Abstract
The objective of the present study was to investigate the antiulcer activity of methanol extract of Oxalis corniculata (whole plant) using pylorus ligation and indomethacin-induced gastric ulceration in Wistar rats. The extract was preliminary evaluated for acute oral toxicity test using Organisation for Economic Co-operation and Development guidelines 423. Further, it was studied for antiulcer potential at the dose levels of 125, 250 and 500 mg/kg. Ranitidine was used as a standard drug (100 mg/kg). Acid secretory parameters like gastric volume, pH, total acidity and free acidity were measured in pylorus ligation model, whereas numbers of ulcers, ulcers score and ulcer index was measured in pylorus ligated and indomethacin treated rats. Pretreatment of test extract significantly (p<0.05) decreased the gastric volume, total acidity, free acidity and increase in the pH of the gastric fluid in pylorus-ligated rats. It also showed significant (p<0.05) decrease in number of ulcers, ulcers score and ulcer index in pylorus ligated and indomethacin treated rats. Results of the study suggest that, the methanol extract of Oxalis corniculata possesses significant antisecretory and antiulcer effects and justify the traditional usage of this herb to treat peptic ulcers.
Keywords: Antiulcer, indomethacin, Oxalis corniculata, pylorus ligation, ranitidine
Peptic ulcer is the most common gastrointestinal tract (GIT) disorder in clinical practice, which affects approximately 5-10% of the people during their life[1]. Gastric hyperacidity and gastroduodenal ulcer is a very common global problem today[2]. The pathophysiology of these disorders has focused on an imbalance between aggressive and protective factors in the stomach, such as acid-pepsin secretion, mucosal barrier, mucus secretion, blood flow, cellular regeneration, prostaglandins and epidermal growth factors[3]. The drugs currently used in the treatment of gastric ulcers are antacids, anticholinergics, proton pump inhibitors and H2-receptor antagonists[4,5]. However, the majority of these drugs produce adverse reactions, such as: hypersensitivity, arrhythmia, impotence, gynecomastia and hematopoietic changes[6]. A search for ulcer treatment that is affordable, effective and devoid of side effects seen in currently available drugs is being intensified and current research has turn to natural resources[7].
Oxalis corniculata is a small procumbent herb belongs to family Oxalidaceae; commonly known as Indian Sorrel in English and ‘Amlapatrika’ in Sanskrit. It is common in moist and cultivated places and warmer parts of India and traditionally used in anaemia, dysentery, diarrhoea, inflamed ulcers, cephalalgia, ophthalmopathy, cardiopathy, and hepatopathy[8–10]. Previous studies have shown that Oxalis corniculata possesses hypoglycemic, antihypertensive, uterine relaxant, antipsychotic, CNS stimulant, smooth muscle relaxant, chronotropic, inotropic effect, antioxidant, antiinflammatory, hepatoprotective, and cardioprotective activities[11–16].
Though the above plant has been used in the treatment of gastrointestinal disorders in the folk medicine, there are no scientific evidences available on its antiulcer potential. Hence, the present work was designed to investigate the antiulcer potential of methanol extract of Oxalis corniculata in pylorus ligation and indomethacin-induced rat ulceration model.
MATERIALS AND METHODS
The whole plant of Oxalis corniculata was collected from in and around the city of Mumbai, Maharashtra, India during the month of August 2007 and dried under shade. The plant was authenticated at the Ramnarayan Ruia College, Mumbai. A voucher specimen (No. 2007/03/23) of the plant material has been kept in our laboratory for future reference.
Preparation of methanol extract:
The dried powdered whole plant of Oxalis corniculata was defatted using petroleum ether (60–80°) and successively extracted with methanol in soxhlet extractor. Extract was filtered through vacuum filter and the filtrate was concentrated in vacuum evaporator. Dried extract was used for the further study. The yield of the extract was found to be 10.16% w/w.
Experimental animals:
Albino rats (Wistar strain) of either sex, weighing 180–200 g were procured from Glenmark Pharmaceuticals, Mhape, Navi Mumbai. Swiss albino mice (20–22 g) of either sex were purchased from Haffkine Biopharmaceuticals, Parel, Mumbai. All the animals were placed in polypropylene cages in a controlled room temperature 22±1° and relative humidity of 60-70% in registered animal house (87/1999/CPCSEA). The animals were maintained on standard pellet diet (Amrut Brand, Sangli, India) and water ad libitum. They were acclimatized to laboratory condition for seven days before commencement of the experiment. Ethical clearance was obtained from Institutional Animal Ethics Committee.
Phytochemical analysis:
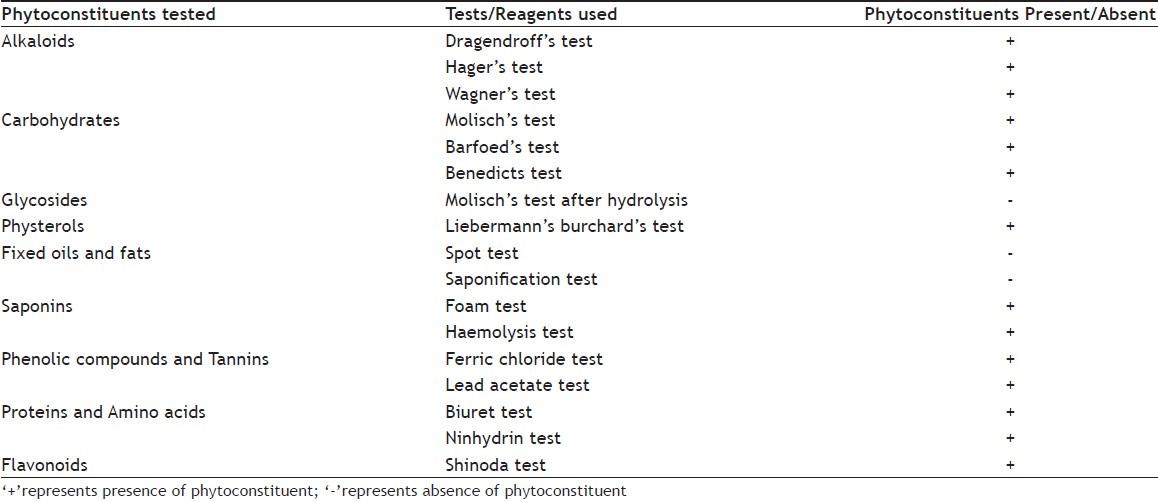
Methanol extract of Oxalis corniculata (MEOC) was studied for phytoconstituents like alkaloids, carbohydrates, glycosides, physterols, fixed oils and fats, saponins, phenolic compounds and tannins, proteins and amino acids, flavonoids using different phytochemical tests[17].
Acute oral toxicity study:
Acute oral toxicity study of MEOC was carried out in Swiss albino mice of both sexes (20-22 g) according to OECD guidelines no 423. Extract at different doses up to 2000 mg/kg was administered orally and animals were observed for behavioral changes, any toxicity and mortality up to 48 h[18].
Pylorus ligation induced gastric ulceration:
The method of Muniappan, et al.[19] was followed with minor alterations. Albino rats of either sex were divided into five groups of six animals each. Animals were fasted for 24 h before the study, but had free access to water. First group served as control and received only distilled water. Second group was treated with ranitidine at the dose of 100 mg/kg. Third, fourth and fifth groups served as treatment groups and administered orally MEOC at the dose levels of 125, 250 and 500 mg/kg, respectively. After 1 h of drugs treatment, they were anesthetized with the help of anesthetic ether; the abdomen was opened by a small midline incision below the xiphoid process. Pyloric portion of the stomach was slightly lifted out and ligated according to method of Shay et al.[20], avoiding traction to the pylorus or damage to its blood supply. The stomach was replaced carefully and the abdominal wall was closed by interrupted sutures. Rats were sacrificed by an over dose of anaesthetic ether after four hours of pyloric ligation. The abdomen was opened, cardiac end of the stomach was dissected out and the contents were drained into a glass tube. The volume of the gastric juice was measured and centrifuged (Superspin, Plasto-crafts, India) at 2000 rpm for 10 min. From the supernatant, aliquots (1 ml of each) were taken for the determination of pH, total and free acidity. The inner surface of free stomach was examined for gastric lesions.
Determination of pH:
An aliquot of 1 ml gastric juice was diluted with 1 ml of distilled water and pH of the solution was measured using pH meter[19].
Determination of total acidity:
An aliquot of 1 ml gastric juice diluted with 1 ml of distilled water was taken into a 50 ml conical flask and two drops of phenolphthalein indicator was added to it and titrated with 0.01N NaOH until a permanent pink colour was observed. The volume of 0.01N NaOH consumed was noted. The total acidity is expressed as meq/l by the formula: n × 0.01 × 36.45 × 1000; Where n is volume of NaOH consumed, 36.45 is molecular weight of NaOH, 0.01 is normality of NaOH, 1000 is the factor (to be represented in litre)[21].
Determination of free acidity:
Instead of phenolphthalein indicator, the Topfer's reagent was used. Aliquot of gastric juice was titrated with 0.01 N NaOH until canary yellow colour was observed. The volume of 0.01 N NaOH consumed was noted. The free acidity was calculated by the same formula for the determination of total acidity[21].
Macroscopic evaluation of stomach:
The stomachs were opened along the greater curvature, rinsed with saline to remove gastric contents and blood clots and examined by a ×5 magnifier lens to assess the formation of ulcers. The number of ulcers was counted. Ulcer scoring was undertaken according to Vogel et al[22]. The scores were: 0= no ulcer, 1= superficial ulcer, 2= deep ulcer, 3= perforation. Ulcer index was measured according to Vogel et al[22] by the formula UI = UN +US +UP ×10 –1;where UI is the ulcer index; UN is the average number of ulcers per animal; US is the average number of severity score; and UP is the percentage of animals with ulcers. The percentage inhibition of ulceration was calculated as (UI Control- UI Test)×100/ UI Control.
Indomethacin-induced gastric ulceration:
The experiment was carried out according to the method of Aguwa et al.[23] and Muriel et al.[24] with a few modifications. Animals were divided into five groups of six rats each. All the animals were fasted for 12 h before the experiment. First group served as control and received distilled water, while second group was treated orally with ranitidine at the dose of 100 mg/ kg. Third, fourth and fifth groups served as test groups and administered orally MEOC at the dose levels of 125, 250 and 500 mg/kg, respectively. After 1 h of drug treatment, Indomethacin (40 mg/kg, p.o.) was given. The animals were sacrificed 5 h after the treatment. Stomach was cut open in the greater curvature and examined by a ×5 magnifier lens to assess the formation of ulcers. The number of ulcers was counted. Ulcer scoring was done by according to Vogel et al.[22] as discussed previously. Ulcer score, ulcer index and % inhibition of ulceration was calculated as before.
Statistical analysis:
The results are expressed as the mean±SD for each group. Statistical differences were evaluated using a One-way analysis of variance (ANOVA) followed by Dunnett's t-test. Results were considered to be statistically significant at p<0.05.
RESULTS
Preliminary phytochemical analysis of MEOC showed the presence of alkaloids, carbohydrates, phytosterols, saponins, phenolic compounds/tannins, proteins, amino acids and flavonoids (Table 1). Acute oral toxicity test of MEOC in Swiss albino mice of both the sexes did not show any behavioral changes, toxic reaction or mortality and found to be safe upto the dose of 2000 mg/kg. LD50 was calculated >2000 mg/kg.
TABLE 1.
PHYTOCHEMICAL ANALYSIS OF MEOC
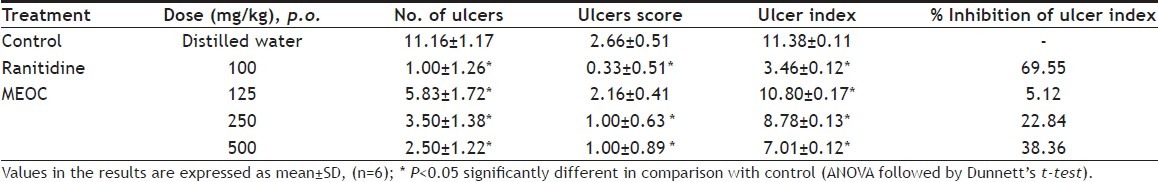
The effect of MEOC on pyloric ligation-induced gastric ulceration is shown in Table 2 and 3. Pretreatment of MEOC at 250 and 500 mg/kg dose levels showed significant (p<0.05) decrease in acid secretary parameters like gastric volume, total and free acidity when compared to control group. pH of the gastric juice was significantly (p<0.05) elevated up to 4.47±0.36 at 500 mg/kg of MEOC. Number of ulcers, ulcers score, and ulcer index was significantly (p<0.05) reduced by MEOC treatment. Inhibition of ulceration index was found to be 5.12%, 22.84% and 38.36% at the dose of 125, 250 and 500 mg/ kg respectively. Ranitidine, standard antiulcer agent showed significant (p<0.05) antisecretory and antiulcer effects as compared to control animals.
TABLE 2.
EFFECT OF MEOC ON GASTRIC CONTENT, pH, TOTAL ACIDITY AND FREE ACIDITY IN PYLORUS LIGATION INDUCED GASTRIC ULCERATION IN RATS
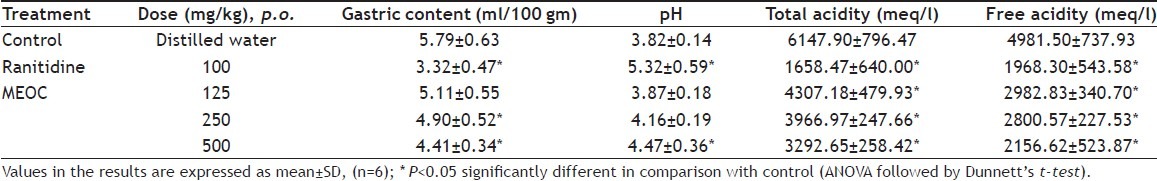
TABLE 3.
EFFECT OF MEOC ON GASTRIC LESIONS IN PYLORUS LIGATION INDUCED GASTRIC ULCERATION IN RATS
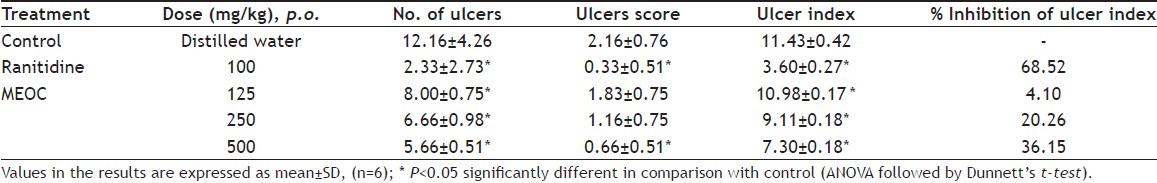
Indomethacin at 40 mg/kg showed superficial, deep ulcers and perforations in the control animals (Table 4). However, animals treated with MEOC at the dose levels of 125, 250 and 500 mg/kg showed significant (p<0.05) reduction in the number of ulcers, ulcer score and ulcer index. It showed 20.26% and 36.15% inhibition of ulceration index at 250 and 500 mg/kg, while ranitidine showed 68.52% ulcer index inhibition in comparison to control group.
TABLE 4.
EFFECT OF MEOC ON GASTRIC LESIONS IN INDOMETHACIN INDUCED GASTRIC ULCERATION IN RATS
DISCUSSION
Peptic ulcer is one of the major gastro-intestinal disorders, which occur due to an imbalance between the offensive (gastric acid secretion) and defensive (gastric mucosal integrity) factors[25]. Although a number of antiulcer drugs such as antacid, H2 receptor antagonist, proton pump inhibitor, cytoprotectives, and prostaglandin analogues are available for treatment of ulcer, all these drugs have side effects and limitations[26]. Natural products are well known as a promising source for the discovery of major new pharmaceuticals. Many natural products are used in folk medicine for the treatment of gastric ulcer[24]. In the present study it has been proved that, Oxalis corniculata is one of such herb, which establishes the antiulcerogenic potency in pylorus ligation and Indomethacin induced gastric ulceration in rats.
Pylorus ligature is an important procedure that shows the possible changes in parameters relative to the gastric content[24]. The causes of gastric ulcer in pyloric ligation are believed to be due to increase in gastric hydrochloric acid secretion and/or stasis of acid, leading to auto digestion of the gastric mucosa and breakdown of the gastric mucosal barrier. These factors are associated with the development of upper gastrointestinal damage including lesions, ulcers and life threatening perforation and hemorrhage[26]. Administration of MEOC at different dose levels significantly decreased gastric secretions, total acidity, free acidity and increased pH of the gastric juice compared to control. It also significantly reduced the number of ulcers, ulcer score, and ulcer index in a dose dependent manner. These results show that, the antiulcer activity of MEOC might be due to its antisecretory effect.
Non-steroidal antiinflammatory drugs (NSAIDs) such as indomethacin, have the ability to cause gastroduodenal ulceration, and this effect is related to the ability of these agents to suppress prostaglandin synthesis[27]. These NSAIDs produce gastric ulceration by inhibiting enzymes cyclooxygenase that promote synthesis of prostaglandins[28]. In the stomach, prostaglandins play a vital protective role, stimulating the secretion of bicarbonate and mucus, maintaining mucosal blood flow, and regulating mucosal cell turnover and repair[29]. Thus, the suppression of prostaglandin synthesis by NSAIDs results in increased susceptibility to mucosal injury and gastric mucosal lesions[30]. In this model, MEOC displayed a significant reduction in mucosal damage in the indomethacin induced ulcer model. These results suggest the possible involvement of prostaglandins and/or mucus in the antiulcer effect of the plant extract.
The preliminary phytochemical analysis of MEOC showed the presence flavonoids. Flavonoids have been reported to possess antiulcer activity in various experimental models[31,32]. Several mechanisms have been proposed to explain gastroprotective effects of flavonoids; these include increase of mucosal prostaglandin content, decrease of histamine secretion from mast cells by inhibition of histidine decarboxylase and inhibition of Helicobacor pylori growth. In addition, flavonoids have been found to be free radical scavenger; free radicals play an important role in ulcerative and erosive lesions of gastrointestinal tract[33]. So, the antiulcer activity of MEOC may be attributed to its flavonoids content. Based on this data, it is suggested that the gastroprotection observed in this study could be related to the presence of flavonoids in the Oxalis corniculata extract.
From the foregoing observations, it could be concluded that methanol extract of Oxalis corniculata (whole plant) possesses significant antisecretory and antiulcer activity. However, further studies are required to evaluate the exact mechanism of action.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the support of Dr. Ganesh Iyer, Botanist, Ramnarayan Ruia College, Mumbai, in authenticating the plant material.
REFERENCES
- 1.Sabiha S, Mohd AA, Asif M, Akhtar M. Role of phenolic compounds in peptic ulcer: An overview. J Pharm Bioallied Sci. 2011;3:361–7. doi: 10.4103/0975-7406.84437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jainu M, Devi CS. Antiulcerogenic and ulcer healing effects of Solanum nigrum (L.) on experimental ulcer models: Possible mechanism for the inhibition of acid formation. J Ethnopharmacol. 2006;104:156–63. doi: 10.1016/j.jep.2005.08.064. [DOI] [PubMed] [Google Scholar]
- 3.Lima ZP, Severi JA, Pellizzon CH, Brito AR, Solis PN, Caceres A, et al. Can the aqueous decoction of mango flowers be used as antiulcer agent? J Ethnopharmacol. 2006;106:29–37. doi: 10.1016/j.jep.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 4.Bighetti AE, Antonio MA, Kohn LK, Rehder VL, Foglio M, Possenti A, et al. Anti-ulcerogenic activity of a crude hydroalcoholic extract and coumarin isolated from Mikania laevigata Schultz Bip. Phytomedicine. 2005;12:72–7. doi: 10.1016/j.phymed.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Lakshimi V, Singh N, Shrivastva S, Mishra SK, Dharmani P, Palit G. Gedunian and photogedunin of Xilocarpus granatum show significant antisecretory effects and protect the gastric mucosa of peptic ulcer in rats. Phytomedicine. 2009;17:569–74. doi: 10.1016/j.phymed.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 6.Chan FK, Leung WK. Peptic ulcer disease. Lancet. 2002;360:933–41. doi: 10.1016/s0140-6736(02)11030-0. [DOI] [PubMed] [Google Scholar]
- 7.Ukwe CV, Ubaka CM, Madusque uche J. Evaluation of the antiulcer activity of Olax subscorpioidea Oliv. Roots in rats. Asian Pac J Trop Med. 2010;3:13–16. [Google Scholar]
- 8.Joshi SG. New Delhi: Oxford and IBH publishing Co. Pvt. Ltd; 2000. Medicinal Plants. [Google Scholar]
- 9.Arya VS. 1st ed. Vol. 4. Hyderabad: Orient Longman Ltd. Publication; 1995. Indian Medicinal Plants. [Google Scholar]
- 10.Kirtikar KR, Basu BD. 2nd ed. Vol. 1. Dehradun: International Book Distributors; 1935. Indian Medicinal Plants. [Google Scholar]
- 11.Tewari PV, Mapa HC, Chaturvedi C. Experimental study of estrogenic activity of certain indigenous drugs. J Res Indian Med Yoga Homeopath. 1976;11:7–12. [Google Scholar]
- 12.Cambie RC, Ash J. Australia: CSIRO; 1994. Fijian Medicinal Plants; pp. 234–5. [Google Scholar]
- 13.Achola KJ, Mwangi JW, Munenge RW. Pharmacological activity of Oxalis corniculata. Int J Pharmacogn. 1995;33:247–9. [Google Scholar]
- 14.Sakat SS, Juvekar AR, Gambhire MN. In vitro antioxidant and antiinflammatory activity of methanol extract of Oxalis corniculata Linn. Int J Pharm Pharm Sci. 2010;2:146–55. [Google Scholar]
- 15.Chetri DR, Parajuli S, Adhikari J. Antihepatopathic plants used by the Lepcha Tribe of the Sikkim and Darjeeling Himalayan Region of India. J Herbs Spices Med Plants. 2008;18:27–35. [Google Scholar]
- 16.Abhilash PA, Nisha P, Prathapan A, Nampoothiri SV, Cherian OL, Sunitha TK, et al. Cardioprotective effects of aqueous extract of Oxalis corniculata in experimental myocardial infarction. Exp Toxicol Pathol. 2011;63:535–40. doi: 10.1016/j.etp.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Farnsworth NR. Biological and phytochemical screening of plants. J Pharm Sci. 1966;55:225–76. doi: 10.1002/jps.2600550302. [DOI] [PubMed] [Google Scholar]
- 18.Paris: Health and Safety Publications; 1996. Guidance document on acute oral toxicity testing Series on testing and assessment No. 423. Organisation for economic co-operation and development, OECD Environment. [Google Scholar]
- 19.Muniappan M, Sundararaj T. Antiinflammatory and antiulcer activities of Bambusa arundinacea. J Ethnopharmacol. 2003;88:161–7. doi: 10.1016/s0378-8741(03)00183-1. [DOI] [PubMed] [Google Scholar]
- 20.Shay H, Komarov SA, Fels SS, Meranze D, Grunstein M, Siplet H. A Simple method for uniform production of gastric ulcers in rats. Gastro-enterol. 1945;5:43–51. [Google Scholar]
- 21.Trease GE, Evans WC. 13th ed. London: Bailliere Tindall Publication; 1992. Text Book of Pharmacognosy. [Google Scholar]
- 22.Vogel HG, Vogel WH. Berlin: Springer Verlag Company; 1997. Activity on the gastrointestinal tract. In: Drug discovery and evaluation (Pharmacological assays) pp. 486–7. [Google Scholar]
- 23.Aguwa CN, Mittal GC. Study of antiulcer activity of aqueous extract of leaves of Pyrenacantha standtii (Family I cacinaceae) using various models of experimental gastric ulcer in rats. Eur J Pharmacol. 1981;74:215–19. doi: 10.1016/0014-2999(81)90533-1. [DOI] [PubMed] [Google Scholar]
- 24.Muriel PB, Marivane L, Edson LM, Mateus FL, João PB, Jairo KB, et al. Evaluation of antiulcer activity of the main phenolic acids found in Brazilian Green Propolis. J Ethnopharmacol. 2008;120:372–7. doi: 10.1016/j.jep.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 25.Hoogerwerf WA, Pasricha PJ. Pharmacotherapy of gastric acidity, peptic ulcers, and gastroesophageal reflux disease. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman and Gilman's The Pharmacological basis of therapeutics. 11th ed. New York: McGraw-Hill Medical Publishing Division; 2006. pp. 967–81. [Google Scholar]
- 26.Kumar A, Singh V, Chaudhary AK. Gastric antisecretory and antiulcer activities of Cedrus deodara (Roxb.) Loud in Wistar rats. J Ethnopharmacol. 2011;134:294–7. doi: 10.1016/j.jep.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 27.Wallace JL. Mechanisms of protection and healing: Current knowledge and future research. Am J Med. 2001;110:19S–22S. doi: 10.1016/s0002-9343(00)00631-8. [DOI] [PubMed] [Google Scholar]
- 28.Kushima H, Nishijima CM, Rodrigues CM, Rinaldo D, Sassa MF, Bauab TM. Davilla elliptica and Davilla nitida: Gastroprotective, anti-inflammatory immunomodulatory and anti-Helicobacter pylori action. J Ethnopharmacol. 2009;123:430–8. doi: 10.1016/j.jep.2009.03.031. [DOI] [PubMed] [Google Scholar]
- 29.Hayllar J, Bjarnason I. NSAIDs, COX-2 inhibitors, and the gut. Lancet. 1995;346:521–2. doi: 10.1016/s0140-6736(95)91378-5. [DOI] [PubMed] [Google Scholar]
- 30.Malfertheiner P, Chan FKL, McColl KEL. Peptic ulcer disease. Lancet. 2009;374:1149–61. doi: 10.1016/S0140-6736(09)60938-7. [DOI] [PubMed] [Google Scholar]
- 31.Parmar NS, Parmar S. Antiulcer potential of flavonoids. Indian J Physiol Pharmacol. 1998;42:343–51. [PubMed] [Google Scholar]
- 32.Kahraman A, Erkasap NF, Köken T, Serteser M, Aktepe F, Erkasap S. The antioxidative and antihistaminic properties of quercetin in ethanol-induced gastric lesions. Toxicology. 2003;183:133–42. doi: 10.1016/s0300-483x(02)00514-0. [DOI] [PubMed] [Google Scholar]
- 33.Francesca B, Angelo AI. The plant kingdom as a source of antiulcer remedies. Phytother Res. 2000;14:581–91. doi: 10.1002/1099-1573(200012)14:8<581::aid-ptr776>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]


