Abstract
Compared with other racial groups, African Americans have a similar prevalence of CKD but are much more likely to progress to ESRD, suggesting that the cost-effectiveness of screening strategies requires dedicated study in this population. Here, we calibrated the CKD Health Policy Model so that it accurately forecasts the higher rates for ESRD observed for African Americans. We then used the calibrated model to estimate the cost-effectiveness of screening for microalbuminuria followed by treatment with angiotensin-converting enzyme inhibitors or angiotensin II-receptor blockers. Incorporating racial differences in risk factors did not fully explain the much higher lifetime incidence of ESRD among African Americans. Thus, to calibrate the model, we applied a 20% increase in the rate of GFR decline at stage 3 and a 60% increase in the rate of GFR decline at stage 4, which resulted in a model that closely reflects lifetime ESRD incidence among African Americans. Compared with usual care, screening African Americans for microalbuminuria at 10-, 5-, 2-, and 1-year intervals had incremental cost-effectiveness ratios of $9000, $11,000, $19,000, and $35,000 per quality-adjusted life year, respectively. Incremental cost-effectiveness ratios for the same screening intervals were higher for non-African Americans: $17,000, $23,000, $44,000, and $81,000 per quality-adjusted life year, respectively. In summary, these models suggest that screening African Americans for microalbuminuria at either 5- or 10-year intervals is highly cost-effective.
CKD is a leading cause of morbidity and high medical costs, affecting an estimated 26 million adults in the United States.1 Persons with CKD but not ESRD accounted for nearly one-sixth of all Medicare expenditures in 2009. CKD may progress to ESRD, which affected >571,000 US adults and cost >$42 billion in 2009.2 Elevated albuminuria is the earliest marker of CKD.3 Previous work has shown that urine dipstick screening for clinical albuminuria (macroalbuminuria) and treatment with angiotensin-converting enzyme (ACE) inhibitors or angiotensin II-receptor blockers (ARBs) is cost-effective in certain populations, even without considering the possible benefits of avoiding non-ESRD costs.4,5 We previously developed and used the CKD Health Policy Model to estimate the cost-effectiveness of screening for low levels of albuminuria (microalbuminuria), an even earlier marker of CKD.6,7 We found this intervention to be cost-effective in populations with diabetes or hypertension, two risk factors for CKD and ESRD.8
African Americans are at particularly high risk for ESRD.8 On the basis of the National Health and Nutrition Examination Survey (NHANES) data, the prevalence of CKD among African Americans is similar to or lower than that of other racial groups.9 However, US Renal Data System (USRDS) data indicate that African Americans have much higher lifetime incidence of ESRD.2 The reasons for this disparity in the progression to ESRD are unclear. The Modification of Diet in Renal Disease (MDRD) study explored the association of GFR slope with certain covariates and found African-American race to be associated with the largest GFR decline coefficient of all variables included in the regression.10 Hsu et al. examined risk factors for CKD using NHANES data and found that African Americans experience higher progression rates unexplained by other risk factors, such as age, sex, diabetes, systolic and diastolic BP, and albuminuria.11 Neither study explains this apparent racial disparity in CKD progression.
Our previous model does well in predicting CKD prevalence and the cumulative incidence of ESRD in the overall US population.7 However, the model does not accurately predict the elevated risk of ESRD among African Americans. The purpose of this paper is to calibrate the CKD Health Policy Model so that it accurately forecasts the observed higher ESRD rates for African Americans, and to use the calibrated model to estimate the cost-effectiveness of microalbuminuria screening for African Americans and non-African Americans.
Results
Effect of African-American Risk Factors
Table 1 shows the effect of African-American risk factors on CKD prevalence and lifetime ESRD incidence using the original model parameters. Observed data (NHANES and USRDS) show that the prevalence of CKD among African Americans is 14.7% and the lifetime incidence of ESRD is 86 per 1000 persons. Our previous nonrace-specific model underpredicts ESRD considerably (39 per 1000 persons). Incorporating race-specific hypertension prevalence results in no increase in lifetime ESRD incidence. Incorporating race-specific diabetes rates results in an increase in lifetime ESRD incidence of only 9 per 1000 persons. Modeling micro- and macroalbuminuria rates observed among African Americans results in an increase in ESRD incidence, but also results in an overprediction of CKD compared with that observed in NHANES data. Incorporating all three African-American risk factor prevalence values causes the model to overpredict CKD prevalence, but still substantially underpredict lifetime ESRD incidence by almost 40% (52 versus 86 per 1000 persons).
Table 1.
Effect of African-American risk factors on model outcomes from initial model
| African-American Risk Factors | Age-Adjusted Prevalence | ESRD Lifetime Incidencea | |||||
|---|---|---|---|---|---|---|---|
| Any CKD | Stage 1 | Stage 2 | Stage 3 | Stage 4 | Stage 5 | ||
| Observed ratesb | 0.147 | 0.033 | 0.040 | 0.062 | 0.005 | — | 86 |
| Model predicted ratesc | |||||||
| Base model | 0.143 | 0.027 | 0.035 | 0.072 | 0.007 | 0.002 | 39 |
| Systolic BP | 0.144 | 0.027 | 0.036 | 0.072 | 0.007 | 0.002 | 39 |
| Diabetes | 0.148 | 0.027 | 0.036 | 0.075 | 0.008 | 0.003 | 48 |
| Albuminuria | 0.181 | 0.046 | 0.052 | 0.073 | 0.007 | 0.003 | 42 |
| All 3 | 0.182 | 0.048 | 0.051 | 0.072 | 0.008 | 0.003 | 52 |
Lifetime incidence of initiating ESRD care per 1000 persons.
Observed stage-specific CKD prevalence is based on the NHANES, 1999–2006. We did not report the estimated prevalence of stage 5 due to the limited sample size in NHANES. ESRD lifetime incidence is based on data from the USRDS.
Stage-specific CKD prevalence and ESRD incidence are based on simulation results from the base model with no race adjustments other than mortality, and with individual African-American risk factors (systolic BP, diabetes, albuminuria), and all three risk factors.
Calibration of Race-Specific CKD Progression
We considered several options to calibrate the model to match external validation data targets for cumulative lifetime ESRD incidence and overall CKD prevalence among African Americans and among non-African Americans. These options included increasing the correlation between initial estimated GFR (eGFR) values and initial albuminuria prevalence, eliminating background screening, and eliminating the 1-year delay in stage 5 before ESRD initiation. Each option resulted in very small effects on ESRD incidence, and because none were supported by external evidence, we ultimately rejected their inclusion.
We ultimately decided to calibrate the model to accelerate CKD progression among African Americans with CKD and conversely to slow the progression of non-African Americans with CKD. The calibration process begins to affect progression beginning at stage 3 (eGFR ≤60 ml/min per 1.73 m2) by applying multiplicative coefficients to the annual GFR decrement value. We initiated calibration at stage 3 because the baseline model slightly overpredicts the prevalence of eGFR ≤60 ml/min per 1.73 m2 for African Americans, and this is also the point at which the model assumes that lower GFR values increase the rate of GFR decline. Increasing the GFR rate of decline by 20% at stage 3 and 60% at stage 4 resulted in the model closely replicating USRDS lifetime ESRD incidence (Table 2). Progression rates for non-African Americans were decreased slightly to keep lifetime ESRD incidence constant for the general population.
Table 2.
Results for African Americans with calibrated CKD progression
| Age-Adjusted Prevalence | ESRD Lifetime Incidencea | ||||||
|---|---|---|---|---|---|---|---|
| CKD | Stage 1 | Stage 2 | Stage 3 | Stage 4 | Stage 5 | ||
| Observed ratesb | 0.147 | 0.033 | 0.040 | 0.062 | 0.005 | — | 86 |
| Model predicted ratesc | |||||||
| Base model | 0.143 | 0.027 | 0.035 | 0.072 | 0.007 | 0.002 | 39 |
| African-American risk factors | 0.182 | 0.048 | 0.051 | 0.072 | 0.008 | 0.003 | 52 |
| African-American risk factors and GFR progression calibration | 0.181 | 0.046 | 0.053 | 0.067 | 0.009 | 0.005 | 82 |
Lifetime incidence of initiating ESRD care per 1000 persons.
Observed stage-specific CKD prevalence is based on the NHANES, 1999–2006. We did not report the estimated prevalence of stage 5 due to the limited sample size in NHANES. ESRD lifetime incidence is based on data from the USRDS.
Stage-specific CKD prevalence and ESRD incidence are based on the simulation results from the base model with no race adjustments other than mortality, and with the African-American risk factors, and with the risk factors and calibrated GFR progression rates.
Cost-Effectiveness
We report cost-effectiveness results as incremental cost-effectiveness ratios (ICERs), in which the change in costs from an intervention is divided by the intervention’s effect on quality-adjusted life years (QALYs). We report total per-person lifetime medical costs for each screening scenario, including costs not directly attributable to CKD. Because most of the population never develops CKD and intervention costs are only a small component of lifetime medical costs, the differences in costs across each scenario appear to be small. However, the incremental cost differences reflect the full cost of the interventions and the effect of the interventions on other medical costs.
Table 3 lists costs, QALYs, ICERs, and lifetime incidence of ESRD results of screening for microalbuminuria by race. For ICERs, we calculated the cost per QALY of the different screening strategies compared with usual care and cost per QALY of one screening strategy compared with the next most effective screening strategy. Costs are rounded to the nearest $100, and ICERs are rounded to the nearest $1000 per QALY. Lifetime incidence of ESRD is reported per 1000 persons. Compared with usual care, screening African Americans at 10-, 5-, 2-, and 1-year intervals has ICERs of $9000, $11,000, $19,000, and $35,000 per QALY, respectively. In all cases, screening among African Americans yields more favorable ICERs than among non-African Americans. Because this analysis includes a higher prevalence of macroalbuminuria, these results are generally more favorable than those found in our earlier analysis for the combined population of all races.8 The ICERs increase with screening frequency compared with the alternative screening frequencies. For African Americans, the ICER increased from $24,000 to $113,000 per QALY when the screening interval shortened from 5 to 2 years. For non-African Americans, the ICER increased from $54,000 to $210,000 per QALY. Lifetime incidence of ESRD among African Americans drops from 82 per 1000 persons under usual care to 79 with 10-year universal screening and 78 with more frequent screening. Part of the beneficial effect of screening is not total avoidance of ESRD, but a delay in ESRD onset. ESRD incidence decreases by negligible amounts with screening intervals <5 years.
Table 3.
Cost-effectiveness and ESRD lifetime incidence results of microalbuminuria screening, by race
| All African Americans | All Non-African Americans | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Costs ($) | QALY | ICER versus Usual ($) | ICER versus Next Most Effective ($) | ESRD Lifetime Incidencea | Costs ($) | QALY | ICER versus Usual ($) | ICER versus Next Most Effective ($) | ESRD Lifetime Incidencea | |
| Usual care | 148,200 | 16.537 | 82 | 150,300 | 18.010 | 35 | ||||
| Universal (yr) | ||||||||||
| 10 | 148,400 | 16.567 | 9000 | 79 | 150,500 | 18.022 | 17,000 | 33 | ||
| 5 | 148,500 | 16.571 | 11,000 | 24,000 | 78 | 150,700 | 18.025 | 23,000 | 54,000 | 33 |
| 2 | 148,900 | 16.574 | 19,000 | 113,000 | 78 | 151,000 | 18.027 | 44,000 | 210,000 | 33 |
| 1 | 149,400 | 16.575 | 33,000 | 635,000 | 78 | 151,700 | 18.027 | 81,000 | 1,571,000 | 33 |
Lifetime incidence of initiating ESRD care per 1000 persons.
Table 4 lists the cost, QALY, ICER, and ESRD incidence results of annual screening for the populations with major risk factors. For African Americans, microalbuminuria screenings for all risk factor subpopulations lead to an ICER below $35,000 per QALY compared with usual care. For non-African Americans, the ICERs are higher for all risk factor subpopulations.
Table 4.
Cost-effectiveness and ESRD lifetime incidence results of microalbuminuria screening, by risk factor
| All African Americans | All Non-African Americans | |||||||
|---|---|---|---|---|---|---|---|---|
| Costs ($) | QALY | ICER versus Usual Care ($) | ESRD Lifetime Incidencea | Costs ($) | QALY | ICER versus Usual Care ($) | ESRD Lifetime Incidencea | |
| Diabetes diagnosed at age 50 yr | ||||||||
| Usual care | 180,300 | 15.195 | 184 | 185,200 | 16.640 | 121 | ||
| Universal (1 yr) | 180,800 | 15.224 | 19,000 | 181 | 186,000 | 16.659 | 43,000 | 119 |
| Hypertension (SBP ≥145) | ||||||||
| Usual care | 153,800 | 16.040 | 110 | 155,800 | 17.394 | 53 | ||
| Universal (1 yr) | 154,600 | 16.079 | 21,000 | 106 | 156,900 | 17.420 | 40,000 | 50 |
| Neither at age 50 yr | ||||||||
| Usual care | 144,100 | 17.438 | 45 | 148,200 | 18.623 | 17 | ||
| Universal (1 yr) | 145,800 | 17.485 | 35,000 | 41 | 149,700 | 18.638 | 106,000 | 15 |
Lifetime incidence of initiating ESRD care per 1000 persons.
Sensitivity Analyses
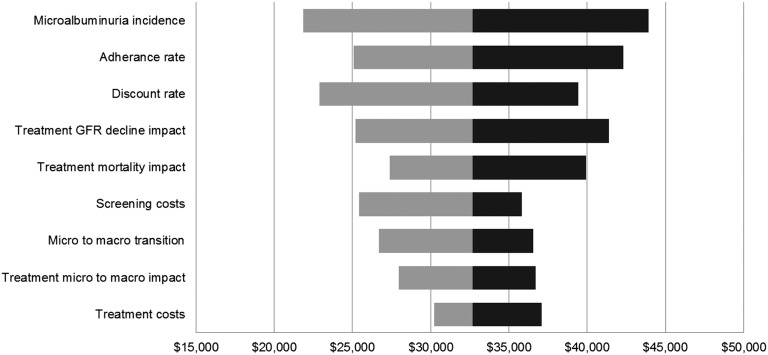
In the one-way sensitivity analysis, we recalculated the cost-effectiveness of microalbuminuria screening for African Americans by increasing and decreasing major parameter point estimates by 25% (Figure 1). We varied the discount rate between 0 and 5%. In a comparison of annual universal microalbuminuria screening versus usual care, all sensitivity analysis scenarios remained under $50,000 per QALY. The results were most sensitive to changes in microalbuminuria incidence rates, treatment adherence rates, and the discount rate. Results were least sensitive to treatment costs, the effect of treatment on slowing progression from micro- to macroalbuminuria, and the normal rate of micro- to macroalbuminuria progression.
Figure 1.
Sensitivity analysis: microalbuminuria screening in African Americans. One-way sensitivity analysis tornado diagram indicates the range of cost-effectiveness ratios associated with a 25% increase and decrease in each parameter value. The baseline cost-effectiveness ratio is $33,000 per QALY.
Discussion
Despite comparable prevalence of early stage CKD, African Americans face a greater risk of ESRD than other racial groups. The mechanism of this increased rate of CKD progression among African Americans is unknown. Our study is the first that we are aware of to use a simulation model to explore the differences in progression between African Americans and non-African Americans.
Our model associates certain risk factors with elevated rates of GFR decline, including diabetes, hypertension, and elevated albuminuria. We adapted the model to reflect the different prevalence of these risk factors by race. We found that racial differences in risk factor prevalence increased the relative progression rates of African Americans, but did not come close to accounting for the substantially higher lifetime ESRD incidence observed among African Americans.
We calibrated the progression of CKD by applying multipliers to increase annual decline in eGFR among African Americans with eGFR ≤60 ml/min per 1.73 m2 and decrease the annual decline in eGFR among non-African Americans with eGFR ≤60 ml/min per 1.73 m2. The eGFR multipliers (+ 20% at stage 3 and + 60% at stage 4 for African Americans) were applied to the total annual eGFR decrement. By incorporating race-specific risk factor prevalence rates and the race-specific multipliers, we were able to closely replicate observed lifetime incidence of ESRD estimated from USRDS data while closely matching prevalence of stages 3, 4, and 5 identified in NHANES data. However, for African Americans, we do overestimate total CKD prevalence, in particular stages 1 and 2.
The study has several limitations. First, simulation modeling extrapolates and synthesizes results from disparate sources and different, typically shorter, periods of observation to build a complete framework of disease natural history. This approach exposes the analysis to risk associated with forecasting events or effects beyond the observed period or applying results to a dissimilar population. Second, limited data exist about the relationship between CKD and the risk factors and complications included in this model, especially for African Americans. Third, we assumed that therapy with ACE/ARBs produces the same relative risk reduction in persons with microalbuminuria and neither diabetes nor hypertension as the therapies produce in persons with microalbuminuria and diabetes and/or hypertension. In studies of ACE/ARB efficacy, most patients have either diabetes or hypertension, so there is limited evidence on the efficacy in patients without these conditions. Fourth, we lacked data to control for differences in access to care between African Americans and other races. Finally, our calibration approach would be more compelling if there were a clear reason why African Americans progress more rapidly than non-African Americans in CKD stages 3 and higher. Genetic differences may play a role.
Although we were unable to account for differential progression rates by race without assuming calibrated GFR multipliers, our close match to the important external validation target of USRDS observed lifetime ESRD rates lends credence to the applicability of our model for estimating the differential cost-effectiveness of screening for microalbuminuria by race. We find that screening African Americans results in favorable cost-effectiveness results, comparable with or even better than the results found when screening the entire diabetes population. This analysis also refines our previous results by more accurately controlling for mortality and effectively increasing the micro- and macroalbuminuria prevalence for all races. As a result, the cost-effectiveness results for screening non-African Americans remain closely in line with previous results for screening the entire population, even though this analysis includes eGFR multipliers to reduce progression rates among non-African Americans with CKD stages 3 and above to yield lower ESRD rates.
This analysis reinforces earlier evidence that the higher prevalence of CKD-associated risk factors among African Americans does not fully explain the much higher incidence of ESRD observed in this population. Comparing our model results to observed CKD stage prevalence and ESRD incidence, we hypothesize that African Americans exhibit accelerated loss of GFR in CKD stages 3, 4, and 5. We calibrated eGFR multipliers such that the model recreates estimated lifetime ESRD incidence rates for African Americans and non-African Americans. This analysis does not seek to explain the causes and mechanisms leading to differential progression rates. The faster GFR rates of decline for AA account for, but do not explain, the higher incidence of ESRD among African Americans.
When considering screening interventions, we find that screening African Americans results in cost-effectiveness ratios better than those estimated for screening non-African-American populations with similar risk factors. We find that screening non-African Americans with diabetes or hypertension remains highly cost-effective; among non-African Americans without risk factors, annual screening exhibits relatively poor cost-effectiveness. For both racial groups, screening less frequently than annually may improve cost-effectiveness ratios.
Concise Methods
Model Overview
We adapted the CKD Health Policy Model, a microsimulation model of the natural history of CKD, by modifying the model to include racial differences (Supplemental Material). The model, described in detail elsewhere,6,7 simulates the natural history of CKD progression among a cohort of persons from age 30 years until age 90 years or death. The model includes seven states: no CKD, CKD stages 1–5, and death. CKD stages are defined by GFR and the presence of kidney damage/albuminuria following Kidney Disease Outcomes Quality Initiative guidelines. Disease parameters are derived from the epidemiologic literature, clinical trials, and a previous cost-effectiveness study by Boulware et al.4
Progression through CKD stages is governed by kidney damage status and eGFR. Our model tracks kidney damage based on persistent microalbuminuria (sustained albumin/creatinine ratio [ACR] between 30 and 299 mg/g) and macroalbuminuria (ACR ≥300 mg/g). We simulated kidney damage by assigning the prevalence of microalbuminuria at age 30 years and then including an annual incidence of persistent microalbuminuria and an annual rate of progression from micro- to macroalbuminuria. Previously, these rates were based on age, sex, diabetes, and hypertension status. eGFR serves as the other primary variable for tracking progression of CKD between stages. In the model, a person is assigned an initial eGFR value and then experiences annual decrements in eGFR based on certain risk factors derived from clinical trials; decrements vary based on diabetes, hypertension, and albuminuria status.4 Annual decline in eGFR increases upon incidence of microalbuminuria and increases again upon transition to macroalbuminuria. Because of a lack of data, we assumed the effect on progression due to a particular risk factor does not vary by race.
Death is simulated by assigning each individual an annual background mortality rate, CKD multipliers reflecting the elevated mortality risk from CKD, cardiovascular disease mortality rates determined by myocardial infarction and stroke events, and ESRD mortality rates. Risk factors (diabetes status, systolic BP and hypertension, left ventricular hypertrophy, total and HDL cholesterol, and smoking status), and medical events (stroke and coronary heart disease, including myocardial infarction and angina) are simulated annually based on probability functions. We assume that patients who survive 1 year in stage 5 begin ESRD.
Incorporating Race-Specific Risk Factor Prevalence and Progression Parameters
For this analysis, we updated the model to account for racial differences between African Americans and non-African Americans. We defined race-specific parameters for risk factors and background mortality rates.
We analyzed 1999–2006 NHANES data to identify the prevalence of micro- and macroalbuminuria based on race, hypertension, diabetes, sex, and age. We estimated persistent microalbuminuria based on rates from Coresh et al.1 We found that African Americans experience higher prevalence of micro- and macroalbuminuria at all ages. We estimated GFR values (baseline distribution and annual rate of decline) with NHANES data using the MDRD equation.
Intervention
This analysis simulates screening for microalbuminuria followed by treatment with ACE inhibitors or ARBs among persons aged ≥50 years. Interventions are defined by the screening rate used and frequency of screening. Screening interventions were compared with a “usual care” scenario that introduces actual annual probabilities of microalbuminuria screening: 23%, 22%, 2%, and 0% for patients with diabetes and hypertension, diabetes only, hypertension only, and neither diabetes nor hypertension, respectively.12 We then introduced population-based screening with 1-, 2-, 5-, or 10-year intervals beginning at age 50 years. The sensitivity of screening is 73% for microalbuminuria and 76% for macroalbuminuria and has a specificity of 96%.13 To account for nonadherence, 75% of persons were assumed to initiate treatment after diagnosis.4 The effect of ACE/ARB therapy on persons with microalbuminuria is assumed to be a 55% reduction in the probability of progression from micro- to macroalbuminuria.14 For persons with macroalbuminuria, treatment is assumed to provide a 32.7% reduction in annual GFR decrement and a 23% reduction in annual mortality rates.15–17 Once patients reach ESRD, costs and mortality are assumed to equal observed USRDS data.
Costs
Early CKD stage costs are derived from cost function estimates for a privately insured population.18 For the 1-year period in stage 5 CKD before ESRD, costs were estimated as a combination of 6 months of stage 4 costs and the costs for the 6 months just before ESRD initiation using costs from USRDS. Estimates from the USRDS 2006 Annual Data Report were used to calculate first and subsequent year ESRD costs.12 Screening costs are based on Centers for Medicare and Medicaid Services physician and laboratory reimbursement rates. Future costs were discounted at a 3% annual rate19; all costs are reported in 2006 US dollars.
QALYs
The summary effectiveness outcome measure was QALYs. We obtained utility values for the different GFR levels from a time trade-off analysis by Gorodetskaya et al.20; the utility values did not vary by race. We included a utility decrement of 0.01 for macroalbuminuria.4 QALYs were discounted 3% annually.
Validation and Calibration
We validated CKD stage progression by comparing average stage prevalence rates from the model to those identified in NHANES data. We also compared lifetime incidence rates of ESRD to rates calculated based on USRDS ESRD initiation counts. We calibrated albuminuria and eGFR parameters concurrently to minimize differences between model output and the external validation targets. Microalbuminuria incidence and micro- to macroalbuminuria transition probabilities were solved such that the model replicated NHANES prevalence rates. This calibration process effectively increases the macroalbuminuria prevalence for African Americans and non-African Americans alike, relative to rates used in our earlier analyses. We solved for eGFR decrement multipliers in stages 3 and 4 such that the model recreates lifetime ESRD incidence rates. We also considered other calibration options.
Disclosures
None.
Supplementary Material
Acknowledgments
This research was supported by funding from the Centers for Disease Control and Prevention (CDC) (Contracts 200-2002-00776 and 200-2008-F-27527). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012040347/-/DCSupplemental.
See related editorial, “Kidney Disease Progression and Screening Cost-Effectiveness among African Americans,” on pages 1915–1916.
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 2.US Renal Data System (USRDS) : USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2011 [Google Scholar]
- 3.National Kidney Foundation : Guideline 5: Assessment of proteinuria. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification and stratification. Am J Kidney Dis 39: S5–S266, 2002 [PubMed] [Google Scholar]
- 4.Boulware LE, Jaar BG, Tarver-Carr ME, Brancati FL, Powe NR: Screening for proteinuria in US adults: A cost-effectiveness analysis. JAMA 290: 3101–3114, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Palmer AJ, Valentine WJ, Chen R, Mehin N, Gabriel S, Bregman B, Rodby RA: A health economic analysis of screening and optimal treatment of nephropathy in patients with type 2 diabetes and hypertension in the USA. Nephrol Dial Transplant 23: 1216–1223, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Hoerger TJ, Wittenborn JS, Segel JE, Burrows NR, Imai K, Eggers P, Pavkov ME, Jordan R, Hailpern SM, Schoolwerth AC, Williams DE, Centers for Disease Control and Prevention CKD Initiative : A health policy model of CKD: 1. Model construction, assumptions, and validation of health consequences. Am J Kidney Dis 55: 452–462, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Hoerger TJ, Wittenborn JS, Segel JE, Burrows NR, Imai K, Eggers P, Paykov ME, Jordan R, Hailpern SM, Schoolwerth AC, Williams DE; Centers for Disease Control and Prevention CKD Initiative: A health policy model of CKD: 2. The cost-effectiveness of microalbuminuria screening. Am J Kidney Dis 55: 463–473, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention : National Chronic Kidney Disease Fact Sheet: General Information and National Estimates on Chronic Kidney Disease in the United States, 2010, Atlanta, GA, US Department of Health and Human Services, Centers for Disease Control and Prevention, 2010 [Google Scholar]
- 9.Clase CM, Garg AX, Kiberd BA: Prevalence of low glomerular filtration rate in nondiabetic Americans: Third National Health and Nutrition Examination Survey (NHANES III). J Am Soc Nephrol 13: 1338–1349, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Hunsicker LG, Adler S, Caggiula A, England BK, Greene T, Kusek JW, Rogers NL, Teschan PE: Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study. Kidney Int 51: 1908–1919, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Hsu CY, Lin F, Vittinghoff E, Shlipak MG: Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol 14: 2902–2907, 2003 [DOI] [PubMed] [Google Scholar]
- 12.US Renal Data System (USRDS) : USRDS 2006 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2006, p 208 [Google Scholar]
- 13.Sarafidis PA, Riehle J, Bogojevic Z, Basta E, Chugh A, Bakris GL: A comparative evaluation of various methods for microalbuminuria screening. Am J Nephrol 28: 324–329, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Strippoli GF, Craig M, Deeks JJ, Schena FP, Craig JC: Effects of angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists on mortality and renal outcomes in diabetic nephropathy: Systematic review. BMJ 329: 828, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agodoa LY, Appel L, Bakris GL, Beck G, Bourgoignie J, Briggs JP, Charleston J, Cheek D, Cleveland W, Douglas JG, Douglas M, Dowie D, Faulkner M, Gabriel A, Gassman J, Greene T, Hall Y, Hebert L, Hiremath L, Jamerson K, Johnson CJ, Kopple J, Kusek J, Lash J, Lea J, Lewis JB, Lipkowitz M, Massry S, Middleton J, Miller ER, 3rd, Norris K, O’Connor D, Ojo A, Phillips RA, Pogue V, Rahman M, Randall OS, Rostand S, Schulman G, Smith W, Thornley-Brown D, Tisher CC, Toto RD, Wright JT, Jr, Xu S, African American Study of Kidney Disease and Hypertension (AASK) Study Group : Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: A randomized controlled trial. JAMA 285: 2719–2728, 2001 [DOI] [PubMed] [Google Scholar]
- 16.The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia) : Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet 349: 1857–1863, 1997 [PubMed] [Google Scholar]
- 17.Ruggenenti P, Perna A, Gherardi G, Garini G, Zoccali C, Salvadori M, Scolari F, Schena FP, Remuzzi G: Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet 354: 359–364, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Smith DH, Nichols GA, Gullion CM, Johnson ES, Keith D: Predicting costs of care in chronic kidney disease: The role of comorbid conditions. Internet J Nephrol 4: 2007
- 19.Gold MR, Siegel JE, Russell LB, Weinstein MC: Cost-Effectiveness in Health and Medicine, New York, Oxford University Press, 1996 [Google Scholar]
- 20.Gorodetskaya I, Zenios S, McCulloch CE, Bostrom A, Hsu CY, Bindman AB, Go AS, Chertow GM: Health-related quality of life and estimates of utility in chronic kidney disease. Kidney Int 68: 2801–2808, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.