Abstract
Calcium oxalate monohydrate crystals are responsible for the kidney injury associated with exposure to ethylene glycol or severe hyperoxaluria. Current treatment strategies target the formation of calcium oxalate but not its interaction with kidney tissue. Because aluminum citrate blocks calcium oxalate binding and toxicity in human kidney cells, it may provide a different therapeutic approach to calcium oxalate-induced injury. Here, we tested the effects of aluminum citrate and sodium citrate in a Wistar rat model of acute high-dose ethylene glycol exposure. Aluminum citrate, but not sodium citrate, attenuated increases in urea nitrogen, creatinine, and the ratio of kidney to body weight in ethylene glycol–treated rats. Compared with ethylene glycol alone, the addition of aluminum citrate significantly increased the urinary excretion of both crystalline calcium and crystalline oxalate and decreased the deposition of crystals in renal tissue. In vitro, aluminum citrate interacted directly with oxalate crystals to inhibit their uptake by proximal tubule cells. These results suggest that treating with aluminum citrate attenuates renal injury in rats with severe ethylene glycol toxicity, apparently by inhibiting calcium oxalate’s interaction with, and retention by, the kidney epithelium.
Ethylene glycol (EG) is a common household poison found in antifreeze, automotive engine coolants, and water-based latex paints. Approximately 5000 accidental or intentional EG ingestions occur per year in the United States, resulting in about 20–30 deaths.1 Acute EG poisoning can result in central nervous system depression, metabolic acidosis, acute renal failure, coma, and death.2 Ethylene glycol itself is nontoxic. However, the end metabolite, oxalate, is insoluble in the presence of calcium and forms oxalate crystals (primarily calcium oxalate monohydrate [COM]) that are deposited in the kidney tissue. Pathologic studies have shown that COM accumulation in the tubule correlates strongly with the degree of proximal tubule cell necrosis and with renal failure.3,4 Experiments using kidney cell cultures have convincingly shown that COM, and not the metabolites glycolate, glyoxylate, or ionic oxalate, is the metabolite responsible for the renal toxicity associated with EG poisoning.5–9 COM crystals can bind to kidney cell membranes and can be internalized by kidney cells,7,10–12 where they induce mitochondrial dysfunction leading to cell death.12–14 The ability to induce cell death is closely linked with the degree of cellular internalization of COM crystals.12
EG is metabolized fairly rapidly, so there is little time between ingestion and the formation of the toxic metabolites; thus, quick and aggressive treatment is required.2,15 With early diagnosis, inhibition of the enzyme alcohol dehydrogenase using fomepizole or ethanol can block the metabolism of EG, effectively preventing the formation of COM. If renal failure has already occurred, long-term hemodialysis (2–6 months) must be used to restore kidney function.2 Primary hyperoxaluria, a genetic disease caused by deficiencies in the glyoxalate-metabolizing enzymes, alanine-glyoxylate aminotransferase (type 1) or glyoxylate reductase/hydroxypyruvate reductase (type 2), also results in COM crystal deposits and ultimately kidney injury.16 Potassium citrate and sodium citrate, which raise the urinary excretion of citrate to chelate calcium and retard the formation of oxalate crystals,17 are used clinically to minimize crystal formation during hyperoxaluria and can be used to treat kidney stone recurrence,18 but neither citrate blocks the toxicity from COM per se.19 As such, for patients with renal damage associated with EG poisoning and primary hyperoxaluria, there are no good therapies to block the effects of COM crystals at the primary site of action in the kidney lumen and the subsequent renal damage.
Aluminum citrate has been shown to be a possible therapeutic agent by blocking COM toxicity in vitro and to operate by a mechanism of action unique from the citrate salts used clinically.19 Of the citrate salts (aluminum, calcium, ammonium, sodium, and potassium) tested against COM-induced cytotoxicity in human proximal tubule (HPT) cells, only aluminum citrate significantly reduces cell death.19 Also, treatment with aluminum chloride does not reduce COM-induced toxicity on kidney cells or erythrocytes, suggesting that efficacy is not due to the aluminum moiety but rather to aluminum complexed with citrate. Aluminum is primarily excreted by the kidneys, and when complexed with citrate, aluminum is freely filtered at the glomerulus and removed from the body.20 For the purposes of treating COM toxicity, the body’s propensity to filter aluminum citrate into the urine is ideal,21 so that it is present at the primary site of action in the proximal tubule lumen of the nephron. Aluminum accumulation has been linked to many diseases, including microcytic anemia, bone disease, and neurologic disorders.22 We are aware that aluminum citrate will probably never be a suitable drug candidate for treating COM toxicities because of the controversy surrounding its potential toxicities, but studies of aluminum citrate’s efficacy and mechanism of action are necessary for developing alternative drug therapies for diseases involving COM, including EG poisoning and severe hyperoxaluria. The purpose of this study was to examine the efficacy of aluminum citrate in treating COM toxicity in a rat model of acute EG poisoning and to understand the mechanism of this inhibition of toxicity.
Results
Aluminum Citrate Interacts with Crystals, Not with Cells, to Block COM Cytotoxicity
Although co-treatment with aluminum citrate decreases COM-induced cytotoxicity,19 the current studies were designed to determine whether aluminum citrate interacts directly with COM or acts on the HPT cells per se to block COM toxicity. Cells that were pretreated with buffer, then treated with aluminum citrate plus COM (COM/AC in Figure 1A) released less lactate dehydrogenase (LDH; 15 U/mg protein, similar to that in control-treated cells), indicating that aluminum citrate blocked COM toxicity. In contrast, cells that were pretreated with aluminum citrate, then with COM (AC/COM in Figure 1A) released as much LDH (55 U/mg protein) as that in the cells treated with COM alone (74 U/mg protein), indicating that aluminum citrate did not affect cells to block toxicity. Similar results were seen when toxicity was assessed by ethidium homodimer-1 uptake (not shown). At these concentrations, aluminum citrate itself has no effect on HPT cell viability.19
Figure 1.
Aluminum citrate interacts directly with COM crystals, and not with HPT cells, to decrease cytotoxicity (A) due to a decrease in internalization of COM crystals by PT cells (B). (A) HPT cells were treated with buffer (control) or with COM alone (735 µg/ml) for 4 hours. Additional cells (COM/AC) were preincubated with buffer for 30 minutes, which was removed before addition of a combination of COM (735 µg/ml) and aluminum citrate (0.8 mmol/L), or cells (AC/COM) were preincubated with aluminum citrate (0.8 mmol/L) for 30 minutes, which was removed before addition of COM (735 µg/ml) for the subsequent 4 hours of incubation. Buffers were collected and analyzed for LDH activity as described in the Concise Methods section. Data represent the group means ± SEM (n=4). *Significant difference from the control group (one-way ANOVA, followed by the Tukey post hoc test, P<0.05). (B) Rat and human PT cells were incubated with [14C]-COM (440 µg/ml) with or without aluminum citrate (0.1–0.4 mmol/L) for 0.5 hour, and internalization of COM was determined as described in the Concise Methods. Data represent the group means ± SEM (n=3). *Significant difference from the control group (COM + 0 mmol/L aluminum citrate) for each respective cell type (one-way ANOVA, followed by the Tukey post hoc test, P<0.05).
Aluminum Citrate Blocks Internalization of COM by PT Cells
Because the internalization of COM by proximal tubule (PT) cells correlates strongly with the degree of toxicity induced by COM,12 we assessed whether aluminum citrate inhibited the internalization of COM by PT cells as a mechanism for decreasing COM toxicity. Data in Figure 1B show that aluminum citrate decreased COM internalization by HPT cells (significant at 0.4 mmol/L) as well as by rat PT cells from F344 and Wistar rats (both significant at 0.1 mmol/L).
Pilot In Vivo Experiments
An EG dose-response experiment using single acute doses from 2 to 6 g/kg was conducted to determine the most appropriate dose of EG that would significantly increase markers of kidney injury. Only the 6-g/kg EG dose resulted in increased kidney to body weight ratio and increased BUN from 48 to 96 hours (Supplemental Figure 1). Therefore, an EG dose of 6 g/kg was used for experiments for the efficacy of aluminum citrate with the endpoint at 72 hours.
A pilot study was also conducted to examine the safety of aluminum citrate and its potential therapeutic utility in treating COM-related kidney injuries. In this study, aluminum citrate (0.2 mmol/kg), administered via intravenous infusion six times in 72 hours, did not produce renal toxicity: no increase in BUN or kidney to body weight ratio (Supplemental Figure 2), no evidence of histopathologic findings (not shown), and no increase in urinary γ-glutamyltransferase excretion (not shown). This dosing schedule of aluminum citrate appeared to slightly reduce kidney injury associated with EG ingestion, but it was significantly more effective than sodium citrate, which enhanced the renal injury (Supplemental Figure 2).
Study of Aluminum Citrate Efficacy
Levels of BUN and plasma creatinine, as well as the kidney to body weight ratio, at 72 hours were used as markers of kidney injury. Treatment with EG alone significantly increased all three measures of injury (P<0.05; Figure 2), whereas intervention with aluminum citrate blocked increases in all three measures (levels in the EG plus aluminum citrate group were not different from those in the control group; P>0.05; Figure 2). In contrast (Figure 3, A and B), sodium citrate administration did not reduce the renal toxicity of EG and instead slightly exacerbated the effects of EG on BUN and kidney to body weight ratio.
Figure 2.
Aluminum citrate reduces markers of kidney injury in EG-treated rats. (A) Kidney to body weight ratio. (B) BUN. (C) Creatinine concentration. Rats were treated with control (water), EG (6 g/kg), or EG plus aluminum citrate (EG/AC; 0.2 mmol/kg repeatedly as described in the Concise Methods section). Data are presented as mean ± SEM, n=6. *Significant difference from control (P<0.05).
Figure 3.
Effect of sodium citrate (NaC) on EG-induced kidney injury (A and B) and on urinary excretion of calcium (C) or oxalate (D) compared with EG treatment only. Rats were treated with control (water), EG (6 g/kg), or EG plus sodium citrate (0.2 mmol/kg repeatedly as described in the Concise Methods section). Data represent the group means ± SEM (n=6, except n=5 for EG/sodium citrate). *Significant difference from control. #Significant difference from control and EG groups. (A) Two-way ANOVA with the Bonferroni post hoc test and (B–D) one-way ANOVA with the Tukey post hoc test, P<0.05.
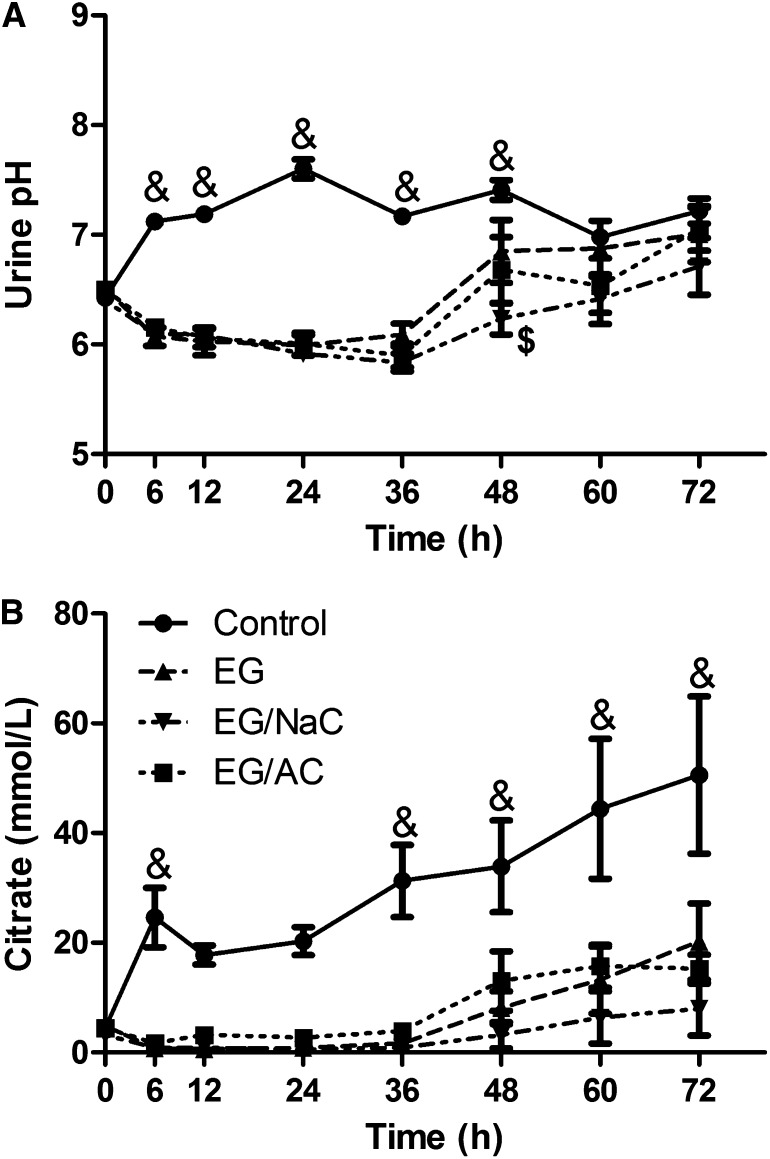
Metabolic acidosis is one of the hallmark signs of EG poisoning that results from the accumulation of glycolate in the blood.23 Urine pH also decreases because of the enhanced excretion of acid metabolites of EG, primarily glycolate. As such, urine pH was significantly decreased in rats treated with EG from 6 to 48 hours after EG dosing, with values returning to control levels by 60 hours for all animals (Figure 4A). Treatment with aluminum citrate or with sodium citrate did not alter the EG-induced decrease in urine pH at any time. Urinary excretion of citrate was reduced in the EG-treated rats from 6 to 36 hours, then increased to pretreatment levels concomitantly with the return of urine pH to normal values (Figure 4B). Neither aluminum citrate nor sodium citrate treatment significantly altered the excretion of citrate in EG-treated rats.
Figure 4.
EG-treated rats show decreased urinary pH (A) and decreased urinary citrate excretion (B), neither of which were altered by aluminum or sodium citrate treatment. Data are presented as mean ± SEM, n=5 for EG/sodium citrate group and n=6 for all other groups. &Significant difference from EG, EG/sodium citrate and EG/aluminum citrate groups. $Significant difference from EG group (P<0.05).
Calcium and Oxalate Excretion
Urine was separated into supernatant and pellet fractions to measure calcium and oxalate in the soluble (ionized) and insoluble (COM) forms, respectively. From 24 through 72 hours, rats treated with EG alone excreted significantly less soluble calcium than did both control rats and rats treated with EG plus aluminum citrate (Figure 5, A and C). Conversely, insoluble calcium excretion was increased above control levels in EG-treated rats at 12 and 24 hours only (Figure 5B). Intervention with aluminum citrate prolonged the elevated excretion of insoluble calcium until the last time point at 72 hours (Figure 3B). The total excretion of insoluble calcium was increased in EG-treated rats compared with controls (about 3.9-fold), and treatment with EG plus aluminum citrate further increased the total excretion of insoluble calcium (about 6.4-fold difference from control) (Figure 5C). Treatment with EG plus sodium citrate increased the excretion of soluble calcium compared with that in control and EG-treated rats (Figure 3C).
Figure 5.
Urinary excretion of calcium in the ionized/supernatant form (A), the COM/pellet form (B), and total calcium excreted over the entire time course of the experiment (C). Data are presented as mean ± SEM, n=6. *Significant difference from control. #Significant difference from both control and EG. $Significant difference from EG only (P<0.05). AC, aluminum citrate.
Urine oxalate concentrations, both soluble and pellet forms, were increased in rats treated with EG or EG plus aluminum citrate compared with controls (Figure 6). At 12 and 24 hours, soluble oxalate excretion was significantly lower in EG plus aluminum citrate–treated rats compared with rats that received EG alone but remained above control levels (P<0.05; Figure 6A). At 36 and 48 hours (Figure 6B), pellet oxalate excretion was significantly increased in EG plus aluminum citrate–treated rats compared with EG-treated rats and was increased compared with controls throughout the entire time course (P<0.05). In fact, the total amount of oxalate excreted in the urine pellet was increased by fourfold with EG treatment and further increased by eightfold with aluminum citrate treatment compared with controls (Figure 6C). Treatment with sodium citrate did not change the excretion of soluble or insoluble oxalate (Figure 3C). These data indicate that aluminum citrate, but not sodium citrate, increased the excretion of crystalline calcium and oxalate after EG exposures.
Figure 6.
Urinary excretion of oxalate in the ionized/supernatant form (A), the COM/pellet form (B), and total oxalate excreted over the entire time course of the experiment (C). Data are presented as mean ± SEM, n=6. *Significant difference from control. #Significant difference from both control and EG.
Histopathologic Findings
To evaluate whether aluminum citrate treatment changed the distribution or retention of COM crystals in the kidney tissues, histopathologic analysis was performed. Viewed under polarized light, more crystals were apparent in the tissues of EG-treated rats than those of EG plus aluminum citrate–treated rats (Figure 7). Crystal retention in the cortical tissue of the kidneys was quantified (Table 1). No crystals were found in control-treated rats, whereas about 7% of tubules in EG-treated rats contained crystals. Treatment with aluminum citrate decreased the percentage of tubules with crystals to about 1%. Consistent with the cortical analysis, aluminum citrate treatment was also associated with decreased crystal retention in the medulla.
Figure 7.
Representative images of hematoxylin and eosin–stained kidney cortical tissue of control, EG and EG plus aluminum citrate–treated rats visualized under polarized light. 100× magnification shows extensive crystal deposition along with swollen tubules in EG-treated rats but not in EG plus aluminum citrate–treated rats. 200× and 400× magnification shows the accumulation of crystals closely associated with tubule epithelium and shows that aluminum citrate treatment appears to reduce the accumulations of large crystal agglomerations compared with EG-treated rats.
Table 1.
Quantification of crystals in kidney cortical and medullary tissue.
| Experimental Group | Cortical Tubules Containing Crystals (%) | Crystals in Medulla |
|---|---|---|
| Control | 0.00±0.00 | 0.17±0.17 |
| EG | 7.25±1.81a | 2.50±0.22 |
| EG/aluminum citrate | 1.26±0.57b | 1.67±0.33 |
The percentage of tubules containing crystals from kidney cortical tissue only was assessed by counting the number of tubules containing crystals from five fields of view per animal and dividing by the average number of tubules per field of view. To assess crystal presence in the medulla, tissues were scored to reflect the relative accumulation of crystals in the medullary tissue: 0 indicated no crystals and 3 indicated extensive amounts of crystals. Data are represented as mean ± SEM, n=6 for control and EG, n=5 for EG/aluminum citrate.
Significant difference from control (P<0.05).
Significant difference from EG (P<0.05).
Discussion
EG poisoning affects thousands of people per year, and without prompt treatment, long-lasting kidney injury is of major concern. For patients with chronic renal insufficiency from EG poisoning due to marked accumulation of COM crystals in the kidney, the only therapy consists of long-term hemodialysis, with no pharmacologic alternatives. Other kidney diseases involving severe hyperoxaluria, such as primary hyperoxaluria, result in increased COM formation and renal deposition leading to kidney injury,24,25 for which there is inadequate pharmacologic treatment. Aluminum citrate has been shown to block the cytotoxicity of COM in human proximal tubule cells in vitro.19 Studies in cultured PT cells have shown that aluminum citrate blocks COM toxicity by interacting with the crystals themselves and not with the cells, thus reducing the internalization of COM by PT cells. Previous studies have shown that the toxicity of COM in these cells is closely related to its internalization.12 Although these in vitro studies have been promising, whether the reduction in cellular crystal retention produced by aluminum citrate was efficacious in an in vivo model of EG poisoning remained unknown before this study.
There are three key steps in the etiology of COM-related kidney injury: COM formation, attachment to the epithelium, and internalization of the crystal by the cell, ultimately resulting in cell death. All three processes are necessary for the development of kidney injury and can be used as targets for therapeutic intervention. Crystal formation is largely governed by the urinary concentrations of various key electrolytes, including calcium, oxalate, magnesium, and citrate, along with urine volume. Increased urinary supersaturation of oxalate and an increased oxalate concentration relative to the other electrolytes correlates with increased likelihood of COM formation.26,27 Other factors, such as cellular injury, presence of inhibitory or promotional macromolecules, or proteins in the tubule and urinary citrate concentration, also regulate calcium oxalate crystal formation.28,29
Alkali citrates, primarily potassium citrate and magnesium potassium citrate, but also sodium citrate, have been widely used to prevent nephrolithiasis, although patient adherence with this therapy is poor because of gastrointestinal side effects.18 These citrates retard crystal formation by complexing with calcium in a soluble form, thus decreasing urinary supersaturation16,30,31 and also by modulating macromolecules associated with crystal formation.32 Indeed, in the present study, co-treatment with sodium citrate increased the excretion of soluble calcium in EG-treated rats, as would be expected from its ability to complex calcium. In contrast, aluminum citrate has a mechanism of action completely different from that of the currently used citrate salts because it interacts with already formed COM to block its binding, uptake, and toxicity by tubular cells.19 In situations with severe hyperoxaluria, such as the metabolism of EG to oxalate, the large increase in urinary supersaturation almost guarantees COM formation. As such, inhibitors of COM formation, such as acidic polyanions (e.g., osteopontin) and alkali citrates will probably not be useful in treating COM-induced kidney injury. The efficacy of aluminum citrate and the lack of efficacy of sodium citrate in these studies confirm that the key mechanism in reducing COM toxicity is blocking the retention of already formed COM, rather than decreasing crystal formation. It is interesting to note that alkaline citrate was given in a human case of EG poisoning to increase oxalate solubility, yet nephrocalcinosis still developed.33
Antidotal therapy using ethanol or fomepizole effectively prevents the formation of the acidic metabolites of EG, blocking both metabolic acidosis and kidney injury.34 Aluminum citrate did not lessen the acidosis (the decreased urine pH) in this study, indicating that the aluminum citrate–induced decreases in kidney toxicity were not related to an inhibition of metabolism of glycolate to oxalate. Also, aluminum citrate did not alter the urinary excretion of EG or of glycolate (not shown), further indicating no changes in EG metabolism. These data demonstrate that aluminum citrate effectively blocked the renal toxicity even in the presence of substantial acidosis, a finding suggesting usefulness in human EG poisonings with acidosis.
Because of the EG-induced acidosis and resulting decreased urine pH, the urinary excretion of citrate was markedly reduced in EG-treated rats, even in those administered aluminum citrate or sodium citrate. Several studies have shown that acidic urine promotes citrate reabsorption,18,35 which could explain the hypocitraturia even in rats treated with citrate. Although citrate administration should produce alkalosis and increase urine pH, the massive amounts of the EG metabolite glycolate that are excreted in the urine apparently overcome the citrate alkalinization.
The enhanced excretion of crystalline calcium and oxalate produced by aluminum citrate in these studies, along with decreased crystal retention in kidney tissues (Table 1 and Figure 7), suggest that aluminum citrate interfered with COM binding to kidney tissue, thus leading to enhanced COM excretion. Although this conclusion is strongly supported by the in vitro studies showing that aluminum citrate blocked accumulation of COM by kidney cells, it is also possible that aluminum citrate could decrease COM retention in kidney tissue by decreasing crystal growth and aggregation in the lumen. For example, aluminum citrate–treated rats might form smaller crystals, which would be less likely to be retained within the kidney. In vitro, aluminum citrate does slow the aggregation of COM in suspension.19 Also, the apparent decrease in size of the crystals deposited in EG plus aluminum citrate–treated rats (Figure 7, compare panel I to panel F) indicates that aluminum citrate might inhibit crystal growth, perhaps enhancing the excretion of small COM crystals that otherwise might attach to other crystals and be retained. Combined with the decreased kidney injury in these animals, the increased urinary excretion of COM showed that aluminum citrate effectively prevented COM-induced kidney injury even in the presence of large amounts of COM.
In this study, we examined the therapeutic efficacy of aluminum citrate in an acute model of EG exposure rather than the chronic EG exposure model that is often used to study long-term hyperoxaluria.36,37 We chose the acute model because it presents the worst-case scenario in assessing efficacy. In acute EG poisoning, there is severe acidosis as well as rapid and massive formation and accumulation of COM in kidney tissues,2 which leads to marked kidney injury within 48 hours (Supplemental Figure 1). One could presume that if aluminum citrate would work in such a scenario, it would probably be effective in a model featuring slower and lesser formation of COM.
In summary, in a rat model of severe EG poisoning, repeated dosing of aluminum citrate can substantially decrease nephrotoxicity by preventing COM crystal attachment to the kidney epithelium, thus enhancing the excretion of crystalline calcium oxalate. Thus, aluminum citrate or another similarly acting small molecule could be a useful adjunct therapy for EG poisoning and severe hyperoxaluria, or may prove useful in treating other COM-related kidney maladies. However, drug therapy containing aluminum will be a controversial treatment because of its link with conditions such as Alzheimer disease, microcytic anemia, and bone disease.38 As such, it is unlikely that aluminum citrate will ever be used treat COM-induced nephrotoxicity. Nevertheless, the unique mechanism of action by which aluminum citrate appears to act (blocking binding of already formed COM to epithelial cells or other binding sites) should prove to be an important target in treating COM-induced nephropathies in the future. Therefore, aluminum citrate can serve as an important tool in understanding interactions between COM and kidney epithelial cells and may lead to the development of safer therapeutics in the future.
Concise Methods
Animal Protocol
Adult male Wistar rats (weight, 425–475 g; Harlan, Indianapolis, IN) were implanted with chronic indwelling jugular catheters 10 days before the start of the experiment to minimize stress to the animal during blood collections and intravenous dosing.39 Catheters were flushed every other day with streptokinase and sterile heparinized saline until the start of the experiment, during which they were flushed after every collection. Animals were housed in metabolic cages for 12 hours before the initial EG dose to collect background urine samples and for the duration of the experiment (72 hours) for continued urine collection. Standard conditions of humidity, temperature (25°C±2°C), and light (12 hours light/12 hours dark) were maintained in the animal room, and all rats were allowed free access to food (normal rat chow) and water. All animal protocols were approved by the Institutional Animal Care Committee (Louisiana State University Health Sciences Center, Shreveport) and were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Urine Collection and Analysis
Urine collections were made over ice to minimize degradation of urinary metabolites. Immediately after collection, the urine volume and pH were recorded and aliquots were stored at −20°C until analysis.
Blood Collection
Approximately 1 mL of blood was collected via the indwelling jugular catheter into heparinized syringes and was immediately placed on ice. Whole blood was transferred to serum separator tubes and centrifuged to isolate the plasma, which was then stored at 4°C until analysis.
Design of Experiment for Efficacy of Aluminum Citrate and Sodium Citrate: Preliminary study
Catheterized male Wistar rats were placed into one of five treatment groups, including water-treated controls (n=6), EG (6 g/kg, n=6), EG plus aluminum citrate (0.2 mmol/kg, n=6), EG plus sodium citrate (0.2 mmol/kg, n=5), or water plus aluminum citrate (0.2 mmol/kg, n=6). EG or water was administered via oral gavage at time 0, and aluminum citrate, sodium citrate, or 0.9% normal saline (for citrate control) was administered via intravenous infusion at 6, 12, 24, 36, 48, and 60 hours. Animals were housed in metabolic cages so that urine was continuously collected at the designated time points, and blood samples were taken via the jugular catheter at times 0, 6, 12, 24, 36, 48, 60, and 72 hours. Urine volume and pH were recorded for each collection. Plasma BUN was measured as described below. At 72 hours, rats were anesthetized with sodium pentobarbital (50 mg/kg, intraperitoneal), the right kidney was collected and fixed in formalin, and the rats were euthanized via pneumothorax.
Design of Experiment for Efficacy of Aluminum Citrate: Main Study
Because preliminary experiments testing the efficacy of aluminum citrate and sodium citrate revealed that six doses of 0.2 mmol/kg aluminum citrate in 72 hours only moderately decreased kidney injury and similar doses of sodium citrate increased kidney injury (Supplemental Figure 2), the main study was designed to increase the total dose of aluminum citrate, which was accomplished by an increased frequency of dosing. Sodium citrate was not included in the main study because the doses in the preliminary study were already injurious. Animals were placed into three treatment groups, including control (n=6), EG (6 g/kg, n=6), and EG plus aluminum citrate (0.2 mmol/kg, n=6). EG or water for controls was gavaged at time 0, and saline (for control and EG groups) or aluminum citrate (154 mmol/L in 0.9% NaCl) was administered by slow intravenous bolus at 2, 6, and then every 6 hours until 72 hours. This schedule doubled the number of aluminum citrate doses, effectively doubling the total dose of aluminum citrate. Urine was collected every 12 hours, and blood was collected every 24 hours. To assess kidney injury, BUN and creatinine were measured in the plasma at 72 hours using the Flex reagent cartridge in the Dimension Vista System (Siemens Healthcare Diagnostics Inc., Newark, DE). At 72 hours, rats were anesthetized with sodium pentobarbital (50 mg/kg, intraperitoneal), the right kidney was collected and fixed in formalin for histopathologic analysis, and the rats were euthanized via pneumothorax.
Animals were monitored multiple times for outward signs of distress, including lack of responsiveness, decreased grooming and feeding behaviors, or respiratory distress. All rats treated with EG (with or without aluminum citrate) appeared intoxicated at the 6-hour time point, with lack of balance and motor control, but returned to normal by 12 hours. One rat, treated with EG alone, was extremely lethargic by 6 hours and by 24 hours had respiratory distress that necessitated euthanasia at 24 hours. All other rats receiving EG, water, or EG plus aluminum citrate showed no such signs of distress and were euthanized at the predetermined 72-hour time point.
Histopathologic Analysis
For each tissue, a 1-mm slice was fixed in 10% neutral buffered formalin, washed and dehydrated in isopropanol and xylene, and then embedded in molten paraffin wax. Sections of 4 µm were cut and stained with hematoxylin and eosin. Tissues were examined with light and polarizing microscopy to visualize tissue necrosis and to look for the presence of calcium oxalate crystals.
Calcium Determinations
Aliquots of urine were sieved using 800-µm nylon mesh to remove large food and fecal matter but allow passage of calcium oxalate crystals. The sieved urine samples were separated into supernatant and pellet fractions by centrifugation at 13,000 g for 10 minutes to isolate the ionized forms of calcium (supernatant) from the crystalline/COM form (pellet). Pellets were then resuspended in equivolume PBS, and 5 µl of 1 N HCl was added to each sample to acidify the fraction and dissolve the crystals. Calcium was measured spectrophotometrically in both the prepared supernatants and pellets according to a published Arsenazo III method.40
Oxalate Determinations
Whole urine samples were separated, as described for calcium determinations, into pellet and supernatant fractions, and oxalate was measured in each using a commercially available kit (Trinity Biotech, St. Louis, MO).
Citrate Determinations
Acidified whole urine samples were analyzed for citrate concentrations by the HPLC method described by Gu et al,41 with the following modifications. Acidified urine, diluted 10-fold in water and mixed with 1 mmol/L tartaric acid (internal standard), was applied to a C18 solid phase extraction column, washed and eluted with 20 mmol/L sulfuric acid. The eluate was then applied to a strong-anion exchange solid-phase extraction column, washed and eluted with 8 mmol/L sulfuric acid. HPLC separation was performed on a Kinetex C18 column (100 × 4.60 mm i.d., 2.6 μm particle size; Phenomenex) using a mobile phase of 20 mmol/L sulfuric acid (adjusted to pH 2.0 using 1 M ammonia) at a flow rate of 0.4 ml/min for 8 minutes, then increased to 1.0 ml/min for 45 min to facilitate the removal of late eluting peaks. Citrate was quantified by ultraviolet absorbance at 210 nm.
Cell Culture
These studies were conducted with cultures of normal HPT and rat PT cells because PT cells are the kidney cells primarily affected at the high levels of oxalate exposure, such as in cases of primary hyperoxaluria or after EG exposure.3,4 HPT cells were isolated from normal human kidney cortex tissue, which was obtained locally, after informed consent for nephrectomy due to tumor or trauma had been secured, or was provided by the Cooperative Human Tissue Network, which is funded by the National Cancer Institute. Tissue was judged normal in macroscopic appearance and then separated by the surgical pathologist. These studies were approved by the Institutional Review Board for Human Research at Louisiana State University Health Sciences Center, Shreveport. HPT cells were isolated by a collagenase-deoxyribonuclease digestion, filtration, and centrifugation technique that produces a suspension of primarily PT cells.42 To further limit the growth of contaminating cells and to enrich the population of PT cells, the suspensions were cultured on collagen-coated flasks in a serum-free mixture of DME/Ham’s F-12 (50/50) media with added growth factors (Insulin-Transferrin-Selenium, EGF, hydrocortisone, triiodothyronine, and l-glutamine) until confluence at 5–7 days, then subcultured to 24-well plates for experiments.42 HPT cells prepared in this way represent a population of cells that retain the properties of the PT, as indicated by enzyme activities, transport functions, hormonal responses, and immunohistochemistry.43,44
Normal rat PT cells were isolated by collagenase-deoxyribonuclease digestion of kidney cortex tissue, obtained under pentobarbital anesthesia from untreated Wistar and Fischer-344 rats. Isolated cells were cultured as described elsewhere45 on rat tail collagen–coated flasks in a serum-free medium and then subcultured for experimentation into 24-well plates.
PT Cell Treatment to Assess Cytotoxicity
Unlabeled COM crystals were prepared as described elsewhere.19 The average size of the COM crystals prepared in this manner ± SD is 2±0.6 μm.46 Free oxalate ion concentrations in the COM resuspensions were negligible, even when incubated for 6 hours at 37°C.5 The ability of externally applied COM to produce cell death in HPT cells was assessed as described previously,6,19 except to test whether aluminum citrate interacted with COM or with cells, HPT cells were preincubated with incubation buffer (in mmol/L, 107 NaCl, 5.3 KCl, 1.9 CaCl2, 1.0 MgCl2, 26.2 NaHCO3, 20 HEPES, 7 glucose, pH 7.4) or aluminum citrate (0.8 mmol/L) for 30 minutes at 37°C. After removal of these solutions, cells that had been pretreated with buffer were subsequently incubated with a mixture containing COM (735 μg/ml) and aluminum citrate (0.8 mmol/L), whereas cells that had been pretreated with aluminum citrate were subsequently treated with COM (735 µg/ml), all for 4 hours at 37°C. After treatment, the buffer was collected to measure LDH activity and then the cells were treated with ethidium homodimer. LDH release into the media by dying cells was assessed spectrophotometrically by the lactate to pyruvate reaction.19 The uptake of ethidium homodimer, which occurs only in damaged cells, was determined as described elsewhere.19
PT Cell Treatment to Quantitate COM Internalization
Internalization of COM was determined using an EDTA treatment described previously.12 [14C]-COM crystals were prepared by mixing an equal volume of 10 mmol/L CaCl2 with 10 mmol/L NaOX that had been spiked with 200,000 cpm of [14C]-oxalate per mL. The resulting labeled crystals were centrifuged at 3200 g, washed three times with deionized water, and resuspended in incubation buffer by gentle sonication to minimize crystal aggregation and produce a uniform particle size. PT cells were exposed to [14C]-COM (440 µg/ml) with or without aluminum citrate (0.1–0.4 mmol/L) for 0.5 hour at 37°C, at which temperature both binding and internalization may occur. After treatment, the cells were rinsed with cold PBS to remove unbound COM and then treated with EDTA (4 mmol/L) for 8 minutes to remove the COM that was bound externally to the cells. After the EDTA treatment, the cells were rinsed with cold PBS to complete the removal of bound radiolabel and the washed cells were solubilized with 0.5% Triton X-100 to determine the amount of internalized label by scintillation analysis.
Statistical Analyses
Differences between treatment groups and time points were assessed with two-way ANOVA with a Bonferroni post hoc test. To compare differences between treatment groups only, one-way ANOVA with a Tukey post hoc test was used. All analyses were performed using GraphPad Prism 5 for Windows. Tests were considered significant if P<0.05.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors would like to thank the National Kidney Foundation of Louisiana’s Francisco Gonzalez Memorial Research Fund for the financial support of the work conducted in this manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012040357/-/DCSupplemental.
References
- 1.Bronstein AC, Spyker DA, Cantilena LR, Jr, Green JL, Rumack BH, Giffin SL: 2009 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 27th Annual Report. Clin Toxicol (Phila) 48: 979–1178, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Jacobsen D, McMartin KE: Methanol and ethylene glycol poisonings. Mechanism of toxicity, clinical course, diagnosis and treatment. Med Toxicol 1: 309–334, 1986 [DOI] [PubMed] [Google Scholar]
- 3.Cruzan G, Corley RA, Hard GC, Mertens JJ, McMartin KE, Snellings WM, Gingell R, Deyo JA: Subchronic toxicity of ethylene glycol in Wistar and F-344 rats related to metabolism and clearance of metabolites. Toxicol Sci 81: 502–511, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Corley RA, Wilson DM, Hard GC, Stebbins KE, Bartels MJ, Soelberg JJ, Dryzga MD, Gingell R, McMartin KE, Snellings WM: Dosimetry considerations in the enhanced sensitivity of male Wistar rats to chronic ethylene glycol-induced nephrotoxicity. Toxicol Appl Pharmacol 228: 165–178, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Guo C, McMartin KE: The cytotoxicity of oxalate, metabolite of ethylene glycol, is due to calcium oxalate monohydrate formation. Toxicology 208: 347–355, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Guo C, Cenac TA, Li Y, McMartin KE: Calcium oxalate, and not other metabolites, is responsible for the renal toxicity of ethylene glycol. Toxicol Lett 173: 8–16, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Khan SR, Byer KJ, Thamilselvan S, Hackett RL, McCormack WT, Benson NA, Vaughn KL, Erdos GW: Crystal-cell interaction and apoptosis in oxalate-associated injury of renal epithelial cells. J Am Soc Nephrol 10[Suppl 14]: S457–S463, 1999 [PubMed] [Google Scholar]
- 8.Schepers MS, van Ballegooijen ES, Bangma CH, Verkoelen CF: Crystals cause acute necrotic cell death in renal proximal tubule cells, but not in collecting tubule cells. Kidney Int 68: 1543–1553, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Thamilselvan S, Hackett RL, Khan SR: Cells of proximal and distal tubular origin respond differently to challenges of oxalate and calcium oxalate crystals. J Am Soc Nephrol 10[Suppl 14]: S452–S456, 1999 [PubMed] [Google Scholar]
- 10.Lieske JC, Swift H, Martin T, Patterson B, Toback FG: Renal epithelial cells rapidly bind and internalize calcium oxalate monohydrate crystals. Proc Natl Acad Sci U S A 91: 6987–6991, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lieske JC, Norris R, Swift H, Toback FG: Adhesion, internalization and metabolism of calcium oxalate monohydrate crystals by renal epithelial cells. Kidney Int 52: 1291–1301, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Hovda KE, Guo C, Austin R, McMartin KE: Renal toxicity of ethylene glycol results from internalization of calcium oxalate crystals by proximal tubule cells. Toxicol Lett 192: 365–372, 2010 [DOI] [PubMed] [Google Scholar]
- 13.McMartin KE, Wallace KB: Calcium oxalate monohydrate, a metabolite of ethylene glycol, is toxic for rat renal mitochondrial function. Toxicol Sci 84: 195–200, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Cao LC, Honeyman TW, Cooney R, Kennington L, Scheid CR, Jonassen JA: Mitochondrial dysfunction is a primary event in renal cell oxalate toxicity. Kidney Int 66: 1890–1900, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Sivilotti ML, Burns MJ, McMartin KE, Brent J, For the Methylpyrazole for Toxic Alcohols Study Group : Toxicokinetics of ethylene glycol during fomepizole therapy: implications for management. Ann Emerg Med 36: 114–125, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Hoppe B, Beck BB, Milliner DS: The primary hyperoxalurias. Kidney Int 75: 1264–1271, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Shall H, Jeon J, Abdel-Aal EA, Khan S, Gower L, Rabinovich Y: A study of primary nucleation of calcium oxalate monohydrate: II. Effect of urinary species. Cryst Res Technol 39: 222–229, 2004 [Google Scholar]
- 18.Mattle D, Hess B: Preventive treatment of nephrolithiasis with alkali citrate—a critical review. Urol Res 33: 73–79, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Guo C, McMartin KE: Aluminum citrate inhibits cytotoxicity and aggregation of oxalate crystals. Toxicology 230: 117–125, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Lote CJ, Willmott K, Wood JA, Thewles A, Freeman M: Renal excretion of aluminium in the rat: Effect of citrate infusion. Hum Exp Toxicol 14: 945–948, 1995 [DOI] [PubMed] [Google Scholar]
- 21.Shirley DG, Lote CJ: Renal handling of aluminium. Nephron Physiol 101: 99–103, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Hewitt CD, Savory J, Wills MR: Aspects of aluminum toxicity. Clin Lab Med 10: 403–422, 1990 [PubMed] [Google Scholar]
- 23.Jacobsen D, Ovrebø S, Ostborg J, Sejersted OM: Glycolate causes the acidosis in ethylene glycol poisoning and is effectively removed by hemodialysis. Acta Med Scand 216: 409–416, 1984 [PubMed] [Google Scholar]
- 24.Coulter-Mackie MB, White CT, Hurley RM, Chew BH, Lange D: Primary hyperoxaluria type 1. In: GeneReviews, edited by Pagon RA, Bird TD, Dolan CR, Stephens K, Adam MP, Seattle, WA, University of Washington, 2011 [Google Scholar]
- 25.Liang L, Chen J, Vittal R, Selvanayagam ZE, McAteer JA, Deng L, Tischfield J, Chin KV, Sahota A: Expression profiling of crystal-induced injury in human kidney epithelial cells. Nephron Physiol 103: 53–62, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Khan SR, Finlayson B, Thomas WC, Jr, Hackett RL: Relationship between experimentally induced crystalluria and relative supersaturation of various stone salts in rats. Urol Res 12: 271–273, 1984 [DOI] [PubMed] [Google Scholar]
- 27.Werness PG, Brown CM, Smith LH, Finlayson B: EQUIL2: a BASIC computer program for the calculation of urinary saturation. J Urol 134: 1242–1244, 1985 [DOI] [PubMed] [Google Scholar]
- 28.Vervaet BA, Verhulst A, De Broe ME, D’Haese PC: The tubular epithelium in the initiation and course of intratubular nephrocalcinosis. Urol Res 38: 249–256, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Khan SR, Kok DJ: Modulators of urinary stone formation. Front Biosci 9: 1450–1482, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Hamm, LL, Hering-Smith, KS: Pathophysiology of hypocitraturic nephrolithiasis. Endocrinol Metab Clin North Am 31: 885–893, 2002 [DOI] [PubMed]
- 31.Pak CY: Citrate and renal calculi: New insights and future directions. Am J Kidney Dis 17: 420–425, 1991 [DOI] [PubMed] [Google Scholar]
- 32.Erwin DT, Kok DJ, Alam J, Vaughn J, Coker O, Carriere BT, Lindberg J, Husserl FE, Fuselier H, Jr, Cole FE: Calcium oxalate stone agglomeration reflects stone-forming activity: Citrate inhibition depends on macromolecules larger than 30 kilodalton. Am J Kidney Dis 24: 893–900, 1994 [DOI] [PubMed] [Google Scholar]
- 33.Stapenhorst L, Hesse A, Hoppe B: Hyperoxaluria after ethylene glycol poisoning. Pediatr Nephrol 23: 2277–2279, 2008 [DOI] [PubMed] [Google Scholar]
- 34.McMartin KE: Antidotes for alcohol and glycol toxicity: Translating mechanisms into treatments. Clin Pharmacol Ther 88: 400–404, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Simpson DP: Citrate excretion: A window on renal metabolism. Am J Physiol 244: F223–F234, 1983 [DOI] [PubMed] [Google Scholar]
- 36.Green ML, Hatch M, Freel RW: Ethylene glycol induces hyperoxaluria without metabolic acidosis in rats. Am J Physiol Renal Physiol 289: F536–F543, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Khan SR, Johnson JM, Peck AB, Cornelius JG, Glenton PA: Expression of osteopontin in rat kidneys: Induction during ethylene glycol induced calcium oxalate nephrolithiasis. J Urol 168: 1173–1181, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Becaria A, Campbell A, Bondy SC: Aluminum as a toxicant. Toxicol Ind Health 18: 309–320, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Goeders NE, Guerin GF: Effects of surgical and pharmacological adrenalectomy on the initiation and maintenance of intravenous cocaine self-administration in rats. Brain Res 722: 145–152, 1996 [DOI] [PubMed] [Google Scholar]
- 40.Leary NO, Pembroke A, Duggan PF: Measuring albumin and calcium in serum in a dual test with the Hitachi 704. Clin Chem 38: 1342–1345, 1992 [PubMed] [Google Scholar]
- 41.Gu Y, Wang GJ, Sun JG, Zhang XY, Sai Y: A new method for plasma citrate determination by reversed-phase high-performance liquid chromatography after ultrafiltration extraction: An example for bioequivalence evaluation of a medicinal endogenous substance. Methods Find Exp Clin Pharmacol 30: 513–520, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Todd JH, McMartin KE, Sens DA: Enzymatic isolation and serum-free culture of human renal cells retaining properties of proximal tubule cells. In: Human Cell Culture Protocols, edited by Jones GE, New York, Humana Press, 1996, pp 431–435 [DOI] [PubMed] [Google Scholar]
- 43.Blackburn JG, Hazen-Martin DJ, Detrisac CJ, Sens DA: Electrophysiology and ultrastructure of cultured human proximal tubule cells. Kidney Int 33: 508–516, 1988 [DOI] [PubMed] [Google Scholar]
- 44.Detrisac CJ, Sens MA, Garvin AJ, Spicer SS, Sens DA: Tissue culture of human kidney epithelial cells of proximal tubule origin. Kidney Int 25: 383–390, 1984 [DOI] [PubMed] [Google Scholar]
- 45.Sikka PK, McMartin KE: Normal rat kidney proximal tubule cells in primary and multiple subcultures. In Vitro Cell Dev Biol Anim 32: 285–291, 1996 [DOI] [PubMed] [Google Scholar]
- 46.Verkoelen CF, Romijn JC, de Bruijn WC, Boevé ER, Cao LC, Schröder FH: Association of calcium oxalate monohydrate crystals with MDCK cells. Kidney Int 48: 129–138, 1995 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.