissima exhibited higher rates of photosynthesis at 45 Pa (4.2 µmol O2 g−1 h−1) and 231 Pa (3.30 µmol O2 g−1 h−1) than at the initial 37 Pa (1.53 µmol O2 g−1 h−1). Likewise, anemones at 231 Pa received more of their respiratory carbon from zooxanthellae (CZAR = 78.2%) than those at 37 Pa (CZAR = 66.6%) but less than anemones at 45 Pa (CZAR = 137.3%). The mitotic index of zooxanthellae was significantly greater in the hypercapnic anemones than in anemones at lower PCO2. Excess zooxanthellae were expelled by their hosts, and cell densities, cell diameters and chlorophyll contents were not significantly different between the groups. The response of A. elegantissima to hypercapnic acidification reveals the potential adaptation of an intertidal, photosynthetic symbiosis for high PCO2.
Key words: Carbon dioxide, Ocean acidification, Anthopleura elegantissima, Zooxanthellae, Metabolic rate, Photosynthesis, CZAR, Intertidal
Introduction
Anthropogenic emissions of carbon dioxide are not only warming the climate but also decreasing the pH of the ocean (Vitousek et al., 1997; Orr et al., 2005). At 39.4 Pa, the present partial pressure of CO2 (PCO2) in the atmosphere is nearly 40% higher than in the prior 800,000 years (Lüthi et al., 2008).
Elevated levels of PCO2 can increase photosynthesis and growth rates of many different kinds of plants (e.g. Kets et al., 2010; Mateos-Naranjo et al., 2010; Moutinho-Pereira et al., 2009; Norikane et al., 2010). In the marine environment, elevated PCO2 stimulates photosynthesis and/or growth rates in macroalgae (Gao et al., 1993; Kübler et al., 1999; Xu et al., 2010) and seagrass (Palacios and Zimmerman, 2007; Jiang et al., 2010). Photosynthesis in some free-living microalgae is positively affected by elevated PCO2 (Beardall et al., 2009; Wu et al., 2010; Van de Waal et al., 2011), but only a few studies have looked at the effects of elevated PCO2 on photosynthesis in the microalgae of alga-cnidarian symbioses. To date, those studies have all been carried out on calcifying symbioses (Schneider and Erez, 2006; Anthony et al., 2008; Crawley et al., 2010).
Many marine ecosystems experience naturally high levels of PCO2, including midwater oceanic oxygen minimum layers (Childress and Seibel, 1998; Paulmier et al., 2011), estuaries (Kempe, 1982; Frankignoulle et al., 1998; Hinga, 2002) and tide pools (Ganning, 1971; Truchot and Duhamel-Jouve, 1980; Morris and Taylor, 1983). Diel cycles of photosynthesis and respiration strongly affect PCO2 in tide pools, both seasonally (Truchot and Duhamel-Jouve, 1980) and on a daily basis (Morris and Taylor, 1983). In isolated tide pools with dense populations of organisms, daytime PCO2 can fall below 1 Pa due to the use of CO2 by photosynthesis; at night, organismal respiration can drive PCO2 values above 355 Pa as CO2 accumulates (Morris and Taylor, 1983). An 8-year study of coastal waters of the northeastern Pacific Ocean found that pH typically varied by 0.24 units over 24 hours and by more than one pH unit annually due to fluctuations in PCO2 (Wootton et al., 2008). The region is also subject to coastal upwelling, which can bring in seawater with PCO2 at 100 Pa (Feely et al., 2008). The combined effects of upwelling with daily and seasonal fluctuations periodically subject coastal organisms to levels of pH and PCO2 that approximate the conditions expected by the year 2300 (Caldeira and Wickett, 2005). Organisms that have evolved adaptations to natural fluctuations of PCO2 may also be able to tolerate persistently high PCO2 and lower pH (Melzner et al., 2009b) which suggest that intertidal organisms may be very successful in Earth's future high-CO2 oceans.
In the eastern North Pacific Ocean, the anemone Anthopleura elegantissima Brandt (Cnidaria: Anthozoa) harbors the dinoflagellate Symbiodinium muscatinei LaJeunesse and Trench (Dinomastigota: Dinophyceae), which is congeneric with symbiotic dinoflagellates found in hermatypic corals (LaJeunesse and Trench, 2000). In its intertidal habitat, Anthopleura elegantissima is regularly exposed to fluctuations of PCO2 and pH due to fresh water input, tidal exchanges, emersion and localized consumption and production of CO2. To better understand how hypercapnia affects metabolic processes in an intertidal, photosynthetic symbiosis, which does not produce a calcium carbonate skeleton, this study examined the metabolic effects of increased PCO2 on A. elegantissima. An experiment was designed to measure a suite of characteristics at naturally-occurring elevated PCO2 levels (45 and 231 Pa) with the lower PCO2 condition serving as a control. This anemone is arguably the most well studied alga-invertebrate symbiosis and much is known about its ecology, genetics, physiology, etc. (Muscatine et al., 1981; Fitt et al., 1982; Ayre and Grosberg, 1995; LaJeunesse and Trench, 2000; Secord and Augustine, 2000; Lewis and Muller-Parker, 2004; Verde and McCloskey, 2007). Anthopleura elegantissima forms aggregations of genetically identical clones through bilateral fission (Ayre and Grosberg, 1995), and experiments examined the effects of elevated PCO2 through paired comparisons of genetically identical but separated clonal couplets.
Results
All anemones thrived during the experimental period, and three individuals among both the 45 Pa specimens and 231 Pa specimens reproduced through bilateral fission. These new clones were combined and treated as single individuals for final measurements. There were no significant differences in mass of the anemones (paired t-test, p>0.4) after 6 weeks of incubations in elevated PCO2, and there were no significant effects of body mass on either Ṗg or ṀO2 over the size range of specimens used in this study (3.33–12.09 g; linear regressions not shown).
Photosynthesis, respiration and CZAR
Significant increases in Ṗg were observed in individuals after six weeks of incubation in 45 and 231 Pa PCO2 (paired t-test, p<0.001 for both groups) (Table 1; Fig. 1A). Furthermore, Ṗg was greater at 45 Pa when compared to their clonemates at 231 Pa (paired t-test, p = 0.03) (Fig. 1A). Significant increases in ṀO2 were also observed in individuals after six weeks of incubation in 45 and 231 Pa PCO2 (paired t-tests, p<0.01 and 0.05, respectively) (Table 1; Fig. 1B). However, there were no significant differences in ṀO2 at 45 Pa PCO2 when compared to their clonemates at 231 Pa PCO2 (paired t-test, p>0.05) (Fig. 1B). As in the case of Ṗg, there were significant increases in daily net photosynthesis after six weeks in 231 Pa PCO2 with even greater increases among anemones in 45 Pa (paired t-test, p<0.001 for both groups) (Table 1; Fig. 1C).
Table 1. Oxygen and carbon flux in Anthopleura elegantissima following one week in PCO2 of 37 Pa and after 6 weeks at 45 and 231 Pa.
CZAR: percentage of carbon provided by zooxanthellae to support animal respiration. Rates are µmol O2 g−1 wet weight h−1. Values are mean ± S.E.
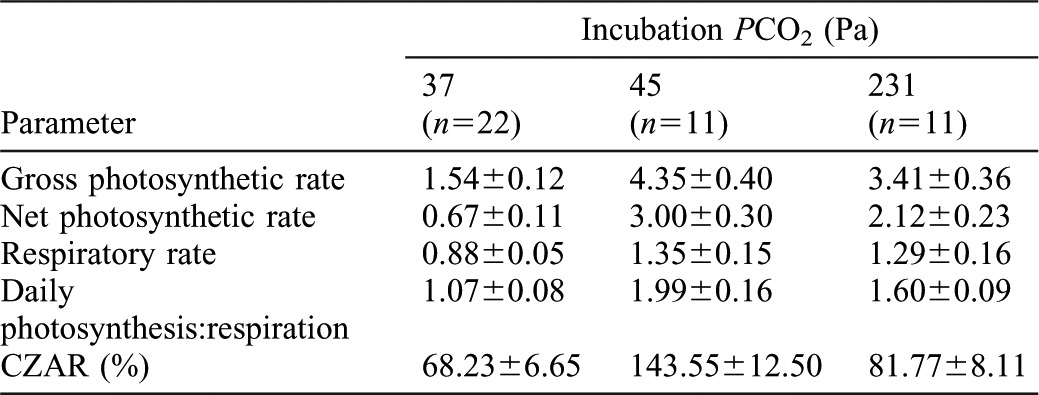
Fig. 1. Mean mass-specific rates of gross photosynthesis (A), respiration (B) and net photosynthesis (C) in Anthopleura elegantissima following incubations of one week at PCO2 of 37 Pa and six weeks at PCO2 of 45 and 231 Pa.
Net photosynthesis was calculated on a 14:24 light:dark basis. Bars of the same color represent the same individuals, while different colors represent genetically identical clones. Error bars represent one standard error; n = 11 for all experiments. Letters designate statistically significant differences between individuals at two different PCO2 (a = p<0.001, b = p<0.01, c = p<0.05) using paired t-tests, while asterisks represents significant differences (* = p<0.05; ** = p<0.01) between clonemates at two different PCO2.
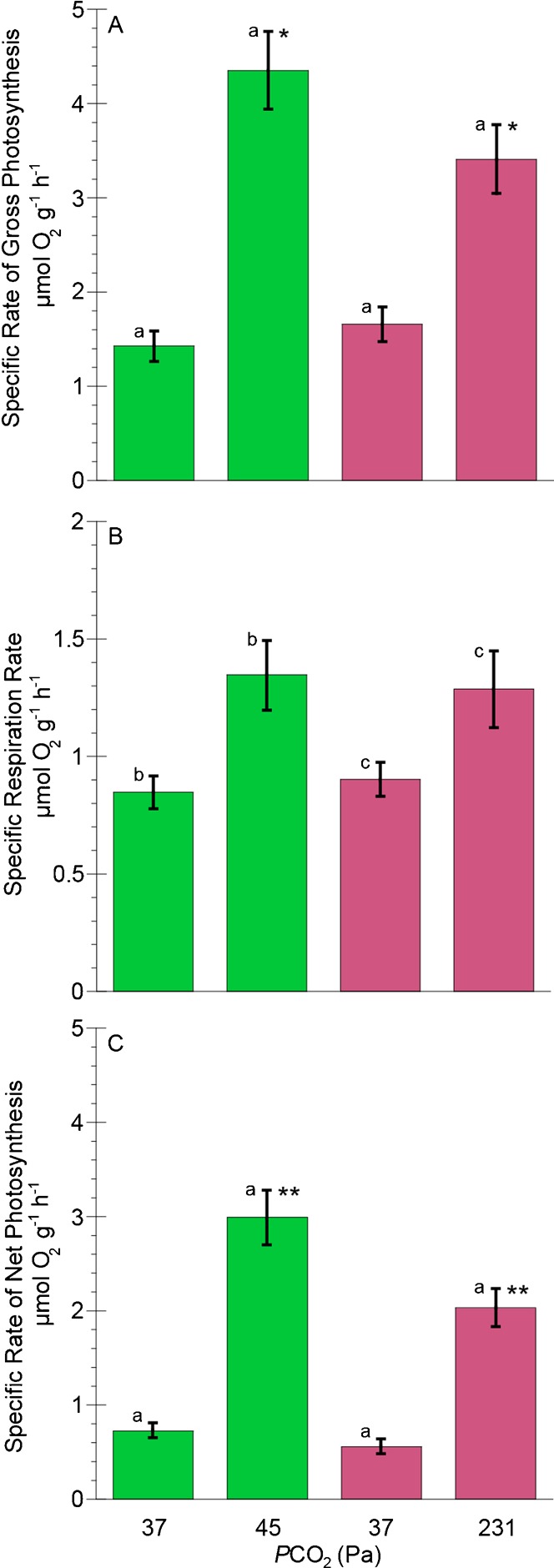
Significant increases in Ṗg:ṀO2 ratios were also observed in individuals after six weeks of incubation in 45 and 231 Pa PCO2 (paired t-tests, p<0.001 and 0.01, respectively) (Table 1; Fig. 2A). Mean Ṗg:ṀO2 ratios were greater in 45 Pa than at 231 Pa PCO2, however the difference was not significant (paired t-test, p = 0.1). The contribution of organic carbon by zooxanthellae to animal respiration (CZAR) (Fig. 2B) was greatest in 45 Pa PCO2 (CZAR = 143.6%). This was significantly higher than after one week at 37 Pa PCO2 (CZAR = 68.2%, paired t-test, p<0.001) and also higher than that of clonemates incubated at 231 Pa PCO2 (CZAR = 81.8%, paired t-test, p<0.01).
Fig. 2. Ratios of photosynthesis to respiration (A) and potential contributions of carbon by zooxanthellae to the animal's respiratory carbon requirements (CZAR) (B) in Anthopleura elegantissima following incubations of one week at PCO2 of 37 Pa and six weeks at PCO2of 45 and 231 Pa.
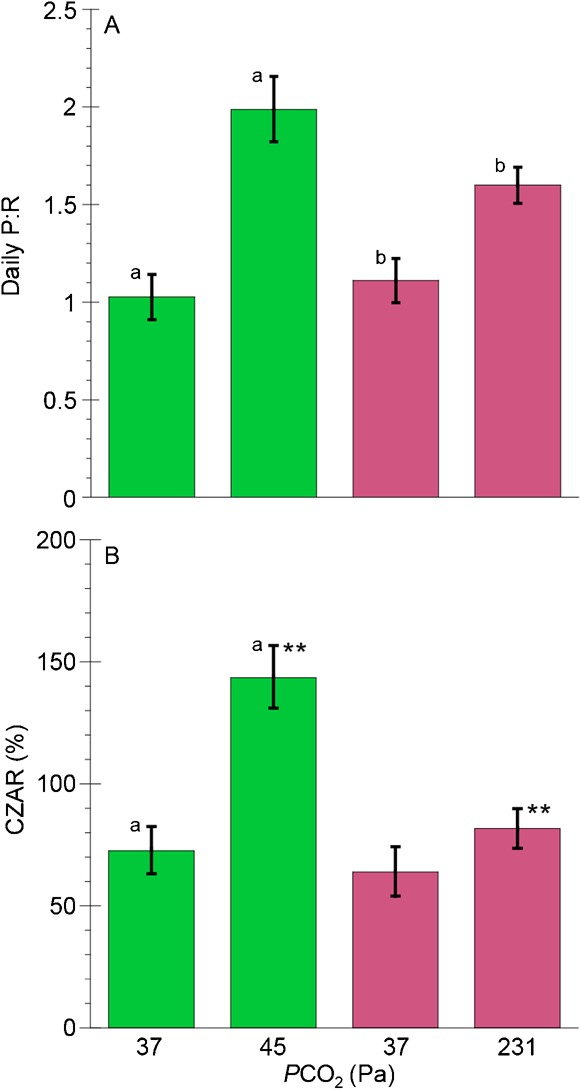
Bars of the same color represent the same individuals, while different colors represent genetically identical clones. Error bars represent ± one standard error; n = 11 for all experiments. Letters designate statistically significant differences between individuals at two different PCO2 (a = p<0.001) using paired t-tests, while asterisks represent significant differences (** = p<0.01) between clonemates at two different PCO2.
Zooxanthellae
There were no significant differences in zooxanthellal cell diameters, cell densities and Chl a concentrations among any of the PCO2 conditions (ANOVA, p>0.05 for all comparisons) (Table 2; Fig. 3). In contrast, the number of dividing zooxanthellal cells (mitotic index) was progressively greater among the three PCO2 (ANOVA, p<0.001) (Fig. 3C). Visual observations of anemones in the highest PCO2 tank revealed that anemones released large amounts of mucus-bound zooxanthellae. When these zooxanthellae were examined under a microscope, a very high ratio of doublet cells was observed. In contrast, specimens in the lowest PCO2 tank released much less mucus that contained zooxanthellae.
Table 2. Characteristics of the zooxanthella Symbiodinium muscatenei in Anthopleura elegantissima following one week in PCO2 of 37 Pa and after 6 weeks at 45 and 231 Pa.
Values are mean ± S.E.; n = 11 for all analyses.
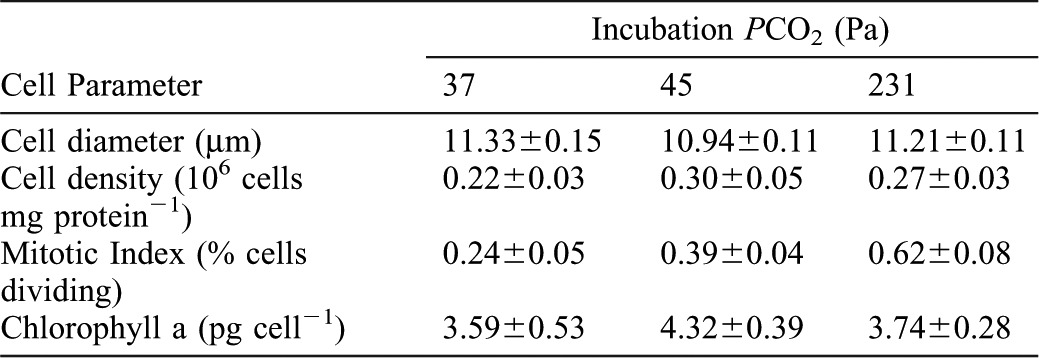
Fig. 3. Characteristics of the zooxanthella, Symbiodinium muscatinei, in Anthopleura elegantissima following incubations of one week at PCO2 of 37 Pa and six weeks at PCO2 of 45 and 231 Pa.
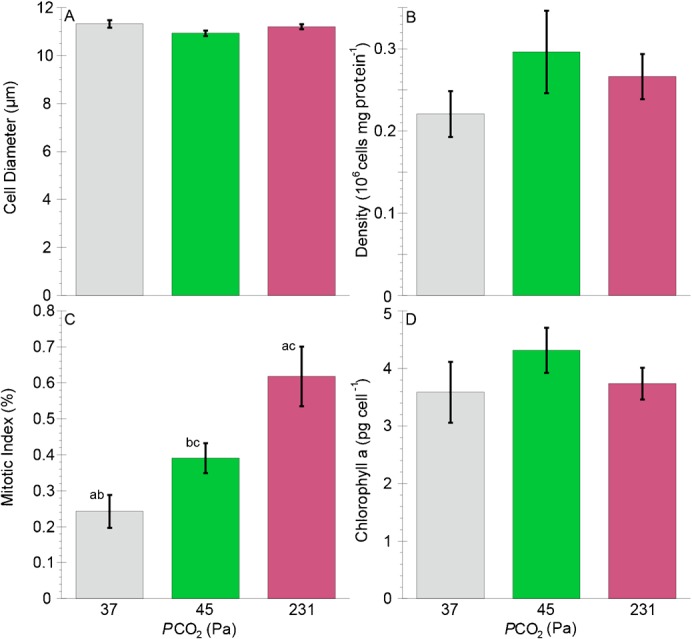
(A) Cell diameter. (B) Cell density. (C) Mitotic index. (D) Chlorophyll a concentration. Bars of the same color represent zooxanthellae from the same individuals, while different color bars represent those from genetically identical clones. Grey bars represent those from different individuals collected at the same time from the same population of anemones. Error bars represent ± one standard error; n = 11 for all measurements. Letters designate statistically significant differences using ANOVA of arcsine-transformed data (not shown) followed by Fisher's LSD post-hoc analyses between individuals (a = p<0.001, b and c = p<0.05).
Discussion
Much work has been done investigating tidepool organisms' strategies to withstand intermittent hypoxia (e.g. Richards, 2011), but investigations distinguishing the effects of hypercapnia are lacking. This study demonstrated that the non-calcifying cnidarian Anthopleura elegantissima and its photosynthetic dinoflagellate Symbiodinium muscatinei can thrive in hypercapnic seawater for 6 weeks. The initial rates of Ṗg and ṀO2 in the current study are similar to those reported by others when adjusted for dry weight/wet weight differences (Shick and Dykens, 1984), however, both Ṗg and ṀO2 increased after 6 weeks under both PCO2 conditions. The consistent feeding schedule in our laboratory study likely contributed to the rise in metabolic activity after six weeks. Such increases are in agreement with previous studies that have shown that laboratory maintenance under conditions of consistent temperature and lighting, low UV exposure, absence of desiccation, and reduced wave stress all contribute to increased physiological performance of anemones (Shick, 1991; Verde and McCloskey, 1996b). In a study by Fitt et al., ṀO2 of well-fed A. elegantissima was twice the rate of starved animals (Fitt et al., 1982); CZAR averaged 13% for fed anemones compared to 45% for starved or newly collected anemones. This suggests that the CZAR in the current study may under-estimate the potential increase in CZAR of wild, underfed anemones living in hypercapnia. These experiments have demonstrated that A. elegantissima can tolerate not only periodic hypercapnia, but thrive for extended periods of time at PCO2 exceeding 5 times normocapnia.
While ṀO2 of anemones was not significantly different between 45 and 231 PCO2 after six weeks, Ṗg of specimens held in 231 PCO2 was lower than that of specimens held in 45 PCO2. The increases in Ṗg at both elevated PCO2 levels offset the much smaller, corresponding increases in ṀO2, thus, the range of P:R ratios (1.52–1.83) and CZAR (78–137%) of specimens held at 45 and 231 Pa PCO2 are greater than the P:R ratios (0.54–0.86) and CZAR (13–126%) reported previously for A. elegantissima in normocapnic conditions (cf. Muller-Parker and Davy, 2001).
To satisfy the needs of zooxanthellae for photosynthesis, animal hosts must actively accumulate dissolved inorganic carbon (DIC) in their tissues. Although some free-living dinoflagellates can use CO2, HCO3−, or both, as their inorganic source of carbon (Hansen et al., 2007), the mechanism whereby DIC is made available to zooxanthellae is still being elaborated (Yellowlees et al., 2008; Venn et al., 2009). It is generally thought that zooxanthellae receive ∼15% of their CO2 from host respiration, and the remaining carbon needs are met by active transport and facilitated diffusion of bicarbonate through host tissues (Allemand et al., 1998; Furla et al., 2005). Even so, zooxanthellae in anemones remain carbon limited under normocapnia (Weis, 1993; Verde and McCloskey, 2007). For A. elegantissima, this study has suggested that CO2 can diffuse through host tissues to reach symbiont cells at elevated PCO2. Although cell size, cell-specific amounts of chlorophyll a and cell densities of S. muscatinei did not change in specimens maintained in the hypercapnic conditions for six weeks, zooxanthellae were affected by increasing PCO2, as evidenced by the pattern of higher mitotic indices. Population densities of zooxanthellae in anthozoans are maintained under host control through the active expulsion of symbionts and chemically-signaled arrest of algal reproduction (Trench, 1987; Baghdasarian and Muscatine, 2000). The high amounts of dividing algal cells observed in excreted mucus in this study indicate the anemone cannot regulate algal reproduction in hypercapnia and an increase in the rate of expulsion to maintain normal densities of rapidly reproducing algal cells is needed in order to avoid toxicity from excess oxidative products (Furla et al., 2005). This suggests that the extra carbon acquired by hypercapnic anemones due to CO2-enhanced photosynthesis was lost to the symbiosis due to expulsion. The small but significant differences in Ṗg and MI between specimens held in 45 and 231 Pa PCO2 indicate that the upper limits of performance in extended hypercapnia were approached at 231 Pa PCO2. It is generally accepted that nutrient limitation is one mechanism that hosts may use to regulate algal symbiont populations (Yellowlees et al., 2008). Under high PCO2 conditions in the present study, A. elegantissima was apparently not able to maintain nutrient-limiting conditions or other photosynthesis- and algal growth-inhibiting mechanisms.
These anemones have a high tolerance for internal hypercapnic conditions, and this trait is shared with animals that have high metabolic rates and need to tolerate high internal PCO2 owing to exercise-induced fluctuations of CO2 (Seibel and Walsh, 2003; Melzner et al., 2009b). This has been demonstrated in active organisms with a high capacity for ion regulation such as teleost fish (Melzner et al., 2009a), brachyuran crabs (Pane and Barry, 2007; Spicer et al., 2007) and cuttlefish (Gutowska et al., 2008). Animals that currently cope with internal hypercapnia may be pre-adapted to survive future increases in environmental PCO2 as well. An environmental study of the species composition at a high PCO2 volcanic vent community off Ischia, Italy, also suggests that non-calcifying alga-invertebrate symbioses may be pre-adapted to hypercapnia (Hall-Spencer et al., 2008). Along a natural pH gradient from 8.2 to 7.4 at this site, gastropods, sea urchins and epiphytic coralline algae were diminished or completely absent in areas below pH 7.7 (Hall-Spencer et al., 2008; Martin et al., 2008). Although several species of symbiont-containing scleractinian corals are common to the region, photosynthetic anemones were the only cnidarians found in zones with elevated PCO2 (Hall-Spencer et al., 2008).
The influence of hypercapnia on photosynthesis in calcifying anthozoans with algal symbionts is variable. The branching coral Acropora intermedia increased rates of photosynthetic productivity (at temperatures increased by 3°C) at moderately increased levels of CO2 (53–71 Pa) (Anthony et al., 2008) similar to the increases observed for A. elegantissima in the current study; however, productivity in the massive coral Porites lobata was diminished (Anthony et al., 2008). Very high PCO2 (101–132 Pa) decreased productivity to near zero in both corals. The authors speculate that the increase in productivity in P. lobata may have resulted directly from an increase in CO2 supply but that at higher concentrations, the effects of hypercapnia were offset by physiological disruption from acidification. As in A. intermedia, A. elegantissima had the highest rate of photosynthesis at moderate levels of PCO2 (45 Pa); however, unlike A. intermedia, A. elegantissima also displayed a higher photosynthetic rate at very high PCO2 (231 Pa) than at 39 Pa. Since A. elegantissima routinely encounters PCO2 of this magnitude in its environment, it appears to be better poised to make the physiological adjustments necessary to support photosynthesis at higher PCO2.
In studies on Acropora formosa, chlorophyll content of zooxanthellae increased but cell density did not following 4 days incubation at 57 Pa PCO2 (Crawley et al., 2010). The Mediterranean coral Cladocora caespitosa displayed no differences in rates of photosynthesis under hypercapnia (∼70 Pa) for one month, but chlorophyll content and density of zooxanthellae increased due to hypercapnia during winter experiments (Rodolfo-Metalpa et al., 2010). None of these studies examined the effects of elevated PCO2 on mitotic indices of zooxanthellae or expulsion rates.
Anthropogenic hypercapnia in marine environments will have various effects on many different kinds of organisms, not only calcifying species (Fabry et al., 2008). The results of the present study suggest that A. elegantissima can tolerate, and possibly benefit from, environmental hypercapnic acidification and highlights the adaptation of A. elegantissima to the broad ranges of PCO2 and pH that are characteristic of both its intertidal habitat and photosynthetic lifestyle. This study used a PCO2 slightly above the current world oceanic mean as a control. Future studies using fluctuating PCO2 levels that more closely represent the natural conditions of this intertidal organism will cast further light on intertidal adaptations to hypercapnia. Much more remains to be understood about mechanisms used by organisms that are “pre-adapted” to high PCO2 due to the demands of life in periodically hypercapnic environments.
Materials and Methods
Specimen collection
Specimens of zooxanthellate Anthopleura elegantissima were collected during April, 2008 from Point Grenville, Washington, USA (47° 18.2′ N, 124° 16.2′ W). This anemone harbors two different types of photosynthetic symbiont, the dinoflagellate Symbiodinium muscatinei and a unicellular trebouxiophycean green alga (Lewis and Muller-Parker, 2004). To exclude green algal symbionts, anemones were collected from colonies at ∼1.5–2.0 m above mean low low water (Secord and Augustine, 2000). Clonemates from contiguous colonies and additional individual specimens were collected and transported to the laboratory at The Evergreen State College, Olympia, Washington in separate, plastic bags filled with seawater. No clonemate displayed acrorhagial aggression toward its respective clonemate: an indication that they are genetically identical (Ayre and Grosberg, 2005).
Specimen maintenance
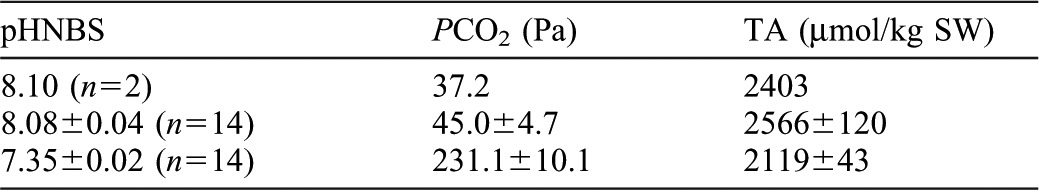
120-l aquaria were prepared with natural seawater (∼27 psu) adjusted up to 30 psu with a combination of Instant Ocean® synthetic seawater and a carbonate-free synthetic seawater (Bidwell and Spotte, 1985) to maintain targeted pH, PCO2 and total alkalinity levels (Table 3). Irradiance was adjusted to ∼660 photosynthetic photon flux (µmol m−2 s−1) and followed a natural spring-summer daily photoperiod (14 h light, 10 h dark) to maximize potential zooxanthellal photosynthesis without risk of photo-inhibition (Fitt et al., 1982; Verde and McCloskey, 2002). Hypercapnia was generated by bubbling CO2 through a reactor constructed of 5-cm PVC pipe containing pegged plastic balls (Bio-BallsTM) to generate turbulence and facilitate dissolution of the gas into the seawater before entering the aquarium. CO2 was delivered through a solenoid valve with a Milwaukee SMS122 pH controller; pH of each aquarium was monitored daily with an Orion Research 601A digital ionalyzer that was calibrated daily with Markson LabSales National Bureau of Standards (NBS) buffers. Total alkalinity of filtered aquarium water was measured in µmol kg−1 seawater as acid neutralizing capacity by titration with 0.2 N HCl using a Gilmont microburet and Gran plot analysis with the USGS web-based Alkalinity Calculator (2008), version 2.20, accessed August, 2008 (http://or.water.usgs.gov/alk). PCO2 was calculated with the CO2SYS Excel macro (Pierrot et al., 2006).
Table 3. Seawater parameters in three incubation conditions used to investigate effects of hypercapnic acidification on the intertidal sea anemone Anthopleura elegantissima.
NBS: National Bureau of Standards, TA: Total Alkalinity. Values are mean ± S.D.
Before being moved to aquaria, anemones were cleaned of debris, blotted dry and weighed. Each individual clonemate was settled into a labeled, 125-ml glass chamber with a stir-bar cage for the duration of the experimental period to minimize disturbance and the risk of damage prior to respiration measurements. Chambers were covered with a 1-cm mesh screen for the first two weeks to prevent anemones from escaping chambers. Several respiration chambers without anemones were maintained at each experimental condition in order to measure background O2 consumption. Damaged individuals and those that failed to adhere to the chamber were not used for experiments.
Anemones were acclimated for 7 days in a 120-l aquarium at 12°C, pH = 8.1, PCO2 = 37.2 Pa; water was recirculated through a filter with an effective pore size of 10 µm. Every day, each chamber was moved within the aquarium to a different position to ensure that all anemones received equivalent irradiance throughout the experimental period. Each specimen was hand fed twice every week from the time of collection until the conclusion of the experiment by alternating shrimp and salmon, each weighing 5% of the specimen's initial wet weight. Chambers were cleaned 24 h after feeding. Aquaria were cleaned of algal growth and water exchanged twice per week to reduce the accumulation of ammonia, nitrate and extracellular proteins. After the initial acclimation period, oral disks of individual anemones (n = 11) were weighed and individually frozen in liquid nitrogen for zooxanthellal measurements to permit comparisons between zooxanthellae at current and experimental conditions. Clonemates were separated so that each individual was maintained in its own chamber in control (45 Pa) or hypercapnic (231 Pa) conditions. The control PCO2 experimental tank represented prevalent, slightly hypercapnic intertidal seawater (45 Pa) and the other was chosen to represent periodic upper-end scenarios of hypercapnic intertidal waters (231 Pa). Mass-specific respiratory (ṀO2) and photosynthetic (Ṗg) rates of each specimen were measured after one week in normocapnic acclimation conditions and after the 6-week experimental period. At the conclusion of the six-week experiment, anemones were blotted dry and weighed. Oral disks and tentacles of each specimen were removed, weighed and frozen in liquid nitrogen for subsequent measurements of zooxanthellae.
Photosynthesis, respiration and CZAR
Mass-specific respiratory (ṀO2) and photosynthetic (Ṗg) rates of each specimen were measured on 11 pairs of clonemates (n = 22 individuals) after one week in normocapnic acclimation conditions and after the 6-week experimental period. Oxygen saturation was measured with Microx TX3 temperature-compensated O2 meters fitted with Type B2 NTH fiber-optic, O2 micro-optodes (Precision Sensing, GmbH, Regensburg) following the methods of Thuesen et al. (Thuesen et al., 2005). Meters were calibrated to 0% O2 with a 5% solution of Na2SO3 and to 100% O2 using oxygen-saturated seawater. Optodes were inserted into the respiration chambers through gas-tight septa. Anemones were fed 48 hours before metabolic measurements to neutralize the effects of specific dynamic action and starvation on metabolic rates (Secor, 2009). To minimize microbial O2 consumption, respiration chambers were cleaned 24 hours before rate measurements and 100 mg l−1 each of streptomycin and ampicillin were added to the test chambers immediately before oxygen measurements. Anemones were sealed into their respiration chambers with seawater at their respective PCO2 with a caged stir-bar to ensure adequate mixing. Chambers were submerged in a circulating water bath at 12°C on stir-plates at 200 rpm. After a 30-minute, lighted acclimation period during normal daylight hours, O2 saturation was measured for 30 minutes in the light followed by 30 minutes of measurement in the dark. Oxygen levels in the respiration chambers stayed within ±20% of normoxia during experiments. Control chambers without anemones were run simultaneously with the same mixtures of antibiotics and seawater at 45 Pa and 231 Pa PCO2. Background rates of microbial O2 consumption within control chambers were <2.5% at each PCO2 condition in light and dark and were subtracted from corresponding anemone rates.
Ṗg to ṀO2 ratios were calculated from the daily gross photosynthetic rate (based on a 14-hour lighted period) relative to the daily respiratory rate. The contribution of carbon from zooxanthellae to animal respiration (CZAR) was based on the ratio of animal (β) and algal (1−β) biomass components (Muscatine et al., 1981) assuming the mean algal biomass ratio of 0.09 = (1−β) calculated for Anthopleura elegantissima (D. M. McKinney, The percent contribution of carbon from zooxanthellae to the nutrition of the sea anemone Anthopleura elegantissima (Coelenterata; Anthozoa), MSc thesis, Walla Walla College, Washington, 1978) (Fitt et al., 1982). Because zooxanthellal respiration cannot be measured in the animal in the light, the daytime algal respiratory rate is estimated from the total dark respiration rate as a ratio of biomass. CZAR was estimated with the formula defined by Muscatine et al. (Muscatine et al., 1981) and modified by Verde and McCloskey (Verde and McCloskey, 1996b; Verde and McCloskey, 2001):
CZAR = [[(0.375*PgO)(PQZ)−1] − [(1−β)(RaeO)(RQae)] − [Cµ]· 100] · [(β)(0.375* RaeO)(RQae)] −1
where daily gross photosynthetic rate (PgO) is equal to the sum of O2 production rate in the light and the O2 consumption rate in the dark for the number of lighted hours per day. The conversion ratio of C to O2 equivalents (12:32) equals 0.375 (Verde and McCloskey, 2001). Daily ṀO2 of the anemone (RaeO) was calculated from the rate of O2 consumption in the dark and extrapolated to 24 h. The photosynthetic quotient (PQz), animal respiratory quotient (RQal) and zooxanthellal respiratory quotient (RQz) were assumed to be 1.1, 0.9 and 1.0, respectively (Verde and McCloskey, 1996b; Verde and McCloskey, 2001). The respiratory quotient of the anemone (RQae) was determined by:
RQae = [(1−β)(RQz)−1 + (β)(RQal)−1]−1
Algal-specific growth rates (μz) were calculated as described in Verde and McCloskey (Verde and McCloskey, 1996b):
μz = (24 · td−1)ln(l + f)
with the duration of cytokinesis td equal to 28 (McCloskey et al., 1996) and f equal to the fraction of cells in the division phase as determined from mitotic index. The zooxanthellal carbon-specific growth rates (Cμ) were determined with the formula supplied by Verde and McCloskey (Verde and McCloskey, 1996a):
Cμ = [(SS)(C·cell−1)(μz)].
Standing stock (SS) was estimated from zooxanthellal cell densities, assuming that 90% of zooxanthellae are harbored in the oral disk and tentacles (Shick, 1991). Carbon per zooxanthellal cell was calculated as reported by Menden-Deuer and Lessard (Menden-Deuer and Lessard, 2000):
pg C·cell−1 = 0.760(cell volume0.819).
Zooxanthellal measurements
Characteristics of Symbiodinium muscatinei were measured to gauge the effects of hypercapnic acidification on the photosynthetic symbionts. Previously frozen oral disks from laboratory-acclimated, non-clonemate anemones and post-experimental clonemates were thawed on ice and individually ground in hand-held, glass tissue-homogenizers with filtered seawater (0.22 µm, 30 psu) at a ratio of approximately 1 tissue: 10 water. Homogenates were separated into 3 aliquots for measurements of protein, chlorophyll and algal parameters. Zooxanthellal cells were compared with the zoochlorellae of Anthopleura xanthogrammica to verify that the symbionts in this study were not the green algal type.
Algal cell counts were performed with a hemocytometer in 10 replicate grid counts per anemone and the number of cells per ml homogenate was normalized to the number of cells per mg protein. Algal cell diameters were measured with an ocular micrometer in replicates of 10 per anemone. Mitotic index was measured as an indicator of zooxanthellal growth and was calculated as a percentage of doublets with a complete cleavage furrow observed per 1000 cells (Wilkerson et al., 1983).
Aliquots of anemone homogenates were desalinated with Millipore Microcon® centrifugal filter units and then diluted to the original concentration with DI water before digestion of the homogenate protein in 5% NaOH to prevent saline interference with the protein assay. Protein concentrations (µmg protein/ml homogenate) were measured with a Thermo Scientific NanoDrop 1000® spectrophotometer against bovine serum albumen standard, and protein density was determined by a modified Lowry Assay (Lowry et al., 1951) according to the NanoDrop protocol on three samples from each anemone.
To measure the concentration of chlorophyll a, 3 replicate homogenates per anemone were centrifuged and resuspended 4 times to remove animal fractions (Muller-Parker et al., 2007). Resuspended zooxanthellae were filtered through GF/C Whatman® filters that were folded and wrapped in foil to freeze at −20°C. Filters were later submerged in 10 ml 90% acetone and stored for 24 h at 4°C for chlorophyll-a extraction (Holm-Hansen and Riemann, 1978). Acetone extracts were analyzed for chlorophyll-a concentration (mg of chlorophyll/ml acetone) with a Turner Designs® 10 AU Fluorometer and converted to pg/zooxanthellal cell.
Statistical analyses
Data were analyzed with JMP Statistical Discovery Software, version 7.0. Paired t-tests were performed on data collected on the same individuals before and after 6-week incubations and between clonemates at each PCO2 to determine if 6 weeks of hypercapnia affected metabolism and photosynthesis. Analyses were carried out on mass-specific rates that were not adjusted for mass. Because symbionts were extracted from non-clonemate animals, ANOVA analyses were performed to examine differences related to zooxanthellae. ANOVA tests were followed with Fisher's LSD post hoc comparisons. CZAR percentage data were not transformed, because data were normally distributed around the mean and not bounded by 100. Mitotic index (%) data were arcsine transformed for ANOVA.
Acknowledgments
We thank D.J. Cox for his assistance in the field and in the lab, and M.A. Gutowska and C. Waters for suggestions about the experimental design of this project. The Quinault Indian Nation allowed access to Quinault lands to collect specimens. C. Barlow and S.H.D. Haddock provided technical assistance, and G. Chin-Leo and F. Melzner made suggestions that improved the manuscript. D. Boltovskoy provided space that facilitated the final stages of this project. This work was supported by an Evergreen State College Foundation Activity Grant.
Footnotes
Competing interests: The authors declare that there are no competing interests.
References
- Allemand D., Furla P., Bénazet-Tambutté S. (1998). Mechanisms of carbon acquisition for endosymbiont photosynthesis in Anthozoa. Can. J. Bot. 76, 925–941 10.1139/b98-086 [DOI] [Google Scholar]
- Anthony K. R. N., Kline D. I., Diaz-Pulido G., Dove S., Hoegh-Guldberg O. (2008). Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc. Natl. Acad. Sci. USA 105, 17442–17446 10.1073/pnas.0804478105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayre D. J., Grosberg R. K. (1995). Aggression, habituation, and clonal coexistence in the sea anemone Anthopleura elegantissima. Am. Nat. 146, 427–453 10.1086/285808 [DOI] [Google Scholar]
- Ayre D. J., Grosberg R. K. (2005). Behind anemone lines: factors affecting division of labour in the social cnidarian Anthopleura elegantissima. Anim. Behav. 70, 97–110 10.1016/j.anbehav.2004.08.022 [DOI] [Google Scholar]
- Baghdasarian G., Muscatine L. (2000). Preferential expulsion of dividing algal cells as a mechanism for regulating algal-cnidarian symbiosis. Biol. Bull. 199, 278–286 10.2307/1543184 [DOI] [PubMed] [Google Scholar]
- Beardall J., Stojkovic S., Larsen S. (2009). Living in a high CO2 world: impacts of global climate change on marine phytoplankton. Plant Ecol. Divers. 2, 191–205 10.1080/17550870903271363 [DOI] [Google Scholar]
- Bidwell J. P., Spotte S. (1985). Artificial Seawaters: Formulas And Methods Boston, MA, USA: Jones and Bartlett Publishers. [Google Scholar]
- Caldeira K., Wickett M. E. (2005). Ocean model predictions of chemistry changes from carbon dioxide emissions to the atmosphere and ocean. J. Geophys. Res. 110, C09S04 10.1029/2004JC002671 [DOI] [Google Scholar]
- Childress J. J., Seibel B. A. (1998). Life at stable low oxygen levels: adaptations of animals to oceanic oxygen minimum layers. J. Exp. Biol. 201, 1223–1232. [DOI] [PubMed] [Google Scholar]
- Crawley A., Kline D. I., Dunn S., Anthony K., Dove S. (2010). The effect of ocean acidification on symbiont photorespiration and productivity in Acropora formosa. Glob. Change Biol. 16, 851–863 10.1111/j.1365-2486.2009.01943.x [DOI] [Google Scholar]
- Fabry V. J., Seibel B. A., Feely R. A., Orr J. C. (2008). Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J. Mar. Sci. 65, 414–432 10.1093/icesjms/fsn048 [DOI] [Google Scholar]
- Feely R. A., Sabine C. L., Hernandez-Ayon J. M., Ianson D., Hales B. (2008). Evidence for upwelling of corrosive “acidified” water onto the continental shelf. Science 320, 1490–1492 10.1126/science.1155676 [DOI] [PubMed] [Google Scholar]
- Fitt W. K., Pardy R. L., Littler M. M. (1982). Photosynthesis, respiration, and contribution to community productivity of the symbiotic sea anemone Anthopleura elegantissima (Brandt, 1835). J. Exp. Mar. Biol. Ecol. 61, 213–232 10.1016/0022-0981(82)90070-3 [DOI] [Google Scholar]
- Frankignoulle M., Abril G., Borges A., Bourge I., Canon C., Delille B., Libert E., Théate J. M. (1998). Carbon dioxide emission from European estuaries. Science 282, 434–436 10.1126/science.282.5388.434 [DOI] [PubMed] [Google Scholar]
- Furla P., Allemand D., Shick J. M., Ferrier-Pagès C., Richier S., Plantivaux A., Merle P. L., Tambutté S. (2005). The symbiotic anthozoan: A physiological chimera between alga and animal. Integr. Comp. Biol. 45, 595–604 10.1093/icb/45.4.595 [DOI] [PubMed] [Google Scholar]
- Ganning B. (1971). Studies on chemical, physical and biological conditions in Swedish rockpool ecosystems. Ophelia 9, 51–105 10.1080/00785326.1971.10430090 [DOI] [Google Scholar]
- Gao K., Aruga Y., Asada K., Kiyohara M. (1993). Influence of enhanced CO2 on growth and photosynthesis of the red algae Gracilaria sp. and G. chilensis. J. Appl. Phycol. 5, 563–571 10.1007/BF02184635 [DOI] [Google Scholar]
- Gutowska M. A., Pörtner H. O., Melzner F. (2008). Growth and calcification in the cephalopod Sepia officinalis under elevated seawater pCO2. Mar. Ecol. Prog. Ser. 373, 303–309 10.3354/meps07782 [DOI] [Google Scholar]
- Hall-Spencer J. M., Rodolfo-Metalpa R., Martin S., Ransome E., Fine M., Turner S. M., Rowley S. J., Tedesco D., Buia M. C. (2008). Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature 454, 96–99 10.1038/nature07051 [DOI] [PubMed] [Google Scholar]
- Hansen P. J., Lundholm N., Rost B. (2007). Growth limitation in marine red-tide dinoflagellates: effects of pH versus inorganic carbon availability. Mar. Ecol. Prog. Ser. 334, 63–71 10.3354/meps334063 [DOI] [Google Scholar]
- Hinga K. R. (2002). Effects of pH on coastal marine phytoplankton. Mar. Ecol. Prog. Ser. 238, 281–300 10.3354/meps238281 [DOI] [Google Scholar]
- Holm-Hansen O., Riemann B. (1978). Chlorophyll a determination: improvements in methodology. Oikos 30, 438–447 10.2307/3543338 [DOI] [Google Scholar]
- Jiang Z. J., Huang X.-P., Zhang J.-P. (2010). Effects of CO2 enrichment on photosynthesis, growth, and biochemical composition of seagrass Thalassia hemprichii (Ehrenb.) Aschers. J. Integr. Plant Biol. 52, 904–913 10.1111/j.1744-7909.2010.00991.x [DOI] [PubMed] [Google Scholar]
- Kempe S. (1982). Valdivia cruise, October 1981: carbonate equilibria in the estuaries of Elbe, Weser, Ems and in the Southern German Bight. Transport Of Carbon And Minerals In Major World Rivers, Part 1. (ed. Degens E T.). Hamburg, Germany: Geologisch-Palaontologischen Institut der Universitat Hamburg. [Google Scholar]
- Kets K., Darbah J. N. T., Sober A., Riikonen J., Sober J., Karnosky D. F. (2010). Diurnal changes in photosynthetic parameters of Populus tremuloides, modulated by elevated concentrations of CO2 and/or O3 and daily climatic variation. Environ. Pollut. 158, 1000–1007 10.1016/j.envpol.2009.09.001 [DOI] [PubMed] [Google Scholar]
- Kübler J. E., Johnston A. M., Raven J. A. (1999). The effects of reduced and elevated CO2 and O2 on the seaweed Lomentaria articulata. Plant Cell Environ. 22, 1303–1310 10.1046/j.1365-3040.1999.00492.x [DOI] [Google Scholar]
- LaJeunesse T. C., Trench R. K. (2000). Biogeography of two species of Symbiodinium (Freudenthal) inhabiting the intertidal sea anemone Anthopleura elegantissima (Brandt). Biol. Bull. 199, 126–134 10.2307/1542872 [DOI] [PubMed] [Google Scholar]
- Lewis L. A., Muller-Parker G. (2004). Phylogenetic placement of “zoochlorellae” (Chlorophyta), algal symbiont of the temperate sea anemone Anthopleura elegantissima. Biol. Bull. 207, 87–92 10.2307/1543583 [DOI] [PubMed] [Google Scholar]
- Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. (1951). Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275. [PubMed] [Google Scholar]
- Lüthi D., Le Floch M., Bereiter B., Blunier T., Barnola J. M., Siegenthaler U., Raynaud D., Jouzel J., Fischer H., Kawamura K. et al. (2008). High-resolution carbon dioxide concentration record 650,000-800,000 years before present. Nature 453, 379–382 10.1038/nature06949 [DOI] [PubMed] [Google Scholar]
- Martin S., Rodolfo-Metalpa R., Ransome E., Rowley S., Buia M. C., Gattuso J. P., Hall-Spencer J. (2008). Effects of naturally acidified seawater on seagrass calcareous epibionts. Biol. Lett. 4, 689–692 10.1098/rsbl.2008.0412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos-Naranjo E., Redondo-Gómez S., Andrades-Moreno L., Davy A. J. (2010). Growth and photosynthetic responses of the cordgrass Spartina maritima to CO2 enrichment and salinity. Chemosphere 81, 725–731 10.1016/j.chemosphere.2010.07.047 [DOI] [PubMed] [Google Scholar]
- McCloskey L. R., Cove T. G., Verde E. A. (1996). Symbiont expulsion from the anemone Anthopleura elegantissima (Brandt) (Cnidaria; Anthozoa). J. Exp. Mar. Biol. Ecol. 195, 173–186 10.1016/0022-0981(95)00079-8 [DOI] [Google Scholar]
- Melzner F., Göbel S., Langenbuch M., Gutowska M. A., Pörtner H.-O., Lucassen M. (2009a). Swimming performance in Atlantic Cod (Gadus morhua) following long-term (4-12 months) acclimation to elevated seawater PCO2. Aquat. Toxicol. 92, 30–37 10.1016/j.aquatox.2008.12.011 [DOI] [PubMed] [Google Scholar]
- Melzner F., Gutowska M. A., Langenbuch M., Dupont S., Lucassen M., Thorndyke M. C., Bleich M., Pörtner H.-O. (2009b). Physiological basis for high CO2 tolerance in marine ectothermic animals: pre-adaptation through lifestyle and ontogeny? Biogeosciences 6, 2313–2331 10.5194/bg-6-2313-2009 [DOI] [Google Scholar]
- Menden-Deuer S., Lessard E. J. (2000). Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnol. Oceanogr. 45, 569–579 10.4319/lo.2000.45.3.0569 [DOI] [Google Scholar]
- Morris S., Taylor A. C. (1983). Diurnal and seasonal variation in physico-chemical conditions within intertidal rock pools. Estuar. Coast. Shelf Sci. 17, 339–355 10.1016/0272-7714(83)90026-4 [DOI] [Google Scholar]
- Moutinho-Pereira J., Gonçalves B., Bacelar E., Cunha J. B., Coutinho J., Correia C. M. (2009). Effects of elevated CO2 on grapevine (Vitis vinifera L.): physiological and yield attributes. Vitis 48, 159–165. [Google Scholar]
- Muller-Parker G., Davy S. K. (2001). Temperate and tropical algal-sea anemone symbioses. Invertebr. Biol. 120, 104–123 10.1111/j.1744-7410.2001.tb00115.x [DOI] [Google Scholar]
- Muller-Parker G., Pierce-Cravens J., Bingham B. L. (2007). Broad thermal tolerance of the symbiotic dinoflagellate Symbiodinium muscatinei (Dinophyta) in the sea anemone Anthopleura elegantissima (Cnidaria) from northern latitudes. J. Phycol. 43, 25–31 10.1111/j.1529-8817.2006.00302.x [DOI] [Google Scholar]
- Muscatine L., McCloskey L. R., Marian R. E. (1981). Estimating the daily contribution of carbon from zooxanthellae to coral animal respiration. Limnol. Oceanogr. 26, 601–611 10.4319/lo.1981.26.4.0601 [DOI] [Google Scholar]
- Norikane A., Takamura T., Morokuma M., Tanaka M. (2010). In vitro growth and single-leaf photosynthetic response of Cymbidium plantlets to super-elevated CO2 under cold cathode fluorescent lamps. Plant Cell Rep. 29, 273–283 10.1007/s00299-010-0820-1 [DOI] [PubMed] [Google Scholar]
- Orr J. C., Fabry V. J., Aumont O., Bopp L., Doney S. C., Feely R. A., Gnanadesikan A., Gruber N., Ishida A., Joos F. et al. (2005). Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437, 681–686 10.1038/nature04095 [DOI] [PubMed] [Google Scholar]
- Palacios S. L., Zimmerman R. C. (2007). Response of eelgrass Zostera marina to CO2 enrichment: possible impacts of climate change and potential for remediation of coastal habitats. Mar. Ecol. Prog. Ser. 344, 1–13 10.3354/meps07084 [DOI] [Google Scholar]
- Pane E. F., Barry J. P. (2007). Extracellular acid-base regulation during short-term hypercapnia is effective in a shallow-water crab, but ineffective in a deep-sea crab. Mar. Ecol. Prog. Ser. 334, 1–9 10.3354/meps334001 [DOI] [Google Scholar]
- Paulmier A., Ruiz-Pino D., Garçon V. (2011). CO2 maximum in the oxygen minimum zone (OMZ). Biogeosciences 8, 239–252 10.5194/bg-8-239-2011 [DOI] [Google Scholar]
- Pierrot D., Lewis E., Wallace. D. W R. (2006). MS Excel Program Developed For CO2 System Calculations. Oak Ridge, Tennessee: U.S. Department of Energy; 10.3334/CDIAC/otg.CO2SYS_XLS_CDIAC105a [DOI] [Google Scholar]
- Richards J. G. (2011). Physiological, behavioral and biochemical adaptations of intertidal fishes to hypoxia. J. Exp. Biol. 214, 191–199 10.1242/jeb.047951 [DOI] [PubMed] [Google Scholar]
- Rodolfo-Metalpa R., Martin S., Ferrier-Pagès C., Gattuso J.-P. (2010). Response of the temperate coral Cladocora caespitosa to mid- and long-term exposure to pCO2 and temperature levels projected for the year 2100 AD. Biogeosciences 7, 289–300 10.5194/bg-7-289-2010 [DOI] [Google Scholar]
- Schneider K., Erez J. (2006). The effect of carbonate chemistry on calcification and photosynthesis in the hermatypic coral Acropora eurystoma. Limnol. Oceanogr. 51, 1284–1293 10.4319/lo.2006.51.3.1284 [DOI] [Google Scholar]
- Secor S. M. (2009). Specific dynamic action: a review of the postprandial metabolic response. J. Comp. Physiol. B 179, 1–56 10.1007/s00360-008-0283-7 [DOI] [PubMed] [Google Scholar]
- Secord D., Augustine L. (2000). Biogeography and microhabitat variation in temperate algal-invertebrate symbioses: zooxanthellae and zoochlorellae in two Pacific intertidal sea anemones, Anthopleura elegantissima and A. xanthogrammica. Invertebr. Biol. 119, 139–146 10.1111/j.1744-7410.2000.tb00002.x [DOI] [Google Scholar]
- Seibel B. A., Walsh P. J. (2003). Biological impacts of deep-sea carbon dioxide injection inferred from indices of physiological performance. J. Exp. Biol. 206, 641–650 10.1242/jeb.00141 [DOI] [PubMed] [Google Scholar]
- Shick J. M. (1991). A Functional Biology Of Sea Anemones London, UK: Chapman and Hall. [Google Scholar]
- Shick J. M., Dykens J. A. (1984). Photobiology of the symbiotic sea anemone Anthopleura elegantissima: photosynthesis, respiration, and behavior under intertidal conditions. Biol. Bull. 166, 608–619 10.2307/1541166 [DOI] [PubMed] [Google Scholar]
- Spicer J. I., Raffo A., Widdicombe S. (2007). Influence of CO2-related seawater acidification on extracellular acid–base balance in the velvet swimming crab Necora puber. Mar. Biol. 151, 1117–1125 10.1007/s00227-006-0551-6 [DOI] [Google Scholar]
- Thuesen E. V., Rutherford L. D., Jr, Brommer P. L., Garrison K., Gutowska M. A., Towanda T. (2005). Intragel oxygen promotes hypoxia tolerance of scyphomedusae. J. Exp. Biol. 208, 2475–2482 10.1242/jeb.01655 [DOI] [PubMed] [Google Scholar]
- Trench R. K. (1987). Dinoflagellates in non-parasitic symbioses. The Biology Of Dinoflagellates (ed. Taylor F J R.), pp. 530–570 London, UK: Blackwell Scientific Publications. [Google Scholar]
- Truchot J. P., Duhamel-Jouve A. (1980). Oxygen and carbon dioxide in the marine intertidal environment: diurnal and tidal changes in rockpools. Respir. Physiol. 39, 241–254 10.1016/0034-5687(80)90056-0 [DOI] [PubMed] [Google Scholar]
- Van de Waal D. B., Verspagen J. M. H., Finke J. F., Vournazou V., Immers A. K., Kardinaal W. E. A., Tonk L., Becker S., Van Donk E., Visser P. M. et al. (2011). Reversal in competitive dominance of a toxic versus non-toxic cyanobacterium in response to rising CO2. ISME J. 5, 1438–1450 10.1038/ismej.2011.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venn A. A., Tambutté E., Lotto S., Zoccola D., Allemand D., Tambutté S. (2009). Imaging intracellular pH in a reef coral and symbiotic anemone. Proc. Natl. Acad. Sci. USA 106, 16574–16579 10.1073/pnas.0902894106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde E. A., McCloskey L. R. (1996a). Carbon budget studies of symbiotic cnidarian anemones - evidence in support of some assumptions. J. Exp. Mar. Biol. Ecol. 195, 161–171 10.1016/0022-0981(95)00078-X [DOI] [Google Scholar]
- Verde E. A., McCloskey L. R. (1996b). Photosynthesis and respiration of two species of algal symbionts in the anemone Anthopleura elegantissima (Brandt) (Cnidaria; Anthozoa). J. Exp. Mar. Biol. Ecol. 195, 187–202 10.1016/0022-0981(95)00080-1 [DOI] [Google Scholar]
- Verde E. A., McCloskey L. R. (2001). A comparative analysis of the photobiology of zooxanthellae and zoochlorellae symbiotic with the temperate clonal anemone Anthopleura elegantissima (Brandt). I. Effect of temperature. Mar. Biol. 138, 477–489 10.1007/s002270000490 [DOI] [Google Scholar]
- Verde E. A., McCloskey L. R. (2002). A comparative analysis of the photobiology of zooxanthellae and zoochlorellae symbiotic with the temperate clonal anemone Anthopleura elegantissima (Brandt) II. Effect of light intensity. Mar. Biol. 141, 225–239 10.1007/s00227-002-0824-7 [DOI] [Google Scholar]
- Verde E. A., McCloskey L. R. (2007). A comparative analysis of the photobiology of zooxanthellae and zoochlorellae symbiotic with the temperate clonal anemone Anthopleura elegantissima (Brandt). III. Seasonal effects of natural light and temperature on photosynthesis and respiration. Mar. Biol. 152, 775–792 10.1007/s00227-007-0737-6 [DOI] [Google Scholar]
- Vitousek P. M., Mooney H. A., Lubchenco J., Melillo J. M. (1997). Human domination of Earth’s ecosystems. Science 277, 494–499 10.1126/science.277.5325.494 [DOI] [Google Scholar]
- Weis V. M. (1993). Effect of dissolved inorganic carbon concentration on the photosynthesis of the symbiotic sea anemone Aiptasia pulchella Carlgren: role of carbonic anhydrase. J. Exp. Mar. Biol. Ecol. 174, 209–225 10.1016/0022-0981(93)90018-J [DOI] [Google Scholar]
- Wilkerson F. P., Muller-Parker G., Muscatine L. (1983). Temporal patterns of cell division in natural populations of endosymbiotic algae. Limnol. Oceanogr. 28, 1009–1014 10.4319/lo.1983.28.5.1009 [DOI] [Google Scholar]
- Wootton J. T., Pfister C. A., Forester J. D. (2008). Dynamic patterns and ecological impacts of declining ocean pH in a high-resolution multi-year dataset. Proc. Natl. Acad. Sci. USA 105, 18848–18853 10.1073/pnas.0810079105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Gao K., Riebesell U. (2010). CO2-induced seawater acidification affects physiological performance of the marine diatom Phaeodactylum tricornutum. Biogeosciences 7, 2915–2923 10.5194/bg-7-2915-2010 [DOI] [Google Scholar]
- Xu Z., Zou D., Gao K. (2010). Effects of elevated CO2 and phosphorus supply on growth, photosynthesis and nutrient uptake in the marine macroalga Gracilaria lemaneiformis (Rhodophyta). Bot. Mar. 53, 123–129 10.1515/bot.2010.012 [DOI] [Google Scholar]
- Yellowlees D., Rees T. A. V., Leggat W. (2008). Metabolic interactions between algal symbionts and invertebrate hosts. Plant Cell Environ. 31, 679–694 10.1111/j.1365-3040.2008.01802.x [DOI] [PubMed] [Google Scholar]


