Abstract
Background
Recent evidence raises the possibility that symptoms of anorexia nervosa (AN) could be related to impaired interoception. Pain is an interoceptive process with well-characterized neuroanatomical pathways that may overlap to a large degree with neural systems that may be dysregulated in AN individuals, such as the insula.
Methods
Functional Magnetic Resonance Imaging (fMRI) was used to assess neural substrates of pain anticipation and processing in ten healthy control women (CW) and 12 individuals recovered from AN (REC AN) in order to avoid the confounding effects of malnutrition. Painful heat stimuli were applied while different colors signaled the intensity of the upcoming stimuli.
Results
REC AN compared to CW showed greater activation within right anterior insula (rAI), dorsolateral prefrontal cortex (dlPFC) and cingulate during pain anticipation, and greater activation within dlPFC and decreased activation within posterior insula during painful stimulation. Greater anticipatory rAI activation correlated positively with alexithymic feelings in REC AN subjects.
Conclusions
REC AN showed a mismatch between anticipation and objective responses, suggesting altered integration and, possibly, disconnection between reported and actual interoceptive state. Alexithymia assessment provided additional evidence of an altered ability to accurately perceive bodily signals in women recovered from anorexia nervosa.
Keywords: Anorexia, insula, pain, anticipation, homeostasis, fmri, interoception, alexithymia, eating disorders, dorsolateral prefrontal
Introduction
Anorexia nervosa (AN) is a debilitating disorder that most commonly affects adolescent females1. AN is often a chronic illness2; 3 that is associated with substantial medical morbidity4 and mortality5; 6. Individuals with AN have puzzling symptoms that are unique to the disorder, such as restricted eating, a relentless drive to lose weight, body image distortions, and denial of illness. However, there are no FDA approved medications or other treatments that reverse core symptoms7–10, and little is known about how such symptoms are encoded in the brain11. Thus a better understanding of the neurobiology of AN is needed in order to develop more efficacious treatments.
Recent theories suggest that AN pathology may relate to a core impairment in interoception12–14, i.e., perceiving and modulating the physiological condition of the body – a process that serves to maintain homeostasis and facilitate adaptive emotion processing15. This assertion is bolstered by studies indicating that AN individuals show altered subjective responses to interoceptive stimuli16–18, such as food, hunger19–22 and physical pain23–28. Moreover, AN individuals often display behavioral traits that could be related to impaired interoception29. Specifically, AN individuals commonly exhibit high levels of alexithymia30, which relates to difficulty describing and identifying feelings31; 32 raising the possibility that AN individuals have an impaired ability to effectively use interoceptive information to appropriately value immediate outcomes.
Pain is a uniquely relevant probe for investigating the function of neural systems relevant to AN symptoms. First, as noted above, most, but not all studies show that ill and recovered AN individuals show maladaptive behavioral responses to experimental pain stimuli. Second, pain is an interoceptive process with well characterized neuroanatomical pathways15; 33, and these pathways overlap to a large degree with the neural systems, such as the insula, that are thought to be dysregulated in AN individuals14. Third, cued pain has a strong anticipatory component34 that may be particularly relevant to probe affective symptoms of AN35; 36. Specifically, anxiety and depression are often comorbid with AN and amplified insula response to the upcoming aversive stimulus has been observed in pathological anxiety36; 37 and mood35 disorders.
The purpose of this study was to use functional magnetic resonance imaging (fMRI) to identify the neural correlates of pain anticipation and processing in women recovered from AN (REC AN) relative to healthy control women (CW) with no history of an eating disorder. We examined recovered AN subjects to avoid the possible confounding effect of starvation and emaciation on pain responses38 and to minimize the interactive metabolic effects on the observed group differences in brain activation, as done in prior research39. We hypothesized that REC AN relative to CW would display dysregulated interoceptive processing during anticipation and processing of heat pain, as evidenced by altered activation within insula and related interoceptive brain circuitry15; 40; 41.
Materials and Methods
Subjects
Twelve women recovered from anorexia nervosa (REC AN) completed this study. To be considered “recovered,” subjects: 1) had maintained at more than 90% of their ideal body weight; 2) reported regular menstrual cycles; and 3) did not use psychoactive medication; and 4) had not binged, purged, or engaged in significant restrictive eating patterns for at least 1 year before the study. These criteria have been used in prior research39; 42; 43. Twelve medically healthy control women (CW) who had regular menstrual cycles since menarche, no history of psychiatric disorders according to a structured clinical interview for DSM-IV (SCID-P), and were comparable to the REC AN women on age (t(21)=3.1, p>0.05), race (χ2=0.88, p>0.05), and BMI (t(21)=0.03, p>0.05) (Table 1) also completed the study. All subjects provided written informed consent to participate in this cross-sectional study, which was approved by the University of California San Diego Human Research Protection Program. Each participant completed the structured clinical interview for DSM-IV SCID-I44, which was administered by trained doctoral level clinicians, to determine current and past eating disorder and other Axis I diagnoses. The final diagnosis was established by consensus meeting with the board-certified psychiatrists (SCM, WHK).
Table 1.
Demographics and Psychological Variables
| CW | REC AN | Stats | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t/χ2 | p | |
| Demographic Variables | ||||||
| Age (yrs) | 24.8 | 6.1 | 29.7 | 6.8 | 3.1 | 0.10 |
| Race | 0.88 | 0.64 | ||||
| Asian | N=1 | N=1 | ||||
| Caucasian | N=9 | N=10 | ||||
| Hispanic | N=0 | N=1 | ||||
| Body Mass Index | 21.9 | 0.73 | 21.9 | 1.65 | 0.03 | 0.97 |
| Age of Onset | - | 13.4 | 2.54 | |||
| Age of Recovery | - | 24.2 | 6.87 | |||
| Disease Duration in years | - | 10.7 | 8.17 | |||
| Years of Recovery | - | 5.5 | 4.45 | |||
| Lifetime Comorbid Diagnosis | ||||||
| Major Depressive Disorder | - | N=7 | ||||
| Any Anxiety Disorder | - | N=6 | ||||
| Obsessive Compulsive Disorder | - | N=3 | ||||
| Alcohol Dependence | - | N=1 | ||||
| Psychological Variables | ||||||
| State Trait Anxiety Inventory (STAI Y) | ||||||
| STAI-Y State Anxiety | 24.2 | 8.2 | 29.0 | 8.3 | 1.6 | 0.13 |
| STAI-Y Trait Anxiety | 27.1 | 7.3 | 34.8 | 10.4 | 2.0 | 0.06 |
| Beck Depression Inventory (BDI) | 1.7 | 3.3 | 3.6 | 4.0 | 1.4 | 0.21 |
| Toronto Alexithymia Scale (TAS-20) & | ||||||
| Difficulty Identifying Feelings | 9.9 | 1.9 | 11.3 | 4 | 0.98 | 0.33 |
| Difficulty Describing Feelings | 9.2 | 2.4 | 10.4 | 4.5 | 0.72 | 0.47 |
| Combined Feelings Score | 19.1 | 4.0 | 21.6 | 7.5 | 0.95 | 0.35 |
| TOTAL | 37.4 | 7.2 | 37 | 9.4 | 0.12 | 0.91 |
data are not available in one REC AN female. REC AN=Recovered Anorexia Nervosa, CW=Control Woman, M=mean, SD=standard deviation
Subjects completed the Toronto Alexithymia Scale-20 (TAS-20), which has three subscales that measure difficulty identifying feelings, difficulty describing feelings and externally orientated thinking31; 32. Specifically, since the difficulty identifying feelings and difficulty describing feelings dimensions of alexithymia have shown high factorial validity and reliability45, and are highly associated with pain sensitivity46; 47, we used these two dimensions to assess alexithymic feelings. All participants also completed the Spielberger State-Trait Anxiety Inventory (STAIY)48 and Beck Depression Inventory (BDI)49 to determine severity of anxiety and depressive symptoms that are generally increased in ill AN. Two CW participants showed extensive noise artifact in the scanner and were removed from the final analysis. Subjects were excluded from the study if they: (1) met DSM-IV criteria for lifetime alcohol or substance dependence; (2) fulfilled DSM-IV criteria for alcohol or substance abuse within 30 days of study participation; (3) used psychotropic medication within the last 4 weeks (or fluoxetine within the last 6 weeks); (4) had irremovable ferromagnetic material; (5) were pregnant or claustrophobic; (6) fulfilled DSM-IV criteria for lifetime bipolar or psychotic disorder, attention deficit hyperactivity disorder; (7) had clinically significant comorbid medical conditions, such as a cardiovascular and/or neurological abnormality; or (8) had a history of current or past chronic pain condition; (9) were left-handed. All participants were unmedicated and were scanned during the first ten days of their menstrual cycles.
Experimental Pain Paradigm (Figure 1)
Figure 1. Experimental Pain Anticipation Paradigm.
Subjects are presented with the blue cross and are cued to anticipate “high pain” (i.e., brief thermal heat temperature that produces high pain sensation) if the color of the cross changes to RED, “low pain” (i.e., brief thermal heat that produces low pain sensation) if the color of the cross changes to GREEN, or “unknown” (i.e., brief thermal heat of either high or low intensity) if the color of the cross changes to YELLOW (50 % probability). Each temperature is delivered for 6 sec.
The paradigm had two temporal conditions (anticipation, stimulus) with the former having three stimulus conditions (anticipation of either high pain, low pain, or unknown pain) and the latter having two stimulus conditions (high pain stimulation or low pain stimulation). High (6sec; 47.5°C) and low (6sec; 45.5°C) thermal pain stimuli that produced moderate and mild pain sensations, respectively, were delivered in a pseudorandom and counterbalanced order by the 9cm2 thermode (Medoc TSA-II, Ramat-Yishai, Israel) that was securely fastened to each subject's left volar forearm. Prior to scanning, all subjects were pre-tested with several non-painful and painful temperature stimuli to ensure that the above temperatures were well tolerated. No group differences in the subjective perception of the administered temperatures were observed during the pre-test. In the scanner, subjects were presented with a BLUE cross and were cued to anticipate “high pain” if the color of the cross changed to RED, to anticipate “low pain” if the color of the cross changed to GREEN, and to anticipate “unknown pain” (either high pain or low pain) if the color of the cross changed to YELLOW (50 % probability). Subjects were instructed that during the task they would receive several thermal heat stimulations that produce high and low pain sensations. A total of 28 (14 high pain, 14 low pain) temperatures were delivered. High temperatures were preceded by the high anticipatory cue (i.e., cross changed from BLUE to RED) 7 times and by the unknown cue (i.e., cross changed from BLUE to YELLOW) 7 times. Likewise low temperatures were preceded by low anticipatory cue (i.e., cross changed from BLUE to GREEN) 7 times and by the unknown cue 7 times.
Post-Task Questionnaire
To verify that both groups had a similar experience with the task, all subjects completed a post-task questionnaire. The following variables were measured: 1) attention to the task (from 0 – “not at all” to 10 – “extreme attention”); 2) experience during the task (from 0 – “very tense” to 10 – “very relaxed”); 3) anticipatory anxiety (from 0 – “not at all” to 10 – “extremely anxious”); 4) perceived pain intensity (0 – “no pain sensation” to 10 – “extreme pain sensation”); and 5) perceived unpleasantness (from 0 – “no unpleasantness” to 10 – “extreme unpleasantness”). Anticipatory anxiety, pain intensity and pain unpleasantness were rated separately for high, low and unknown pain cues, i.e., subjects provided separate ratings for each type of stimulus. Because task demands may interact with pain perception50, within-scan ratings were not employed in this study, and prior research has shown that average within- and post-scan pain ratings do not differ significantly51.
fMRI Protocol
Two fMRI runs (309 brain volumes/run) sensitive to blood oxygenation level-dependent (BOLD) contrast52 were collected for each subject using 3.0 Tesla GE scanner (T2*-weighted echo planar imaging, TR=2000 ms, TE=32ms, flip angle= 90, FOV=23cm, 64×64 matrix, 30 2.6-mm 1.4-mm gap axial slices) while they performed the paradigm described above (Figure 1). FMRI acquisitions were time-locked to the onset of the task. During the same experimental session, a high-resolution T1-weighted image (FSPGR, TR=8ms, TE=3ms, TI=450 ms, flip angle=12, FOV=25cm, 172 sagittal slices, 1×0.97×0.97 mm3 voxels) was obtained for anatomical reference.
fMRI Statistical Analysis
All imaging data were analyzed with the Analysis of Functional NeuroImages (AFNI) software package53. Preprocessed time series data for each individual were analyzed using a multiple regression model consisting of three anticipation-related and two stimulus-related regressors. Anticipation-related regressors consisted of: 1) Anticipation of high pain; 2) Anticipation of low pain; and 3) Anticipation of unknown pain (i.e., either high pain or low pain) (see Figure 1). Stimulus-related regressors consisted of: 1) Application of high pain; and 2) Application of low pain. Seven additional regressors were included in the model as nuisance regressors: one outlier regressor to account for physiological and scanner noise (i.e., the ratio of brain voxels outside of 2SD of the mean at each acquisition), each individual's white matter regressor to account for signal that is not spatially specific, three movement regressors to account for residual motion (in the roll, pitch, and yaw directions), and regressors for baseline and linear trends to account for signal drifts. Voxels were resampled to 4×4×4 mm. A Gaussian filter with a full width-half maximum of 4mm was applied to the voxel-wise percent signal change data to account for individual variation in the anatomical landmarks. Data from each subject were normalized to Talairach coordinates54. Primary contrasts between regression coefficients from the AFNI program 3dDeconvolve were entered into two-sample t-tests. Since all three cues (high, low, unknown) signaled an upcoming painful stimulation, linear combinations of the three anticipation-related regressors and the two stimulus-related regressors were created to maximize statistical power and reliability. In order to examine whether REC AN compared to CW demonstrated abnormal brain activation during anticipation and processing of pain, the following data were compared between groups: 1) BOLD activation during pain anticipation; and 2) BOLD activation during pain stimulation. Whole brain activations within each group are reported in a Supplement. A threshold adjustment method based on Monte-Carlo simulations was used to guard against identifying false positive areas of activation55. Based on the whole brain analysis using a 4mm Gaussian filter, an a priori voxel-wise probability of p< 0.05 in a cluster of 704 mm3 resulted in an a posteriori cluster-wise probability of p <0.05. The average percent signal in areas that survived this whole brain threshold/cluster method was extracted for each condition. Based on prior literature showing the specific role of anterior insula in anticipatory pain processing and interoception15; 35; 41, greater alexithymia in individuals with acute eating disorders30, and a positive relationship between increased feelings scores on alexithymia scale and increased sensitivity to pain56, we conducted an exploratory post-hoc analysis examining the relationship between insula activation and the combined alexithymic feelings score. All post-hoc statistical analyses were performed with PASWStatistics17.0 (SPSS, Chicago, IL).
RESULTS
Post-Scan Ratings and Psychological Variables
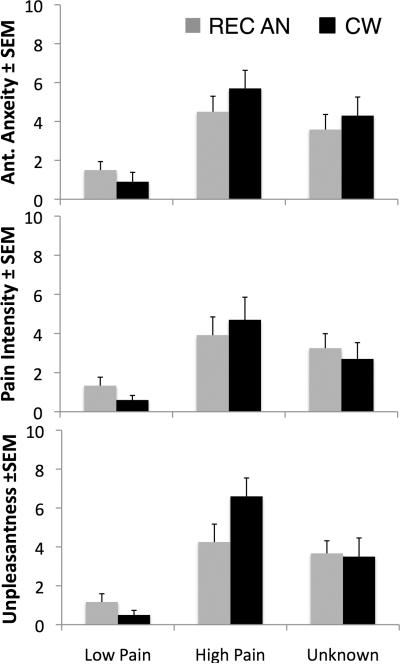
Both groups perceived the high temperature stimuli as more painful than the low temperature stimuli, and both temperatures were rated as painful and unpleasant (Figure 2). Repeated measures ANOVA with type (high, low, unknown) as the within-subject repeated measure and group as the between-subject factor showed no significant main effect of group on subjects' ratings of anticipatory anxiety (F(2,19)=0.259; p=0.616), pain intensity (F(2,19)=0.034, P=0.855) or pain unpleasantness (F(2,19)=0.405; P=0.532). A significant type × group interaction was observed for unpleasantness ratings (F(2,19)= 4.428; p=0.026) but not for anxiety (F(2,19)=1.329, p=0.288) or intensity ratings (F(2,19)= 1.483; p=0.252) (Figure 2). Both groups also reported paying good attention to the task (Mean ± SEM; REC AN: 8.6±0.5 CW: 7.9±0.6, t(20)=0.925; p=0.37) and were similarly relaxed during the task (Mean ± SEM: REC AN: 7.4±0.7; CW: 7.7±0.6; t(20)=0.822; p=0.42). This suggests that the observed differences in brain activation were not due to different subjective experiences between the groups. Furthermore, the groups were not statistically different in their depressive and anxiety symptoms or in the degree of alexithymia (Table 1).
Figure 2. Post-Scan Subjective Temperature Ratings.
Subjects rated the average anticipatory anxiety, average perceived pain intensity and average perceived unpleasantness of each cue and each temperature stimulus after the functional scan. The unknown cue was followed by low and high pain at 50% probability. No significant between-group differences were observed in subjective ratings of anticipatory anxiety (F(2,20)=0.259; p=0.616) or temperatures (F's(2,20)<0.5, P>0.5).
Within-scanner Movement
Since between-group differences in movement parameters during scanning may present a potential confound, we calculated peak and average movement in each subject and compared these variables between the two groups. We found no significant between-group differences in these variables in our sample (t's < 0.2, p's > 0.8).
Whole Brain fMRI Analysis
Pain Anticipation
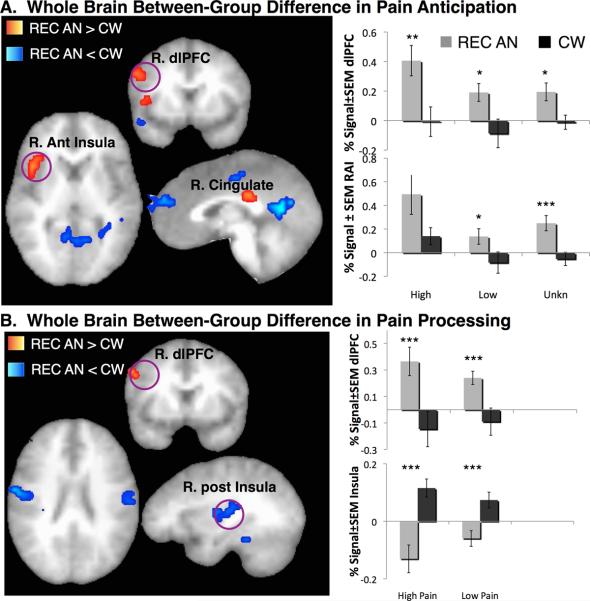
Whole brain analysis of the between-group differences during pain anticipation revealed that REC AN compared to CW showed significantly higher activation within right anterior insula (rAI), right cingulate, and right dorsolateral prefrontal (dlPFC) cortex, and significantly lower activation within left posterior cingulate, left dorsomedial prefrontal (dmPFC) and premotor cortices, as well as within several clusters within temporal and occipital regions (Table 2; Figure 3a).
Table 2.
BETWEEN GROUP T-TEST: PAIN ANTICIPATION
| BRAIN REGION | VOLUME | Talairach | T-VAL | ||
|---|---|---|---|---|---|
| μL | X | Y | Z | ||
| REC AN > CW | |||||
| Right AI | 1728 | 39 | 16 | 6 | 3.64 |
| Right Cingulate | 1024 | 2 | −26 | 26 | 4.36 |
| Right dlPFC | 960 | 51 | 8 | 32 | 4.46 |
| REC AN < CW | |||||
| Left PCC | 5120 | −5 | −59 | 14 | 4.05 |
| Left dmPFC | 3840 | −9 | 60 | 20 | 4.6 |
| Right STG | 2432 | 46 | −62 | 19 | 3.64 |
| Left Premotor Cortex | 1024 | −41 | −23 | 36 | 3.8 |
| Left Paracentral Lobule | 1024 | −3 | −18 | 46 | 3.26 |
| Left Parahippocampal Gyrus | 896 | −23 | −20 | −15 | 4.12 |
| Right MTG | 768 | 48 | 1 | −15 | 3.99 |
| Right Linugal Gyrus | 1664 | 13 | −53 | 3 | 4.58 |
AI = anterior insula, dlPFC= dorsolateral prefrontal cortex, PCC = posterior cingulate cortex, dmPFC = dorsomedial prefrontal cortex, STG = superior temporal gyrus, MTG = medial temporal gyrus, REC AN=Recovered Anorexia Nervosa, CW=Control Woman
Figure 3. Significant Between-group Differences in BOLD activation.
Whole brain analysis of the between-group differences during A. Pain Anticipation showed increased right anterior insula (rAI), cingulate and dorsolateral prefrontal cortex (dlPFC) in women recovered from anorexia nervosa (REC AN) compared to healthy control women (CW) (see Table 2 for further details). Bar graphs indicate percent signal changes within rAI and r. dlPFC during anticipation of low, high and unknown pain. Whole brain analysis of the between-group differences during B. Pain Processing showed increased right dlPFC in REC AN compared to CW women (see Table 3 for further details). Bar graphs indicate percent signal changes within r. dlPFC and r. mid-posterior insula during anticipation of low, high and unknown pain. Left = Right. REC AN = recovered, CW = control women. * < 0.05, ** < 0.01, *** < 0.005.
Pain Stimulation
Whole brain analysis of the between-group differences during pain stimulation revealed that REC AN compared to CW showed significantly higher activation within right dlPFC, and significantly lower activation within right mid-posterior insula, left anterior cingulate cortex, left parahippocampal gyrus, bilateral post-central gyri, as well as within several clusters in the occipital regions (Table 3; Figure 3b).
Table 3.
BETWEEN GROUP T-TEST: PAIN STIMULATION
| BRAIN REGION | VOLUME | Talairach | T-VAL | ||
|---|---|---|---|---|---|
| μL | X | Y | Z | ||
| REC AN > CW | |||||
| Right dlPFC | 768 | 51 | 0 | 36 | 3.37 |
| REC AN < CW | |||||
| Right mid-post Insula | 3136 | 28 | −19 | 11 | 5.10 |
| Left ACC | 1984 | −9 | −12 | 44 | 4.67 |
| Right Postcentral Gyrus | 1088 | 53 | −8 | 24 | 3.55 |
| Left Postcentral Gyrus | 1152 | −55 | −10 | 17 | 4.60 |
| Left Parahippocampal Gyrus | 832 | −20 | −23 | −10 | 3.45 |
| Right Lingual Gyrus | 1728 | 12 | −55 | 1 | 3.80 |
| Left Lingual Gyrus | 1344 | −11 | −54 | −4 | 3.78 |
| Right Fusiform | 704 | 27 | −37 | −17 | 3.88 |
dlPFC = dorsolateral prefrontal cortex, ACC = anterior cingulate cortex, REC AN=Recovered Anorexia Nervosa, CW=Control Woman
Total Grey Matter Volumes
In order to ensure that the observed group differences in functional activation were not due to cerebral volume loss in the REC AN population, we conducted gray matter partial volume extraction, which showed no significant differences between the groups in the total grey matter volumes (F(1,20)= 1.136, p=0.299), which is consistent with our prior work57.
Exploratory Brain-Behavior Correlations
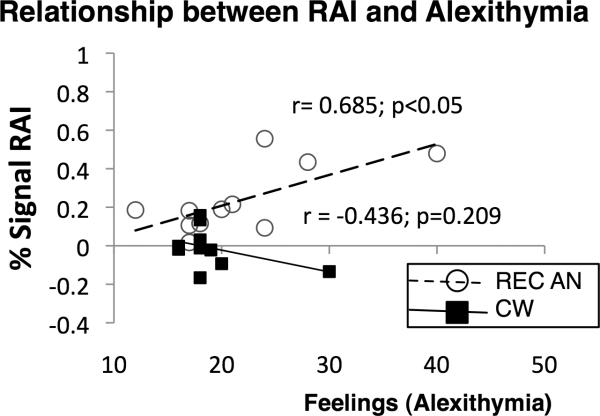
An exploratory analysis examining the relationship between anticipatory rAI activation and alexithymic feelings, showed significant positive correlations between rAI activity during pain anticipation and alexithymic feelings in the REC AN group (r = 0.685, p < 0.05). This correlation was not significant in CW group (r = −0.436, p = 0.209) (Figure 4). The strength of the correlation between rAI activation and alexithymic feelings differed significantly between the groups (p=0.013). Note that this relationship remained significant after removing the CW subject with the highest alexithymia score. To further ensure that outliers were not driving the relationship between rAI activation and alexithymic feelings that was observed in our exploratory analysis, non-parametric Spearman correlations between alexithymia and rAI activation were performed, which produced similar results (REC AN: rho=0.602, p<0.05; CW: rho=−0.460, p = 0.18). No significant correlations were observed between alexithymic feelings and pain-related activation within posterior insula (REC AN: r=−0.16, p=0.63; CW: r=−0.407, p=0.243). Furthermore, no significant correlations were observed between rAI activation and anticipatory anxiety in our sample (r's <0.3, p's >0.05).
Figure 4. Brain-Behavior Correlations.
Significant positive correlations were found between rAI activity during pain anticipation (see Table 2 for details) and alexithymic feelings score in the combined group (r = 0.460, p < 0.05) and in women recovered from anorexia nervosa (REC AN) (r = 0.685, p < 0.05). This correlation was not significant in healthy control women (CW) (r = −0.436, p = 0.209). The strength of the correlation between rAI activation and alexithymic feelings differed significantly between the groups (p=0.013). Note that this relationship remained significant after removing the CW subject with the highest alexithymia score. To further ensure that outliers were not driving the relationship between rAI activation and alexithymic feelings non-parametric Spearman correlations between alexithymia and rAI activation were performed, which produced similar results (REC AN: rho=0.602, p<0.05; CW: rho=−0.460, p = 0.18).
Discussion
Three main findings were observed in this study, which was the first to use neuroimaging to investigate the neural basis of pain anticipation and processing in recovered AN. First, when anticipating painful stimuli, REC AN compared to CW individuals showed more activation of rAI and right dlPFC. Second, when experiencing pain, REC AN compared to CW individuals showed less activation of right posterior insula and more activation of right dlPFC. Third, a positive post-hoc correlation between rAI activation and alexithymia (i.e., the inability to identify one's own feelings and emotions) was observed in REC AN individuals, and not in CW. Taken together, these findings suggest a functional brain basis of altered interoceptive processing in AN12; 13.
As expected58, both groups activated the rAI during pain anticipation (see Supplement), but the activation was significantly greater in the REC AN group. The rAI plays a critical role in health and psychopathology16; 59 by perceiving and modulating the physiological condition of the body, and processing homeostatic emotions such as hunger, thirst, “air hunger” and pain15. Neuroanatomical and functional brain imaging studies suggest that the anterior insula is an integrator of interoceptive, cognitive and emotional experiences, and is a neural substrate for emotional salience60, as well as interoceptive40 and emotional awareness15; 16; 41. Therefore, increased rAI activation during anticipation of thermal heat pain in REC AN may suggest an amplified stress response to the upcoming aversive interoceptive stimulus, similar to what is observed in pathological anxiety36; 37; 61; 62 and mood35 disorders. However, our REC AN subjects did not rate the upcoming painful stimulation as subjectively more aversive, i.e., subjective experiences during pain anticipation were rated similarly by both groups. Importantly, during pain stimulation, REC AN subjects showed less activation in the right mid-posterior insula, again despite a similar reported subjective experience of painful stimuli. Current evidence suggests that the posterior insula encodes objective thermosensory information, whereas the middle and anterior insula integrate thermosensory information with emotionally salient stimuli from all sensory modalities15; 16; 41; 63; 64. The observed mismatch between subjective experiences (ratings) and objective responses (brain activation) in REC AN potentially points to the abnormal integration and, possibly, disconnection between reported and actual interoceptive state. This interpretation is consistent with prior research indicating that AN is associated with a reduced capacity to accurately perceive bodily signals13; 65, a deficit that seems to persist even after recovery.
This interpretation is further supported by our exploratory analyses. While the REC AN did not significantly differ from CW in alexithymic feelings, a positive correlation between alexithymic feelings and rAI activation during pain anticipation was observed in the REC AN group, such that the REC AN subjects who displayed the greatest deficits in identifying and describing feelings and emotions also showed the highest anticipatory rAI activation. Furthermore, this relationship was specific to REC AN group and was not evident in the CW group in our study. Prior literature shows negative correlation between activity within anterior insula during emotional processing tasks and alexithymia scores in healthy controls66; 67, which is consistent with the idea that these individuals have a reduced capacity to experience emotion. However, when a person is classified as highly alexithymic, the correlation between right insula activation and alexithymia scores becomes positive, suggesting that highly alexithymic people are able to experience emotion, in fact, they show hyperarousal to the emotion, but are unable to effectively appraise and identify the emotion68. We posit that in our REC AN individuals similar process is taking place, i.e., the REC AN individuals are more distressed by the upcoming painful stimuli but instead of lack of cognitive awareness of their physiological feedback they intentionally suppress it. Although the results of these exploratory analyses should be interpreted with caution, we think these findings show direct clinical relevance, since treatments directed at improving awareness of emotions may benefit those with anorexia.
DLPFC activation was higher in the REC AN group during pain anticipation and pain processing. The medial and lateral prefrontal cortex is involved in regulating responses to emotional stimuli, as evidenced in prior work by the increased activation of these areas during reappraisal of pain35; 69; 70, and during placebo analgesia71. fMRI studies have demonstrated altered prefrontal activation in ill and recovered AN subjects. Wagner et al. (2007) found that REC AN individuals compared to healthy controls showed increased dlPFC activation to a monetary choice task39. Zastrow et al. (2009)72 reported that ill AN subjects showed enhanced responses in dorsal cognitive circuitry during performance of a sets-hifting paradigm, suggesting that altered prefrontal activation may be independent of state. Uher and colleagues found increased prefrontal activity to food images in participants recovered from AN compared to currently ill AN patients and to healthy controls73. Thus, these findings suggest that REC AN individuals show increased motivation to control stimuli that are perceived as (or expected to be) aversive. Taken together, heightened rAI and dlPFC activation during pain anticipation, potentially due to higher distress of the upcoming pain in REC AN may result in the increased attempts to modulate both anticipatory stress and sensory pain experience in these women. This model is consistent with heightened dlPFC activation and reduced insula activation during pain stimulation. The interpretation that REC AN women may be attempting to control their brain responses to pain in an attempt to achieve a specific subjective experience is consistent with clinical observations of AN individuals who tend to be overcontrolled and inhibited – behaviors that persist after recovery74. Our exploratory post-hoc correlations are consistent with this notion (see Supplement). Interestingly, we observed decreased activation in medial prefrontal cortex and posterior cingulate during pain anticipation in REC AN when compared to CW. These regions correspond closely to the default network, which is often deactivated during cognitively demanding tasks75. This seems to further support the idea that the REC AN participants were particularly engaged during pain anticipation.
We found that subjective experiences associated with pain anticipation and perception were similar in the REC AN and CW groups. Although we did not measure heat pain thresholds in this study, testing prior to scanning showed no between group differences in subjective ratings of non-painful and painful stimuli, thus temperatures used were identical in both groups. There is a substantial, but inconsistent literature, regarding responses to experimental pain in subjects with eating disorders. When compared to healthy control participants, individuals currently ill with either AN or bulimia nervosa show decreased thermal pain sensitivity23–26; 28; 76, and either decreased27 or no change in mechanical pain sensitivity24; 28. Limited data are available regarding whether abnormal experimental pain perception persists after recovery from AN. One earlier study measured heat pain thresholds in recovered anorexic in-patients and found similar thresholds to those in healthy female controls77. Another study measured heat pain thresholds in AN individuals before and immediately after weight gain, then again six months after weight restoration and found that thresholds increased six month after weight restoration but not upon immediate weight gain25. Consistent with these two studies, the REC AN women in our study did not show reduced subjective pain perception, as evidenced by equally intense thermal stimuli being rated similarly by both groups. Our data therefore suggest that although subjective experiences during stimulation and anticipation were similar in REC AN and CW, functional activation of neurocircuitry involved in pain perception and anticipation was altered in women recovered from AN.
This study had several important limitations. First, the sample size of the current study was modest, and therefore, the results require replication. Furthermore, we cannot exclude the possibility that the observed results were partially related to the presence of prior comorbid psychiatric conditions, which were present in most of the REC AN sample in this study. In addition, we did not conduct comprehensive assessment of thermal sensitivity in our subjects. Even though we observed no between group differences during brief pre-testing of non-painful and painful stimuli, and the temperatures used during scanning were rated comparably by both groups (Figure 2), we have no information about pain thresholds in our subjects. Finally, we cannot infer from this cross sectional study whether pain sensitivity changed after recovery or whether the observed alterations in functional brain activity observed in REC AN individuals represent pre-existing vulnerability factors or residual symptoms of AN. Future longitudinal studies should explore how changes in pain sensitivity and/or emotional awareness relate to one's capacity to recover from anorexia nervosa. Despite these limitations, the current findings may contribute to the neurobiological understanding of AN, and have important implications for the development of new treatments that modify individuals' ability to modify and modulate their interoceptive state.
Supplementary Material
Acknowledgments
Funding This study was supported by the National Institute of Mental Health (MH042984; WHK). Additional salary support was provided by a Veterans Health Administration Clinical Science R&D Career Development Award (SCM) and NIMH (MH080003; IAS).
Footnotes
Financial Disclosures/Conflict of Interest None of the authors report conflict of interest. Dr. Kay received funding from Astra Zeneca. All other authors report no financial disclosures with this work.
References
- 1.American Psychiatric Association . Diagnostic and statistical manual of mental Disorders. 4th Edition, text revision (DSM-IV-TR) (4th ed.) American Psychiatric Asociation; Washington, DC: 2000. [Google Scholar]
- 2.Klein DA, Walsh BT. Eating disorders. Int Rev Psychiatry. 2003;15(3):205–216. doi: 10.1080/0954026031000136839. [DOI] [PubMed] [Google Scholar]
- 3.Herzog DB. Eating disorders. New threats to health. Psychosomatics. 1992;33(1):10–15. doi: 10.1016/S0033-3182(92)72015-5. [DOI] [PubMed] [Google Scholar]
- 4.McKenzie JM, Joyce PR. Hospitalization for anorexia nervosa. International Journal of Eating Disorders. 1992;11(3):235–241. [Google Scholar]
- 5.Sullivan PF. Mortality in anorexia nervosa. Am J Psychiatry. 1995;152(7):1073–1074. doi: 10.1176/ajp.152.7.1073. [DOI] [PubMed] [Google Scholar]
- 6.Keel PK, Dorer DJ, Eddy KT, Franko D, Charatan DL, Herzog DB. Predictors of mortality in eating disorders. Arch Gen Psychiatry. 2003;60(2):179–183. doi: 10.1001/archpsyc.60.2.179. [DOI] [PubMed] [Google Scholar]
- 7.NICE, Eating disorders—core interventions in the treatment and management of anorexia nervosa, bulimia nervosa and related eating disorders. 2004 from http://www.nice.org.uk. [PubMed]
- 8.Attia E, Schroeder L. Pharmacologic treatment of anorexia nervosa: where do we go from here? Int J Eat Disord. 2005;37(Suppl):S60–63. doi: 10.1002/eat.20133. discussion S87-69. [DOI] [PubMed] [Google Scholar]
- 9.Bulik CM, Berkman ND, Brownley KA, Sedway JA, Lohr KN. Anorexia nervosa treatment: a systematic review of randomized controlled trials. Int J Eat Disord. 2007;40(4):310–320. doi: 10.1002/eat.20367. [DOI] [PubMed] [Google Scholar]
- 10.Jimerson DC, Wolfe BE, Brotman AW, Metzger ED. Medications in the treatment of eating disorders. Psychiatr Clin North Am. 1996;19(4):739–754. doi: 10.1016/s0193-953x(05)70378-6. [DOI] [PubMed] [Google Scholar]
- 11.Van den Eynde F, Treasure J. Neuroimaging in Eating Disorders and Obesity: Implications for Research. Child and adolescent psychiatric clinics of North America. 2009;18(1):95–115. doi: 10.1016/j.chc.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 12.Kaye WH, Fudge JL, Paulus M. New insights into symptoms and neurocircuit function of anorexia nervosa. Nat Rev Neurosci. 2009;10(8):573–584. doi: 10.1038/nrn2682. [DOI] [PubMed] [Google Scholar]
- 13.Pollatos O, Kurz A-L, Albrecht J, Schreder T, Kleemann AM, Sch^pf V. Reduced perception of bodily signals in anorexia nervosa. Eating Behaviors. 2008;9(4):381–388. doi: 10.1016/j.eatbeh.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Nunn K, Frampton I, Fuglset TS, Torzsok-Sonnevend M, Lask B. Anorexia nervosa and the insula. Med Hypotheses. 2011;76(3):353–357. doi: 10.1016/j.mehy.2010.10.038. [DOI] [PubMed] [Google Scholar]
- 15.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat.Rev.Neurosci. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 16.Craig AD. Interoception and emotion: a neuroanatomical perspective. In: Lewis M, Haviland-Jones JM, Barrett LF, editors. Handbook of Emotions. 3rd ed. Guilford Publications; New York: 2008. pp. 272–288. [Google Scholar]
- 17.Mayer EA, Naliboff BD, Craig AD. Neuroimaging of the brain-gut axis: from basic understanding to treatment of functional GI disorders. Gastroenterology. 2006;131(6):1925–1942. doi: 10.1053/j.gastro.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Craig AD. A new view of pain as a homeostatic emotion. Trends Neurosci. 2003;26(6):303–307. doi: 10.1016/s0166-2236(03)00123-1. [DOI] [PubMed] [Google Scholar]
- 19.Heilbrun AB, Jr., Flodin A. Food cues and perceptual distortion of the female body: implications for food avoidance in the early dynamics of anorexia nervosa. J Clin Psychol. 1989;45(6):843–851. doi: 10.1002/1097-4679(198911)45:6<843::aid-jclp2270450603>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 20.Wagner A, Aizenstein H, Mazurkewicz L, Fudge J, Frank GK, Putnam K, et al. Altered insula response to taste stimuli in individuals recovered from restricting-type anorexia nervosa. Neuropsychopharmacology. 2008;33(3):513–523. doi: 10.1038/sj.npp.1301443. [DOI] [PubMed] [Google Scholar]
- 21.Yoshikatsu N, Toshikiyo K. Perception of hunger to insulin-induced hypoglycemia in anorexia nervosa. International Journal of Eating Disorders. 2001;29(3):354–357. doi: 10.1002/eat.1030. [DOI] [PubMed] [Google Scholar]
- 22.Santel S, Baving L, Krauel K, Munte TF, Rotte M. Hunger and satiety in anorexia nervosa: fMRI during cognitive processing of food pictures. Brain Res. 2006;1114(1):138–148. doi: 10.1016/j.brainres.2006.07.045. [DOI] [PubMed] [Google Scholar]
- 23.Lautenbacher S, Pauls AM, Strian F, Pirke KM, Krieg JC. Pain perception in patients with eating disorders. Psychosom Med. 1990;52(6):673–682. doi: 10.1097/00006842-199011000-00008. [DOI] [PubMed] [Google Scholar]
- 24.de Zwaan M, Biener D, Bach M, Wiesnagrotzki S, Stacher G. Pain sensitivity, alexithymia, and depression in patients with eating disorders: Are they related? Journal of Psychosomatic Research. 1996;41(1):65–70. doi: 10.1016/0022-3999(96)00088-8. [DOI] [PubMed] [Google Scholar]
- 25.Bar K-JMD, Boettger SMD, Wagner GPD, Wilsdorf C, Gerhard UJMD, Boettger MKMD, et al. Changes of Pain Perception, Autonomic Function, and Endocrine Parameters During Treatment of Anorectic Adolescents. Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45(9):1068–1076. doi: 10.1097/01.chi.0000227876.19909.48. [DOI] [PubMed] [Google Scholar]
- 26.Papezova H, Yamamotova A, Uher R. Elevated pain threshold in eating disorders: physiological and psychological factors. J Psychiatr Res. 2005;39(4):431–438. doi: 10.1016/j.jpsychires.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Raymond NC, Faris PL, Thuras PD, Eiken B, Howard LA, Hofbauer RD, et al. Elevated pain threshold in anorexia nervosa subjects. Biol Psychiatry. 1999;45(10):1389–1392. doi: 10.1016/s0006-3223(98)00177-2. [DOI] [PubMed] [Google Scholar]
- 28.Pauls AM, Lautenbacher S, Strian F, Pirke KM, Krieg JC. Assessment of somatosensory indicators of polyneuropathy in patients with eating disorders. Eur Arch Psychiatry Clin Neurosci. 1991;241(1):8–12. doi: 10.1007/BF02193748. [DOI] [PubMed] [Google Scholar]
- 29.Herbert BM, Herbert C, Pollatos O. On the relationship between interoceptive awareness and alexithymia: Is interoceptive awareness related to emotional awareness? J Pers. 2011 doi: 10.1111/j.1467-6494.2011.00717.x. [DOI] [PubMed] [Google Scholar]
- 30.Henrik K, Markus S, Suzanne F, Harald CT, Joern von W. Alexithymia and facial emotion recognition in patients with eating disorders. International Journal of Eating Disorders. 2006;39(3):245–251. doi: 10.1002/eat.20228. [DOI] [PubMed] [Google Scholar]
- 31.Bagby RM, Taylor GJ, Parker JD. The Twenty-item Toronto Alexithymia Scale--II. Convergent, discriminant, and concurrent validity. J Psychosom Res. 1994;38(1):33–40. doi: 10.1016/0022-3999(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 32.Bagby RM, Parker JD, Taylor GJ. The twenty-item Toronto Alexithymia Scale--I. Item selection and cross-validation of the factor structure. J Psychosom Res. 1994;38(1):23–32. doi: 10.1016/0022-3999(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 33.Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55(3):377–391. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 34.Ploner M, Lee MC, Wiech K, Bingel U, Tracey I. Prestimulus functional connectivity determines pain perception in humans. Proceedings of the National Academy of Sciences. 2010;107(1):355. doi: 10.1073/pnas.0906186106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strigo IA, Simmons AN, Matthews SC, Craig AD, Paulus MP. Association of major depressive disorder with altered functional brain response during anticipation and processing of heat pain. Arch Gen Psychiatry. 2008;65(11):1275–1284. doi: 10.1001/archpsyc.65.11.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simmons A, Strigo I, Matthews SC, Paulus MP, Stein MB. Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biol Psychiatry. 2006;60(4):402–409. doi: 10.1016/j.biopsych.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 37.Simmons AN, Paulus MP, Thorp SR, Matthews SC, Norman SB, Stein MB. Functional activation and neural networks in women with posttraumatic stress disorder related to intimate partner violence. Biol Psychiatry. 2008;64(8):681–690. doi: 10.1016/j.biopsych.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lautenbacher S, Pauls AM, Strian F, Pirke KM, Krieg JC. Pain sensitivity in anorexia nervosa and bulimia nervosa. Biol Psychiatry. 1991;29(11):1073–1078. doi: 10.1016/0006-3223(91)90249-l. [DOI] [PubMed] [Google Scholar]
- 39.Wagner A, Aizenstein H, Venkatraman VK, Fudge J, May JC, Mazurkewicz L, et al. Altered reward processing in women recovered from anorexia nervosa. Am J Psychiatry. 2007;164(12):1842–1849. doi: 10.1176/appi.ajp.2007.07040575. [DOI] [PubMed] [Google Scholar]
- 40.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat.Neurosci. 2004;7(2):189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 41.Craig AD. How do you feel [mdash] now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 42.Oberndorfer TA, Kaye WH, Simmons AN, Strigo IA, Matthews SC. Demand-specific alteration of medial prefrontal cortex response during an inhibition task in recovered anorexic women. Int J Eat Disord. doi: 10.1002/eat.20750. [DOI] [PubMed] [Google Scholar]
- 43.Wagner A, Aizenstein H, Venkatraman VK, Bischoff-Grethe A, Fudge J, May JC, et al. Altered striatal response to reward in bulimia nervosa after recovery. Int J Eat Disord. 2009 doi: 10.1002/eat.20699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.First M,B, Spitzer R,L, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. Biometrics Research, New York State Psychiatric Institute; (SCID-I/P) New York: 2002. [Google Scholar]
- 45.Kooiman CG, Spinhoven P, Trijsburg RW. The assessment of alexithymia: A critical review of the literature and a psychometric study of the Toronto Alexithymia Scale-20. Journal of Psychosomatic Research. 2002;53(6):1083–1090. doi: 10.1016/s0022-3999(02)00348-3. [DOI] [PubMed] [Google Scholar]
- 46.Lumley MA, Smith JA, Longo DJ. The relationship of alexithymia to pain severity and impairment among patients with chronic myofascial pain: Comparisons with self-efficacy, catastrophizing, and depression. Journal of Psychosomatic Research. 2002;53(3):823–830. doi: 10.1016/s0022-3999(02)00337-9. [DOI] [PubMed] [Google Scholar]
- 47.Lumley MA, Radcliffe AM, Macklem DJ, Mosley-Williams A, Leisen JCC, Huffman JL, et al. Alexithymia and Pain in Three Chronic Pain Samples: Comparing Caucasians and African Americans. Pain Medicine. 2005;6(3):251–261. doi: 10.1111/j.1526-4637.2005.05036.x. [DOI] [PubMed] [Google Scholar]
- 48.Spielberger CD. Manual for the State-Trait Anxiety Inventory (Form Y) 1983 [Google Scholar]
- 49.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 50.Seminowicz DA, Davis KD. A re-examination of pain-cognition interactions: implications for neuroimaging. Pain. 2007;130(1-2):8–13. doi: 10.1016/j.pain.2007.03.036. [DOI] [PubMed] [Google Scholar]
- 51.Christenfeld N. Memory for pain and the delayed effects of distraction. Health Psychol. 1997;16(4):327–330. doi: 10.1037//0278-6133.16.4.327. [DOI] [PubMed] [Google Scholar]
- 52.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc.Natl.Acad.Sci.U.S.A. 1990;87(24):9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput.Biomed.Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 54.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme; New York: 1988. [Google Scholar]
- 55.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson.Med. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 56.Katz J, Martin AL, Page MG, Calleri V. Alexithymia and fear of pain independently predict heat pain intensity ratings among undergraduate university students. Pain Res Manag. 2009;14(4):299–305. doi: 10.1155/2009/468321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wagner A, Greer P, Bailer UF, Frank GK, Henry SE, Putnam K, et al. Normal brain tissue volumes after long-term recovery in anorexia and bulimia nervosa. Biol Psychiatry. 2006;59(3):291–293. doi: 10.1016/j.biopsych.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 58.Ploghaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PM, et al. Dissociating pain from its anticipation in the human brain. Science. 1999;284(5422):1979–1981. doi: 10.1126/science.284.5422.1979. [DOI] [PubMed] [Google Scholar]
- 59.Shin LM, Liberzon I. The Neurocircuitry of Fear, Stress, and Anxiety Disorders. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Strigo IA, Simmons AN, Matthews SC, Grimes EM, Allard CB, Reinhardt LE, et al. Neural Correlates of Altered Pain Response in Women with Posttraumatic Stress Disorder from Intimate Partner Violence. Biological Psychiatry. 2010;68(5):442–450. doi: 10.1016/j.biopsych.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 62.Moeller-Bertram T, Keltner J, Strigo IA. Pain and post traumatic stress disorder - Review of clinical and experimental evidence. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.04.028. In Press, Uncorrected Proof. [DOI] [PubMed] [Google Scholar]
- 63.Craig AD, Chen K, Bandy D, Reiman EM. Thermosensory activation of insular cortex. Nat.Neurosci. 2000;3(2):184–190. doi: 10.1038/72131. [DOI] [PubMed] [Google Scholar]
- 64.Craig AD. Pain mechanisms: labeled lines versus convergence in central processing. Annu.Rev.Neurosci. 2003;26:1–30. doi: 10.1146/annurev.neuro.26.041002.131022. [DOI] [PubMed] [Google Scholar]
- 65.Merwin RM, Zucker NL, Lacy JL, Elliott CA. Interoceptive awareness in eating disorders: Distinguishing lack of clarity from non-acceptance of internal experience. Cognition & Emotion. 2009 [Google Scholar]
- 66.Bird G, Silani G, Brindley R, White S, Frith U, Singer T. Empathic brain responses in insula are modulated by levels of alexithymia but not autism. Brain. 2010;133(Pt 5):1515–1525. doi: 10.1093/brain/awq060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Silani G, Bird G, Brindley R, Singer T, Frith C, Frith U. Levels of emotional awareness and autism: an fMRI study. Soc Neurosci. 2008;3(2):97–112. doi: 10.1080/17470910701577020. [DOI] [PubMed] [Google Scholar]
- 68.Kano M, Hamaguchi T, Itoh M, Yanai K, Fukudo S. Correlation between alexithymia and hypersensitivity to visceral stimulation in human. Pain. 2007;132(3):252–263. doi: 10.1016/j.pain.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 69.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 70.Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126(Pt 5):1079–1091. doi: 10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- 71.Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303(5661):1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 72.Zastrow A, Kaiser S, Stippich C, Walther S, Herzog W, Tchanturia K, et al. Neural correlates of impaired cognitive-behavioral flexibility in anorexia nervosa. Am J Psychiatry. 2009;166(5):608–616. doi: 10.1176/appi.ajp.2008.08050775. [DOI] [PubMed] [Google Scholar]
- 73.Uher R, Brammer MJ, Murphy T, Campbell IC, Ng VW, Williams SC, et al. Recovery and chronicity in anorexia nervosa: brain activity associated with differential outcomes. Biol Psychiatry. 2003;54(9):934–942. doi: 10.1016/s0006-3223(03)00172-0. [DOI] [PubMed] [Google Scholar]
- 74.Srinivasagam NM, Kaye WH, Plotnicov KH, Greeno C, Weltzin TE, Rao R. Persistent perfectionism, symmetry, and exactness after long-term recovery from anorexia nervosa. Am J Psychiatry. 1995;152(11):1630–1634. doi: 10.1176/ajp.152.11.1630. [DOI] [PubMed] [Google Scholar]
- 75.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van EDC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc.Natl.Acad.Sci.U.S.A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yamamotova A, Papezova H, Uher R. Modulation of thermal pain perception by stress and sweet taste in women with bulimia nervosa. Neuro Endocrinol Lett. 2009;30(2):237–244. [PubMed] [Google Scholar]
- 77.Krieg J. r.-C., Roscher S, Strian F, Pirke K-M, Lautenbacher S. Pain sensitivity in recovered anorexics, restrained and unrestrained eaters. Journal of Psychosomatic Research. 1993;37(6):595–601. doi: 10.1016/0022-3999(93)90054-j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.