Abstract
The hazards of prescribing many drugs, including side-effects, drug-drug interactions and difficulties of compliance have long been recognized as particular problems when prescribing. This study estimates the rate and factors associated with potential drug-drug interactions in prescriptions from wards of An Iranian General Hospital. Data were retrieved from the pharmacy of a general hospital (200 beds) during one year period 2010. Potential drug-drug interaction were identified using a computerized drug-drug interaction database system (Prescription Analyzer 2000, Sara Rayane Co., Iran). Patients of both genders and 15 years-old or more were included in this study. Prescriptions with two or more drugs prescribed were selected during one year period 2010. Gender number of drugs and therapeutic drug classes on prescriptions were explored as associated factors to drug-drug interaction. The overall prevalence of potential drug-drug interaction was 20.3%. The risks of severe potential drug interactions were relatively high and the rate of potential drug-drug interaction was significantly higher in women (60.6%) and the patients aged over 60 years old (57.1%). The frequency of the potentially severe drug-drug interaction was 10.8% with digoxin-furosemide as the most common interacting pair (5.91%). A positive correlation was found between drug-drug interaction, patient's age, number of drugs and drugs acting on cardiovascular system. So cardiology women inpatients, age more then 60 years old, and patients prescribed digoxin and angiotensin-converting enzyme inhibitors should be closely monitored for adverse outcomes from drug-drug interaction.
Keywords: General hospital, Iran, potential drug-drug interactions, moderate, severe
Adverse drug events affect millions of patients each year and are responsible for up to 5% of hospital admissions[1,2]. They also pose an enormous financial burden, with an estimated cost of more than $16000 per hospitalization[3–5]. While some adverse drug events are unpredictable (such as anaphylaxis from an unrecognized allergy), many others can be anticipated and prevented. Drug-drug interactions (DDIs) are a particularly important type of adverse drug events because they are often predictable based on previous reports, clinical studies, and an understanding of pharmacologic principles[6–13]. Some adverse drug events have life-threatening consequences and may prompt the removal of popular medications from the marketplace[14–21].
Adverse consequences of drug interactions have been shown in various studies. The prevalence of important DDIs varied in different countries. Studies conducted in various countries report rates of potential DDIs ranging from 1 to 66[7,8,10,14–16,22–24].
Different factors are associated with the occurrence of potential DDIs. Polypharmacy is now common, and carries a high risk of DDIs and drug-disease interactions. These may cause adverse effects, or the therapeutic effects of the combined medicines may change, with serious consequences for health. In the United States 25% of ambulatory patients taking drug combinations were at risk for clinically important interactions[25]. A European study of 1601 ambulatory elderly patients, taking an average of seven different drugs, found that 46.0% were at risk for at least one clinically important potential DDI[22]. Furthermore, it has been reported that about 40% of hospitalized patients had at least one potential drug-disease interaction[26]. Also the risk of potential drug interaction is higher in old patients[17,22,23,27]. DDIs cause 4.8% of hospitalizations attributed to drugs in the elderly[3].
It is possible that other risk factors for potential interactions exist, and these should be identified to establish successful methods for improving prescription practices. The prevalence of DDIs and the factors associated with it is not determined in inpatients′ prescriptions in Iran. So the aim of this study was to estimate the prevalence and the factors associated with potential DDI in adult inpatients′ prescriptions from wards of a general hospital in Zarand, Iran.
A retrospective study was performed using data of the prescriptions held at the pharmacy of an Iranian general hospital which is supervised by Social Insurance Organization (an Iranian general insurance organization). The hospital is a 200-bed general institution including different wards (internal, pediatric, surgery, obstetrics and gynecology) which is also a referral centre for hospital care in Zarand region with an estimated population of 150 000. Patients of both genders and 15 years-old or more were included in this study. Prescriptions with two or more drugs prescribed were selected during one year period 2010. All drug groups were accepted. Only one prescription from each patient during his/her hospitalization at ward during the study period was included.
Prescriptions with one or more potential DDI were identified by using a computerized DDI database system (Prescription Analyzer 2000, Sara Rayane Co., Iran). To estimate rates, results were expressed as odds ratios. All drugs were classified with Anatomical- Therapeutic-Chemical Classification (ATC code, level one - WHO, 2004). A total of 1000 randomly selected prescriptions were analyzed. Handwritten prescriptions with two or more drugs prescribed at wards during a one-year period (2010) were analyzed for potential DDI by using the information recorded on standard prescription forms. Only one prescription from each patient during his/her hospitalization at ward during the study period was included. The prescription forms include, patient characteristics [gender, age (more than 15 years old)], the number of drugs/prescription, drug name (generic or brand), and therapeutic drug classes. This protocol was approved by the Ethics Committee of Vice Chancellor of Research, Kerman University of Medical Sciences, Kerman, Iran (K/88/47).
DDIs were sorted by clinical relevance. Drug interactions are rated mild when they are not of clinical importance, or the effect of the interaction has not yet been established. Moderate interaction can cause possible changes in the therapeutic effects, or may cause adverse effects, but can be avoided adjusting the individual drug doses. A severe DDI is defined as drug interactions which can cause potential adverse effects; individual dose adjustment is difficult in these cases. Potential DDIs is defined as the moderate and severe drug interactions[7].
Demographic data of patients and other data of prescriptions were presented as mean± standard deviation and percentage. Student's t, χ 2(qui square) and Fisher's exact tests were performed. Probability (P) values of 0.05 or less were considered statistically significant. The data were processed and presented using EPI info statistical program software.
From 1000 prescriptions retrieved from hospital pharmacy for data analysis, at least 418 prescriptions (41.8%) were of inpatients of male ward (p=0.039). The average age of inpatients was 58.3±20.3 years old (range 15-89 years old).
The total number of medicines prescribed to the patients was 6400 with an average of 6.4±1.3 drugs per patient. The most frequent drugs prescribed were active on the cardiovascular system (38.6%). The next most common class of drugs was active on the infections and parasitic disease (25.8%); followed by drugs addressing gastrointestinal problems (18.7%), drugs active on the musculoskeletal and joint disorders (17.2%) and, finally, drugs affecting pulmonary diseases (9.6%) (Table 1).
TABLE 1.
RELATIONSHIP BETWEEN PATIENT'S AND PRESCRIPTION'S CHARACTERISTICS AND POTENTIAL DRUG-DRUG INTERACTIONS IN AN IRANIAN GENERAL HOSPITAL
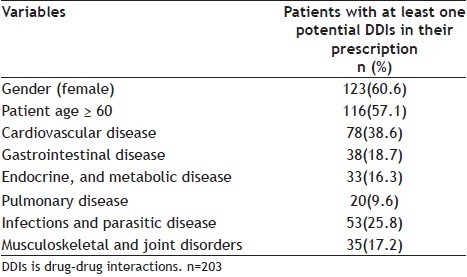
The overall prevalence of DDI was 20.3%, which was significantly higher for inpatients who were women (χ2=9.1, p=0.003) or patients who were 60 years old or more (χ2=112.1, p=0.0) (Table 1).
About 22 (10.8%) of drug interactions were recorded in patients who were prescribed drug combinations with severe DDIs that should be avoided (Table 2. Most of drug interactions were drugs combinations of mild type 92 (45.3%) followed by moderate type 89 (43.8%) (Table 2). Severe DDIs were recorded in patients prescribed drugs combating cardiovascular diseases (10.8%). There was a significant statistical difference between age groups for DDIs and most of DDIs were in patients who were 60 years old or more was significant (χ 2=177, p=0.00).
TABLE 2.
POTENTIAL DRUG-DRUG INTERACTIONS IN AN IRANIAN GENERAL HOSPITAL
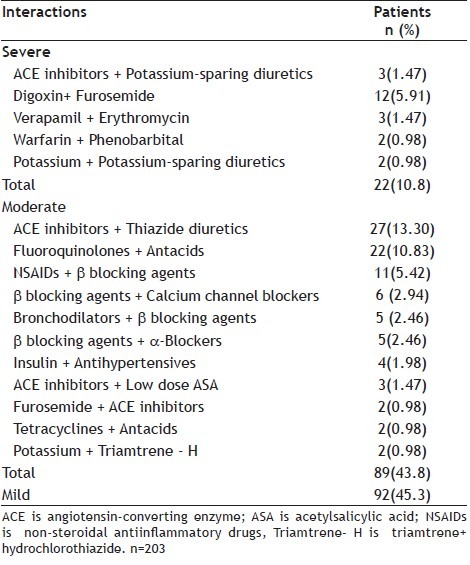
The type of drug combinations showing DDIs are shown in Table 2. The most frequent severe drug interactions were the combination of digoxin and furosemide (5.91%) followed by the combination of angiotensin converting enzyme inhibitors (ACEIs) + potassium-sparing diuretics (1.47%). The combination of ACEIs + thiazide diuretics (13.3%) was the most frequent moderate drug interactions followed by the combination of, fluoroquinolones + antacids (10.83%) and non-steroidal antiinflammatory drugs (NSAIDs) and β blocking agents (5.42%).
Various studies have shown that potential drug-drug interactions are frequent when patients receive multiple prescriptions. This is true for both ambulatory and hospitalized patients, and, in many cases, causes adverse effects and changes in therapeutic efficacies of the combined medicines, with consequent poor control of the diseases under treatment[14,15,24]. The results of this study showed that the occurrence of potential DDI was significantly higher in women and the patients aged over 60 years old, cardiology patients and having digoxin prescribed which is comparable to other studies[23,27].
In the present study, we found that the total frequency of potential drug-drug interactions (both severe and moderate DDIs) in prescriptions in general hospital in Zarand City was almost 59.1%, which is higher than the frequency in Europe[22] (46.0%) , in ambulatory patients over 59 years of age in the United States (25.0%)[25], Thailand (49.7%)[17] and a Brazilian teaching hospital (49.7%)[15]. Severe DDIs were recorded in patients prescribed drugs combating cardiovascular diseases (10.8%). The frequency of severe drug interactions (10.8%) (which should be avoided) in our work, is almost comparable with some other studies the type D (very severe DDIs) frequency was 10.0% in an European study)[22]. However it is significantly higher the value recorded in north Italy (4.7%)[29].
Digoxin and ACEIs were the most commonly prescribed drugs which showed severe and dangerous DDIs. These data are comparable with many other studies which reported that drugs with a narrow therapeutic range or low therapeutic index such as digitals and warfarin. ACEIs are more likely to be the objects for serious drug interactions, especially in elderly patients[3,23,25,27,30]. Object drugs for moderate DDIs were ACEIs (15.75%), β blocking agents (13.3%), fluoroquinolones (12.8%) and antacids (10.8%) which is comparable to some other studies[27,31–33].
Fluoroquinolones adverse effects and interactions can be considered as mild, moderate and severe, and their incidence is irrespective of the gender. Fluoroquinolones can interact with a variety of drugs: antacids, non-steroid antirheumatics, xanthines, warfarin, and others[31,33].
It is possible to conclude that the high frequency of prescription of drugs with potential drug interactions is not acceptable in clinical practice; the easiest way to reduce the frequency of them is to decrease the number of medicines prescribed. Nevertheless, sometimes it is difficult to reduce the number of drugs prescribed for patients with multiple chronic conditions; therefore, to lower the frequency of potential interactions it could be necessary to make a careful selection of therapeutic alternatives, and in cases without other options, patients should be continuously monitored to identify adverse events. It is recommended that the health professionals along with the pharmacist, has a duty to aware the nurses and the patients for the signs and risk of possible side effects.
We should declare the limitations of our study. We used administrative data. We had no direct measure of drug levels, renal function, or adherence to medications, and the accuracy of the dispensed and used drugs. Also the data shows the possible drug interactions only in internal medicine, surgery, obstetrics and gynecology departments, however, the data did not include cardiology, intensive care units and psychiatric patients which are more prone to reveal potential DDIs[16,28].
ACKNOWLEDGMENTS
This study was supported by Pharmaceutical Research Center at the Kerman University of Medical Sciences.
REFERENCES
- 1.Einarson TR. Drug-related hospital admissions. Ann Pharmacother. 1993;27:832–40. doi: 10.1177/106002809302700702. [DOI] [PubMed] [Google Scholar]
- 2.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279:1200–5. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 3.Becker ML, Kallewaard M, Caspers PW, Visser LE, Leufkens HG, Stricker BH. Hospitalisations and emergency department visits due to drug-drug interactions: a literature review. Pharmacoepidemiol Drug Saf. 2007;16:641–51. doi: 10.1002/pds.1351. [DOI] [PubMed] [Google Scholar]
- 4.Jha AK, Kuperman GJ, Rittenberg E, Teich JM, Bates DW. Identifying hospital admissions due to adverse drug events using a computer-based monitor. Pharmacoepidemiol Drug Saf. 2001;10:113–9. doi: 10.1002/pds.568. [DOI] [PubMed] [Google Scholar]
- 5.Shad MU, Marsh C, Preskorn SH. The economic consequences of a drug-drug interaction. J Clin Psychopharmacol. 2001;21:119–20. doi: 10.1097/00004714-200102000-00027. [DOI] [PubMed] [Google Scholar]
- 6.Bobb A, Gleason K, Husch M, Feinglass J, Yarnold PR, Noskin GA. The epidemiology of prescribing errors: the potential impact of computerized prescriber order entry. Arch Intern Med. 2004;164:785–92. doi: 10.1001/archinte.164.7.785. [DOI] [PubMed] [Google Scholar]
- 7.Doubova SV, Reyes-Morales H, Torres-Arreola Ldel P, Suarez-Ortega M. Potential drug-drug and drug-disease interactions in prescriptions for ambulatory patients over 50 years of age in family medicine clinics in Mexico City. BMC Health Serv Res. 2007;7:147. doi: 10.1186/1472-6963-7-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janchawee B, Owatranporn T, Mahatthanatrakul W, Chongsuvivatwong V. Clinical drug interactions in outpatients of a university hospital in Thailand. J Clin Pharm Ther. 2005;30:583–90. doi: 10.1111/j.1365-2710.2005.00688.x. [DOI] [PubMed] [Google Scholar]
- 9.Kaushal R, Bates DW, Landrigan C, McKenna KJ, Clapp MD, Federico F, et al. Medication errors and adverse drug events in pediatric inpatients. JAMA. 2001;285:2114–20. doi: 10.1001/jama.285.16.2114. [DOI] [PubMed] [Google Scholar]
- 10.Klarin I, Wimo A, Fastbom J. The association of inappropriate drug use with hospitalisation and mortality: a population-based study of the very old. Drugs Aging. 2005;22:69–82. doi: 10.2165/00002512-200522010-00005. [DOI] [PubMed] [Google Scholar]
- 11.McCabe BJ. Prevention of food-drug interactions with special emphasis on older adults. Curr Opin Clin Nutr Metab Care. 2004;7:21–6. doi: 10.1097/00075197-200401000-00005. [DOI] [PubMed] [Google Scholar]
- 12.McDonnell PJ, Jacobs MR. Hospital admissions resulting from preventable adverse drug reactions. Ann Pharmacother. 2002;36:1331–6. doi: 10.1345/aph.1A333. [DOI] [PubMed] [Google Scholar]
- 13.Peterson JF, Bates DW. Preventable medication errors: identifying and eliminating serious drug interactions. J Am Pharm Assoc (Wash) 2001;41:159–60. doi: 10.1016/s1086-5802(16)31243-8. [DOI] [PubMed] [Google Scholar]
- 14.Becker ML, Caspers PW, Kallewaard M, Bruinink RJ, Kylstra NB, Heisterkamp S, et al. Determinants of potential drug-drug interaction associated dispensing in community pharmacies in the Netherlands. Pharm World Sci. 2007;29:51–7. doi: 10.1007/s11096-006-9061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cruciol-Souza JM, Thomson JC. A pharmacoepidemiologic study of drug interactions in a Brazilian teaching hospital. Clinics. 2006;61:515–20. doi: 10.1590/s1807-59322006000600005. [DOI] [PubMed] [Google Scholar]
- 16.Heininger-Rothbucher D, Bischinger S, Ulmer H, Pechlaner C, Speer G, Wiedermann CJ. Incidence and risk of potential adverse drug interactions in the emergency room. Resuscitation. 2001;49:283–8. doi: 10.1016/s0300-9572(00)00363-4. [DOI] [PubMed] [Google Scholar]
- 17.Janchawee B, Wongpoowarak W, Owatranporn T, Chongsuvivatwong V. Pharmacoepidemiologic study of potential drug interactions in outpatients of a university hospital in Thailand. J Clin Pharm Ther. 2005;30:13–20. doi: 10.1111/j.1365-2710.2004.00598.x. [DOI] [PubMed] [Google Scholar]
- 18.Koh TW. Risk of torsades de pointes from oral erythromycin with concomitant carbimazole (methimazole) administration. Pacing Clin Electrophysiol. 2001;24:1575–6. doi: 10.1046/j.1460-9592.2001.01575.x. [DOI] [PubMed] [Google Scholar]
- 19.Monahan BP, Ferguson CL, Killeavy ES, Lloyd BK, Troy J, Cantilena LR., Jr Torsades de pointes occurring in association with terfenadine use. JAMA. 1990;264:2788–90. [PubMed] [Google Scholar]
- 20.Wynn RL. Erythromycin and ketoconazole (Nizoral) associated with terfenadine (Seldane)-induced ventricular arrhythmias. Gen Dent. 1993;41:27–9. [PubMed] [Google Scholar]
- 21.Wysowski DK, Bacsanyi J. Cisapride and fatal arrhythmia. N Engl J Med. 1996;335:290–1. doi: 10.1056/NEJM199607253350416. [DOI] [PubMed] [Google Scholar]
- 22.Bjorkman IK, Fastbom J, Schmidt IK, Bernsten CB. Drug-drug interactions in the elderly. Ann Pharmacother. 2002;36:1675–81. doi: 10.1345/aph.1A484. [DOI] [PubMed] [Google Scholar]
- 23.Cruciol-Souza JM, Thomson JC. Prevalence of potential drug-drug interactions and its associated factors in a Brazilian teaching hospital. J Pharm Pharm Sci. 2006;9:427–33. [PubMed] [Google Scholar]
- 24.Merlo J, Liedholm H, Lindblad U, Bjorck-Linne A, Falt J, Lindberg G, et al. Prescriptions with potential drug interactions dispensed at Swedish pharmacies in January 1999: Cross sectional study. BMJ. 2001;323:427–8. doi: 10.1136/bmj.323.7310.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Costa AJ. Potential drug interactions in an ambulatory geriatric population. Fam Pract. 1991;8:234–6. doi: 10.1093/fampra/8.3.234. [DOI] [PubMed] [Google Scholar]
- 26.Lindblad CI, Artz MB, Pieper CF, Sloane RJ, Hajjar ER, Ruby CM, et al. Potential drug-disease interactions in frail, hospitalized elderly veterans. Ann Pharmacother. 2005;39:412–7. doi: 10.1345/aph.1E467. [DOI] [PubMed] [Google Scholar]
- 27.Juurlink DN, Mamdani M, Kopp A, Laupacis A, Redelmeier DA. Drug-drug interactions among elderly patients hospitalized for drug toxicity. JAMA. 2003;289:1652–8. doi: 10.1001/jama.289.13.1652. [DOI] [PubMed] [Google Scholar]
- 28.Weih M, Bachmeier C, Degirmenci U, Sojer R, Kreil S, Thurauf N, et al. Assessment of Possible Drug-Drug Interactions in Psychopharmacotherapy after Hospital Discharge using an Interactive Database. Fortschr Neurol Psychiatr. 2011;79:92–6. doi: 10.1055/s-0029-1245778. [DOI] [PubMed] [Google Scholar]
- 29.Magro L, Conforti A, Del Zotti F, Leone R, Iorio ML, Meneghelli I, et al. Identification of severe potential drug-drug interactions using an Italian general-practitioner database. Eur J Clin Pharmacol. 2008;64:303–9. doi: 10.1007/s00228-007-0394-1. [DOI] [PubMed] [Google Scholar]
- 30.Kohler GI, Bode-Boger SM, Busse R, Hoopmann M, Welte T, Boger RH. Drug-drug interactions in medical patients: effects of in-hospital treatment and relation to multiple drug use. Int J Clin Pharmacol Ther. 2000;38:504–13. doi: 10.5414/cpp38504. [DOI] [PubMed] [Google Scholar]
- 31.Sepcic K, Perkovic O, Turel I, Sepcic J. [Adverse effects and interactions of fluoroquinolones] Lijec Vjesn. 2009;131:74–80. [PubMed] [Google Scholar]
- 32.Navare HA, Frye RF, Cooper-Dehoff RM, Shuster JJ, Hall K, Schmidt SO, et al. Atenolol exposure and risk for development of adverse metabolic effects: a pilot study. Pharmacotherapy. 2010;30:872–8. doi: 10.1592/phco.30.9.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ansari J. Drug interaction and pharmacist. J Young Pharm. 2010;2:326–31. doi: 10.4103/0975-1483.66807. [DOI] [PMC free article] [PubMed] [Google Scholar]


