Abstract
Overexpression of soluble urokinase receptor (suPAR) causes pathology in animal models similar to primary FSGS, and one recent study demonstrated elevated levels of serum suPAR in patients with the disease. Here, we analyzed circulating suPAR levels in two cohorts of children and adults with biopsy-proven primary FSGS: 70 patients from the North America–based FSGS clinical trial (CT) and 94 patients from PodoNet, the Europe-based consortium studying steroid-resistant nephrotic syndrome. Circulating suPAR levels were elevated in 84.3% and 55.3% of patients with FSGS patients in the CT and PodoNet cohorts, respectively, compared with 6% of controls (P<0.0001); inflammation did not account for this difference. Multiple regression analysis suggested that lower suPAR levels associated with higher estimated GFR, male sex, and treatment with mycophenolate mofetil. In the CT cohort, there was a positive association between the relative reduction of suPAR after 26 weeks of treatment and reduction of proteinuria, with higher odds for complete remission (P=0.04). In the PodoNet cohort, patients with an NPHS2 mutation had higher suPAR levels than those without a mutation. In conclusion, suPAR levels are elevated in geographically and ethnically diverse patients with FSGS and do not reflect a nonspecific proinflammatory milieu. The associations between a change in circulating suPAR with different therapeutic regimens and with remission support the role of suPAR in the pathogenesis of FSGS.
FSGS is an important cause of ESRD in children and adults.1–3 It can occur as a primary disorder, consequent to genetic mutations in specific podocyte proteins, secondary to a wide range of medical problems, or as a consequence of adaptive changes to reduced kidney mass.4,5 Intensive research for >50 years has focused mainly on genetic, immunologic, and systemic causes of FSGS but the cause of primary disease remains elusive.
In recent years, there has been a great deal of investigation into the role of permeability factors in the pathogenesis of proteinuria and the development of primary FSGS. Proposed candidate molecules include hemopexin, vascular endothelial growth factor, and cardiotrophin-like cytokine-1.6–8 However, the detection of increased levels of most circulating permeability factors is generally not specific for a given glomerular disorder, and the activity or expression of these factors may be increased in patients with FSGS and minimal change nephrotic syndrome, two disorders with markedly disparate responses to therapy and long-term prognosis.9
Soluble urokinase receptor (suPAR), the soluble form of urokinase plasminogen-type activator receptor, has been reported to be elevated in a number of diseases including cancer and infection in a nonspecific manner. However, its underlying mechanism of action in these conditions and its clinical value either in diagnosis or prognosis is still far from clear.10 Recently, Wei et al. reported the molecular identity of a putative permeability factor in FSGS.11 Approximately two-thirds of patients with primary FSGS had increased suPAR. Moreover, the circulating suPAR concentration was mostly normal in other forms of primary glomerular diseases including minimal change nephrotic syndrome, idiopathic membranous nephropathy, or preeclampsia.11 The investigators related the abnormal suPAR level to the pathogenesis of FSGS based on both in vitro and in vivo studies, which demonstrated suPAR binding to and, more importantly, activation of β3 integrin on podocytes. This interaction led to alterations in the morphology (foot process effacement) and function of podocytes, which resulted in proteinuria and initiation of FSGS.11
The prevalence of elevated circulating suPAR levels in patients with primary FSGS has not been systematically evaluated in any other large patient cohort. In addition, it is unclear if the circulating suPAR is modulated by immunosuppressive therapy. Therefore, in this report, we detail suPAR levels in two discrete well described cohorts of children and young adults with biopsy-proven primary FSGS—the North America–based FSGS clinical trial (CT), and the Europe-based consortium for study of steroid-resistant nephrotic syndrome (PodoNet).
Results
Elevated Circulating suPAR Levels in Distinct FSGS Cohorts
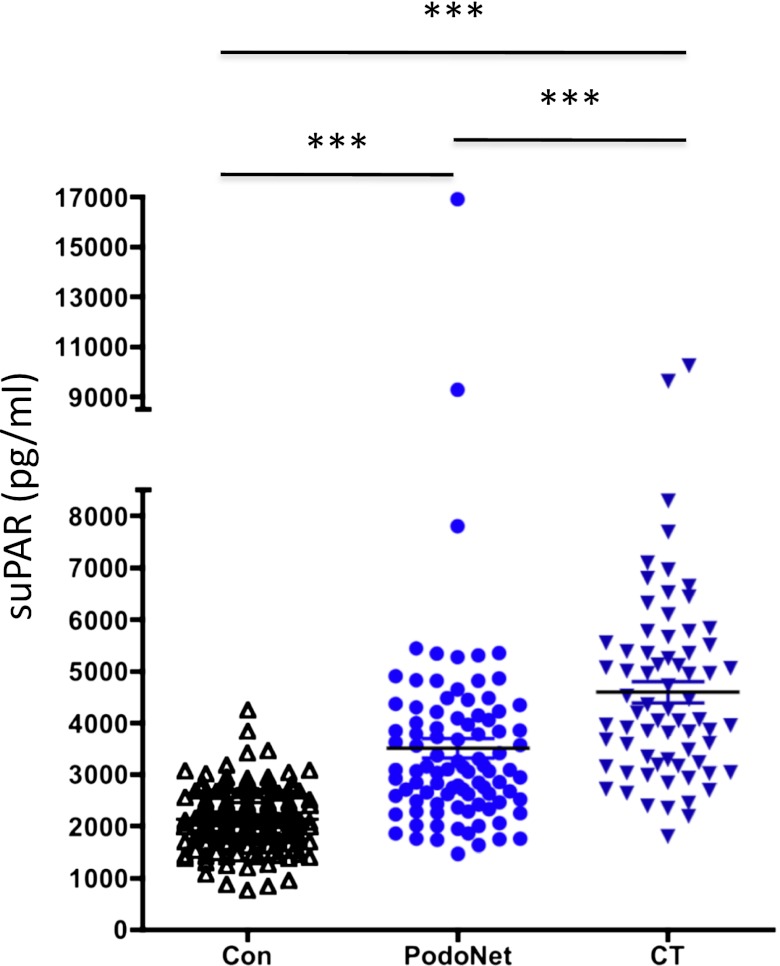
Circulating suPAR was measured in 150 control participants, 70 patients with primary FSGS from the FSGS CT cohort, and 94 patients with primary FSGS from PodoNet. Control participants were age and sex matched for FSGS patients. Compared with control participants, the serum suPAR levels in FSGS patients were markedly increased in both FSGS cohorts (Figure 1). We set 3000 pg/ml as a cut-off value, which is based on our own recent dataset11 as well as on a reported normal mean serum suPAR level of 2710 pg/ml.12 Using 3000 pg/ml as a threshold level, the baseline circulating suPAR concentration was elevated in 84.3% of FSGS patients in the FSGS CT cohort compared with 55.3% in the PodoNet cohort. The mean suPAR level was higher in the FSGS CT cohort than in the PodoNet cohort (4588±203 versus 3497±195 pg/ml; P<0.0001). The mean serum creatinine was significantly higher in patients enrolled into the FSGS CT cohort than in PodoNet cohort, which might partially account for the difference of suPAR between the two groups (see below).
Figure 1.
Serum suPAR levels in distinct primary FSGS cohorts and control participants. ***P<0.001 for CT FSGS cohort versus control, PodoNet FSGS cohort versus control, and CT versus PodoNet. Con, control.
C-Reactive Protein in FSGS Patients
C-reactive protein (CRP) is an acute phase reactant that increases 4–6 hours after an inflammatory trigger, such as viral or bacterial infections. In most cases in which suPAR is proposed as a marker of inflammation, CRP and suPAR are positively correlated with each other.13,14 In parallel with the suPAR measurements, serum CRP was determined to assess the inflammatory status in a randomly selected subset of patients from both the FSGS CT and PodoNet cohorts as well as in controls. The average CRP levels were generally low, indicating minimal inflammation, and there was no difference between controls and FSGS patients (Figure 2A). This is consistent with the classification of FSGS as a noninflammatory renal disease. Moreover, there was no correlation between CRP and respective suPAR levels in either controls (Figure 2B) or FSGS patients (Figure 2C). In addition, serum samples from pediatric patients with mild infection and high CRP levels were randomly selected for suPAR measurement and suPAR was found to be <3000 pg/ml in 9 of 11 patients with an average of 2401±195 pg/ml (Figure 2D). These data suggest that inflammation is not a relevant factor in determining the elevated suPAR levels noted in the two FSGS patient cohorts.
Figure 2.
Serum CRP and its respective suPAR in FSGS patients and controls. (A) Serum CRP levels in FSGS patients. Control and FSGS patients from CT and PodoNet were determined for serum CRP concentration. Mean CRP was at low risk for inflammation and there was no difference between FSGS patients and controls. (B) Serum CRP did not correlate to its respective serum suPAR level in controls. (C) Serum CRP did not correlate to its respective suPAR in FSGS patients. (D) Patients with mild infection had high serum CRP level but presented with low suPAR concentration (n=11). Con, control.
Characteristics of FSGS CT Patients
Circulating suPAR levels, measured from the serum collected at baseline and after 26 weeks of treatment, were analyzed for correlation with demographic variables and serum albumin, estimated GFR (eGFR) and proteinuria (urine protein/creatinine ratio [Up/c]) at the two time points. Multiple regression analysis showed that baseline suPAR level was significantly correlated with eGFR (P<0.0001) and black race (P=0.04), whereas suPAR level at week 26 after treatment was significantly associated with baseline eGFR (P=0.01) and age at sampling (P=0.01) (Table 1). To explore the effect of treatment on circulating suPAR levels, samples were analyzed in patients who were randomly assigned to either cyclosporine A (CSA) or mycophenolate mofetil (MMF)/dexamethasone arm of the trial. At baseline, there was no difference between the patients in the two treatment arms with regard to age at disease onset, age at sampling, sex, race, Up/c, serum creatinine, eGFR, and serum albumin (Table 2) as well as circulating suPAR levels as indicated by univariate analysis. However, after 26 weeks of trial treatment, the mean suPAR level was significantly higher in patients assigned to CSA compared with the MMF arm (Figure 3A). When examining the alteration from baseline to week 26, suPAR levels were increased in the CSA arm, but decreased in the MMF arm and the changes in suPAR levels between the two arms were significantly different (Figure 3B). These differences remained significant after controlling for age, sex, race, and the baseline levels of suPAR, eGFR, albumin, and Up/c (Table 3).
Table 1.
Multiple regression analysis of suPAR in FSGS patients
| Parameter | CT | PodoNet | PodoNet + CT | |||||
|---|---|---|---|---|---|---|---|---|
| suPAR at Baseline | suPAR in Treatment | suPAR in Treatment | suPAR in Treatment | |||||
| β ± SEM | P | β ± SEM | P | β ± SEM | P | β ± SEM | P | |
| Serum albumin at baseline (g/dl) | −47±357 | 0.90 | −637±480 | 0.19 | ||||
| eGFR at baseline (ml/min per 1.73 m2) | −17±4 | <0.0001 | −16±6 | 0.01 | ||||
| Up/c at baseline (g/g) | −42±77 | 0.59 | −130±91 | 0.16 | ||||
| Black race | −903±420 | 0.035 | −617±441 | 0.17 | −291±475 | 0.54 | ||
| Age at sampling (yr) | −34±22 | 0.12 | −65±23 | 0.01 | −45±43 | 0.30 | −42±21 | 0.04 |
| Male sex | −320±374 | 0.40 | −535±370 | 0.15 | −737±408 | 0.08 | −592±278 | 0.04 |
| MMF treatment | −374±366 | 0.31 | −725±411 | 0.08 | −872±563 | 0.13 | −689±331 | 0.04 |
| Serum albumin in treatment (g/dl) | 439±441 | 0.32 | 169±226 | 0.46 | −53±154 | 0.73 | ||
| eGFR in treatment (ml/min per 1.73 m2) | −8±8 | 0.29 | −7±2 | 0.004 | −7±2 | 0.001 | ||
| Genetic/familial | 1076±448 | 0.02 | 1072±411 | 0.01 | ||||
| Up/c (g/g) in treatment | 156±95 | 0.11 | ||||||
| CT cohort | 2965±753 | 0.0001 | ||||||
| eGFR by cohort (ml/min per 1.73 m2)a | −9±5 | 0.07 | ||||||
Interaction between cohorts and eGFR in treatment.
Table 2.
Characteristics of FSGS CT cohort, univariate analysis
| Parameter | CSA (n=35) | MMF (n=35) | P |
|---|---|---|---|
| Age onset (yr) | 18.43±1.76 | 17.29±1.65 | 0.60 |
| Age at sampling (yr) | 19.89±1.79 | 18.26±1.64 | 0.47 |
| Female, n (%) | 13 (37.14) | 18 (51.42) | 0.28 |
| Black race, n (%) | 11 (31) | 12 (34) | 0.50 |
| Up/c (g/g) | 5.41±0.77 | 4.59±0.53 | 0.40 |
| Serum albumin (g/dl) | 3.07±0.15 | 3.13±0.13 | 0.90 |
| Serum creatinine (mg/dl) | 1.18±0.12 | 1.00±0.09 | 0.25 |
| eGFR (ml/min per 1.73 m2) | 118.20±10.34 | 122.60±9.24 | 0.53 |
| suPAR (pg/ml) | 4721±319.9 | 4311±224.4 | 0.36 |
Figure 3.
Characteristics of serum suPAR in the CT FSGS cohort. (A) Circulating suPAR levels at baseline and 26 weeks after treatment (**P<0.01). (B) Therapy-associated changes of suPAR levels (*P<0.05). (C) suPAR levels in patients who achieved complete remission at 26 weeks, and stabilized for at least 6 months (n=5). (D) suPAR levels in patients who achieved complete remission at 26 weeks, but proteinuria came back at 52 weeks (n=4). (E) suPAR responders were associated with a substantial decrease in Up/c. Responders were FSGS patients with high suPAR at baseline, but dropped to <3000 pg/ml after 26-week therapy, whereas nonresponders were patients whose suPAR levels remained high (†P<0.05 for responders versus nonresponders).
Table 3.
Multiple regression analysis of absolute and relative change in suPAR from baseline to week 26 in FSGS CT
| Parameter | Absolute Changea | Relative Changeb | ||
|---|---|---|---|---|
| β ± SEM | P | β ± SEM | P | |
| Age at sampling (yr) | 31±16 | 0.06 | 0.4±0.4 | 0.32 |
| Male sex | 266±274 | 0.34 | 6.1±6.8 | 0.37 |
| Black race | −181±317 | 0.57 | −4.5±7.9 | 0.57 |
| Up/c at baseline (g/g) | 20±56 | 0.72 | 0.2±1.4 | 0.87 |
| Serum albumin at baseline (g/dl) | 289±260 | 0.27 | 6.6±6.5 | 0.31 |
| eGFR at baseline (ml/min per 1.73 m2) | 8±3 | 0.01 | 0.1±0.1 | 0.16 |
| suPAR at baseline (pg/ml) | 0.4±0.1 | 0.0002 | 0.008±0.002 | 0.001 |
| MMF treatment | 713±269 | 0.01 | 20.8±6.7 | 0.003 |
suPAR at baseline suPAR at week 26.
100×(suPAR at baseline suPAR at week 26)/suPAR at baseline.
With regard to the relationship between suPAR and proteinuria, serum suPAR was not correlated to Up/c at baseline or after 26-week treatment (Table 1). As indicated in Table 3, the absolute change in suPAR between weeks 0 and 26 was strongly associated with baseline eGFR and suPAR level and MMF treatment. The relative change in suPAR level over this 26-week period was related only to baseline suPAR level and MMF treatment. Neither the baseline nor the change in suPAR level from week 0 to week 26 predicted the outcome in the FSGS CT cohort based on the trial’s original six-ordinal scale.15 However, using multiple regression analysis, investigation of the relative changes of serum suPAR from baseline to week 26 revealed a positive association with both the absolute change (P=0.01) and percentage reduction (P=0.003) in the Up/c between the two time points when proteinuria was assessed as a continuous variable and complete or partial remission were based on the definitions of Troyanov16 (Table 4). A logistic regression analysis that controlled for age, sex, race, eGFR, Up/c, and suPAR at baseline indicated greater odds for complete remission (Up/c ≤0.2 g/g achieved at least once from weeks 26 to 78) with each 10% reduction in suPAR concentration (odd ratios, 1.44; 95% confidence interval, 1.02–2.03; P=0.04) (Table 4).
Table 4.
Multiple regression analysis of Up/c change and complete remission in FSGS CT patients
| Parameter | Absolute Change | Relative Change | Complete Remission | |||
|---|---|---|---|---|---|---|
| β ± SEM | P | β ± SEM | P | OR (95% CI) | P | |
| Relative change in suPARa | 0.03±0.01 | 0.01 | 0.80±0.25 | 0.003 | 1.44 (1.02–2.03) | 0.04 |
| Age at sampling | −0.04±0.04 | 0.36 | −1.16±0.81 | 0.16 | 0.90 (0.81–1.00) | 0.05 |
| Male sex | −0.36±0.65 | 0.58 | −11.42±13.77 | 0.41 | 0.34 (0.08–1.55) | 0.16 |
| Black race | −0.79±0.74 | 0.29 | −13.47±15.79 | 0.40 | 0.35 (0.05–2.56) | 0.30 |
| suPAR at baseline (pg/ml) | −0.00±0.00 | 0.30 | −0.01±0.00 | 0.26 | 1.00 (0.99–1.00) | 0.15 |
| Up/cat baseline (g/g) | 0.60±0.09 | <0.0001 | −0.01±1.84 | 0.99 | 0.78 (0.60–1.01) | 0.06 |
| eGFR at baseline (ml/min per 1.73 m2) | −0.02±0.01 | 0.03 | −0.34±0.16 | 0.04 | 0.99 (0.97–1.01) | 0.40 |
| MMF treatment | −0.62±0.67 | 0.36 | −27.26±14.33 | 0.06 | 0.12 (0.02–0.64) | 0.01 |
For per 10% reduction in suPAR.
Of the 70 patients who were available for suPAR analysis, 9 achieved complete remission at week 26 regardless of treatment. In these cases, suPAR was increased from baseline to week 26 in the four patients whose proteinuria recurred at week 52 (Figure 3C), whereas suPAR level was decreased in those five patients who achieved stable remission through at least week 26 (Figure 3C). The same pattern was observed in patients who achieved complete remission at week 52. Alternatively, patients were stratified into the responders based on changes in suPAR levels (i.e., those patients whose serum suPAR was high at baseline but dropped to below 3000 pg/ml in response to 26 weeks of therapy) and the nonresponders whose suPAR remained high (≥3000 pg/ml) after 26 weeks of treatment. In total, there were nine responders, six of whom were from the MMF arm and three were from the CSA arm. There was no difference of Up/c between responders and nonresponders at baseline. However, Up/c was decreased dramatically from the start (6.43±1.84 g/g) to the end of treatment (0.33±0.15 g/g; P<0.001 versus baseline) and remained stable up to 78 weeks (0.61±0.25 g/g; P<0.001 versus baseline) in suPAR responders, whereas Up/c was decreased by <40% for suPAR nonresponders (4.95±0.49 g/g at baseline versus 3.06±0.62 g/g at the end of the treatment; P<0.001) (Figure 3E).
In the FSGS CT cohort, none of the patients analyzed for suPAR levels had a disease causing genetic mutation in NPHS2, INF2, or PLCE1, precluding an assessment of the relationship between suPAR levels and hereditary causes of FSGS.
Characteristics of PodoNet FSGS Patients
In the PodoNet cohort, multiple regression analysis showed that circulating suPAR levels were significantly correlated with eGFR (P=0.004), and were marginally associated with sex (Table 1) in that female patients had higher suPAR levels than male patients (Figure 4A). If the data from both the North American and European FSGS cohorts were combined while the patients were on treatment, the serum suPAR level was significantly and inversely correlated with age at sampling, eGFR, male sex and MMF therapy; all of these variables were associated with significantly lower suPAR concentrations (Table 1). There were a substantial number of familial cases and FSGS patients with a defined genetic mutation in podocin (NPHS2) in the PodoNet cohort. Therefore, we stratified the cohort into two subgroups, familial/genetic versus nongenetic FSGS. Although there was no difference with regard to age at disease onset, age at sampling, sex distribution, eGFR, or serum albumin level between the two subgroups (Table 5), serum suPAR levels were higher in the familial/genetic group compared with patients with nongenetic primary FSGS (Figure 4B and Supplemental Figure 1), and the difference remained significant after adjustment for other covariates (Table 1).
Figure 4.
Characteristics of serum suPAR in PodoNet FSGS cohort. (A) Female FSGS patients had higher levels of suPAR compared with male FSGS patients. (B) Familial FSGS patients and/or FSGS patients who had NHPS2 mutation were associated with higher suPAR levels as indicated by univariate analysis. (C) MMF treatment was associated with lower suPAR levels. (D) Patients received MMF plus prednisone possessed lower suPAR levels compared with those received calcineurin inhibitor and prednisone (*P<0.05). NM, therapy other than MMF; MP, MMF plus prednisone; CP, calcineurin inhibitor plus prednisone.
Table 5.
Characteristics of PodoNet cohort by genetics and treatment, univariate analysis
| Parameter | Genetics | Treatment | ||||
|---|---|---|---|---|---|---|
| Genetic/Familial (n=28) | Nongenetic (n=66) | P | MMF (n=17) | Others (n=77) | P | |
| Age onset (yr) | 5.24±0.78 | 6.41±0.56 | 0.22 | 4.83±0.99 | 6.41±0.51 | 0.17 |
| Age at sampling (yr) | 8.36±1.06 | 10.53±0.63 | 0.06 | 11.07±1.29 | 9.62±0.60 | 0.22 |
| Female, n (%) | 20 (71.42) | 36 (54.54) | 0.13 | 8 (47.05) | 48 (62.33) | 0.13 |
| Proteinuria (g/m2 per day) | 3.27±0.76 | 1.68±0.35 | 0.03 | 1.59±0.68 | 2.25±0.39 | 0.18 |
| Serum albumin (g/dl) | 2.96±0.21 | 3.43±0.13 | 0.07 | 3.21±0.31 | 3.31±0.12 | 0.86 |
| Serum creatinine (mg/dl) | 1.15±0.33 | 0.58±0.07 | 0.02 | 0.82±0.13 | 0.74±0.07 | 0.66 |
| eGFR (ml/min per 1.73 m2) | 119.8±21.11 | 132.9±10.43 | 0.09 | 91.60±10.82 | 112.2±10.90 | 0.89 |
In contrast to the CT cohort, treatment in PodoNet was at the discretion of the attending physicians. Patients were grouped according to the medication they received at the time of blood sampling (i.e., MMF versus other drugs, such as calcineurin inhibitors). On the basis of demographic features or laboratory findings, there was no difference overall between those who were treated with MMF and those who did not receive this medication (Table 5). However, circulating suPAR levels were significantly lower in the MMF-treated group (Figure 4C). In contrast, no difference of suPAR level was observed when patients with and without calcineurin inhibitor treatment were compared. Finally, the circulating suPAR levels were significantly lower in patients who received MMF and prednisone compared with patients who received calcineurin inhibitors and prednisone (Figure 4D).
Discussion
We reported that high serum levels of suPAR are specifically associated with primary FSGS and chronic overexpression of suPAR leads to an FSGS-like nephropathy in mice.11 The major findings in this study are as follows: (1) the circulating suPAR levels were markedly elevated in the majority of patients with primary FSGS in two distinct cohorts including children and adults; (2) the elevation of suPAR in FSGS patients was not related to inflammation because concurrent CRP levels were low and not different than in normal controls; (3) MMF therapy was associated with a lower serum level of suPAR; (4) a sustained decline in suPAR levels over the course of 26 weeks of treatment was associated with both an absolute and relative reduction in proteinuria, and with greater odds for complete remission; and (5) serum suPAR levels were higher in familial cases including those with a defined podocin mutation.
Serum suPAR level was elevated in 55%–84% of patients with FSGS in these two distinct cohorts. The fact that 15%–45% of the patients in the two cohorts had normal suPAR levels suggests that primary FSGS is a heterogeneous disorder and that additional factors might exist that contribute to proteinuria. Concurrent analysis of CRP revealed minimal or low level of inflammation in both controls and FSGS patients. Taken together, these observations are consistent with our previous report11 and indicate that a high level of serum suPAR is a characteristic feature in the majority of patients with primary FSGS. It is possible that patients with primary FSGS express higher levels of suPAR in response to a distinct pathologic stimulus other than or in addition to a primary inflammatory instigator. This is consistent with the independent relationship between suPAR and CRP observed in FSGS. It is also worth noting that inflammation-driven suPAR displays less activity for stimulation of podocyte β3 integrin compared with FSGS-associated suPAR (unpublished results, Jochen Reiser 2012). More studies are needed to understand the mechanisms behind the differences in pathogenic strength of various suPAR forms.
Multivariate analysis revealed an inverse correlation of suPAR to eGFR in both FSGS cohorts. In addition to the de novo increase, we speculate that serum suPAR levels may also rise as GFR declines. This might contribute to the difference in mean suPAR level between the FSGS CT and PodoNet cohorts, namely the younger age of the European group and the higher serum creatinine in the FSGS CT cohort. A similar scenario has been observed with fibroblast growth factor 23 in which its accumulation stems from overproduction during kidney disease and is also correlated to eGFR.16 The differences in genetic and racial demographics—that is, the much higher percentage (33%) of African American participants in FSGS CT cohort—could also contribute to the suPAR distinction between the two cohorts. However, further research is warranted to explain variations in circulating suPAR levels in different patient populations.
Female patients had higher levels of suPAR in both cohorts combined and in PodoNet alone. Sex differences have also been documented in another study that reported elevated suPAR levels,17 and this phenomenon warrants further study.
In the FSGS CT cohort, there was a strong positive association of the percentage of suPAR reduction over 26 weeks of treatment with both the absolute as well as the percent reduction in proteinuria. Decreasing suPAR is also associated with greater odds of achieving complete remission.18 These findings are based on analysis of proteinuria as a continuous rather than a discrete variable, as was done in the evaluation of efficacy in the FSGS CT cohort.19 We acknowledge that our findings from the FSGS CT are exploratory in nature because suPAR was assayed in a subset of the total study cohort and patients could be withdrawn from the study if there was no evidence of response after 26 weeks. Thus, the patients with samples beyond that time point are subject to bias.
The changes in circulating suPAR level that we documented in response to treatment may reflect the underlying effect of the agents on net suPAR production. This is reinforced by the observation that a decline in the suPAR level to <3000 pg/ml within the first 6 months of treatment was predictive of a sustained remission during the subsequent 12 months. In the latter scenario, in addition to its role in the pathogenesis of FSGS, suPAR might also be useful as a biomarker of glomerular disease activity. More studies, preferably prospective in nature and inclusive of sensitivity and specificity data, will be required to evaluate whether changes in the circulating level of suPAR can be utilized as a biomarker to predict outcome or monitor response to treatment.
Multiple regression analyses of both FSGS cohorts indicate lower levels of suPAR associated with combined MMF/dexamethasone treatment compared with cyclosporine. These are agents that are widely used to treat primary FSGS.19,20 MMF therapy was associated with a significant decline in suPAR levels in contrast to the calcineurin inhibitor, which caused a nonsignificant increase in serum suPAR concentration. This finding is buttressed by the cross-sectional data from the PodoNet cohort, in which patients treated with MMF exhibited lower suPAR levels than those receiving calcineurin inhibitor or other therapy. Thus, although calcineurin inhibitors may have direct beneficial effects on the podocyte in patients with FSGS,21 MMF could have a different biologic effect on cells producing circulating factor(s). It will be worthwhile to study the differences in drug effects on suPAR further and to define if MMF and cyclosporine act synergistically in the treatment of glomerular diseases such as FSGS.
The clinical differences in pathogenesis, response to treatment, and recurrence of disease after transplantation between genetic and nongenetic cases of primary FSGS have been a steady topic of discussion.22,23 Carraro et al. analyzed five patients with autosomal recessive steroid-resistant nephrotic syndrome (SRNS) (NPHS2) for serum glomerular permeability activity (Palb), and found high pretransplant Palb in all cases, equivalent to values observed in idiopathic FSGS.24 In the PodoNet cohort, circulating suPAR levels were higher in familial FSGS and FSGS with documented NPHS2 mutations, and the difference remained significant after adjustment for other covariates. Our results justify larger cohort studies, with more comprehensive assessment of FSGS-associated mutations including TRPC6. Moreover, documentation of high levels of suPAR in patients with genetic forms of FSGS warrants further work to identify interventions that can safely inhibit the action of suPAR at the glomerular capillary wall and lower urinary protein excretion in these cases. For example, galactose, which is being tested in the Novel Therapies for Resistant FSGS clinical trial (DK70341),25,26 may act by binding to suPAR and/or preventing its interaction with β3 integrin in the podocyte.
In conclusion, after the initial identification of suPAR as a circulating permeability factor in primary FSGS, this study of two distinct FSGS cohorts confirms that a high circulating level of suPAR is characteristic of the majority of patients with this glomerular disease. Moreover, the elevated suPAR levels are not a reflection of a nonspecific proinflammatory milieu. Although additional studies are warranted, this study suggests the potential additional role of suPAR as an independent biomarker of FSGS disease progression and/or response to different therapeutic interventions.
Concise Methods
FSGS CT Cohort
The FSGS CT was a randomized controlled study that compared the efficacy of CSA with the combination of MMF and dexamethasone. Key inclusion criteria were age 2–40 years, eGFR >40 ml/min per 1.73 m2, biopsy-proven FSGS, and resistance to corticosteroid therapy. Exclusion criteria included secondary FSGS, obesity, or prior treatment with the experimental therapies. All patients received prednisone, 0.3 mg/kg per dose every other day (maximum 15 mg), for the first 6 months of the treatment period. Losartan was provided to those patients who were intolerant of the angiotensin converting enzyme inhibitor lisinopril for the entire treatment period. The experimental therapy was provided for 52 weeks. The primary outcome was based on the achievement of remission of proteinuria during the first 52 weeks after randomization. Complete remission was defined as normalization of proteinuria with the Up/c <0.2 g/g in a first morning urine sample. Partial remission was defined as a combination of a Up/c between 0.2 and 2 g/g and ≥50% decline in Up/c from the baseline value. The main secondary outcome was based on the level of proteinuria at 78 weeks, 6 months after discontinuation of the study drugs. Patients were seen 11 times during the treatment period and BP was measured and blood and urine were obtained at each visit to determine serum creatinine, eGFR, albumin, and cholesterol concentrations and proteinuria.27 The serum samples collected at baseline and 26 weeks on the experimental treatment from a group of randomly selected patients (n=35 in each arm) were retrieved from the National Institutes of Diabetes and Digestive and Kidney Diseases Biorepository for suPAR measurement. In this study, complete remission was defined as Up/c ≤0.2 g/g observed at least once from weeks 26 to 78.
PodoNet Cohort
PodoNet is a Europe-based consortium for clinical, genetic, and experimental study of SRNS. The inclusion criteria are children (age 0–18 years) with SRNS based on management protocols at the participating medical centers and adults with familial SRNS. Patients who were included in this study had biopsy-proven FSGS (n=94). The treatment of patients was clinically determined and managed by their attending physician. In these patients, serum was retrieved for measurement of the suPAR concentration.
Control Participants
The pediatric controls (n=110, 55 of whom were female; age 0–18 years) were either healthy Caucasian participants recruited from local primary and high schools in Rostock or children presenting at the University Children’s Hospital Rostock for diagnostic workup either before minor surgery or secondary to noninflammatory diseases like epilepsy and orthostatic complaints. These participants were not fully characterized and there may be some unidentified selection bias in defining normal suPAR and CRP levels. However, this study excluded children with growth disorders, a history of recent fracture or malnutrition, acute infections, elevated serum concentration of CRP (≥5 mg/L), or creatinine (≥2 SD) at time of enrollment, as well as those with metabolic disorders, chronic inflammatory diseases, and renal or hepatic disease. In addition, previous data indicate that there is no difference in CRP levels in Caucasian and African-American patients.28 This study was approved by the Hospital Ethics Committee (HV-2009–003), and informed consent was obtained from parents and/or participants, if appropriate.29,30 The adult controls (n=40; age 16–52 years) were obtained from healthy blood donors through ProMeddx.
suPAR and CRP Assay
The measurement of serum suPAR and CRP was performed using a Human uPAR or CRP Quantikine ELISA kit (R&D Systems Inc), respectively. Standards were run three times to calculate the intra-assay coefficient of variation (CV). The mean and SD for standard 1, standard 2, and so forth were used to derive the CV for each before averaging the CV of each standard. The interassay CV was derived by calculating the mean and SD for standard 1 (e.g., measurement day 1 and day 2), standard 2 (day 1 and day 2), and so forth to derive the CV for each and then average the CV. Both the intra-assay and interassay CVs were <5% for suPAR and <5.5% for CRP.
Statistical Analyses
Data are expressed as mean ± SEM for continuous variables. The demographic and clinical characteristics of patient and control participants were compared using the t test, or the chi-squared test for categorical variables. Multiple linear or logistic regression analyses were performed to evaluate the association between circulating suPAR and the variables of interest while controlling for age, sex, and other potential confounders with SPSS software. All statistical tests were two tailed and P values <0.05 were considered significant.
Disclosures
J.R. and C.W. are inventors on issued (J.R.) or pending patents (J.R. and C.W.) regarding novel antiproteinuric technologies. They stand to gain royalties from their commercialization.
Supplementary Material
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (DK073495 and DK089394 to J.R., U01-DK063385 to the FSGS CT), the Halpin Foundation (American Society of Nephrology research grant to C.W.), and La fondazione la Nuova speranza (to G.M.G.).
Parts of this work were presented as an abstract during the Annual Meeting of the American Society of Nephrology, November 8–13, 2011, in Philadelphia, Pennsylvania.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012030302/-/DCSupplemental.
References
- 1.Benchimol C: Focal segmental glomerulosclerosis: Pathogenesis and treatment. Curr Opin Pediatr 15: 171–180, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Korbet SM: Treatment of primary focal segmental glomerulosclerosis. Kidney Int 62: 2301–2310, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Boyer O, Moulder JK, Somers MJ: Focal and segmental glomerulosclerosis in children: A longitudinal assessment. Pediatr Nephrol 22: 1159–1166, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Barisoni L, Schnaper HW, Kopp JB: Advances in the biology and genetics of the podocytopathies: Implications for diagnosis and therapy. Arch Pathol Lab Med 133: 201–216, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santín S, Bullich G, Tazón-Vega B, García-Maset R, Giménez I, Silva I, Ruíz P, Ballarín J, Torra R, Ars E: Clinical utility of genetic testing in children and adults with steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol 6: 1139–1148, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCarthy ET, Sharma M, Savin VJ: Circulating permeability factors in idiopathic nephrotic syndrome and focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 5: 2115–2121, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Cattran D, Neogi T, Sharma R, McCarthy ET, Savin VJ: Serial estimates of serum permeability activity and clinical correlates in patients with native kidney focal segmental glomerulosclerosis. J Am Soc Nephrol 14: 448–453, 2003 [DOI] [PubMed] [Google Scholar]
- 8.McCarthy ET, Sharma M, Sharma R, Falk RJ, Jennette JC: Sera from patients with collapsing focal segmental glomerulosclerosis increase albumin permeability of isolated glomeruli. J Lab Clin Med 143: 225–229, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Savin VJ, Sharma M: Plasma “factors” in recurrent nephrotic syndrome after kidney transplantation: Causes or consequences of glomerular injury? Am J Kidney Dis 54: 406–409, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Reiser J, Wei C, Tumlin J: Soluble urokinase receptor and focal segmental glomerulosclerosis. Curr Opin Nephrol Hypertens 21: 428–432, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Wei C, El Hindi S, Li J, Fornoni A, Goes N, Sageshima J, Maiguel D, Karumanchi SA, Yap HK, Saleem M, Zhang Q, Nikolic B, Chaudhuri A, Daftarian P, Salido E, Torres A, Salifu M, Sarwal MM, Schaefer F, Morath C, Schwenger V, Zeier M, Gupta V, Roth D, Rastaldi MP, Burke G, Ruiz P, Reiser J: Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med 17: 952–960, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao W, Wang Z, Bai X, Xi X, Ruan C: Detection of soluble urokinase receptor by immunoradiometric assay and its application in tumor patients. Thromb Res 102: 25–31, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Koch A, Voigt S, Kruschinski C, Sanson E, Dückers H, Horn A, Yagmur E, Zimmermann H, Trautwein C, Tacke F: Circulating soluble urokinase plasminogen activator receptor is stably elevated during the first week of treatment in the intensive care unit and predicts mortality in critically ill patients. Crit Care 15: R63, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yilmaz G, Köksal I, Karahan SC, Mentese A: The diagnostic and prognostic significance of soluble urokinase plasminogen activator receptor in systemic inflammatory response syndrome. Clin Biochem 44: 1227–1230, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Gipson DS, Trachtman H, Kaskel FJ, Radeva MK, Gassman J, Greene TH, Moxey-Mims MM, Hogg RJ, Watkins SL, Fine RN, Middleton JP, Vehaskari VM, Hogan SL, Vento S, Flynn PA, Powell LM, McMahan JL, Siegel N, Friedman AL: Clinical trials treating focal segmental glomerulosclerosis should measure patient quality of life. Kidney Int 79: 678–685, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyngbæk S, Sehestedt T, Marott JL, Hansen TW, Olsen MH, Andersen O, Linneberg A, Madsbad S, Haugaard SB, Eugen-Olsen J, Jeppesen J: CRP and suPAR are differently related to anthropometry and subclinical organ damage [published online ahead of print March 27, 2012]. Int J Cardiol doi:10.1016/j.ijcard.2012.03.040 [DOI] [PubMed] [Google Scholar]
- 17.Isakova T, Wahl P, Vargas GS, Gutiérrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CA, Lash JP, Hsu CY, Leonard MB, Wolf M: Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79: 1370–1378, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Troyanov S, Wall CA, Miller JA, Scholey JW, Cattran DC, Toronto Glomerulonephritis Registry Group : Focal and segmental glomerulosclerosis: Definition and relevance of a partial remission. J Am Soc Nephrol 16: 1061–1068, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Burgess E: Management of focal segmental glomerulosclerosis: Evidence-based recommendations. Kidney Int Suppl 70: S26–S32, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Ponticelli C, Passerini P: Other immunosuppressive agents for focal segmental glomerulosclerosis. Semin Nephrol 23: 242–248, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Faul C, Donnelly M, Merscher-Gomez S, Chang YH, Franz S, Delfgaauw J, Chang JM, Choi HY, Campbell KN, Kim K, Reiser J, Mundel P: The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med 14: 931–938, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Billing H, Müller D, Ruf R, Lichtenberger A, Hildebrandt F, August C, Querfeld U, Haffner D: NPHS2 mutation associated with recurrence of proteinuria after transplantation. Pediatr Nephrol 19: 561–564, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Caridi G, Bertelli R, Carrea A, Di Duca M, Catarsi P, Artero M, Carraro M, Zennaro C, Candiano G, Musante L, Seri M, Ginevri F, Perfumo F, Ghiggeri GM: Prevalence, genetics, and clinical features of patients carrying podocin mutations in steroid-resistant nonfamilial focal segmental glomerulosclerosis. J Am Soc Nephrol 12: 2742–2746, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Carraro M, Caridi G, Bruschi M, Artero M, Bertelli R, Zennaro C, Musante L, Candiano G, Perfumo F, Ghiggeri GM: Serum glomerular permeability activity in patients with podocin mutations (NPHS2) and steroid-resistant nephrotic syndrome. J Am Soc Nephrol 13: 1946–1952, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Savin VJ, McCarthy ET, Sharma R, Charba D, Sharma M: Galactose binds to focal segmental glomerulosclerosis permeability factor and inhibits its activity. Transl Res 151: 288–292, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Trachtman H, Vento S, Gipson D, Wickman L, Gassman J, Joy M, Savin V, Somers M, Pinsk M, Greene T: Novel therapies for resistant focal segmental glomerulosclerosis (FONT) phase II clinical trial: Study design. BMC Nephrol 12: 8, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gipson DS, Trachtman H, Kaskel FJ, Greene TH, Radeva MK, Gassman JJ, Moxey-Mims MM, Hogg RJ, Watkins SL, Fine RN, Hogan SL, Middleton JP, Vehaskari VM, Flynn PA, Powell LM, Vento SM, McMahan JL, Siegel N, D’Agati VD, Friedman AL: Clinical trial of focal segmental glomerulosclerosis in children and young adults. Kidney Int 80: 868–878, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anuurad E, Tracy RP, Pearson TA, Beckett L, Berglund L: Comparison of C-reactive protein and metabolic syndrome as cardiovascular risk factors in African-Americans and European-Americans. Am J Cardiol 103: 523–527, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wigger M, Schaible J, Muscheites J, Kundt G, Haffner D, Fischer DC: Fetuin-A serum concentrations in healthy children. Ann Clin Biochem 46: 511–513, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Fischer DC, Mischek A, Wolf S, Rahn A, Salweski B, Kundt G, Haffner D: Paediatric reference values for the C-terminal fragment of fibroblast-growth factor-23, sclerostin, bone-specific alkaline phosphatase and isoform 5b of tartrate-resistant acid phosphatase [published online ahead of print September 14, 2012]. Ann Clin Biochem doi:10.1258/acb.2012.011274 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.