Abstract
Multiple studies demonstrate a strong independent association between CKD and cardiovascular events including death, heart failure, and myocardial infarction. This review focuses on recent clinical studies that expand this spectrum of adverse cardiovascular events to include ventricular arrhythmias and sudden cardiac death. In addition, experimental models suggest structural remodeling of the heart and electrophysiologic changes in this population. These processes may explain the increased arrhythmic risk in kidney disease and aid in identifying patients who are at higher risk for sudden cardiac death. Finally, we review here the data to support the use of pharmacologic and device-based therapies for both the primary and secondary prevention of sudden cardiac death.
CKD affects approximately 13% of adults in the United States.1–3 Its incidence continues to rise as the population ages, and obesity, hypertension, and diabetes increase in prevalence. The elevated risk of cardiovascular morbidity and mortality in this population is well established and often precedes progression to ESRD and dialysis.4–7 Historically, the cardiovascular death associated with CKD had been attributed to the complications of atherosclerotic disease.8 A substantial proportion of cardiac deaths, however, is not directly linked to myocardial infarction (MI), stroke, or heart failure suggesting the presence of other processes contributing to cardiovascular mortality.9–11 Recently, kidney dysfunction has been evaluated as an independent risk factor for sudden cardiac death (SCD), which has been adjudicated as a distinct endpoint in various cohort studies and clinical trials. This review highlights the epidemiology of kidney disease and SCD, potential mechanisms for this association, and management strategies to reduce the burden of this fatal event.
SCD
SCD refers to an unexpected death from a cardiovascular cause with or without structural heart disease. In general, SCD events are defined as those that either are preceded by a witnessed collapse, occur within 1 hour of an acute change in clinical condition, or occur not more than 24 hours since the deceased individual was known to be in his or her usual state of health.12,13 The incidence of SCD in the United States ranges between 180,000 and 450,000 cases annually.14 Despite major advances in cardiopulmonary resuscitation15 and postresuscitation care, survival to hospital discharge after cardiac arrest remains poor.16 Survival to hospital discharge was recently estimated to be only 7.9% among out-of-hospital cardiac arrests that were treated by emergency medical services personnel.17 In addition, the majority of SCDs occur at home, where the event is often unwitnessed.18,19 The prognosis from cardiac arrests is even worse in patients with kidney dysfunction in which survival probability decreases with a declining GFR.20 Among patients with ESRD who have a witnessed cardiac arrest at an outpatient dialysis facility, more than three-quarters are not discharged alive from the hospital.21
Coronary heart disease (CHD) or congestive heart failure (CHF) markedly increases the risk of SCD in the population.22,23 Both left ventricular dysfunction and New York Heart Association functional class are important risk factors for SCD and have been incorporated as diagnostic and clinical parameters that guide the placement of implantable cardioverter-defibrillators (ICDs) for the primary prevention of SCD.24 The majority of patients who suffer a cardiac arrest, however, will not have had a left ventricular ejection fraction (LVEF) <35% documented before SCD and thus would not have qualified for an ICD.13,25,26 In order to address effectively this public health dilemma, intermediate or other vulnerable subgroups of the population need to be identified so that preventive and management strategies can be evaluated. In addition, an understanding of the mechanisms underlying SCD in well defined subgroups may help to provide additional insight into this condition across the entire population.
Nondialysis-Dependent CKD and SCD
Initial studies demonstrating an increased risk of SCD among patients with kidney disease stem from subgroup analyses of clinical trials designed to evaluate the efficacy of ICDs. The Multicenter Automatic Defibrillator Implantation Trial-II (MADIT-II), which evaluated the benefit of prophylactic ICD therapy in patients with a previous MI and a LVEF of ≤35%,27 investigated the risk of SCD among patients with CKD. Among participants treated with optimal medical therapy only, the risk for SCD was 17% higher for every 10 ml/min per 1.73 m2 decrement in the estimated GFR (eGFR).28 Similarly, in the Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure trial,29 which demonstrated the benefit of cardiac resynchronization therapy in reducing death or hospitalization in patients with advanced heart failure and conduction disease, kidney dysfunction was associated with a 67% greater risk of SCD during the 16-month follow-up period.30 Similar studies in more intermediate risk populations with CHD and without heart failure also demonstrate an independent association between kidney dysfunction and SCD (Table 1).31,32 Despite these findings, the presence of heart failure, systolic dysfunction, and/or coronary disease that were required for entry into these studies precluded an understanding of whether kidney dysfunction was a marker of severity of cardiac disease or an independent risk factor for SCD.
Table 1.
Studies evaluating an association between kidney disease and SCD
| Study (Year of Publication) | Primary Study Inclusion Criteria, (n) | Notable Exclusions | Subgroups According to Degree of Kidney Failure (n) (If Published)a | Findings | Risk for SCD |
|---|---|---|---|---|---|
| MADIT-II (2006)28 | 1223 patients | NYHA class IV, ESRD, Cr >3.0 | eGFR ≥60, n=756 | Successive 10-unit decrements in eGFR impose a 17% increased risk of SCD | High |
| LVEF ≤30% | eGFR 35–59, n=387 | ||||
| History of MI | eGFR <35, n=80 | ||||
| UPMC (2006)102 | 230 patients | None | Cr <1.0, Cr 1–1.4, Cr >1.4 | Cr >1.4 poses a 6-fold greater likelihood of appropriate ICD shock within the first year of implantation when compared with Cr <1.0 | High |
| ICD for primary or secondary prevention | |||||
| COMPANION (2006)30 | 1519 patients | Recent MI or coronary intervention | The presence of renal dysfunction was not defined | Renal dysfunction poses a 70% increased risk of SCD | High |
| LVEF ≤35% | |||||
| HERS (2008)31 | 2760 women | NYHA class III/IV, ESRD | eGFR >60, n=1027 | eGFR <40 is associated with a 3-fold increased risk of SCD relative to an eGFR >60 | Intermediate |
| History of CHDb | eGFR 40–60, n=1503 | ||||
| eGFR <40, n=230 | |||||
| DDCD (2009)32 | 19,440 patients | None | eGFR ≥60, n=14,652 | Successive 10-unit decrements in eGFR impose an 11% increased risk of SCD | Intermediate |
| History of CHDc | eGFR 15–59, n=4364 | ||||
| eGFR <15, n=175 | |||||
| CHS (2010)33 | 4465 patients, ≥65 yr | Prevalent cardiovascular diseased | Cystatin C tertile 1: eGFR ≥86, n=1579; | Successive tertiles of declining eGFR are associated with 0.1%, 0.25%, and 0.32% yearly risk of SCD, respectively | Low |
| Cystatin C tertile 2: eGFR 69–85, n=1582; | |||||
| Cystatin C tertile 3: eGFR ≤68, n=1304 |
MADIT-II, Multicenter Automated Defibrillator Implantation Trial II; NYHA, New York Heart Association; UPMC, University of Pittsburgh Medical Center; COMPANION, Comparison of Medical Therapy, Pacing and Defibrillation in Heart Failure; HERS, Heart and Estrogen/Progestin Replacement Study; DDCD, Duke Databank for Cardiovascular Disease; CHS, Cardiovascular Health Study.
Cr, creatinine (mg/dl), or eGFR, estimated GFR (ml/min per 1.73 m2).
Defined as history of MI, coronary artery bypass graft surgery, percutaneous coronary intervention, or CHD on angiography.
Defined as significant CHD on angiography.
Defined as history of MI or CHF.
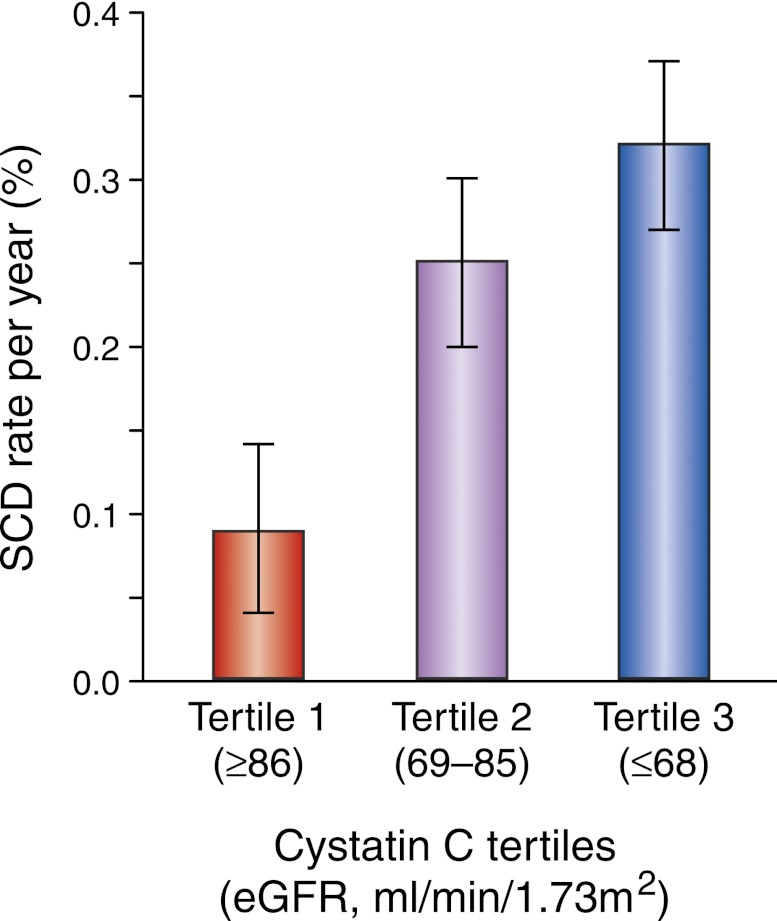
Population-based studies have attempted to understand the risk of SCD among participants with kidney disease by minimizing the confounding effects of prevalent cardiovascular disease. Among 4465 community-based participants from the Cardiovascular Health Study without a history of heart failure or MI, the incidence of SCD was >2.5-fold higher with lower levels of kidney function (Figure 1).33 Further analysis from this study also utilized both creatinine and cystatin C measures to identify a subgroup with preclinical kidney disease defined as a creatinine-based eGFR ≥60 ml/min per 1.73 m2 and cystatin C ≥1.0 mg/L. After multivariate adjustment, SCD risk is twice as likely in the preclinical kidney disease group compared with the normal kidney function referent group (creatinine-based eGFR ≥60 ml/min per 1.73 m2 and cystatin C <1.0 mg/L). These findings suggest that even mild reductions in kidney function increase risk of SCD, especially within susceptible populations such as the elderly.
Figure 1.
Cystatin C–based eGFR and SCD. The SCD rate increased across cystatin C tertiles: 0.10% per year, 0.25% per year, and 0.32% per year.33
ESRD and SCD
The majority of cardiovascular-related deaths in ESRD are attributable to SCD events.20,34 Administrative data suggest that arrhythmic deaths and cardiac arrests in ESRD patients, combined, represent approximately 22% of all deaths.20,35 Prospective, dialysis cohorts have corroborated these findings. In the Choices for Healthy Outcomes in Caring for ESRD trial, a total of 658 deaths occurred in 1041 dialysis participants over 8 years of follow-up. Among these 658 deaths, 146 were due to SCD (SCD rate of 1.8% per year).36 In addition, a high incidence of SCD over a 5-year longitudinal follow-up (SCD rate of 4.9% per year) was observed in a prospective cohort study of Chinese patients receiving chronic peritoneal dialysis.37 Despite the slight variations in annualized rates of SCD, approximately 20%–25% of all-cause mortality was attributed to SCD. This relative risk is almost identical to that reported by the US Renal Data System (USRDS) in which 25% of all-cause death among peritoneal dialysis patients and 27% of all-cause death among hemodialysis patients in the United States were attributed to cardiac arrest.20
Finally, the strong association between ESRD and SCD extends to the pediatric population as well. In a retrospective analysis of nearly 1400 deaths among ESRD patients aged 0–30 years from the USRDS, cardiac arrests and arrhythmias comprised the majority of cardiac-related deaths, which occurred at a rate >2% per year.38 These findings suggest that mechanisms aside from atherosclerotic disease and CHF are responsible for triggering fatal arrhythmias in the ESRD population.
Possible Mechanisms
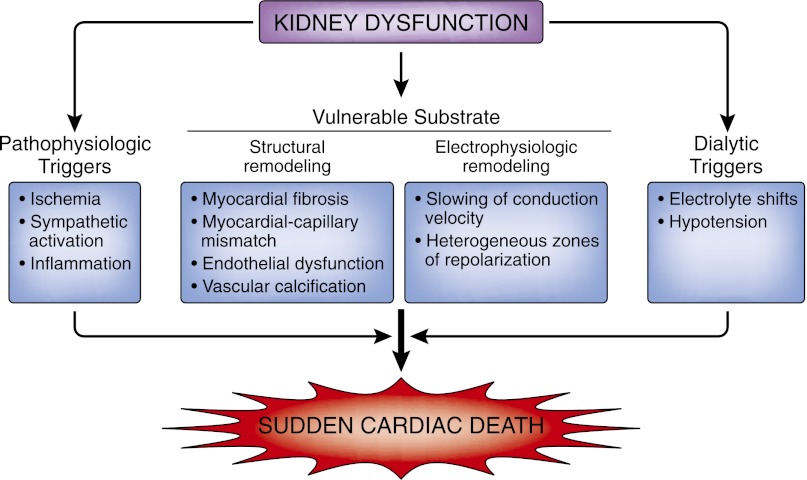
The pathophysiology of SCD is complex and is believed to require the interaction between a transient event and underlying substrate. This process induces electrical instability and ventricular arrhythmias followed by hemodynamic collapse. An understanding of the mechanisms inciting these events may help clarify when the interaction between a triggering event and the underlying substrate proves harmful. Structural and electrophysiologic remodeling of the heart, vascular calcification and fibrosis, autonomic dysregulation, and volume and electrolyte shifts are some of the underlying processes thought to explain the increased predisposition for SCD in people with CKD (Figure 2). Finally, although some of the studies supporting the proposed mechanisms discussed below have been conducted in people with CKD who are not on renal replacement therapy, the majority of data come from the ESRD population.
Figure 2.
Overview of SCD and kidney dysfunction.
Kidney disease induces adverse cardiac remodeling that includes left ventricular hypertrophy (LVH) and cardiac fibrosis. Several clinical studies, including ones that have enrolled participants with mild to moderate reductions in eGFR, demonstrate an independent association between CKD and LVH.39–42 Specifically, there is a progressive increase in the prevalence of LVH and left ventricular mass as eGFR declines. In addition, among participants with more advanced kidney disease on dialysis, contrast-enhanced magnetic resonance imaging demonstrates a diffuse pattern of gadolinium uptake suggestive of fibrosis and a nonischemic cardiomyopathy.43 The pathogenesis of these conditions is considered to be multifactorial, and the presence of commonly associated comorbidities such as hypertension, diabetes, and anemia explain only part of the left ventricular remodeling.44–46 The molecular basis for these changes includes activation of growth factors, proto-oncogenes, plasma noradrenalin, cytokines, and angiotensin II. These factors regulate intracellular processes that accelerate cardiac hypertrophy, myocardial fibrosis, and apoptosis.47,48 Both LVH and cardiac fibrosis have been implicated in increasing the risk for sustained ventricular arrhythmias and the predisposition to SCD.49–53
Kidney disease is also associated with vascular disease including calcification and stiffening of the blood vessels.54–57 Declines in eGFR and endothelial dysfunction are inter-related processes that diminish vascular elasticity and subsequently increase ischemic events. Human studies demonstrate that an impaired endothelium-dependent vasodilatory response is associated with mild kidney dysfunction.58,59 If untreated, these interdependent conditions progress and establish a cyclical relationship that results in further kidney and vascular damage. Subsequent remodeling and sclerosis of the vessels can compromise the perfusion reserve and increase the risk for ischemic events,60 which are a common trigger for the initiation of arrhythmias. In the setting of ESRD, vascular remodeling is even more pronounced because calcium-phosphate deposition may further exacerbate vascular integrity.56,61 Elevated phosphate concentrations and an increase in the calcium-phosphate product contribute to myocardial and vessel calcification as well as plaque instability, and increase SCD risk by 20%–30%.62
Structural changes can alter the electrophysiologic properties of the myocardium. Fibrosis disrupts the normal myocardial architecture and results in a slowing of conduction velocity across the diseased tissue.63 This pathology can form heterogeneous zones of conduction and repolarization that can sustain a re-entrant arrhythmia such as ventricular tachycardia.51,53,64 These structural changes impair cardiac conduction, delay ventricular activation, and create late potentials in the terminal portion of the QRS complex. Furthermore, these low amplitude signals, which can be detected using a specialized signal-averaged electrocardiographic recording, have been identified in approximately 25% of dialysis patients.65 Several studies have also evaluated QT dispersion, which reflects nonhomogeneous recovery of ventricular excitability and is calculated as the difference between the longest and shortest QT interval in a standard 12-lead electrocardiogram. The QT dispersion is maximally elevated in the postdialysis period66–68 and reflects a greater susceptibility to arrhythmias.
In addition to scar-based re-entrant arrhythmias that require heterogeneous zones of conduction, kidney dysfunction also increases the risk of automatic or trigger-based arrhythmias.69 These rhythms are sensitive to adrenergic activity. Human studies demonstrate that ESRD increases the rate of sympathetic nerve discharge, which is mediated by an afferent signal arising in diseased kidneys.70 This enhanced autonomic tone in the setting of electrophysiologic remodeling explains the basis for an increased frequency of premature ventricular complexes that occur in >75% of ESRD patients during and shortly after dialysis sessions.71 Sympathetic activity in these patients likely reflects a more serious pathophysiologic state because it correlates with an overall increased risk of death and cardiovascular events.72
Ventricular arrhythmias and SCD in ESRD patients may be related to the timing of dialysis. An inability to maintain homeostasis predisposes these patients to adverse events especially after a long interdialytic interval. Cardiac arrhythmias and SCD are more common on Mondays and Tuesdays after hemodialysis-free weekends, and during the 12 hours after initiation of an hemodialysis session.73–76 These findings suggest that major shifts in BP, electrolytes, and volume may induce triggers that result in arrhythmias.
Therapeutic Strategies to Prevent SCD
Few clinical trials have been designed specifically to evaluate the efficacy of therapeutics and interventions for SCD prevention in the CKD population. The approach to reducing SCD risk in this group relies on data obtained from other high-risk populations and subgroup analyses of clinical trials.
Pharmacologic Agents
Pharmacologic agents used to treat comorbid conditions such as hypertension, diabetes, hyperlipidemia, and coronary disease are known to reduce the risk of severe cardiovascular complications including death. Because the pathways linking kidney disease to arrhythmic events and SCD are complex and likely involve high BP, dyslipidemia, and glucose intolerance, aggressive treatment of these common conditions may protect partially these patients from SCD.
β Adrenergic Antagonists (β-Blockers)
β-blockers are antiarrhythmic and anti-ischemic agents that not only reduce the risk of hospitalizations and death but also the risk of SCD.77–79 Randomized controlled trials providing this evidence have generally excluded individuals with CKD.80,81 In a recent meta-analysis that evaluated the efficacy of β-blockers in CKD patients with heart failure, there was a 34% relative reduction in cardiovascular mortality in treated patients compared with placebo.82 Although SCD was not a separate adjudicated outcome in these analyses, the effect on cardiovascular death likely was a result of an effect on both sudden and nonsudden cardiovascular events. Limited data also exist on the effect of β-blocker therapy in CKD patients without heart failure and in patients receiving dialysis. In dialysis the hypotensive side effects of β-blockers may be exacerbated by marked fluctuations in extracellular fluid volume. Adequately powered prospective randomized control trials assessing the effects of β-blockers in a broader population of individuals with advanced CKD are necessary.
Statins
Statins lower cholesterol and have antioxidant properties that help attenuate endothelial dysfunction, prevent plaque rupture in vessel walls, and inhibit platelet aggregation and thrombus formation. Statin therapy associates with significant reductions in all-cause mortality in a series of clinical trials.83–85 These benefits were paralleled by a reduction in SCD incidence; however, limited data exist on the reduction of this specific outcome. This class of medications appears to be effective and safe for the secondary prevention of cardiovascular events in individuals with mild kidney disease. Retrospective analysis from the Cholesterol and Recurrent Events study, which was a randomized trial of pravastatin versus placebo in individuals with previous MI, identified 1711 participants (approximately 41% of the study population) with mild kidney disease.86 Pravastatin resulted in a 28% reduction in the primary endpoint of cardiovascular death or MI. Similar findings were documented in a meta-analysis that included 25,017 participants with CKD not requiring dialysis from 26 randomized controlled trials: statins decreased both the risk of all-cause mortality and cardiovascular events by nearly 20% each.87 Although SCD was not a separate, adjudicated outcome in these studies, their benefit in reducing cardiovascular mortality among higher risk participants with CHD is well established and likely reflects prevention of both sudden and nonsudden cardiac death.
Several clinical trials have evaluated the role of statins in the dialysis population. Most recently, The Study of Heart and Renal Protection (SHARP) randomized 9270 patients with CKD to ezetimibe plus simvastatin or placebo. At the time of randomization, approximately one-third of the participants were receiving maintenance dialysis.88 After a median follow-up of 4.9 years, patients randomized to an ezetimibe/ simvastatin combination experienced a 17% reduction in major atherosclerotic events compared with the placebo group. Further subgroup analysis did not demonstrate a difference in the reduction of atherosclerotic events between study participants receiving chronic dialysis and those who were not. These findings suggest that statins and a subsequent lowering of the LDL may be beneficial in a wide range of patients with CKD including those on dialysis. These results differ from two other placebo-controlled statin trials in dialysis populations that did not demonstrate a reduction in cardiovascular death, MI and stroke,89,90 although secondary analyses from the AURORA study suggest some benefit from rosuvastatin treatment in dialysis patients with diabetes.91 This failure to achieve statistical significance in primary trials may have derived from the smaller number of participants enrolled in these trials. The SHARP study also noted that statin therapy was safe in patients with kidney disease. There was no excess elevation of hepatic transaminases, hepatitis, gallstones, or pancreatitis.
Renin-Angiotensin-Aldosterone System Blockers
Dysregulation of the renin-angiotensin-aldosterone system (RAAS) comprises a fundamental abnormality in cardiorenal disorders. Experimental evidence suggests that hyperaldosteronism may both result from and contribute to kidney injury.92 Animal models with kidney damage demonstrate a 10-fold increase in plasma aldosterone concentrations that precede the onset of hypertension, proteinuria, and glomerulosclerosis. More recently, epidemiologic studies demonstrate that elevated aldosterone concentrations are an independent risk factor for SCD in patients with coronary atherosclerosis and reduced eGFR.93 These findings provide additional justification for the use of RAAS inhibitors in patients with kidney disease.
Medications that inhibit activation of RAAS are known to provide survival benefits in patients with CHF or MI.94 The benefits of angiotensin-converting enzyme inhibitors extend to patients with mild kidney dysfunction.95 In the Heart Outcomes and Prevention Evaluation study, treatment with ramipril reduced cardiovascular events, including cardiovascular mortality, to a similar extent as patients with normal kidney function. Mineralocorticoid receptor blockers such as spironolactone and eplerenone are also known to reduce SCD by 20%–30% in clinical trials enrolling patients with heart failure.96 These benefits extend to the subgroup with mild renal insufficiency; however, no trials have evaluated whether mineralocorticoid receptor blockers reduce SCD risk in patients with kidney disease alone and without heart failure.
ICDs
The American Heart Association’s recommendations for ICD implantation have evolved from identifying patients who have survived a cardiac arrest to high-risk populations for the primary prevention of SCD.24 For this later group, large clinical trials demonstrate that ICDs reduce mortality when implanted in those with clinical heart failure and a LVEF ≤35%.27,97 These clinical trials excluded participants with advanced kidney disease and ESRD; as a result, only registry-based and single-center data are available to inform ICD implantation in these patients.
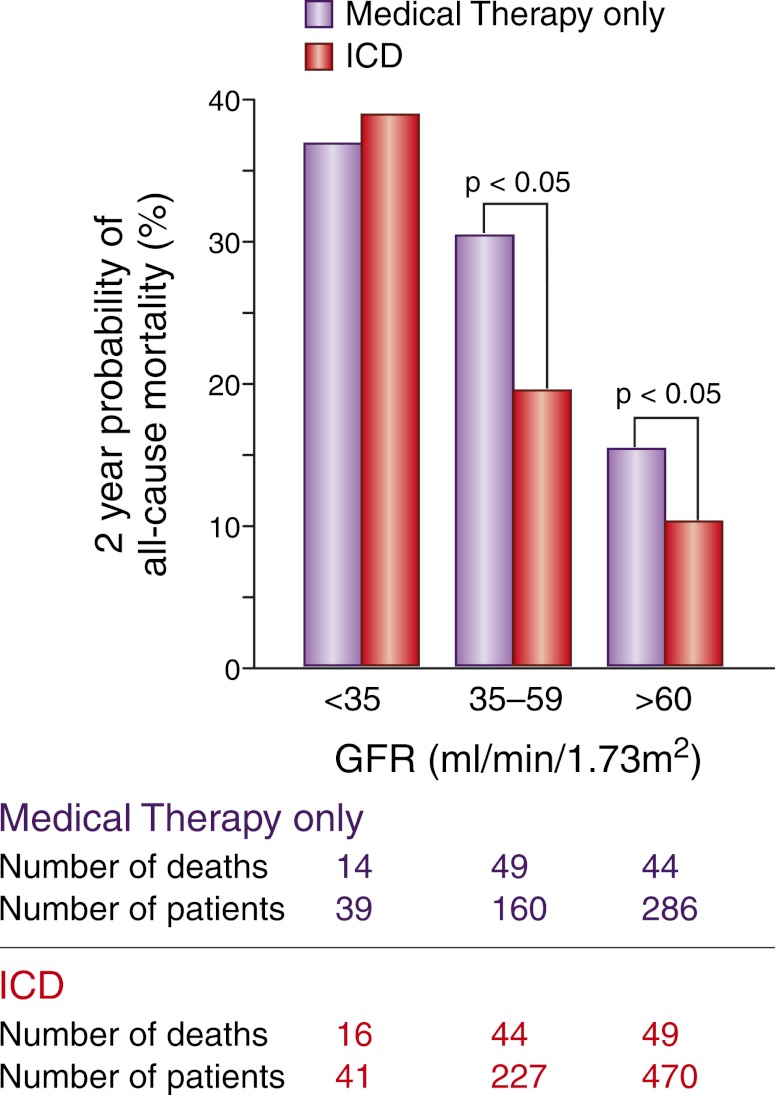
ICDs appear to reduce mortality risk in heart failure patients with CKD who are not on hemodialysis. A retrospective analysis from MADIT-II identified nearly 500 study participants (approximately 40% of the study population) with CKD. In participants with an eGFR ≥35 ml/min per 1.73 m2, the risks of all-cause mortality and SCD were significantly lower in the ICD group than in the conventional therapy group after 2 years of follow-up28 (Figure 3). No significant difference was found in either mortality or SCD risk between the two treatment groups for an eGFR <35 ml/min per 1.73 m2. The similar risk of SCD in the ICD and conventionally treated groups at an eGFR <35 ml/min per 1.73 m2 suggests reduced ICD responsiveness in this population with more advanced kidney disease.
Figure 3.
Mortality rates according to kidney function and ICD status. In this subgroup analysis from the MADIT-II study, mortality rates increased across eGFR categories in both the ICD and conventional treatment groups. In addition, mortality was significantly lower in the ICD group compared with the conventional therapy group in patients with an eGFR >35 ml/min per 1.73 m2.28
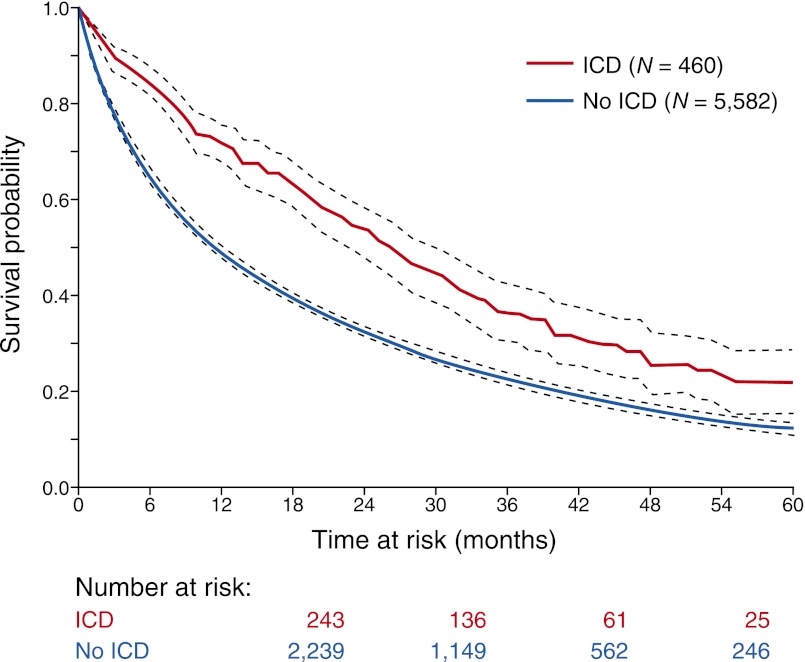
ICDs appear efficacious in dialysis patients who have survived an episode of sudden cardiac arrest.35,98 ESRD patients, identified from the Medicare database, who underwent ICD implantation within 30 days of the sudden cardiac arrest episode had a 42% reduction in death over a 5-year period than a matched sample of patients who did not undergo ICD implantation after experiencing cardiac arrest35 (Figure 4). Uncertainty, however, remains in defining the optimal use of ICD placement for primary prevention in these patients. ESRD patients have a high mortality risk even after ICD implantation,98,99 averaging 45 deaths per 100 patient-years of follow-up. According to a recent analysis from the USRDS that evaluated outcomes in nearly 10,000 dialysis patients undergoing ICD implantation over a 12-year period, the majority of cardiovascular deaths were arrhythmic.98 This group of ESRD patients was also more likely to receive appropriate therapies for ventricular fibrillation or ventricular tachycardia compared with their non-ESRD counterparts.100–102 This discordance between appropriate ICD shocks and increased arrhythmic mortality suggests that patients with severe kidney dysfunction may be intermittently refractory to ICD therapies because of the unique metabolic derangements imparted by renal failure and dialytic treatment.103,104 Defibrillation thresholds have also been reported to be higher in this group101 and may increase the risk of ICD nonresponsiveness. In addition, the high risk of all-cause mortality in ICD patients on dialysis may be explained by competing risks from other life-threatening disease process that the ICD would not be expected to effect. Finally, ESRD patients have a high risk of periprocedural complications; approximately 5% of devices became infected during each year of follow-up.98,105 This complication can result in sepsis that is refractory to antibiotic therapy, infective endocarditis, and often requires extraction of the entire ICD system.
Figure 4.
Mortality rates in ESRD patients who had a history of cardiac arrest. “Number at risk” reflects the number of patients who survived to each time interval (i.e., 12, 24, 36, and 48 months). The 1-, 2-, 3-, 4-, and 5-year survival in the ICD group was 71%, 53%, 36%, 25%, and 22%, respectively; in the no-ICD group, it was 49%, 33%, 23%, 16%, and 12% (P<0.001), respectively.35
The elevated risk for both sudden and nonsudden deaths in ESRD patients and concerns for procedural-based complications related to ICD implantation emphasize the need for prospective clinical trials. The Implantable Cardioverter Defibrillators in Dialysis Patients trial has been approved in the Netherlands and aims to evaluate whether ICD therapy in dialysis patients aged 55–80 years will reduce the risk of SCD compared with patients with no ICDs.106 Until more definitive data are available, ICD implantation in dialysis patients requires a thorough review of the individual’s history for sudden cardiac arrest, presence of heart failure, left ventricular dysfunction, functional status, and a consideration of other medical comorbidities.
This review has clarified that CKD patients are at an increased risk of sudden cardiac arrest, an outcome that is often fatal and carries a poor prognosis. Structural and electrophysiologic remodeling of the heart, vascular disease, and autonomic dysregulation are some of the underlying processes identified to explain the increased predisposition for SCD in people with CKD. Translational studies provide some insights into potential targets for identifying CKD patients who may be at higher risk for SCD events. Future studies will need to identify novel approaches toward arrhythmic risk stratification in CKD patients and evaluate whether treatment with medical and interventional therapies is effective and safe.
Disclosures
None.
Acknowledgments
R.D. is supported by National Institutes of Health Grant K23DK089118.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, Eckardt KU, Nahas ME, Jaber BL, Jadoul M, Levin A, Powe NR, Rossert J, Wheeler DC, Lameire N, Eknoyan G: Chronic kidney disease as a global public health problem: Approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int 72: 247–259, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Nissenson AR, Pereira BJ, Collins AJ, Steinberg EP: Prevalence and characteristics of individuals with chronic kidney disease in a large health maintenance organization. Am J Kidney Dis 37: 1177–1183, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Coresh J, Byrd-Holt D, Astor BC, Briggs JP, Eggers PW, Lacher DA, Hostetter TH: Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. J Am Soc Nephrol 16: 180–188, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH: Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 164: 659–663, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Foley RN, Parfrey PS, Sarnak MJ: Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol 9[Suppl]: S16–S23, 1998 [PubMed] [Google Scholar]
- 6.Parfrey PS, Foley RN: The clinical epidemiology of cardiac disease in chronic renal failure. J Am Soc Nephrol 10: 1606–1615, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Muntner P, He J, Hamm L, Loria C, Whelton PK: Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J Am Soc Nephrol 13: 745–753, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Lindner A, Charra B, Sherrard DJ, Scribner BH: Accelerated atherosclerosis in prolonged maintenance hemodialysis. N Engl J Med 290: 697–701, 1974 [DOI] [PubMed] [Google Scholar]
- 9.Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, Siscovick DS, Stehman-Breen C: Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med 352: 2049–2060, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Sarnak MJ, Katz R, Stehman-Breen CO, Fried LF, Jenny NS, Psaty BM, Newman AB, Siscovick D, Shlipak MG, Cardiovascular Health Study : Cystatin C concentration as a risk factor for heart failure in older adults. Ann Intern Med 142: 497–505, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Deo R, Fyr CL, Fried LF, Newman AB, Harris TB, Angleman S, Green C, Kritchevsky SB, Chertow GM, Cummings SR, Shlipak MG, Health ABC study : Kidney dysfunction and fatal cardiovascular disease—an association independent of atherosclerotic events: Results from the Health, Aging, and Body Composition (Health ABC) study. Am Heart J 155: 62–68, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Fishman GI, Chugh SS, Dimarco JP, Albert CM, Anderson ME, Bonow RO, Buxton AE, Chen PS, Estes M, Jouven X, Kwong R, Lathrop DA, Mascette AM, Nerbonne JM, O’Rourke B, Page RL, Roden DM, Rosenbaum DS, Sotoodehnia N, Trayanova NA, Zheng ZJ: Sudden cardiac death prediction and prevention: Report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation 122: 2335–2348, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, Priori SG, Quinones MA, Roden DM, Silka MJ, Tracy C, Smith SC, Jr, Jacobs AK, Adams CD, Antman EM, Anderson JL, Hunt SA, Halperin JL, Nishimura R, Ornato JP, Page RL, Riegel B, Blanc JJ, Budaj A, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL, American College of Cardiology/American Heart Association Task Force. European Society of Cardiology Committee for Practice Guidelines. European Heart Rhythm Association. Heart Rhythm Society : ACC/AHA/ESC 2006 Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death): Developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation 114: e385–e484, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Roger VL, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J, American Heart Association Statistics Committee and Stroke Statistics Subcommittee : Heart disease and stroke statistics—2010 update: A report from the American Heart Association. Circulation 121: e46–e215, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Field JM, Hazinski MF, Sayre MR, Chameides L, Schexnayder SM, Hemphill R, Samson RA, Kattwinkel J, Berg RA, Bhanji F, Cave DM, Jauch EC, Kudenchuk PJ, Neumar RW, Peberdy MA, Perlman JM, Sinz E, Travers AH, Berg MD, Billi JE, Eigel B, Hickey RW, Kleinman ME, Link MS, Morrison LJ, O’Connor RE, Shuster M, Callaway CW, Cucchiara B, Ferguson JD, Rea TD, Vanden Hoek TL: Part 1: Executive summary: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 122[Suppl 3]: S640–S656, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Hallstrom AP, Ornato JP, Weisfeldt M, Travers A, Christenson J, McBurnie MA, Zalenski R, Becker LB, Schron EB, Proschan M, Public Access Defibrillation Trial Investigators : Public-access defibrillation and survival after out-of-hospital cardiac arrest. N Engl J Med 351: 637–646, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP, Rea T, Lowe R, Brown T, Dreyer J, Davis D, Idris A, Stiell I, Resuscitation Outcomes Consortium Investigators : Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA 300: 1423–1431, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Vreede-Swagemakers JJ, Gorgels AP, Dubois-Arbouw WI, van Ree JW, Daemen MJ, Houben LG, Wellens HJ: Out-of-hospital cardiac arrest in the 1990’s: A population-based study in the Maastricht area on incidence, characteristics and survival. J Am Coll Cardiol 30: 1500–1505, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Straus SM, Bleumink GS, Dieleman JP, van der Lei J, Stricker BH, Sturkenboom MC: The incidence of sudden cardiac death in the general population. J Clin Epidemiol 57: 98–102, 2004 [DOI] [PubMed] [Google Scholar]
- 20.National Institute of Diabetes and Digestive and Kidney Diseases : 2006 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, Cardiovascular Special Studies, 2006 [Google Scholar]
- 21.Davis TR, Young BA, Eisenberg MS, Rea TD, Copass MK, Cobb LA: Outcome of cardiac arrests attended by emergency medical services staff at community outpatient dialysis centers. Kidney Int 73: 933–939, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Kannel WB, Cupples LA, D’Agostino RB: Sudden death risk in overt coronary heart disease: The Framingham Study. Am Heart J 113: 799–804, 1987 [DOI] [PubMed] [Google Scholar]
- 23.Stevenson WG, Stevenson LW, Middlekauff HR, Saxon LA: Sudden death prevention in patients with advanced ventricular dysfunction. Circulation 88: 2953–2961, 1993 [DOI] [PubMed] [Google Scholar]
- 24.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Faxon DP, Halperin JL, Hiratzka LF, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura RA, Ornato JP, Page RL, Riegel B, Tarkington LG, Yancy CW, American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) American Association for Thoracic Surgery. Society of Thoracic Surgeons : ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): Developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation 117: e350–e408, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Myerberg R, Castellanos A: Cardiac arrest and sudden death. In: Heart Disease: A Textbook of Cardiovascular Medicine, edited by Braunwald E, Philadelphia, Saunders, 1997, pp 742–779 [Google Scholar]
- 26.Stecker EC, Vickers C, Waltz J, Socoteanu C, John BT, Mariani R, McAnulty JH, Gunson K, Jui J, Chugh SS: Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: Two-year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol 47: 1161–1166, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML, Multicenter Automatic Defibrillator Implantation Trial II Investigators : Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 346: 877–883, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Goldenberg I, Moss AJ, McNitt S, Zareba W, Andrews ML, Hall WJ, Greenberg H, Case RB, Multicenter Automatic Defibrillator Implantation Trial-II Investigators : Relations among renal function, risk of sudden cardiac death, and benefit of the implanted cardiac defibrillator in patients with ischemic left ventricular dysfunction. Am J Cardiol 98: 485–490, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM, Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators : Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 350: 2140–2150, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Saxon LA, Bristow MR, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, Feldman AM, Galle E, Ecklund F: Predictors of sudden cardiac death and appropriate shock in the Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Trial. Circulation 114: 2766–2772, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Deo R, Lin F, Vittinghoff E, Tseng ZH, Hulley SB, Shlipak MG: Kidney dysfunction and sudden cardiac death among women with coronary heart disease. Hypertension 51: 1578–1582, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Pun PH, Smarz TR, Honeycutt EF, Shaw LK, Al-Khatib SM, Middleton JP: Chronic kidney disease is associated with increased risk of sudden cardiac death among patients with coronary artery disease. Kidney Int 76: 652–658, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deo R, Sotoodehnia N, Katz R, Sarnak MJ, Fried LF, Chonchol M, Kestenbaum B, Psaty BM, Siscovick DS, Shlipak MG: Cystatin C and sudden cardiac death risk in the elderly. Circ Cardiovasc Qual Outcomes 3: 159–164, 2010 [DOI] [PMC free article] [PubMed]
- 34.Levey AS, Beto JA, Coronado BE, Eknoyan G, Foley RN, Kasiske BL, Klag MJ, Mailloux LU, Manske CL, Meyer KB, Parfrey PS, Pfeffer MA, Wenger NK, Wilson PW, Wright JT, Jr: Controlling the epidemic of cardiovascular disease in chronic renal disease: What do we know? What do we need to learn? Where do we go from here? National Kidney Foundation Task Force on Cardiovascular Disease. Am J Kidney Dis 32: 853–906, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Herzog CA, Li S, Weinhandl ED, Strief JW, Collins AJ, Gilbertson DT: Survival of dialysis patients after cardiac arrest and the impact of implantable cardioverter defibrillators. Kidney Int 68: 818–825, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Parekh RS, Plantinga LC, Kao WH, Meoni LA, Jaar BG, Fink NE, Powe NR, Coresh J, Klag MJ: The association of sudden cardiac death with inflammation and other traditional risk factors. Kidney Int 74: 1335–1342, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Wang AY, Lam CW, Chan IH, Wang M, Lui SF, Sanderson JE: Sudden cardiac death in end-stage renal disease patients: A 5-year prospective analysis. Hypertension 56: 210–216, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Parekh RS, Carroll CE, Wolfe RA, Port FK: Cardiovascular mortality in children and young adults with end-stage kidney disease. J Pediatr 141: 191–197, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Cerasola G, Nardi E, Mulè G, Palermo A, Cusimano P, Guarneri M, Arsena R, Giammarresi G, Carola Foraci A, Cottone S: Left ventricular mass in hypertensive patients with mild-to-moderate reduction of renal function. Nephrology (Carlton) 15: 203–210, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Levin A, Thompson CR, Ethier J, Carlisle EJ, Tobe S, Mendelssohn D, Burgess E, Jindal K, Barrett B, Singer J, Djurdjev O: Left ventricular mass index increase in early renal disease: Impact of decline in hemoglobin. Am J Kidney Dis 34: 125–134, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Paoletti E, Bellino D, Cassottana P, Rolla D, Cannella G: Left ventricular hypertrophy in nondiabetic predialysis CKD. Am J Kidney Dis 46: 320–327, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Moran A, Katz R, Jenny NS, Astor B, Bluemke DA, Lima JA, Siscovick D, Bertoni AG, Shlipak MG: Left ventricular hypertrophy in mild and moderate reduction in kidney function determined using cardiac magnetic resonance imaging and cystatin C: The multi-ethnic study of atherosclerosis (MESA). Am J Kidney Dis 52: 839–848, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mark PB, Johnston N, Groenning BA, Foster JE, Blyth KG, Martin TN, Steedman T, Dargie HJ, Jardine AG: Redefinition of uremic cardiomyopathy by contrast-enhanced cardiac magnetic resonance imaging. Kidney Int 69: 1839–1845, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Cioffi G, Tarantini L, Frizzi R, Stefenelli C, Russo TE, Selmi A, Toller C, Furlanello F, de Simone G: Chronic kidney disease elicits excessive increase in left ventricular mass growth in patients at increased risk for cardiovascular events. J Hypertens 29: 565–573, 2011 [DOI] [PubMed] [Google Scholar]
- 45.Schroeder AP, Kristensen BO, Nielsen CB, Pedersen EB: Heart function in patients with chronic glomerulonephritis and mildly to moderately impaired renal function. An echocardiographic study. Blood Press 6: 286–293, 1997 [DOI] [PubMed] [Google Scholar]
- 46.Hunter JJ, Chien KR: Signaling pathways for cardiac hypertrophy and failure. N Engl J Med 341: 1276–1283, 1999 [DOI] [PubMed] [Google Scholar]
- 47.Amann K, Kronenberg G, Gehlen F, Wessels S, Orth S, Münter K, Ehmke H, Mall G, Ritz E: Cardiac remodelling in experimental renal failure—an immunohistochemical study. Nephrol Dial Transplant 13: 1958–1966, 1998 [DOI] [PubMed] [Google Scholar]
- 48.Mall G, Huther W, Schneider J, Lundin P, Ritz E: Diffuse intermyocardiocytic fibrosis in uraemic patients. Nephrol Dial Transplant 5: 39–44, 1990 [DOI] [PubMed] [Google Scholar]
- 49.Haider AW, Larson MG, Benjamin EJ, Levy D: Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol 32: 1454–1459, 1998 [DOI] [PubMed] [Google Scholar]
- 50.Reinier K, Dervan C, Singh T, Uy-Evanado A, Lai S, Gunson K, Jui J, Chugh SS: Increased left ventricular mass and decreased left ventricular systolic function have independent pathways to ventricular arrhythmogenesis in coronary artery disease. Heart Rhythm 8: 1177–1182, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yan AT, Shayne AJ, Brown KA, Gupta SN, Chan CW, Luu TM, Di Carli MF, Reynolds HG, Stevenson WG, Kwong RY: Characterization of the peri-infarct zone by contrast-enhanced cardiac magnetic resonance imaging is a powerful predictor of post-myocardial infarction mortality. Circulation 114: 32–39, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Schmieder RE, Hilgers KF, Schlaich MP, Schmidt BM: Renin-angiotensin system and cardiovascular risk. Lancet 369: 1208–1219, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Roes SD, Borleffs CJ, van der Geest RJ, Westenberg JJ, Marsan NA, Kaandorp TA, Reiber JH, Zeppenfeld K, Lamb HJ, de Roos A, Schalij MJ, Bax JJ: Infarct tissue heterogeneity assessed with contrast-enhanced MRI predicts spontaneous ventricular arrhythmia in patients with ischemic cardiomyopathy and implantable cardioverter-defibrillator. Circ Cardiovasc Imaging 2: 183–190, 2009 [DOI] [PubMed] [Google Scholar]
- 54.Pai AS, Giachelli CM: Matrix remodeling in vascular calcification associated with chronic kidney disease. J Am Soc Nephrol 21: 1637–1640, 2010 [DOI] [PubMed] [Google Scholar]
- 55.Briet M, Collin C, Karras A, Laurent S, Bozec E, Jacquot C, Stengel B, Houillier P, Froissart M, Boutouyrie P, Nephrotest Study Group : Arterial remodeling associates with CKD progression. J Am Soc Nephrol 22: 967–974, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu MC, Shi M, Zhang J, Quiñones H, Griffith C, Kuro-o M, Moe OW: Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol 22: 124–136, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shroff R, Shanahan CM: Klotho: An elixir of youth for the vasculature? J Am Soc Nephrol 22: 5–7, 2011 [DOI] [PubMed] [Google Scholar]
- 58.Perticone F, Maio R, Tripepi G, Zoccali C: Endothelial dysfunction and mild renal insufficiency in essential hypertension. Circulation 110: 821–825, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Perticone F, Maio R, Perticone M, Sciacqua A, Shehaj E, Naccarato P, Sesti G: Endothelial dysfunction and subsequent decline in glomerular filtration rate in hypertensive patients. Circulation 122: 379–384, 2010 [DOI] [PubMed] [Google Scholar]
- 60.Kingwell BA, Waddell TK, Medley TL, Cameron JD, Dart AM: Large artery stiffness predicts ischemic threshold in patients with coronary artery disease. J Am Coll Cardiol 40: 773–779, 2002 [DOI] [PubMed] [Google Scholar]
- 61.Schlieper G, Aretz A, Verberckmoes SC, Krüger T, Behets GJ, Ghadimi R, Weirich TE, Rohrmann D, Langer S, Tordoir JH, Amann K, Westenfeld R, Brandenburg VM, D’Haese PC, Mayer J, Ketteler M, McKee MD, Floege J: Ultrastructural analysis of vascular calcifications in uremia. J Am Soc Nephrol 21: 689–696, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK: Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol 12: 2131–2138, 2001 [DOI] [PubMed] [Google Scholar]
- 63.Waldo AL, Plumb VJ, Arciniegas JG, MacLean WA, Cooper TB, Priest MF, James TN: Transient entrainment and interruption of the atrioventricular bypass pathway type of paroxysmal atrial tachycardia. A model for understanding and identifying reentrant arrhythmias. Circulation 67: 73–83, 1983 [DOI] [PubMed] [Google Scholar]
- 64.Schmidt A, Azevedo CF, Cheng A, Gupta SN, Bluemke DA, Foo TK, Gerstenblith G, Weiss RG, Marbán E, Tomaselli GF, Lima JA, Wu KC: Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation 115: 2006–2014, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morales MA, Gremigni C, Dattolo P, Piacenti M, Cerrai T, Fazi A, Pelosi G, Vergassola R, Maggiore Q: Signal-averaged ECG abnormalities in haemodialysis patients. Role of dialysis. Nephrol Dial Transplant 13: 668–673, 1998 [DOI] [PubMed] [Google Scholar]
- 66.Patel RK, Mark PB, Halliday C, Steedman T, Dargie HJ, Cobbe SM, Jardine AG: Microvolt T-wave alternans in end-stage renal disease patients—associations with uremic cardiomyopathy. Clin J Am Soc Nephrol 6: 519–527, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morris ST, Galiatsou E, Stewart GA, Rodger RS, Jardine AG: QT dispersion before and after hemodialysis. J Am Soc Nephrol 10: 160–163, 1999 [DOI] [PubMed] [Google Scholar]
- 68.Lorincz I, Mátyus J, Zilahi Z, Kun C, Karányi Z, Kakuk G: QT dispersion in patients with end-stage renal failure and during hemodialysis. J Am Soc Nephrol 10: 1297–1302, 1999 [DOI] [PubMed] [Google Scholar]
- 69.Brotman DJ, Bash LD, Qayyum R, Crews D, Whitsel EA, Astor BC, Coresh J: Heart rate variability predicts ESRD and CKD-related hospitalization. J Am Soc Nephrol 21: 1560–1570, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Converse RL, Jr, Jacobsen TN, Toto RD, Jost CM, Cosentino F, Fouad-Tarazi F, Victor RG: Sympathetic overactivity in patients with chronic renal failure. N Engl J Med 327: 1912–1918, 1992 [DOI] [PubMed] [Google Scholar]
- 71.Multicentre, cross-sectional study of ventricular arrhythmias in chronically haemodialysed patients. Gruppo Emodialisi e Patologie Cardiovasculari. Lancet 2: 305–309, 1988 [PubMed] [Google Scholar]
- 72.Zoccali C, Mallamaci F, Parlongo S, Cutrupi S, Benedetto FA, Tripepi G, Bonanno G, Rapisarda F, Fatuzzo P, Seminara G, Cataliotti A, Stancanelli B, Malatino LS: Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end-stage renal disease. Circulation 105: 1354–1359, 2002 [DOI] [PubMed] [Google Scholar]
- 73.Bleyer AJ, Russell GB, Satko SG: Sudden and cardiac death rates in hemodialysis patients. Kidney Int 55: 1553–1559, 1999 [DOI] [PubMed] [Google Scholar]
- 74.Bleyer AJ, Hartman J, Brannon PC, Reeves-Daniel A, Satko SG, Russell G: Characteristics of sudden death in hemodialysis patients. Kidney Int 69: 2268–2273, 2006 [DOI] [PubMed] [Google Scholar]
- 75.Foley RN, Gilbertson DT, Murray T, Collins AJ: Long interdialytic interval and mortality among patients receiving hemodialysis. N Engl J Med 365: 1099–1107, 2011 [DOI] [PubMed] [Google Scholar]
- 76.Perl J, Chan CT: Timing of sudden death relative to the hemodialysis procedure. Nat Clin Pract Nephrol 2: 668–669, 2006 [DOI] [PubMed] [Google Scholar]
- 77.The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): A randomised trial. Lancet 353: 9–13, 1999 [PubMed] [Google Scholar]
- 78.Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 353: 2001–2007, 1999 [PubMed] [Google Scholar]
- 79.Brophy JM, Joseph L, Rouleau JL: Beta-blockers in congestive heart failure. A Bayesian meta-analysis. Ann Intern Med 134: 550–560, 2001 [DOI] [PubMed] [Google Scholar]
- 80.Charytan D, Kuntz RE: The exclusion of patients with chronic kidney disease from clinical trials in coronary artery disease. Kidney Int 70: 2021–2030, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Coca SG, Krumholz HM, Garg AX, Parikh CR: Underrepresentation of renal disease in randomized controlled trials of cardiovascular disease. JAMA 296: 1377–1384, 2006 [DOI] [PubMed] [Google Scholar]
- 82.Badve SV, Roberts MA, Hawley CM, Cass A, Garg AX, Krum H, Tonkin A, Perkovic V: Effects of beta-adrenergic antagonists in patients with chronic kidney disease: A systematic review and meta-analysis. J Am Coll Cardiol 58: 1152–1161, 2011 [DOI] [PubMed] [Google Scholar]
- 83.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S). Lancet 344: 1383–1389, 1994 [PubMed] [Google Scholar]
- 84.Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, McKillop JH, Packard CJ, West of Scotland Coronary Prevention Study Group : Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med 333: 1301–1307, 1995 [DOI] [PubMed] [Google Scholar]
- 85.The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group : Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 339: 1349–1357, 1998 [DOI] [PubMed] [Google Scholar]
- 86.Tonelli M, Moyé L, Sacks FM, Kiberd B, Curhan G, Cholesterol and Recurrent Events (CARE) Trial Investigators : Pravastatin for secondary prevention of cardiovascular events in persons with mild chronic renal insufficiency. Ann Intern Med 138: 98–104, 2003 [DOI] [PubMed] [Google Scholar]
- 87.Navaneethan SD, Pansini F, Perkovic V, Manno C, Pellegrini F, Johnson DW, Craig JC, Strippoli GF: HMG CoA reductase inhibitors (statins) for people with chronic kidney disease not requiring dialysis. Cochrane Database Syst Rev (2): CD007784, 2009 [DOI] [PubMed] [Google Scholar]
- 88.Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J, Neal B, Jiang L, Hooi LS, Levin A, Agodoa L, Gaziano M, Kasiske B, Walker R, Massy ZA, Feldt-Rasmussen B, Krairittichai U, Ophascharoensuk V, Fellström B, Holdaas H, Tesar V, Wiecek A, Grobbee D, de Zeeuw D, Grönhagen-Riska C, Dasgupta T, Lewis D, Herrington W, Mafham M, Majoni W, Wallendszus K, Grimm R, Pedersen T, Tobert J, Armitage J, Baxter A, Bray C, Chen Y, Chen Z, Hill M, Knott C, Parish S, Simpson D, Sleight P, Young A, Collins R, SHARP Investigators : The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): A randomised placebo-controlled trial. Lancet 377: 2181–2192, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wanner C, Krane V, März W, Olschewski M, Mann JF, Ruf G, Ritz E, German Diabetes and Dialysis Study Investigators : Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 353: 238–248, 2005 [DOI] [PubMed] [Google Scholar]
- 90.Fellström BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, Chae DW, Chevaile A, Cobbe SM, Grönhagen-Riska C, De Lima JJ, Lins R, Mayer G, McMahon AW, Parving HH, Remuzzi G, Samuelsson O, Sonkodi S, Sci D, Süleymanlar G, Tsakiris D, Tesar V, Todorov V, Wiecek A, Wüthrich RP, Gottlow M, Johnsson E, Zannad F, AURORA Study Group : Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med 360: 1395–1407, 2009 [DOI] [PubMed] [Google Scholar]
- 91.Holdaas H, Holme I, Schmieder RE, Jardine AG, Zannad F, Norby GE, Fellström BC, AURORA study group : Rosuvastatin in diabetic hemodialysis patients. J Am Soc Nephrol 22: 1335–1341, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Greene EL, Kren S, Hostetter TH: Role of aldosterone in the remnant kidney model in the rat. J Clin Invest 98: 1063–1068, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tomaschitz A, Pilz S, Ritz E, Grammer T, Drechsler C, Boehm BO, März W: Association of plasma aldosterone with cardiovascular mortality in patients with low estimated GFR: The Ludwigshafen Risk and Cardiovascular Health (LURIC) Study. Am J Kidney Dis 57: 403–414, 2011 [DOI] [PubMed] [Google Scholar]
- 94.Domanski MJ, Exner DV, Borkowf CB, Geller NL, Rosenberg Y, Pfeffer MA: Effect of angiotensin converting enzyme inhibition on sudden cardiac death in patients following acute myocardial infarction. A meta-analysis of randomized clinical trials. J Am Coll Cardiol 33: 598–604, 1999 [DOI] [PubMed] [Google Scholar]
- 95.Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S: Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: The HOPE randomized trial. Ann Intern Med 134: 629–636, 2001 [DOI] [PubMed] [Google Scholar]
- 96.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J, Randomized Aldactone Evaluation Study Investigators : The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 341: 709–717, 1999 [DOI] [PubMed] [Google Scholar]
- 97.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH, Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators : Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 352: 225–237, 2005 [DOI] [PubMed] [Google Scholar]
- 98.Charytan DM, Patrick AR, Liu J, Setoguchi S, Herzog CA, Brookhart MA, Winkelmayer WC: Trends in the use and outcomes of implantable cardioverter-defibrillators in patients undergoing dialysis in the United States. Am J Kidney Dis 58: 409–417, 2011 [DOI] [PubMed] [Google Scholar]
- 99.Sakhuja R, Keebler M, Lai TS, McLaughlin Gavin C, Thakur R, Bhatt DL: Meta-analysis of mortality in dialysis patients with an implantable cardioverter defibrillator. Am J Cardiol 103: 735–741, 2009 [DOI] [PubMed] [Google Scholar]
- 100.Robin J, Weinberg K, Tiongson J, Carnethon M, Reddy M, Ciaccio C, Quadrini M, Hsu J, Fan J, Choi P, Kadish A, Goldberger J, Passman R: Renal dialysis as a risk factor for appropriate therapies and mortality in implantable cardioverter-defibrillator recipients. Heart Rhythm 3: 1196–1201, 2006 [DOI] [PubMed] [Google Scholar]
- 101.Wase A, Basit A, Nazir R, Jamal A, Shah S, Khan T, Mohiuddin I, White C, Saklayen M, McCullough PA: Impact of chronic kidney disease upon survival among implantable cardioverter-defibrillator recipients. J Interv Card Electrophysiol 11: 199–204, 2004 [DOI] [PubMed] [Google Scholar]
- 102.Hreybe H, Ezzeddine R, Bedi M, Barrington W, Bazaz R, Ganz LI, Jain S, Ngwu O, London B, Saba S: Renal insufficiency predicts the time to first appropriate defibrillator shock. Am Heart J 151: 852–856, 2006 [DOI] [PubMed] [Google Scholar]
- 103.Sims JJ, Miller AW, Ujhelyi MR: Regional hyperkalemia increases ventricular defibrillation energy requirements: Role of electrical heterogeneity in defibrillation. J Cardiovasc Electrophysiol 11: 634–641, 2000 [DOI] [PubMed] [Google Scholar]
- 104.Ujhelyi MR, Sims JJ, Miller AW: Induction of electrical heterogeneity impairs ventricular defibrillation: An effect specific to regional conduction velocity slowing. Circulation 100: 2534–2540, 1999 [DOI] [PubMed] [Google Scholar]
- 105.Aggarwal A, Wang Y, Rumsfeld JS, Curtis JP, Heidenreich PA, National Cardiovascular Data Registry : Clinical characteristics and in-hospital outcome of patients with end-stage renal disease on dialysis referred for implantable cardioverter-defibrillator implantation. Heart Rhythm 6: 1565–1571, 2009 [DOI] [PubMed] [Google Scholar]
- 106.de Bie MK, van Dam B, Gaasbeek A, van Buren M, van Erven L, Bax JJ, Schalij MJ, Rabelink TJ, Jukema JW: The current status of interventions aiming at reducing sudden cardiac death in dialysis patients. Eur Heart J 30: 1559–1564, 2009 [DOI] [PubMed] [Google Scholar]