Abstract
Polypharmacy is common in drug prescriptions of chronic kidney disease patients. A study of the prescription patterns of drugs with potential interactions would be of interest to prevent drug related adverse events. A prospective observational study of six months (Dec 2009-May 2010) was carried out among the chronic kidney disease patients admitted to the nephrology ward of a South Indian tertiary care hospital. The pattern and rates of drug-drug interactions seen in the prescriptions of these patients was studied. Among the 205 prescriptions included, a total of 474 interactions were reported, making 2.7 interactions per prescription with incidence rates of 76.09%. Around 19.62% of interactions were of major severity. Most common interactions were found between ascorbic acid and cyanocobalamine (12.45%), clonidine and metoprolol (3.80%) respectively. Hypo or hypertension (31.65%), decreased drug efficacy (29.11%) and hypo or hyperglycemia (14.14%), were the most commonly reported clinical outcomes of the drug interactions. Cardiovascular drugs (calcium channel blockers and beta blockers; 52%) constitute the major class of drugs involved in interactions. As most of the interactions had a delayed onset, long term follow-up is essential to predict the clinically significant outcomes of these interactions. Hence, drug interactions are commonly seen in the prescriptions of chronic kidney disease patients which can lead to serious adverse events if not detected early. Need for collaboration with a clinical pharmacist and electronic surveillance, which are absent in developing countries like India, is emphatic.
Keywords: Chronic kidney disease, drug-drug interaction, drug interaction, drug related problems, end-stage renal disease
Health-care professionals, such as physicians, nurses, and clinical pharmacists, in both inpatient and outpatient settings are increasingly confronted with a growing number of patients with chronic kidney disease (CKD) and end-stage renal disease (ESRD)[1]. CKD is becoming increasingly common in the community as a result of our aging population and rising incidence of diabetes[2].
Medical care for CKD patients is complex due to widespread co-morbidities and major risk factors for CKD. The progression of CKD and the deterioration of kidney function from stage 1 to more severe stages can be slowed by optimal treatment of underlying comorbidities and risk factors, which can be accomplished with lifestyle modifications and/or different pharmacological interventions that address the treatment of hypertension, diabetes mellitus and hyperlipidemia[1,3].
Although the exact incidence and prevalence rates are not available, it is estimated that one out of 10,000 people suffer from CKD in India and around 100 thousand new patients develop ESRD in India annually. In addition to this, higher number of patients are requiring renal replacement therapies such as dialysis and renal transplantation[3].
ESRD patients who are on hemodialysis have complex drug regimens and receive nearly 10 to 12 medications daily, many of which requires multiple doses/day. Due to polypharmacy, frequent medication adjustments on dialysis versus non- dialysis days, medically unstable nature of the disease and restricted life styles, these patients are at high risk for developing drug related problems (DRPs) and nonadherence. With such a large number of medications, there is an increased risk for drug interactions (DI)[3,4].
The plethora of pharmaceutical options must be balanced with the potential risk of multiple medication use. The risks include, but are not limited to, adverse effects, drug-drug interactions (DDIs), drug-disease interactions and inappropriate dosing regimens. Henceforth, DRPs are associated with significant morbidity, impaired quality of life, mortality, and are primary drivers of hospital admissions and health care costs[5,6].
The mechanism of an interaction can be important in predicting the time course of an interaction, and provides a way to minimize the risk of an adverse outcome[7]. Even though, DDIs are considered as preventable medication related problems, studies found that up to 11% of patients experience symptoms associated with DDI and these are responsible for nearly 2.8% of hospital admissions[8]. Monitoring of potential DIs may improve the quality of prescribing and dispensing and it might form a basis for education focused on appropriate prescribing[9].
The present study was carried out in a 1475 bedded tertiary care teaching hospital. Department of Nephrology is one of the speciality departments with a provision of separate dialysis unit and kidney transplantation facility. A pilot study was performed on utilization pattern of drugs in this unit. The study results of the pilot study had shown that majority of the patients were having CKD and its comorbidities (71%). The average number of drugs per prescription was 7.49±3.25; indicated polypharmacy. The most commonly prescribed therapeutic classes of drugs were drugs for cardiovascular system (92%), drugs for gastrointestinal system (68%), antidiabetic drugs (34%), drugs for infections (50%), immunomodulators (8%), nutritional supplements (100%), steroids (16%) and drugs acting centrally (13%)[10]. Among these classes of drugs quantitative measurement of DDIs was not made and hence there was a need to study the rates of occurrence, severity and outcomes. Data obtained from this study will be helpful for the nephrologists to manage CKD patients effectively. Moreover, study results help to intervene, prevent and reduces the morbidity of the adverse effects of a DDI.
MATERIALS AND METHODS
A prospective, observational study was carried out in a South Indian tertiary care hospital. It is a 1475 bedded multi-speciality teaching firm. It is one of the largest medical institutions in India providing health care to many Indians. Nephrology department is a speciality unit consisting of a male ward, female ward and semi-special ward along with separate 14-station dialysis facility and kidney transplantation unit.
The duration of the current study is six months i.e., from December 2009 to May 2010. The study is evaluated and approved by the Committee of Ethics of the University. All the patients admitted during the study duration were followed from the day of admission to the day of discharge and all the new prescriptions issued for hospitalized patients were collected. Along with the medication information, even the medical data and patient demographics were also noted in a drug interaction recording and documentation form. The usage of the medications of every individual patient was cross-checked with the nurse's administration record.
Outcome measures:
To look for potential DDIs among the prescription medications, every combination of prescribed drug was analyzed using the Thomson Reuters Micromedex® DrugReax® system[11]. Each of the potential DDI was then analyzed using descriptive statistical measures (proportions, means, median, range and standard deviation) using SPSS version 11.0. These DDIs were then measured for incidence rates and clinical outcomes. They were also categorized according to different parameters such as severity (minor, moderate, major), time of onset (rapid, delayed), classification and for levels of significance (from 1 to 5)[12]. The definitions of onset, severity, significance rating, and documentation of Micromedex® DrugReax® system were accepted for use in the study, as follows[11]:
Criteria for level of significance:
Each of the potential drug-drug interactions was categorized according to their level of significance. The level of significance relates to the type and magnitude of the effect and, subsequently, to the necessity of monitoring the patients or altering therapy to avoid potentially adverse consequences.
The primary factors that define level of clinical significance include significance rating, the time of onset of the interactions, and the documentation that an interaction occurs clinically. The following discussion defines the guidelines used to designate these factors.
Criteria for significance rating:
A significance rating of one to five was assigned to each interactions and the definition for these number ratings is given as: (1) The reaction is severe and a well documented interactions; (2) The reaction is occurring with moderate severity and the documentation is suspected; (3) The reaction is minor and with suspected documentation; (4) The severity of the interactions will be major or moderate with possible documentation; (5) The reaction is no more than unlikely or possible documentation.
Criteria for onset:
How rapidly the clinical effects of an interaction can occur determines the urgency with which preventive measures should be initiated to avoid the consequences of the interaction. It classified as: Rapid: The effect will be evident within 24 h of administration of the interacting drugs; Delayed: The effect will not be evident within 24 h of concomitant administration. Effects may take days or weeks of concomitant administration to appear.
Criteria for severity:
The potential severity of the interaction is important in assessing the risk vs. benefit of the treatment and that of therapeutic alternatives. With appropriate dosage adjustment or modification of the administration schedule, the negative effects of several interactions can be avoided. Three degrees of severity are defined as; Minor: The effects are usually mild; consequences may be bothersome or unnoticeable but should not significantly affect the therapeutic outcome; Moderate: The effects may cause deterioration in the patient's clinical status and additional treatment, hospitalization or extension of hospital stay may be necessary; Major: The effects are potentially life threatening or capable of causing permanent damage.
Criteria for documentation
Documentation determines the degree of confidence that an interaction can cause an altered clinical response. This scale represents the editorial group's evaluation of the quality and clinical relevance of the primary literature supporting the occurrence of an interaction. However, multiple factors can influence whether even a well-documented interaction occurs in a particular patient. The documentation does not address the incidence or frequency of the interaction; it is also independent of the potential severity of the effect of the interaction. Established: Proven to occur in well-controlled studies; Probable: Very likely but not proven clinically; Suspected: May occur; some good data; needs more study; Possible: Could occur, but data are very limited; Unlikely: Doubtful; no good evidence of an altered clinical effect.
RESULTS
Demography status of study population:
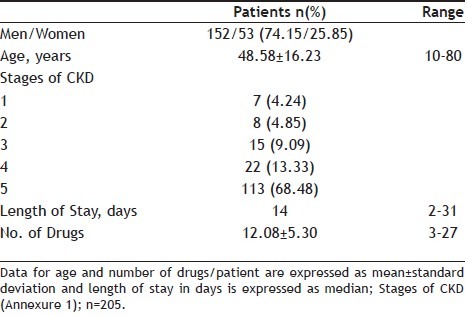
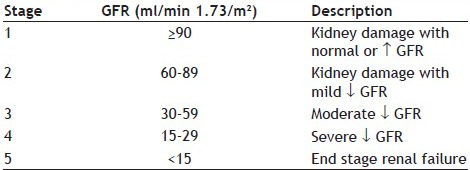
A total of 205 patients were included in the study. Among them, 156 (76.09%) study subjects had shown potential DDIs. Mean age of the study population was 48.58±16.23 years and majority of population were men (74.15%). The average number of medications used per day was 12.08±6.30 with 3-27 medicines/day. Majority of the study population were of stage 5 renal failure and the median duration of hospital stay was found to be 14 days (Table 1).
TABLE 1.
DEMOGRAPHIC CHARACTERISTICS OF THE CHRONIC KIDNEY DISEASE PATIENTS WHO HAD SHOWN POTENTIAL DRUG-DRUG INTERACTION
Characteristics of DDIs:
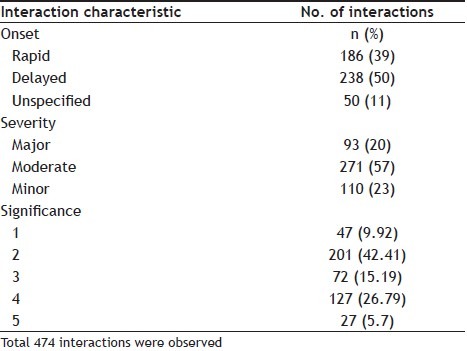
Among the 156 patients, 474 drug interactions were identified with 2.7 interactions per patient. Out of which, 238 (50%) of DI were of delayed onset and 186 (39%) were of rapid onset. According to severity classification, 93 (20%) interactions were severe, 271 (57%) were moderate and 110 (23%) were minor. Among 474 DDI, 240 (50.63%) were of pharmacodynamic DI and 222 (46.84%) were of pharmacokinetic.
Of the 474 DDI, 47 (9.92%) belong to level 1 significance which are of severe type, 201 (42.41%) were of level 2 i.e. moderate, 72 (15.19%) were of level 3 which are minor in nature, 127 (26.79%) were of level 4 which are of mild/moderate level, and 27 (5.7%) were of level 5 which are uncertain/unlikely (Table 2).
TABLE 2.
CLINICAL SIGNIFICANCE OF DRUG-DRUG INTERACTION IN 205 CHRONIC KIDNEY DISEASE PATIENTS
ANNEXURE 1.
STAGES OF CHRONIC KIDNEY DISEASE[17]
Classes of drugs involved in DDI:
Among different categories of drugs involved in DDI, 429 (52%) of them were by cardiovascular drugs, antidiabetic drugs were included in 66 (8%) of drug interactions, antimicrobial agents were involved in 95 (11.52%), gastrointestinal drugs involved in 25 (3.03%), respiratory drugs in 16 (1.94%) while miscellaneous drugs (vitamins and minerals) were involved in 194 (23.52%) DDI.
The most common class of drugs involved in DDI among the cardiovascular drugs were betablockers 168 (39.16%) and calcium channel blockers 87 (20.28%). Fluroquinolones constituted 42 (44.2%) majority of DDI among the antimicrobials. Among vitamins 59 (89.3%) were constituted by ascorbic acid and vitamin B12 and among the antidiabetics, insulin was involved in 44 (66.6%) of DDIs.
Frequency of DDI:
The frequency of DDI for each drug combination ranged from 1 to 59. The most frequently prescribed drug combinations with potential DDI were ascorbicacid/cyanocobalamine [(59 prescriptions (12.45%), clonidine/metoprolol (18 patients (3.80%), amlodipine/metoprolol (16 prescriptions (3.38%), insulin/metoprolol (14 prescriptions (2.95%)].
Outcomes of DDI:
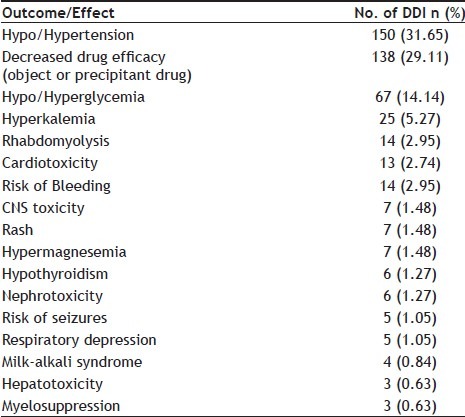
Among the 474 potential drug interactions, when classified according to the resulting effect, the frequencies of the outcomes of the DDIs was found that 150 (31.65%) may lead to hypotension or hypertension, 67 (14.14%) hypoglycemia or hyperglycemia, 25 (5.27%) hyperkalemia, 14 (2.95%) rhabdomyolysis, 14 (2.95%) increased risk of bleeding, 13 (2.74%) cardiotoxicity, 7 (1.48%) CNS toxicity, 7 (1.48%) hypermagnesemia, 7 (1.48%) rash, 6 (1.27%) nephrotoxicity, 6 (1.27%) hypothyroidism, 5 (1.05%) respiratory depression, 5 (1.05%) risk of seizures, 4 (0.84%) milk-alkali syndrome, 3 (0.63%) myelosuppression, 3 (0.63%) hepatotoxicity and 138 (29.11%) reduction in drug efficacy i.e. either object or precipitant drug (Table 3).
TABLE 3.
FREQUENCY OF THE DIFFERENT OUTCOMES OF DRUG-DRUG INTERACTION (DDI) (N=474)
Analyzing the medications involved in interactions that result in possibly increased risk of hypotensive or hypertensive episodes (n=150), 108 (72%) were related to beta blockers with calcium channel blockers. Amongst the interactions that have hypoglycemia or hyperglycemia as possible effects (n=67), 30 (44.8%) were related to betablockers with insulin and/or oral hypoglycemic agents, and 13 (19.4%) involved quinolones with insulin and/or hypoglycemic agents.
In relation to interactions that would lead to hyperkalemia (n=25), 10 (40%) were associated with angiotensin converting enzyme inhitors (ACE inhibitors) with potassium sparing diuretic agents or potassium supplements. Rhabdomyolysis (n=14), could be a result of atorvastatin with quinolones: 8 (57.14%), or quinolones with corticosteroids: 6 (42.8%).
Among the medications involved in interactions that would potentially cause increased risk of bleeding (n=14), 6 (43%) were associated with clopidogrel with anticoagulants. Of the interactions that have cardiotoxicity (n=13), 7 (53.8%) were related to digoxin with levothyroxine and 5 (38.4%) by fluconazole with amlodipine. DDIs associated with hypermagnesemia (n=7), 5 (71.4%) were related to antacids.
The DDIs that would result in rashes (n=7), 5 (71.4%) were related to penicillins with allopurinol. Those having a risk of nephrotoxicity (n=6), 3 (50%) were related to aminoglycosides and 3 (50%) with calcium channel blockers. All drug combinations of levothyroxine (n=6), 6 (100%) were related to hypothyroidism.
Among the interactions involved with risk of seizures (n=5), 4 (80%) were associated with phenytoin. Immunosuppressants 3 (100%) were associated with myelosuppression (n=3). Regarding decreased drug efficacy of either an object drug or a precipitant drug (n=128), 70 (54.6%) were associated with vitamins and 25 (19.5%) with quinolones.
DISCUSSION
Current study highlights the detection of DDI prevalence using a computer program to check prescriptions of inpatients of nephrology ward of a South Indian tertiary care hospital. In the present study, we found that the frequency of DDI in medications of CKD patients was found to be 76.09%. The large variations in the reported frequencies in different studies[13] are generally due to methodological considerations, such as the use of different definitions for clinically significant DDIs, difference in study designs and difference in ways of measuring the frequency or incidence of DDIs. In a study done on prescriptions for 624 ambulatory patients of a family clinic in Mexico City by Dubova et al.[13], it was it was found that 80% of the patients had prescriptions implying one or more potential DDIs and 3.8% of patients were prescribed drug combinations that should be avoided. Whereas, in another study done in a cohort of Dutch nursing home residents by Dijk et al.[14], it was found that 32% of the 2,335 patients were exposed to one or more combinations of drugs that could lead to clinically adverse outcomes. So, the rates of occurrence of DDIs vary with different studies. This reinforces for the problems relating to administering drug treatment to renal failure patients, its consequences and the need for continuous efforts to optimize drug therapy.
The median number of medications/patient administered in the study population was 11 indicating polypharmacy which is a major risk factor for DDI. A study done by Glintborg, et al.[15], among 200 patients found that a median number of drugs used were 8 with a range of 1 to 24, and they state that polypharmacy is a predictable risk factor for medication errors.
Most of the DDI were found to be of delayed in onset and pharmacokinetic in nature which is comparable to Nazari et al.[16]; which implies that if there was an interaction occurring during the concomitant administration it may not manifest itself immediately and if these drugs were to be continued for the patient on an outpatients basis then this could potentially lead to decreased efficacy of drugs leading to therapeutic failures and potential adverse events etc. Hence the duration of concomitant drug use should also be taken into account when prescribing these interacting drugs. The high proportion of DDI being at a level of 2 or 4 on a scale of 5 of significance rating means that patient's life could be at risk and in such cases the physician and nursing staff should keep the patient under close surveillance since they are mostly delayed in onset.
The medicines commonly involved in DDI were those of daily use to treat patients with chronic disorders. Beta blockers, calcium channel blockers, diuretics, digoxin, antiarrhythmic agents, phenytoin, central action analgesics, antipsychotic agents, hypoglycemic agents, and quinolones are frequently mentioned in the lists of potential drug interactions. Many of these drug interactions can be monitored and avoided, by means of serum dosage adjustments (as in the case with azathioprine and enalapril or metformin with trimethoprim), glycemic control (hypoglycemic agents with quinolones) or by means of clinical or laboratory control.
Studies like the present one provides a list of the most frequent drug interactions in CKD patients; may serve as a warning for the interdisciplinary team concerning the reactions that may occur due to an interaction, as well as a support material for physicians to choose alternative medication, dose adjustment and patient monitoring. The easiest way to reduce the frequency of them is to decrease the number of medicines prescribed. Nevertheless, sometimes it's difficult to reduce the number of drugs prescribed for patients with multiple chronic conditions; therefore, to lower the frequency of potential interactions it could be necessary to make a careful selection of therapeutic alternatives, and in cases without other options, patients should be continuously monitored to identify adverse events. The clinical pharmacist is of paramount importance in this process to provide information for a better decision, improving quality of treatment and reducing risks for the CKD patients.
REFERENCES
- 1.USRDS 2008 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda. MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. 2008.
- 2.Clark L, Prescott G, Fluck N, Simpson W, Smith W, Macleod A. Identifying Individuals with CKD: Are we overestimating the number? XLIV Congress of the European Renal Association European Dialysis and Transplant Association (ERA-EDTA), 2007. Nephrol Dial Transplant. 2007;22(Suppl 6):71–90. [Google Scholar]
- 3.Sathvik BS, Mangasuli S, Narahari MG, Gurudev KC, Parthasarathi G. Medication knowledge of hemodialysis patients and influence of clinical pharmacist provided education on their knowledge. Indian J Pharm Sci. 2007;69:232. [Google Scholar]
- 4.Koch-Weser J, Greenblatt DJ. Drug interactions in clinical perspective. Eur J Clin Pharmacol. 1977;11:405–8. doi: 10.1007/BF00562929. [DOI] [PubMed] [Google Scholar]
- 5.Lafleur J, Beth C, Gunning K, Oderda L, Steinvoort C, Oderda GM. Prevalence of Drug-Related Problems and Cost-Savings Opportunities in Medicaid High Utilizers Identified by a Pharmacist-Run Drug Regimen Review Center. J Manag Care Pharm. 2006;12:677–85. doi: 10.18553/jmcp.2006.12.8.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olyaei AJ, Bennett WM. Drug Dosing in the Elderly Patients with Chronic Kidney Disease. Clin Geriatr Med. 2009;25:459–527. doi: 10.1016/j.cger.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Hansten PD. Drug interaction management. Pharm World Sci. 2003;25:94–7. doi: 10.1023/a:1024077018902. [DOI] [PubMed] [Google Scholar]
- 8.Mahmood M, Malone DC, Skrepnek GH, Abarka J, Armstrong EP, Murphy JE, et al. Potential drug-drug interactions within veterans affairs medical centers. Am J Health Syst Pharm. 2007;64:1500–5. doi: 10.2146/ajhp060548. [DOI] [PubMed] [Google Scholar]
- 9.Merlo J, Liedholm H, Lindblad U, Bjorck-Linne A, Falt J, Lindberg G, et al. Prescriptions with potential drug interaction dispensed at Swedish pharmacies in January 1999: Cross sectional study. Br Med J. 2001;323:427–8. doi: 10.1136/bmj.323.7310.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rama M, Leelavathi DA, Prabhu RA. Drug utilization pattern in patients with renal failure in Nephrology unit of Tertiary care Hospital.Indian Pharmaceutical Association Convention 2010. Indian J Pharm Sci. 2010;72:276–82. [Google Scholar]
- 11.Klasko RK, editor. DRUG-REAX® System [Internet database].Greenwood Village, Colo: Thomson Micromedex. Updated periodically, 2011 [Google Scholar]
- 12.Tatro DS. St. Louis, MO : Wolters Kluwer Health; 2003. Drug Interaction Facts; pp. XI–XVI. [Google Scholar]
- 13.Doubova SV, Reyes-Morales H, Torres-Arreola LP, Suarez-Ortega M. Potential drug-drug and drug-disease interactions in prescriptions for ambulatory patients over 50 years of age in family medicine clinics in Mexico City. BMC Health Serv Res. 2007;7:147. doi: 10.1186/1472-6963-7-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dijk KV, De-Vires CS, Van-Den-Berg PB, Brouwers JR, Van-Den-Berg LT. Occurrence of Potential Drug-Drug Interactions in Nursing Home Residents. Int J Pharm Pract. 2001;9:45–54. [Google Scholar]
- 15.Glintborg B, Andersen SE Dalhoff K. Drug-drug interactions among recently hospitalised patients-frequent but mostly clinically insignificant. Eur J Clin Pharmacol. 2005;61:675–81. doi: 10.1007/s00228-005-0978-6. [DOI] [PubMed] [Google Scholar]
- 16.Nazari MA, Moqhadam NK. Evaluation of Pharmacokinetic drug interactions in Prescriptions of Intensive Care Unit in a teaching hospital. Iran J Pharm Res. 2006;5:215–8. [Google Scholar]
- 17.Kidney Disease: Improving Global Outcomes. KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation and treatment of Hepatitis C in chronic kidney disease. Kidney Int. 2008;73(Suppl 109):S1–S99. doi: 10.1038/ki.2008.81. [DOI] [PubMed] [Google Scholar]


