Abstract
Vitamin D and its analogs have antiproteinuric activity and podocytes express the vitamin D receptor, but whether vitamin D signaling in podocytes accounts for this renoprotection is unknown. To investigate this question, we used the 2.5 kb podocin promoter to target Flag-tagged human vitamin D receptor (hVDR) to podocytes in DBA/2J mice. After the induction of diabetes with streptozotocin, transgenic mice had less albuminuria than wild-type controls. In transgenic mice, a low dose of the vitamin D analog doxercalciferol prevented albuminuria, markedly attenuated podocyte loss and apoptosis, and reduced glomerular fibrosis, but it had little effect on the progression of diabetic nephropathy in wild-type mice. Moreover, reconstitution of VDR-null mice with the hVDR transgene in podocytes rescued VDR-null mice from severe diabetes-related renal damage. In culture, 1,25-dihydroxyvitamin D suppressed high-glucose–induced apoptosis of podocytes by blocking p38- and ERK-mediated proapoptotic pathways. Taken together, these data provide strong evidence that vitamin D/VDR signaling in podocytes plays a critical role in the protection of the kidney from diabetic injury.
Podocytes play a key role in the regulation of glomerular filtration in the kidney. The foot processes of podocytes are an integral part of the glomerular filtration barrier that keeps proteins and other large molecules from being filtered into the urine. Podocytes synthesize proteins that are key components of the slit diaphragm formed between adjacent interdigitating foot processes that functions as the major size- and charge-selective barrier to protein leakage.1 Therefore, podocyte injury, loss, or death leads to albuminuria, a major risk factor for the progression of CKD, renal failure, cardiovascular events, and death.2
A body of literature has documented the antiproteinuric activity of vitamin D and its analogs.3 Vitamin D insufficiency is associated with increased prevalence of albuminuria in the general population.4 High prevalence of vitamin D deficiency is common in patients with CKD,5 mainly as a result of renal dysfunction and abnormal vitamin D metabolism.6 A number of recent randomized clinical trials have confirmed the antiproteinuric activity of vitamin D analogs in diabetic patients with CKD.7,8 Potent antiproteinuric activity of vitamin D has also be demonstrated in a variety of animal models of kidney disease.3 Treatment with 1,25-dihydroxyvitamin D (1,25(OH)2D3) or activated vitamin D analogs reduced albuminuria and prevented podocyte injury in 5/6 nephrectomized rats,9–11 puromycin aminonucleoside-induced podocyte apoptosis,12 and adriamycin-induced nephropathy.13 We reported that vitamin D analog therapy reduced albuminuria and prevented podocyte loss in experimental models of type 1 and type 2 diabetes.14–16 We also showed that podocytes express the vitamin D receptor (VDR) that is highly inducible by 1,25(OH)2D3,17 1,25(OH)2D3 transcriptionally stimulated the expression of nephrin, a key slit diaphragm protein synthesized by podocytes,18 and deletion of VDR in mice led to early onset and robust albuminuria in diabetic state.19 Together these data suggest that podocytes may be a key antiproteinuric target of vitamin D20; however, no study has directly addressed the renoprotective role of podocyte VDR signaling. In this study, we used transgenic approaches to address this important question. Our data provide strong evidence that podocyte VDR signaling protects podocytes from hyperglycemia-induced apoptosis and prevents diabetic nephropathy.
Results
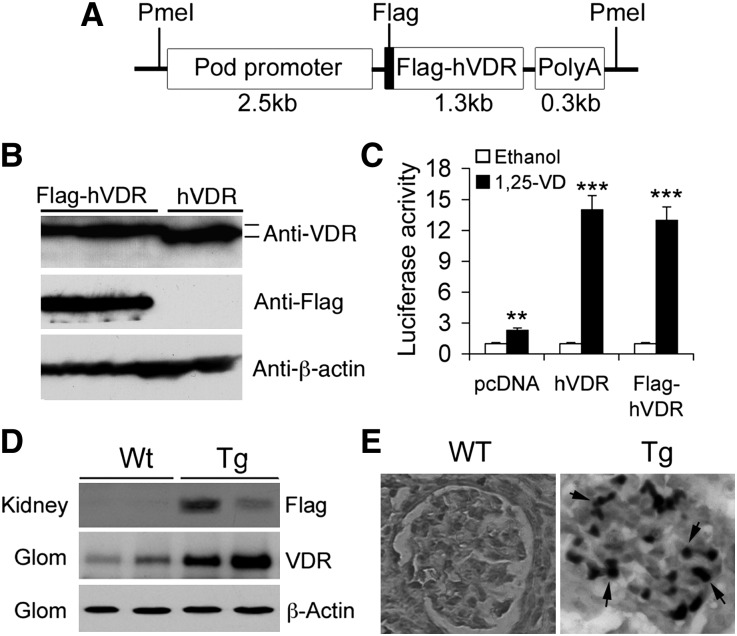
We used the 2.5 kb human podocin gene (NEPH2) promoter to target human VDR (hVDR) to podocytes in transgenic (Tg) mice (Figure 1A). This podocin gene promoter has been well documented for its podocyte specificity in driving transgene expression.21 To distinguish the hVDR transgene from the endogenous mouse VDR, we tagged the hVDR with a Flag sequence at the N-terminus (Figure 1A) so that the hVDR transgene could be detected using anti-Flag antibody (Figure 1B). Luciferase reporter assays in VDRE-Luc plasmid-transfected HEK293 cells validated the transactivating activity of Flag-hVDR in response to 1,25(OH)2D3 stimulation (Figure 1C). The purified 4.1 kb PmeI DNA construct (Figure 1A) was microinjected into fertilized embryos isolated from pregnant female DBA/2J mice, a genetic background known to be susceptible to diabetic renal injury.14,16,22 PCR-based genotyping identified 3 positive pups of 62 born from the microinjection, and cross of these founder lines with DBA/2J mice resulted in germline transmission in lines 5 and 12. This study focused on line 5. Similar phenotypes were observed in line 12.
Figure 1.
Generation of podocyte-specific hVDR transgenic mice. (A) Schematic illustration of podocin-Flag-hVDR-polyA DNA construct used for microinjection. (B) HEK293 cells were transfected with pcDNA-hVDR or pcDNA-Flag-hVDR and cell lysates were analyzed by Western blotting. Anti-VDR antibody recognizes both hVDR and Flag-hVDR, whereas anti-Flag antibody recognizes only Flag-hVDR, but not hVDR. (C) HEK293 cells cotransfected with p3xVDRE-Luc and pcDNA, pcDNA-hVDR, or pcDNA-Flag-hVDR are treated with ethanol or 1,25(OH)2D3. Luciferase reporter assays show that both hVDR and Flag-hVDR respond to 1,25(OH)2D3 stimulation and have the same activity. **P<0.01; ***P<0.001 versus corresponding ethanol, n=3. (D) Western blot analysis of total kidney lysates and purified glomerular (Glom) lysates from WT and Tg offspring, using anti-VDR antibodies or anti-Flag antibodies as indicated. (E) Immunostaining with anti-Flag antibodies shows Flag-positive (dark stain, arrows) podocytes in the glomerulus of Tg mice. Original magnification, ×400.
Western blot analysis with anti-Flag antibodies confirmed the expression of Flag-hVDR in the kidney of Tg offspring (Figure 1D), which explained the increased VDR levels detected with anti-VDR antibodies in glomerular lysates from Tg mice relative to wild-type (WT) counterparts (Figure 1D). Immunostaining of kidney sections with anti-Flag antibodies revealed Flag-positive podocytes in Tg glomerulus but not in WT mice (Figure 1E), and the Flag-positive cells in the glomerulus are also Wilms tumor 1 (WT1)–positive (Supplemental Figure 1). The hVDR transgene was not detectable in other tissues examined. Together these data demonstrate the establishment of podocyte-specific hVDR Tg mouse lines.
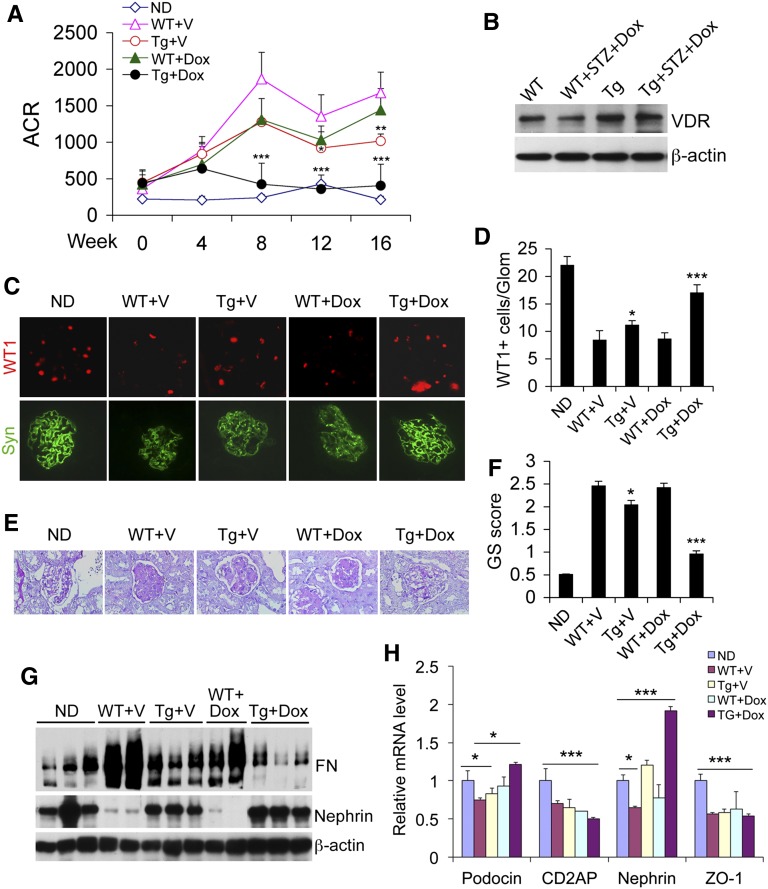
We subjected WT and Tg mice to streptozotocin (STZ) treatment to induce diabetes as reported previously.14,16 The mice developed hyperglycemia (blood glucose >400 mg/dl) at 3 weeks after STZ injection. At this point the mice were treated with vehicle (V) (60:40 propylene glycol/H2O) or a low dose of vitamin D analog doxercalciferol (Dox) (30 ng/kg, injected intraperitoneally three times per week) for 16 weeks before sacrifice. This dose was one-tenth of the dose that we used previously to prevent albuminuria in diabetic WT mice.16 Vehicle-treated WT mice developed robust progressive albuminuria during the 16 weeks, and the low dose Dox treatment had little effects on the development of albuminuria in WT mice. In contrast, baseline vehicle-treated Tg mice exhibited significantly reduced albuminuria compared with WT+V mice, and the low dose Dox almost completely blocked albuminuria in Tg mice (Figure 2A) without altering the blood glucose levels and body weight (Table 1). Western blot showed that the reduced glomerular VDR levels in diabetic WT mice could not be reversed by the low-dose Dox treatment (Figure 2B).
Figure 2.
Reduced albuminuria and glomerular injury in transgenic mice. (A) Urinary albumin to creatinine ratio (ACR) over time. *P<0.05; **P<0.01; ***P<0.001 versus WT+V. n=7–9 for each group. (B) Western blot analysis of glomerular VDR protein levels in WT and Tg mice in nondiabetic and diabetic states. (C) Immunostaining of kidney sections from different mice with anti-WT1 (red) or antisynaptopodin (green) antibodies. (D) Semiquantitation of WT1-positive cells per glomerulus. (E) PAS staining of kidney sections showing accumulation of extracellular matrix in the glomeruli. (F) Glomerulosclerotic score based on PAS staining. *P<0.05; ***P<0.001 versus WT+V or WT+Dox. (G) Western blot analyses of glomerular lysates with antibodies against fibronectin (FN) and nephrin. (H) Real-time RT-PCR quantitation of slit diaphragm proteins podocin, CD2AP, nephrin, and ZO-1. *P<0.05; **P<0.01; ***P<0.001. Original magnification, ×400 in C; ×200 in E. ND, nondiabetic; Glom, glomerulus; GS, glomerulosclerotic.
Table 1.
Blood glucose levels and body weight during the treatment period
| Mouse and Treatment | Blood Glucose (mg/dl) | Body Weight (g) | |||
|---|---|---|---|---|---|
| Week 0 | Week 4 | Week 16 | Week 0 | Week 16 | |
| ND | 141.6±15.4 | 135.6±13.5 | 138.4±12.3 | 21.3±0.96 | 34.96±1.02 |
| WT+V | 134.7±22.7 | 427.2±169.9 | >600 | 21.98±0.84 | 19.38±2.2 |
| Tg+V | 144±4.3 | 425.6±136.4 | >600 | 22.54±1.25 | 21.77±3.09 |
| WT+Dox | 143±4.3 | 617.2±69.8 | >600 | 19.43±1.79 | 16.41±2.94 |
| Tg+Dox | 136±17.7 | 589.7±73.9 | >600 | 19.34±1.63 | 17.17±1.08 |
Values are expressed as mean ± SD. ND, nondiabetic control. n=7–9 for each group.
Immunostaining of kidney sections with antibodies against WT1 (nuclear staining) and synaptopodin (cytoplasmic and foot process staining) demonstrated significant podocyte loss in WT+V and WT+Dox mice and to a less extent in Tg+V mice relative to nondiabetic mice, but podocyte loss was markedly attenuated in Tg+Dox mice (Figure 2C), which was confirmed by semiquantitation of WT1-positive cells in the glomeruli (Figure 2D). Periodic acid–Schiff (PAS) staining demonstrated various degrees of glomerulosclerosis in WT+V, WT+Dox, Tg+V, and Tg+Dox kidneys, with Tg+Dox mice showing the most attenuated glomerular fibrosis (Figure 2, E and F). Western blot analyses showed that glomerular fibronectin levels were substantially elevated, and nephrin levels markedly reduced, in WT+V and WT+Dox kidneys compared with the nondiabetic kidneys, but the accumulation of fibronectin was clearly lessened and nephrin levels preserved in Tg+V and Tg+Dox mice (Figure 2G). Hyperglycemia-induced decline of slit diaphragm proteins podocin and nephrin was reversed in Tg+Dox mice (Figure 2H), the latter consistent with our recent report that nephrin is transcriptionally upregulated by vitamin D.18
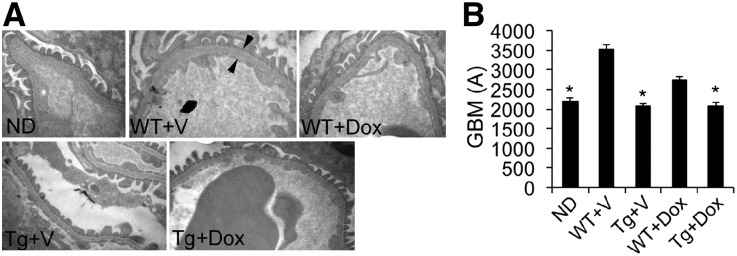
Electron microscopic examination revealed thickening of the glomerular basement membrane (GBM), a marker of glomerular filtration barrier dysfunction, in WT+V and WT+Dox mice, and hVDR overexpression prevented GBM thickening and preserved the morphology of podocyte foot processes (Figure 3, A and B).
Figure 3.
Electron microscopy analysis of the glomerular filtration barrier. (A) Electron micrographs of the glomerular filtration barrier from different mice as indicated. Arrows indicate the width of the GBM. (B) Quantitation of the thickness of the GBM from different mice. *P<0.05 versus WT+V. Original magnification, ×25,000 in A.
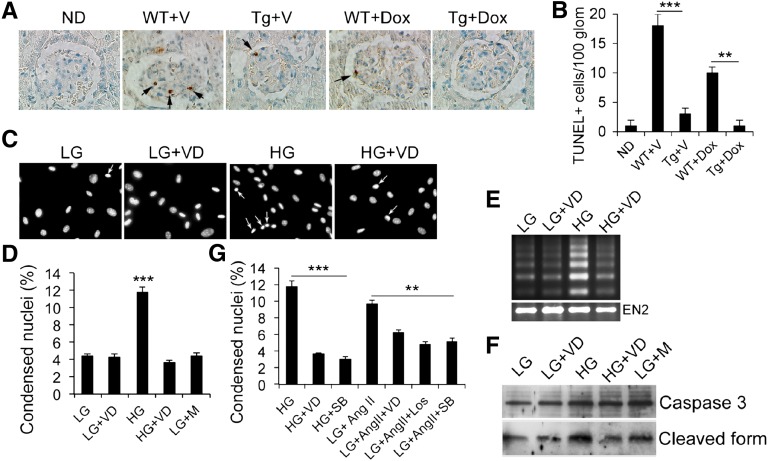
We further explored the renoprotective mechanism of the podocyte VDR signaling. Chronic hyperglycemia induced podocyte apoptosis, demonstrated by terminal deoxynucleotidyl transferase–mediated deoxyuridine 5′-triphosphate nick-end labeling (TUNEL) staining, in WT kidneys with or without Dox treatment, and podocyte hVDR overexpression attenuated podocyte apoptosis in Tg mice with or without Dox treatment, with more attenuation seen in Dox-treated kidneys (Figure 4, A and B). Consistently, exposure to high glucose (HG) (30 mM) induced apoptosis in cultured podocytes, manifested by increased nucleic condensation (Figure 4, C and D), DNA fragmentation (Figure 4E), and caspase 3 activation (Figure 4F), and these changes were markedly attenuated in the presence of 1,25(OH)2D3 treatment (Figure 4, C–F). HG-induced nuclear condensation was also blocked by p38 mitogen-activated protein kinase (MAPK) inhibitor SB203580 (Figure 4G). Angiotensin (Ang) II could induce podocyte nuclear condensation under low-glucose (LG) (5 mM) culture condition, and this effect could be blocked by AT1 receptor blocker losartan as expected as well as by SB203580 and 1,25(OH)2D3 (Figure 4G). These observations suggest that 1,25(OH)2D3 inhibits podocyte apoptosis by targeting p38 activation.
Figure 4.
Podocyte VDR signaling inhibits podocyte apoptosis. (A) TUNEL staining of kidney sections from different mice showing the glomerular TUNEL-positive podocytes. (B) Semiquantitation of TUNEL-positive cells per 100 glomeruli. **P<0.01; ***P<0.001. (C) Hoechst 33342 staining of podocyte nuclei under different cultural conditions as indicated. Arrows indicate condensed nuclei. (D) Semiquantitative data showing percentage of condensed nuclei under different cultural conditions. ***P<0.001 versus the rest. (E) DNA fragmentation assays. (F) Western blots showing full-length and cleaved 17 kD caspase 3 under different conditions. (G) Semiquantitation of condensed nuclei showing that p38 inhibition by SB203580 attenuates podocyte apoptosis induced by HG or AngII. **P<0.01; ***P<0.001. Glom, glomeruli; VD, 1,25-dihyroxyvitamin D; M, mannitol; SB, SB203580; Los, losartan. Original magnification, ×400 in A; ×200 in C.
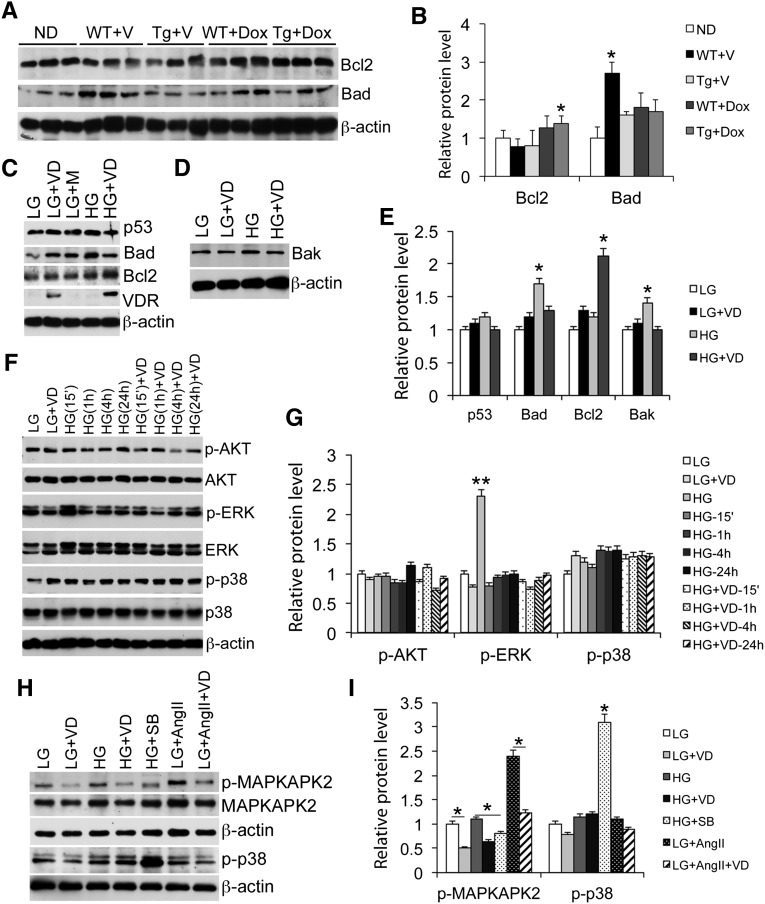
The Bcl2 family members play important roles in apoptosis. Western blot analyses showed that glomerular Bcl2 (antiapoptotic) was moderately reduced, and Bad (proapoptotic) levels increased, in WT mice, which were reversed in Tg mice (Figure 5, A and B). Because podocyte content only accounts for a small portion of the glomerular lysates, making it difficult to directly analyze glomerular podocytes, we directly examined podocyte cultures to assess the molecular mechanism. Although exposure of podocyte culture to HG had little effects on p53, HG induced Bad and Bak (proapoptotic), and these changes were diminished in the presence of 1,25(OH)2D3 pretreatment (Figure 5, C–E). 1,25(OH)2D3 also increased Bcl2 levels (Figure 5, C and E). This is consistent with the notion that 1,25(OH)2D3-VDR signaling inhibits podocyte apoptosis. We further examined several intracellular signaling pathways known to promote apoptosis. Whereas HG and 1,25(OH)2D3 had little effect on Akt phosphorylation (Figure 5, F and G), HG quickly induced extracellular signal-regulated kinases (ERK) phosphorylation, which was inhibited when the cells were pretreated with 1,25(OH)2D3 (Figure 5, F and G). HG also moderately induced p38MAPK phosphorylation, and this was not affected by 1,25(OH)2D3 (Figure 5, F–I); however, like p38 inhibitor SB203580 that had no effect on p38 phosphorylation but inhibited its activity to phosphorylate MAPK-activated protein kinase 2 (MAPKAPK2), a downstream substrate p38 kinase,23 1,25(OH)2D3, also inhibited HG-induced MAPKAPK2 phosphorylation (Figure 5, H and I). Moreover, AngII is known to activate p38 to induce apoptosis,24 and 1,25(OH)2D3 also blocked AngII-induced MAPKAPK2 phosphorylation (Figure 5, H and I). These data are consistent with the podocyte nuclear condensation data reported in Figure 4G, indicating that 1,25(OH)2D3 inhibits p38 activity and p38-mediated signaling in podocyte apoptosis.
Figure 5.
Podocyte VDR signaling inhibits proapoptotic pathways. (A and B) Western blots (A) and densitometric quantitation (B) showing Bcl2 and Bad levels in glomerular lysates isolated from different mice. *P<0.05 versus the rest. (C–E) Western blots (C and D) and quantitation (E) showing levels of p53, Bad, Bcl2, and VDR in podocyte lysates under different cultural conditions as indicated. *P<0.05; **P<0.01 versus the rest. (F and G) Time course changes (F) and quantitation (G) in the levels of phospho-AKT, AKT, phospho-ERK, ERK, phospho-p38, and p38 in podocytes cultured in LG and HG conditions at different times (15 minutes to 24 hours) after 1,25-dihydroxyvitamin D treatment. **P<0.01 versus the rest. (H and I) Western blots (H) and quantitation (I) showing phosphorylation of MAPKAPK2 and p38 under different conditions. *P<0.05. VD, 1,25-dihydroxyvitamin D; M, mannitol; SB, SB203580. All Western blots are representative of at least three independent experiments.
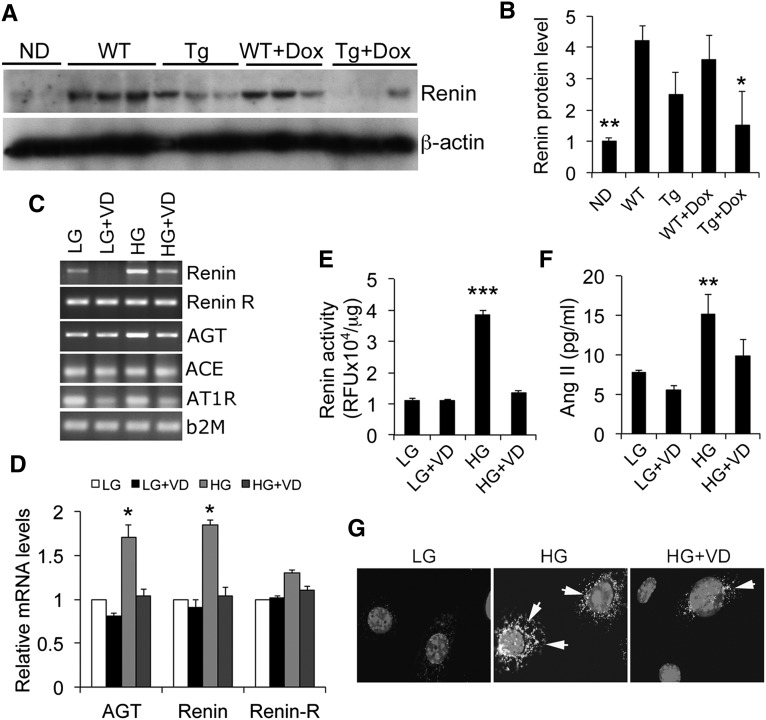
The intrarenal renin-angiotensin system (RAS) plays a key role in diabetic renal injury.25 AngII, the effector of the RAS, is known to promote podocyte apoptosis in vitro and in vivo.26,27 Glomerular renin was upregulated in diabetic WT mice, and the hVDR transgene blocked the increase in renin, particularly in the presence of Dox treatment (Figure 6, A and B). In podocyte cultures, HG induced the expression of renin and angiotensinogen (Figure 6, C and D). As a result, intracellular renin activity (Figure 6E) and released extracellular AngII levels in the media (Figure 6F) were also markedly increased by HG exposure. These inductions were blocked by 1,25(OH)2D3 (Figure 6, C–F). Moreover, HG-induced AT1 receptor expression in podocytes was also attenuated by 1,25(OH)2D3 (Figure 6G). These data suggest that the 1,25(OH)2D3-VDR signaling inhibits the RAS activation in podocytes to suppress HG-induced podocyte apoptosis.
Figure 6.
Podocyte VDR signaling inhibits the RAS. (A and B) Western blots (A) and quantitation (B) showing renin protein levels in glomerular lysates obtained from different mice. *P<0.05 versus WT, Tg, and WT+Dox; **P<0.01 versus the rest. (C) RT-PCR analysis of the RAS components in podocyte cultures. (D) Real-time RT-PCR assessment of RAS components in podocyte cultures. *P<0.05 versus the rest. (E) Cellular lysate renin activity in podocytes cultured in different conditions. (F) AngII levels in the media of podocyte cultures. **P<0.01; ***P<0.001 versus the rest. (G) Immunostaining of podocyte cultures with anti-AT1 receptor antibodies. Arrows indicate AT1 receptor staining surrounding the nuclei. Original magnification, ×200.
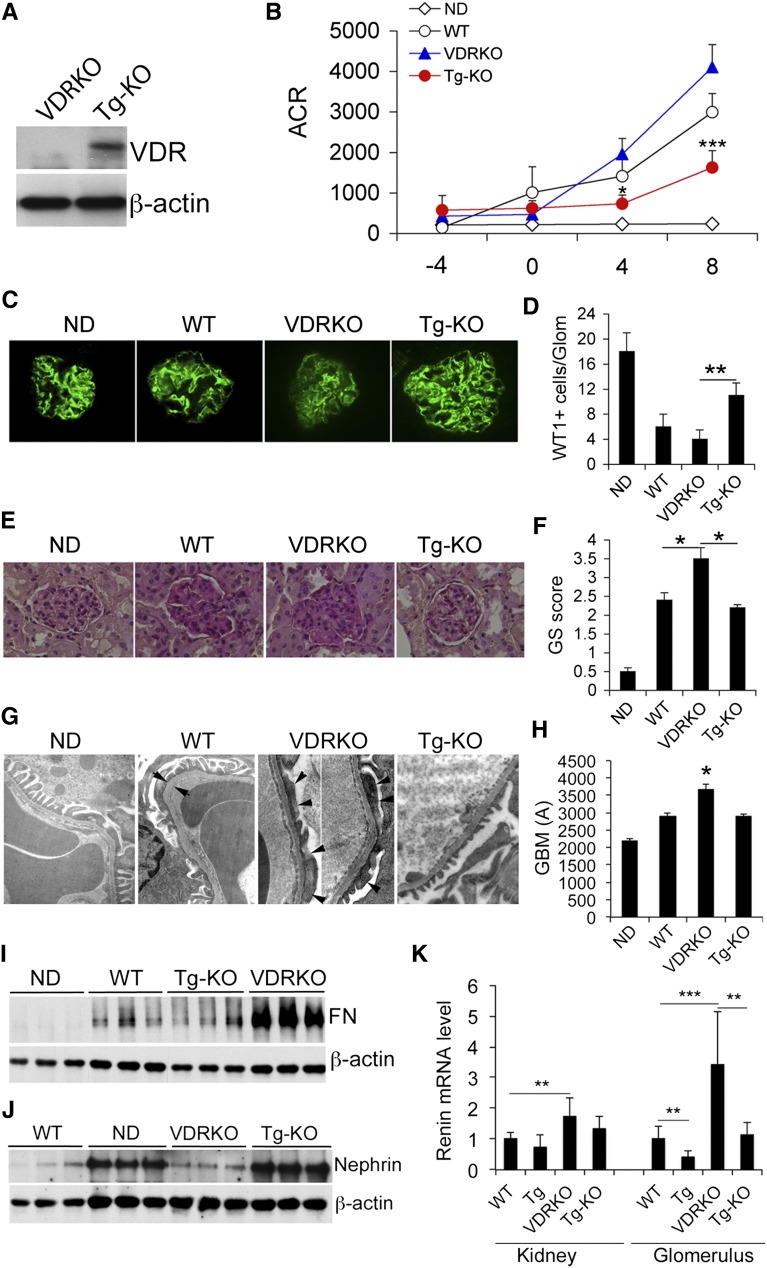
We reported previously that VDR-null (VDRKO) mice developed much more severe albuminuria and renal damage than WT mice in diabetes.19 To further assess the renoprotective role of podocyte VDR signaling, we asked whether the podocyte-specific hVDR transgene was able to rescue VDRKO mice from developing the severe diabetic renal injury. Transgenic VDRKO (Tg-KO) mice in which only podocytes were reconstituted with hVDR in the VDR-null genetic background were obtained through crossing of Tg and VDRKO mice in DBA/2J background. As expected, VDR expression was detected in the glomerular lysates of Tg-KO mice (Figure 7A). In the STZ diabetes model, VDRKO mice developed more severe albuminuria than WT mice as reported previously19; interestingly, albuminuria was markedly attenuated in Tg-KO mice (Figure 7B). Diabetes-induced podocyte loss (Figure 7, C and D) and glomerular sclerosis (Figure 7, E and F) were also more severe in VDRKO mice compared with WT mice, and these phenotypes were ameliorated in Tg-KO mice (Figure 7, C–F). Electron microscopy examination of the glomerular filtration barrier revealed severe effacement of podocyte foot processes and thickened GBM in VDRKO mice, and these abnormalities were attenuated in Tg-KO mice (Figure 7, G and H). The dramatic increase in glomerular fibronectin levels and decrease in nephrin levels seen in VDRKO mice were mostly reversed in Tg-KO mice (Figure 7, I and J). Reconstitution of the hVDR transgene in podocytes was able to attenuate renin induction in the glomerulus, but the effect was not detectable if assayed using whole kidney RNAs (Figure 7K). This is expected because hVDR is not expressed outside the glomerulus. Collectively these data demonstrate that by reconstituting VDR signaling only in podocytes could the severe renal injury phenotype seen in VDRKO mice be partly rescued, confirming the critical role of podocyte VDR signaling in renal protection.
Figure 7.
The hVDR transgene rescues VDR-null mice from developing severe renal injury. (A) Glomerular VDR protein levels in VDRKO and Tg-KO mice. (B) Urinary ACR in different mice as indicated. *P<0.05; ***P<0.001 versus VDRKO; n=6–7 for each genotype. (C) Synaptopodin immunostaining of kidney sections from different mice. (D) Semiquantitation of WT1-positive cells per glomerulus. **P<0.01. (E) PAS staining of kidney sections. (F) Glomerulosclerotic score of kidney sections. *P<0.05. (G) Electron microscopic examination of glomerular filtration barrier. Arrows indicate the width of the GBM; arrowheads indicate effacement of foot processes. (H) GBM thickness. *P<0.05 versus the rest. (I) Glomerular levels of fibronectin. (J) Glomerular levels of nephrin in different mice, both assessed by Western blotting. (K) RT-PCR quantitation of renin transcript in whole kidney or in glomeruli of different mice. **P<0.01; ***P<0.001. n=7–10 for each genotype. Original magnification, ×400 in C; ×200 in E; ×25,000 in G. GS, glomerulosclerotic; ND, nondiabetic.
Discussion
Although the renoprotective role of vitamin D has been well documented in recent years, the underlying protective mechanism remains elusive. In this study, we provided strong evidence that the VDR signaling in podocytes has potent renoprotective activities against diabetic nephropathy, and the podocyte VDR at least in part mediates the antiproteinuric action of 1,25(OH)2D3 and its analogs. The central mechanistic basis of this protection appears to be the inhibition of podocyte apoptosis induced by hyperglycemia. The data reported here strongly support the speculation that podocytes are key renoprotective targets of vitamin D actions.20
We assessed the role of the podocyte VDR signaling by a transgenic approach in which hVDR expression was specifically targeted to podocytes using the well characterized podocyte-specific NEPH2 gene promoter. The Flag-tag on hVDR allowed for distinction of the hVDR transgene from the endogenous mouse VDR. We estimated that the NEPH2 promoter-driven hVDR expression increased the podocyte VDR level by about 50% over the WT level. This moderate increase, as we demonstrated, was sufficient to render the Tg kidney resistant to diabetic injury at baseline and more upon vitamin D analog treatment even at a low dose that had no protective effects in WT mice. The hVDR transgene-mediated protective effects were manifested by blockade of albuminuria and attenuation of podocyte damage and glomerular sclerosis. At the molecular level, hyperglycemia-induced decline of slit diaphragm proteins and induction of extracellular matrix proteins were partially reversed, and the activation of the RAS was blocked in Tg mice. Importantly the proapoptotic pathway was partially inhibited. Given the complexity of the diabetic damage process, some of these protective effects of vitamin D might be secondary, but together these data provide novel mechanistic explanations for the renoprotective activity of VDR signaling reported in the literature.3
Podocyte detachment, due to apoptotic and nonapoptotic reasons, leads to podocyte loss and ultimately to proteinuria. Hyperglycemia may damage podocytes by nonapoptotic mechanisms, but podocyte apoptosis is thought to be one major cause for podocyte loss in diabetic nephropathy.28 The suppression of podocyte apoptosis seen in Tg mice could be recapitulated in podocyte culture, allowing for more in-depth investigations into the underlying molecular mechanism. As reported previously,29 our data showed that exposure of podocytes to HG-induced program cell death, manifested by increased nuclear condensation and DNA fragmentation. These phenomena were markedly ameliorated in the presence of 1,25(OH)2D3. In the HG cultural condition, caspase 3 is activated and HG also induced proapoptotic protein Bad and Bak and reduced antiapoptotic protein Bcl2. These effects were attenuated by 1,25(OH)2D3. For the upstream signaling pathways that have been reported to promote apoptosis, we found that HG induced activation of ERK and p38 MAPK, but not that of Akt. HG-induced activation of p38 and ERK has been documented in podocytes and mesangial cells to cause renal injury.29,30 1,25(OH)2D3 abrogated ERK phosphorylation and inhibited p38 activity by yet unknown mechanisms. This regulation may be direct or indirect via RAS/AngII (see below), because AngII is known to active ERK and p38 signaling pathways. We speculate that vitamin D inhibits podocyte apoptosis by blocking these signaling pathways (Figure 8). VDR activation was recently shown to decrease renal inflammation and podocyte apoptosis in diabetic nephropathy models,31 and the anti-inflammatory activity of vitamin D may also contribute to the inhibition of podocyte apoptosis. Moreover, a recent elegant study demonstrated the involvement of a CD2AP-dendrin-cathepsin L pathway in the regulation of podocyte cytoskeletal structure and survival.32 This work shows that slit diaphragm injury allows for nuclear translocation of the transcription factor dendrin from the plasma membrane, promoting cathepsin L expression. Cathepsin L acts to alter podocyte actin cytoskeletal organization and renders podocytes hypersensitive to TGF-β proapoptotic signals. The relationship between VDR and this pathway is unknown. Given the role of VDR signaling in the protection of the slit diaphragm, its interaction with this pathway warrants further exploration. It is plausible that vitamin D may regulate podocyte function and survival by targeting this newly identified pathway.
Figure 8.
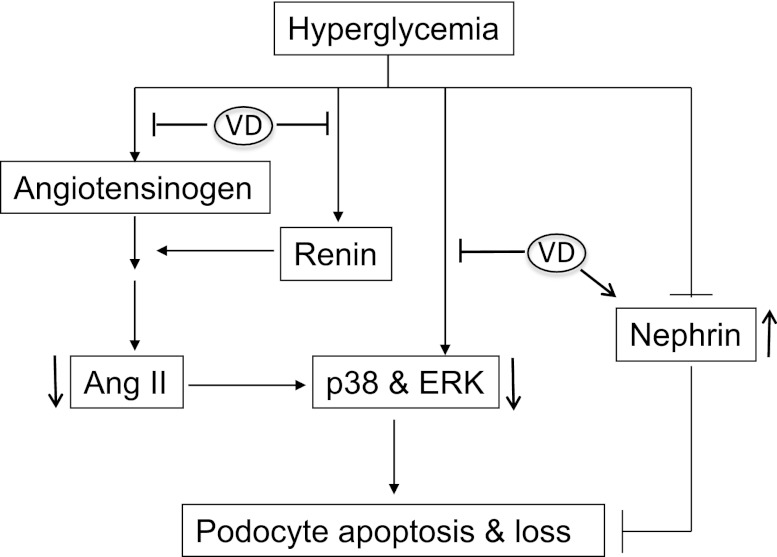
Vitamin D targets pathways that are involved in apoptosis of podocytes. Hyperglycemia activates p38 MAPK- and ERK-mediated signaling pathways to induce podocyte apoptosis. 1,25-dihydroxyvitamin D (VD) inhibits the induction of angiotensinogen and renin by HG, leading to suppression of AngII production. VD may also suppress p38 or ERK activation via an AngII-independent mechanism. VD upregulation of nephrin also contributes to the protection of podocytes.
The RAS plays a key role in diabetic renal injury. The proapoptotic activity of AngII toward podocytes has been documented in previous studies.27 Interestingly, podocytes express all components of the RAS that can be activated by HG,33,34 suggesting that the activation of the local RAS in podocytes is able to induce apoptosis of these cells by an autocrine or paracrine fashion. In Tg mice, the induction of renin in the glomeruli by hyperglycemia was attenuated, and the high glomerular renin was reversed by the hVDR transgene in Tg-KO mice (Figure 7G). Indeed, HG was able to activate the local RAS in podocytes by induction of renin, angiotensinogen and AT1 receptor, leading to increased production of AngII. In the presence of 1,25(OH)2D3, HG-induced activation of the RAS in podocytes was reversed. Therefore, it is clear that vitamin D protects podocytes through blockade of the local RAS activation (Figure 8). Moreover, the induction of nephrin by 1,25(OH)2D3, as described previously,18 also contributes to the protection of podocytes (Figure 8). In addition, vitamin D analogs have also been reported to block Wnt/β-catenin signaling in podocytes to ameliorate podocyte damage.13 Therefore, it is likely that the podocyte VDR signaling regulates multiple pathways to protect the podocytes.
The observation that a low dose of Dox treatment protected the Tg mice but not the WT mice attests to the importance of the podocyte VDR signaling. The VDR mediates the activity of 1,25(OH)2D3 and its activated analogs. Thus when 1,25(OH)2D3 levels are constant, the abundance of VDR in cells largely determines the hormonal activity. VDR expression is highly inducible in podocytes by 1,25(OH)2D3, but the baseline podocyte VDR expression level is usually low.18 Therefore, VDR expression in podocytes needs to be upregulated by the vitamin D hormone to be renoprotective. It is possible that the low-dose Dox was insufficient to induce the endogenous VDR in podocytes in WT mice (Figure 2B), but in Tg mice the hVDR is constitutively expressed in podocytes, which is sufficient to transduce the activity of Dox and the endogenous vitamin D hormone leading to protection.
The rescue of VDR-null mice by the hVDR transgene further confirms the renoprotective property of the podocyte VDR signaling. In Tg-KO mice, only podocytes express hVDR, whereas the rest of the body is VDR deleted. In this VDR-null background, the systemic level of 1,25(OH)2D3, which is mainly produced from the proximal tubular cells in the kidney, is extremely high (>10 fold the normal level) because of the lack of feedback suppression.35,36 In Tg-KO mice this high level of 1,25(OH)2D3 was not able to act on cells except the podocytes. Therefore, the rescue of diabetic renal injury observed in the Tg-KO mice is a very compelling piece of evidence that supports the importance of the podocyte VDR signaling in renoprotection. Taken together, these data demonstrate podocytes as a key therapeutic target in vitamin D therapy of CKD.
Concise Methods
Generation of Transgenic Lines
To tag hVDR, the Flag nucleotide sequence GATTACAAGGATGACGATGACAAG was PCR-added to the N-terminus of hVDR cDNA coding sequence. The Flag-hVDR sequence (1.3 kb) was then placed under the control of the 2.5 kb human podocin gene (NPHS2) promoter (provided by Dr. Larry Holzman, University of Michigan),21 followed by SV40 T antigen poly(A) sequence (Figure 1A). The 4.1 kb PmeI-PmeI DNA construct was purified for microinjection performed by the Transgenic Mouse Core Facility at the University of Chicago, using fertilized embryo in DBA/2J background. Pups born from the microinjection were screened by PCR-based genotyping using hVDR-specific primers. Transgene-positive mice were crossed with DBA/2J mice to obtain germ line transmission. To obtain VDR-null mice in DBA/2J background, VDR(+/−) mice in C57BL/6 background35 were backcrossed with DBA/2J mice for 10 generations before being used for interbreeding to generate VDR(−/−) mice for this study.
Diabetic Animal Models and Treatment
Six-week-old male mice were made diabetic by intraperitoneal injection of 35 mg/kg of STZ per day freshly prepared in 10 mM citrate buffer (pH 4.2) for 5 consecutive days as previously described.14,16 Three weeks after STZ treatment the diabetic mice were treated with vehicle (60:40 propylene glycol/H2O) or Dox at 30 ng/kg (injected intraperitoneally three times per week) for 10–16 weeks. Nondiabetic mice without drug treatment served as control. Blood glucose was monitored biweekly using CONTOUR glucometers (Bayer). Spot urine was collected monthly, and urinary albumin and creatinine levels were determined using commercial kits as reported previously.14,16 Immediately after sacrifice, kidneys were harvested for histologic and biochemical analyses. All mice were fed the 2018 Teklad rodent diet containing 1.5 IU/g of a vitamin D3 and 38 μg/g of cholecalciferol and exposed to a 12:12-hour light-dark cycle. The Institutional Animal Care and Use Committee at the University of Chicago approved all animal studies.
Histology and Immunostaining
Freshly dissected kidneys were fixed overnight with 4% formaldehyde in PBS (pH 7.2) and processed as described.14,16 Kidney sections were stained with PAS. Semiquantitative scoring of glomerular sclerosis was performed using a five-grade method described previously.14,16 For immunostaining, frozen tissues in optimal cutting temperature (OCT) compound were cut into 5-μm sections. The sections were stained with antibodies against synaptopodin conjugated with Alexa 647, or with anti-WT1 antibodies (Santa Cruz Biotechnology), followed by incubation with Cy3-conjugated second antibody. Antigens were visualized with a fluorescence microscope.
TUNEL Staining
TUNEL staining was performed with the ApopTag Plus Peroxidase In Situ Apoptosis Detection Kit (Chemicon) according to the manufacturer’s instructions. TUNEL-positive cells were semiquantified by randomly counting 100 glomeruli in each mouse.
Podocyte Culture
Conditionally immortalized mouse podocytes, kindly provided by Dr. Peter Mundel (Albert Einstein College of Medicine), were cultured as previously described.37 Briefly, podocytes were grown under permissive conditions at 33°C with RPMI 1640 containing 10% FBS, 50 U/ml γ-IFN, and 100 U/ml penicillin/streptomycin. The γ-IFN concentration was gradually reduced to 10 U/ml in successive passages. Cells were allowed to differentiate in γ-IFN–free media at 37°C for 14 days with media changes every other day. Differentiated podocytes were serum starved in RPMI containing 0.5% FBS and low D-glucose (5.5 mM, LG) for 24 hours before being cultured in RPMI with 0.5% FBS and high D-glucose (30 mM, HG) or mannitol (24.4 mM mannitol + 5.5 mM D-glucose) in the presence or absence of 50 nM 1,25(OH)2D3 or 20 μM p38 inhibitor SB203580. In some experiments, the cells were treated with 100 nM AngII in LG condition for 24 hours. Cells were harvested with Trizol for RNA assays and with radioimmunoprecipitation assay (RIPA) buffer for protein assays.
AngII Assays
AngII levels in the media of podocyte cultures were determined using a Human/Mouse/Rat Angiotensin II Enzyme Immunoassay Kit (Ray Biotech Inc) according to the manufacturer’s instructions.
Renin Activity Assays
Renin activity in podocyte lysates was determined using a Fluorometric Sensolyte 520 Mouse Renin Assay Kit (AnaSpec Inc) following the manufacturer’s protocol.
DNA Fragmentation Assays
Podocyte genomic DNA was purified using the DNeasy DNA purification system (Qiagen, Valencia, CA). DNA ladders were assayed by ligase-mediated PCR using a commercial kit (Clontech, Palo Alto, CA) according to the manufacturer’s instructions. Briefly, genomic DNA was ligated to PCR primer sequences by T4 DNA ligase (18 hours at 16°C). Twenty nanograms of ligated DNA was used as the template for PCR (23 cycles at 94°C for 1 minute/72°C for 3 minutes). PCR products were resolved on 1.3% agarose gel by electrophoresis and visualized by ethidium bromide staining. PCR amplification of gene En-2 (26 cycles at 94°C for 1 minute/70°C for 1 minute) served as the internal control.
Western Blotting
Kidneys, cells, and isolated glomeruli were lysed in RIPA buffer with a cocktail of proteinase inhibitors (Roche). Protein concentrations were determined using a bicinchoninic acid protein assay reagent kit (Pierce). Proteins were separated by SDS-PAGE and Western blotting was carried out as previously described,38 using the following antibodies: nephrin (Fitzgerald Industries International Inc); fibronectin and actin (Sigma); active caspase 3, angiotensinogen, VDR, renin, Bad, Bcl2, p53, and Bax (Santa Cruz Biotechnology); and ERK, p-ERK, p38, p-p38, Akt, p-Akt, p-MAPKAPK-2, and MAPKAPK-2 (Cell Signalling Technology). Secondary antibody was horseradish peroxidase–conjugated anti-rabbit IgG (Pierce Biotechnology), and signals were detected using SuperSignal West Dura Extended Duration Substrate (Pierce). The relative amount of proteins was quantified using UNSCAN-IT gel analysis software (version 5.3; Silk Scientific, Orem, UT).
RT-PCR
Total RNAs were isolated using Trizol reagents (Invitrogen, Carlsbad, CA). First-strand cDNAs were synthesized from 2 μg RNAs using Moloney murine leukemia virus RT and hexanucleotide random primers. The first-strand cDNAs served as the template for the PCR performed using a Bio-Rad DNA Engine. Real-time RT-PCR was performed in an Applied Biosystems 7900 Real Time PCR System using SYBR green PCR reagent kits (Applied Biosystems, Foster City, CA) as described previously.19 β-2 microglobulin served as the internal controls.
Electron Microscopy
Tissue blocks of kidney cortex were fixed in 2% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) and then postfixed in 1% osmium tetroxide. Electron microscopy analyses were performed as described previously.14
Statistical Analyses
Data were presented as means ± SEM. Statistical comparisons were carried out using the unpaired two-tailed t test or one-way ANOVA as appropriate, with P<0.05 considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health Grant HL085793 and a Nephrology Research Fellowship from Genzyme Corporation. Generation of transgenic mice was supported in part by CTSA Grant UL1RR024999 from the National Center for Research Resources. The University of Chicago Transgenic Mouse Core Facility was supported in part by National Institutes of Health Grant 5P30CA014599-36.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012040383/-/DCSupplemental.
References
- 1.Tryggvason K, Patrakka J, Wartiovaara J: Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med 354: 1387–1401, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Keane WF, Eknoyan G: Proteinuria, albuminuria, risk, assessment, detection, elimination (PARADE): A position paper of the National Kidney Foundation. Am J Kidney Dis 33: 1004–1010, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Li YC: Renoprotective effects of vitamin D analogs. Kidney Int 78: 134–139, 2010 [DOI] [PubMed] [Google Scholar]
- 4.de Boer IH, Ioannou GN, Kestenbaum B, Brunzell JD, Weiss NS: 25-Hydroxyvitamin D levels and albuminuria in the Third National Health and Nutrition Examination Survey (NHANES III). Am J Kidney Dis 50: 69–77, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL: Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int 71: 31–38, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Li YC: Vitamin D: Roles in renal and cardiovascular protection. Curr Opin Nephrol Hypertens 21: 72–79, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alborzi P, Patel NA, Peterson C, Bills JE, Bekele DM, Bunaye Z, Light RP, Agarwal R: Paricalcitol reduces albuminuria and inflammation in chronic kidney disease: A randomized double-blind pilot trial. Hypertension 52: 249–255, 2008 [DOI] [PubMed] [Google Scholar]
- 8.de Zeeuw D, Agarwal R, Amdahl M, Audhya P, Coyne D, Garimella T, Parving HH, Pritchett Y, Remuzzi G, Ritz E, Andress D: Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): A randomised controlled trial. Lancet 376: 1543–1551, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Kuhlmann A, Haas CS, Gross ML, Reulbach U, Holzinger M, Schwarz U, Ritz E, Amann K: 1,25-Dihydroxyvitamin D3 decreases podocyte loss and podocyte hypertrophy in the subtotally nephrectomized rat. Am J Physiol Renal Physiol 286: F526–F533, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Freundlich M, Quiroz Y, Zhang Z, Zhang Y, Bravo Y, Weisinger JR, Li YC, Rodriguez-Iturbe B: Suppression of renin-angiotensin gene expression in the kidney by paricalcitol. Kidney Int 74: 1394–1402, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Mizobuchi M, Morrissey J, Finch JL, Martin DR, Liapis H, Akizawa T, Slatopolsky E: Combination therapy with an angiotensin-converting enzyme inhibitor and a vitamin D analog suppresses the progression of renal insufficiency in uremic rats. J Am Soc Nephrol 18: 1796–1806, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Xiao H, Shi W, Liu S, Wang W, Zhang B, Zhang Y, Xu L, Liang X, Liang Y: 1,25-Dihydroxyvitamin D(3) prevents puromycin aminonucleoside-induced apoptosis of glomerular podocytes by activating the phosphatidylinositol 3-kinase/Akt-signaling pathway. Am J Nephrol 30: 34–43, 2009 [DOI] [PubMed] [Google Scholar]
- 13.He W, Kang YS, Dai C, Liu Y: Blockade of Wnt/β-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J Am Soc Nephrol 22: 90–103, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Z, Zhang Y, Ning G, Deb DK, Kong J, Li YC: Combination therapy with AT1 blocker and vitamin D analog markedly ameliorates diabetic nephropathy: Blockade of compensatory renin increase. Proc Natl Acad Sci U S A 105: 15896–15901, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deb DK, Sun T, Wong KE, Zhang Z, Ning G, Zhang Y, Kong J, Shi H, Chang A, Li YC: Combined vitamin D analog and AT1 receptor antagonist synergistically block the development of kidney disease in a model of type 2 diabetes. Kidney Int 77: 1000–1009, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Deb DK, Kong J, Ning G, Wang Y, Li G, Chen Y, Zhang Z, Strugnell S, Sabbagh Y, Arbeeny C, Li YC: Long-term therapeutic effect of vitamin D analog doxercalciferol on diabetic nephropathy: Strong synergism with AT1 receptor antagonist. Am J Physiol Renal Physiol 297: F791–F801, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Zhou J, Minto AW, Hack BK, Alexander JJ, Haas M, Li YC, Heilig CW, Quigg RJ: Altered vitamin D metabolism in type II diabetic mouse glomeruli may provide protection from diabetic nephropathy. Kidney Int 70: 882–891, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Deb DK, Wang Y, Zhang Z, Nie H, Huang X, Yuan Z, Chen Y, Zhao Q, Li YC: Molecular mechanism underlying 1,25-dihydroxyvitamin D regulation of nephrin gene expression. J Biol Chem 286: 32011–32017, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Z, Sun L, Wang Y, Ning G, Minto AW, Kong J, Quigg RJ, Li YC: Renoprotective role of the vitamin D receptor in diabetic nephropathy. Kidney Int 73: 163–171, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Li YC: Podocytes as target of vitamin D. Curr Diabetes Rev 7: 35–40, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB: Two gene fragments that direct podocyte-specific expression in transgenic mice. J Am Soc Nephrol 13: 1561–1567, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Qi Z, Fujita H, Jin J, Davis LS, Wang Y, Fogo AB, Breyer MD: Characterization of susceptibility of inbred mouse strains to diabetic nephropathy. Diabetes 54: 2628–2637, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Stokoe D, Campbell DG, Nakielny S, Hidaka H, Leevers SJ, Marshall C, Cohen P: MAPKAP kinase-2; a novel protein kinase activated by mitogen-activated protein kinase. EMBO J 11: 3985–3994, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Z, Ma JY, Kerr I, Chakravarty S, Dugar S, Schreiner G, Protter AA: Selective inhibition of p38alpha MAPK improves cardiac function and reduces myocardial apoptosis in rat model of myocardial injury. Am J Physiol Heart Circ Physiol 291: H1972–H1977, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Carey RM, Siragy HM: The intrarenal renin-angiotensin system and diabetic nephropathy. Trends Endocrinol Metab 14: 274–281, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Jia J, Ding G, Zhu J, Chen C, Liang W, Franki N, Singhal PC: Angiotensin II infusion induces nephrin expression changes and podocyte apoptosis. Am J Nephrol 28: 500–507, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding G, Reddy K, Kapasi AA, Franki N, Gibbons N, Kasinath BS, Singhal PC: Angiotensin II induces apoptosis in rat glomerular epithelial cells. Am J Physiol Renal Physiol 283: F173–F180, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Shankland SJ: The podocyte’s response to injury: Role in proteinuria and glomerulosclerosis. Kidney Int 69: 2131–2147, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Susztak K, Raff AC, Schiffer M, Böttinger EP: Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 55: 225–233, 2006 [PubMed] [Google Scholar]
- 30.Tsiani E, Lekas P, Fantus IG, Dlugosz J, Whiteside C: High glucose-enhanced activation of mesangial cell p38 MAPK by ET-1, ANG II, and platelet-derived growth factor. Am J Physiol Endocrinol Metab 282: E161–E169, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Sanchez-Niño MD, Bozic M, Córdoba-Lanús E, Valcheva P, Gracia O, Ibarz M, Fernandez E, Navarro-Gonzalez JF, Ortiz A, Valdivielso JM: Beyond proteinuria: VDR activation reduces renal inflammation in experimental diabetic nephropathy. Am J Physiol Renal Physiol 302: F647–F657, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Yaddanapudi S, Altintas MM, Kistler AD, Fernandez I, Möller CC, Wei C, Peev V, Flesche JB, Forst AL, Li J, Patrakka J, Xiao Z, Grahammer F, Schiffer M, Lohmüller T, Reinheckel T, Gu C, Huber TB, Ju W, Bitzer M, Rastaldi MP, Ruiz P, Tryggvason K, Shaw AS, Faul C, Sever S, Reiser J: CD2AP in mouse and human podocytes controls a proteolytic program that regulates cytoskeletal structure and cellular survival. J Clin Invest 121: 3965–3980, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Durvasula RV, Shankland SJ: Activation of a local renin angiotensin system in podocytes by glucose. Am J Physiol Renal Physiol 294: F830–F839, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Yoo TH, Li JJ, Kim JJ, Jung DS, Kwak SJ, Ryu DR, Choi HY, Kim JS, Kim HJ, Han SH, Lee JE, Han DS, Kang SW: Activation of the renin-angiotensin system within podocytes in diabetes. Kidney Int 71: 1019–1027, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Li YC, Pirro AE, Amling M, Delling G, Baron R, Bronson R, Demay MB: Targeted ablation of the vitamin D receptor: An animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci U S A 94: 9831–9835, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, Kawakami T, Arioka K, Sato H, Uchiyama Y, Masushige S, Fukamizu A, Matsumoto T, Kato S: Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet 16: 391–396, 1997 [DOI] [PubMed] [Google Scholar]
- 37.Mundel P, Reiser J, Zúñiga Mejía Borja A, Pavenstädt H, Davidson GR, Kriz W, Zeller R: Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 236: 248–258, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Li YC, Bolt MJG, Cao L-P, Sitrin MD: Effects of vitamin D receptor inactivation on the expression of calbindins and calcium metabolism. Am J Physiol Endocrinol Metab 281: E558–E564, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.