Abstract
MicroRNA-494 mediates apoptosis and necrosis in several types of cells, but its renal target and potential role in AKI are unknown. Here, we found that microRNA-494 binds to the 3′UTR of activating transcription factor 3 (ATF3) and decreases its transcription. In mice, overexpression of microRNA-494 significantly attenuated the level of ATF3 and induced inflammatory mediators, such as IL-6, monocyte chemotactic protein-1, and P-selectin, after renal ischemia/reperfusion, exacerbating apoptosis and further decreasing renal function. Activation of NF-κB mediated this proinflammatory response. In this ischemia/reperfusion model, urinary levels of microRNA-494 increased significantly before the rise in serum creatinine. In humans, urinary microRNA-494 levels were 60-fold higher in patients with AKI than normal controls. In conclusion, upregulation of microRNA-494 contributes to inflammatory or adhesion molecule-induced kidney injury after ischemia/reperfusion by inhibiting expression of ATF3. Furthermore, microRNA-494 may be a specific and noninvasive biomarker for AKI.
Ischemia/reperfusion (I/R) injury in the kidneys results in cell death and scar formation,1 which ultimately lead to congestive renal failure, and inflammation is the key mechanism resulting in organ damage after renal I/R injury.2 I/R injury induces a programmed stress response and expression of many transcriptional regulators, including activating transcription factor 3 (ATF3). Previous study has shown that ATF3-associated histone deacetylases 1 deacetylated histones, which resulted in the condensation of chromatin structure, interference with NF-κB binding, and inhibition of inflammatory gene transcription after I/R injury.3 Nonetheless, ATF3 gene regulation has not been extensively explored in the kidneys.
MicroRNAs are small 22- to 25-nucleotide-long noncoding RNA molecules that negatively regulate translation of target mRNAs. MicroRNAs (miRNAs) normally bind to the 3′-untranslated region (3′UTR) of their target mRNAs, leading to translation inhibition and/or mRNA degradation.4 One specific miRNA, miR-494, was initially identified as one of the upregulated miRNAs in human retinoblastoma tissues and Waldenström macroglobulinemia cells.5,6 Increased levels of miR-494 were observed in many conditions, such as myocardial and microvascular endothelial cells, in type 2 diabetic Goto–Kakizaki rats7 or ex vivo I/R mouse hearts.8 In contrast, miR-494 was downregulated in head and neck squamous cell carcinoma primary tissues.9 As for the physiologic functions of miR-494, almost all focus has been on cancer research.10,11 In the heart, experiments with transgenic mice overexpressing cardiac-specific miR-494 showed that elevation of miR-494 improved the post-I/R recovery of cardiac function and suppressed I/R-triggered cardiomyocyte apoptosis and necrosis.8 However, whether miR-494 plays an important role in the regulation of renal I/R injury is unknown.
In the present study, we identified ATF3 as a target of miR-494, and overexpression of miR-494 by lentivirus infection decreased ATF3 expression, aggravated inflammation, and resulted in decreased kidney function in mice. We also showed that elevated levels of urinary miR-494 after I/R precede the increases in serum creatinine levels. Our data suggest that miR-494 contributes to inflammatory cytokine gene expression by inhibiting ATF3, and I/R-induced kidney injury releases miR-494 into the urine, which could be a reliable and measurable indicator for AKI patients.
Results
3′UTR of ATF3 Was a Direct Target of miR-494
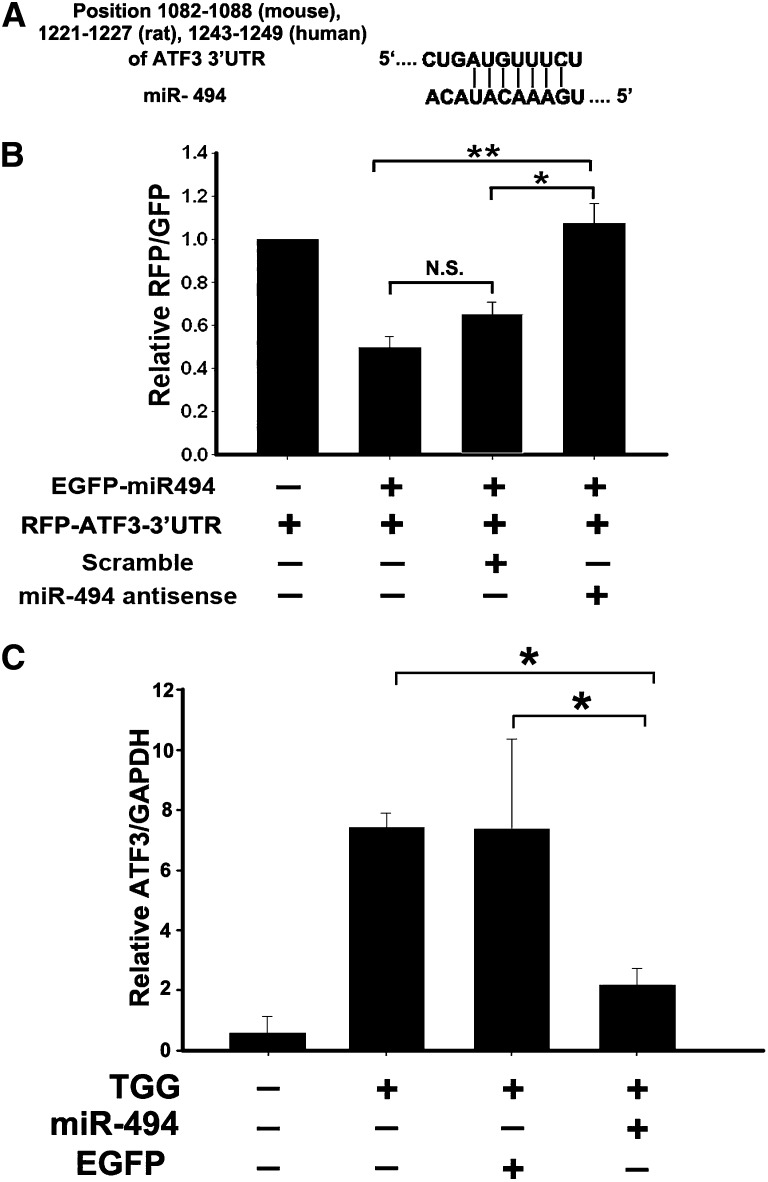
Using TargetScan (www.targetscan.org), we identified that nucleotides 1082–1088 of the 3′UTR of ATF3 from mouse, nucleotides 1221–1227 of the 3′UTR of ATF3 from rat, and nucleotides 1243–1249 of the 3′UTR of ATF3 from human all are complementary to seed sequences of miR-494 (Figure 1A). To study the direct interaction between miR-494 and ATF3 transcription, the 3′UTR of ATF3 downstream of the fluorescent reporter gene was cloned into the red fluorescent protein C1 (pRFP-C1) vector (RFP-ATF3–3′UTR); precursors of miR-494 were constructed into enhanced green fluorescent protein (pEGFP) plasmids (pEGFP-pre miR-494). Normal rat kidney (NRK)-52E cells were transiently cotransfected with both RFP-ATF3–3′UTR and pEGFP-pre miR-494, resulting in significant inhibition of fluorescent activity compared with RFP-ATF3–3′UTR transfection only. The fluorescent activity was reversed and returned to control level by miR-494 antisense transfection (Figure 1B). Furthermore, ATF3 expression induced by thapsigargin was inhibited by miR-494 transfection compared with the EGFP control plasmid or sham transfections (Figure 1C). These observations confirm that miR-494 binds to ATF3 3′UTR and inhibits ATF3 transcription in vitro.
Figure 1.
Overexpression of miR-494 inhibits ATF3 expression in vitro. (A) Schematic representation of the putative miR-494 target sites in the 3′UTR of ATF3 of mouse, rat, or human. (B) ATF3 3′UTR activity assay. Fluorescent constructs containing EGFP–miR-494 and RFP-ATF3–3′UTR plasmids were cotransfected into 293T cells with or without scrambling or antisense plasmids. Fluorescent activity was determined 24 hours after transfection. The ratio of normalized sensor to control fluorescent activity is shown. The data are expressed as means ± SEM of three independent experiments. *P<0.05, **P<0.01. N.S., no significant difference. (C) Real-time PCR detected marked induction of miR-494, which dramatically decreased ATF3 levels in the NRK-52E cells induced by ATF3 inducer thapsigargin (TGG). The relative expression of the ATF3 was normalized to glyceraldehydes-3-phosphate dehydrogenase. *P<0.05 (n=3).
miR-494 Was Induced Earlier than ATF3 in Mouse I/R Renal Failure Model
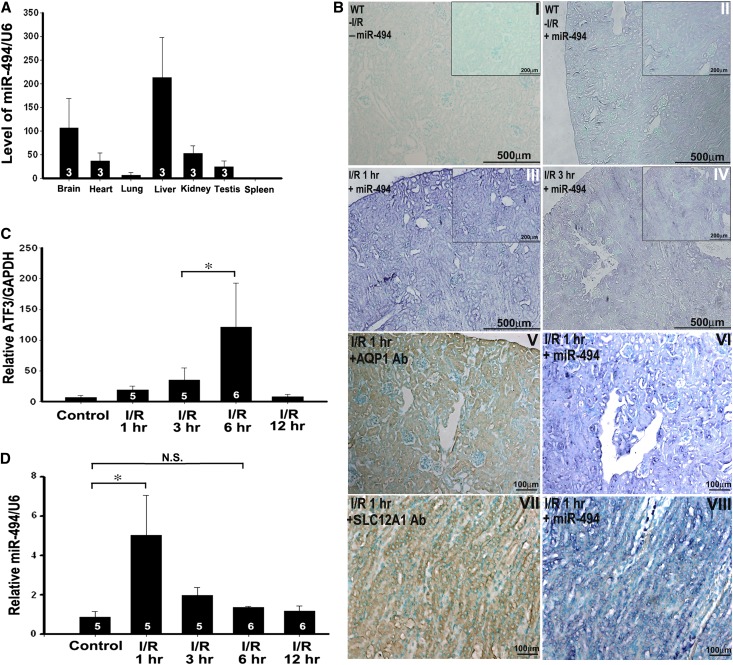
Previous study has shown the protective role of ATF3 in I/R-induced kidney injury.3 The binding between ATF3 and miR-494, which was shown in the in vitro experiment, was further investigated in the kidneys of the mouse I/R model. Using semiquantitative real-time PCR, we found that miR-494 was expressed highly in the liver and brain, moderately in the kidneys, testes, and heart, and lowest in the lung and spleen (Figure 2A). Interestingly, in situ hybridization revealed miR-494 staining (showing purple staining) on both tubular epithelial and glomerular cells under normal circumstances (Figure 2B, I versus II); however, expression of miR-494 was predominantly within the tubular epithelial cytosol after I/R injury (Figure 2B, III, IV, V, VI, VII, and VIII). Real-time PCR indicated that ATF3 mRNA levels were significantly increased 6 hours after reperfusion, but miR-494 expression levels were increased as early as 1 hour after I/R injury (Figure 2, C and D), suggesting that miR-494 is a more upstream regulator than ATF3. These observations indicate that the ischemic-responsive gene miR-494 is expressed earlier than the ATF3 gene and that miR-494 may function as a transcriptional regulator to ATF3 after renal I/R injury.
Figure 2.
The expression of miR-494 is ubiquitous in mice tissues and induced by I/R injury in the kidneys. (A) miRNAs were reverse-transcribed using miR-494 and U6 RNA-specific primers, and real-time PCR was performed as described in Concise Methods. The relative expression of the mature miR-494 was normalized to U6 RNA. Data are given as means ± SEM of triplicates. (B) The localization of miR-494 in the mouse kidney after I/R injury was detected by in situ hybridization. Paraffin-fixed sections of mouse kidney were hybridized with the digoxigenin-labeled miR-494 probe, proximal and distal tubular marker (aquaporin 1 [AQP1]), and Henle's loop marker (sodium-potassium-chloride cotransporter isoform 2 [SLC12A1]), and nuclei staining was stained by Contrast green. Figures are representative of three experiments performed on different days. Scale bar, 100, 200, and 500 μm as indicated. (C and D) Time course of the expression levels of both miR-494 and ATF3 in the kidneys after I/R injury. *P<0.05 (n=3, 5, or 6 mice per group as shown in the diagram). N.S., no significant difference.
Overexpression of miR-494 Negatively Correlated with ATF3 Levels in Mice
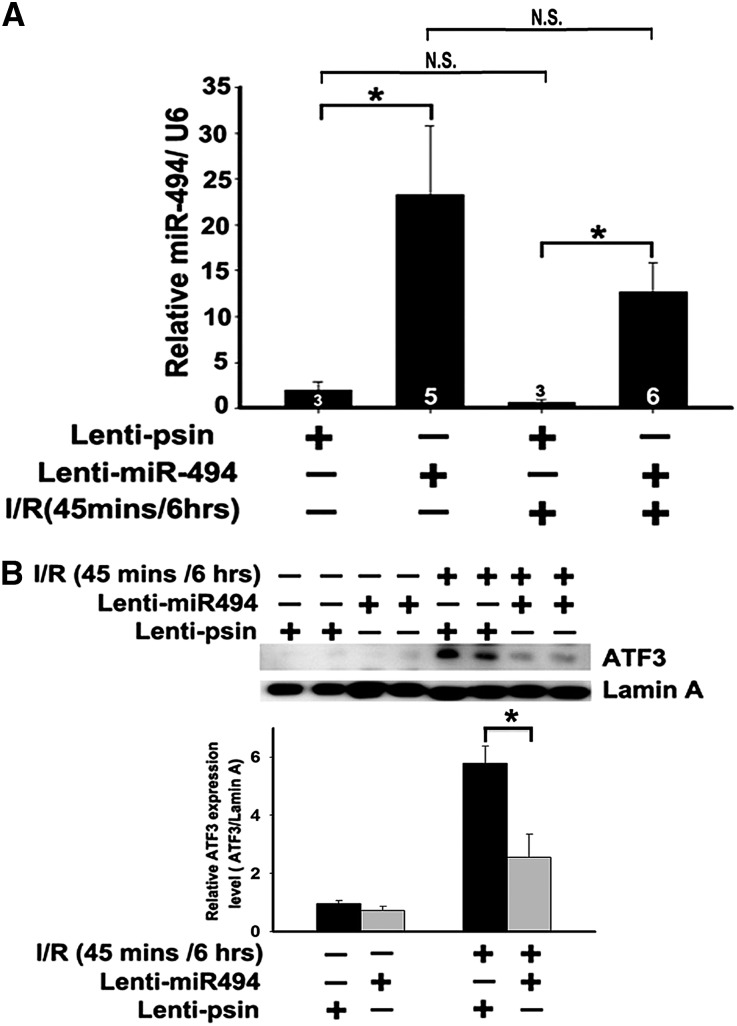
Regardless of whether the pathophysiological role of miR-494 in I/R injury is through inhibition of the ATF3 pathway, the gain of function of lentivirus-mediated gene transfer was used to overexpress miR-494 in the kidneys of sham or I/R mice. As shown in Figure 3, regardless of the types of surgery, miR-494 expression was greater in the kidney tissue infused with lentivirus containing miR-494 compared with the lenti-pSin (self-inactivating vector), and the degree of miR-494 overexpression by lentivirus also seemed to exceed its induction during I/R injury. In addition, as miR-494 increased, translocation levels of ATF3, which reflected transcriptional activity, were decreased in the kidneys after I/R (Figure 3B).
Figure 3.
Overexpression of miR-494 inhibits ATF3 translocation in mice. (A) Quantitative analysis of miR-494 level after the kidney was infused with lenti–miR-494 or lenti-pSin control (n=3, 5, or 6 mice per group as shown in the diagram). Bilateral renal arterial clamping was for 45 minutes, and miR-494 level was measured 6 hours after reperfusion. (B) The protein levels of ATF3 in the mouse kidneys after infusion with lenti–miR-494 or lenti-pSin after I/R injury. *P<0.05 (n=3). N.S., no significant difference.
Role of miR-494 in I/R-Induced Renal Function and Apoptotic Response
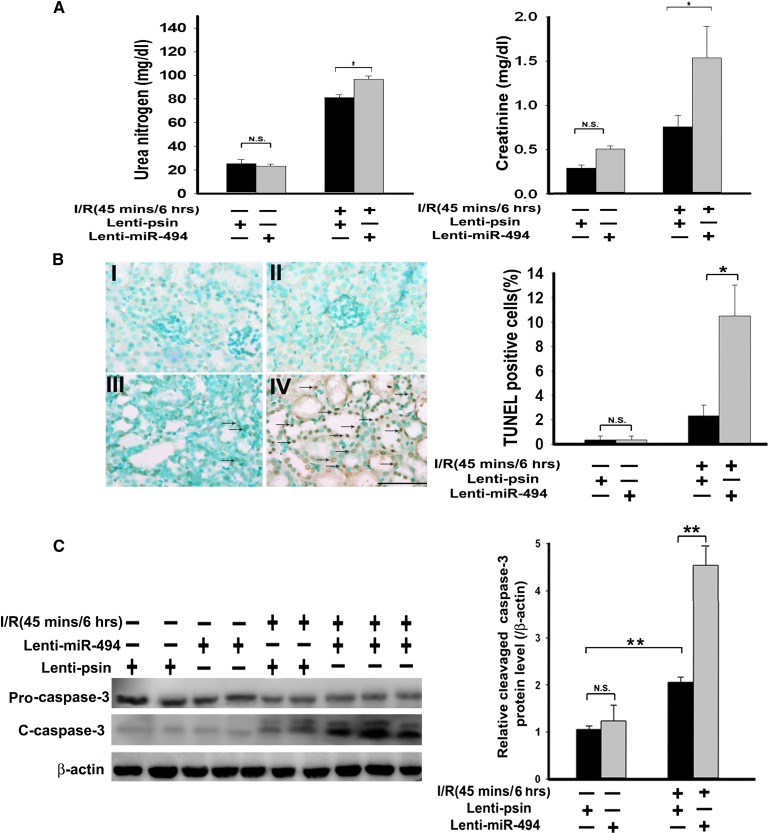
To further address the pathologic involvement of miR-494 in I/Rinjury, we compared the renal function (measured by BUN and creatinine) of mice with or without overexpression of either miR-494 or antisense miR-494 in the presence or absence of renal I/R. As shown in Figure 4A, overexpression of miR-494 significantly increased I/R-induced renal dysfunction compared with no overexpression and the sham group. In contrast, overexpression of antisense miR-494 in mice attenuated I/R-induced renal dysfunction compared with lenti-pSin control group (Supplemental Figure 1). Enhanced renal dysfunction with overexpression of miR-494 was accompanied by increased numbers of apoptotic terminal deoxynucleotidyl transferase-mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) -positive tubular epithelial cells (Figure 4B) and elevated activity of the cleaved active form of caspase-3 (Figure 4C). Likewise, renal I/R-induced apoptotic response was rescued (by suppressing caspase-3 activation) by in vivo lentivirus-mediated antisense miR-494 gene transfer into the kidneys (Supplemental Figure 2). Together, our data suggest that miR-494 and ATF3 play important pathophysiological roles in the kidneys after I/R.
Figure 4.
miR-494 overexpression decreases kidney function and increases renal apoptosis in mice. (A) Kidney functions assessed with or without miR-494 infusion after I/R injury. Bilateral renal arteries were clamped for 45 minutes, and serum urea nitrogen and creatinine levels were measured 6 hours after reperfusion or sham surgery. Values are means ± SEM; n=7 animals/group. *P<0.05 compared with control groups. (B) Apoptotic kidney cells in mice infused with or without miR-494 using in vivo TUNEL staining. Without (I and III) or with (II and IV) infused miR-494, mice underwent a sham operation (I and II) or 45 minutes of renal clamping to induce ischemia followed by 6 hours of reperfusion (III and IV). TUNEL staining of representative kidney sections from each experimental group are shown. Colocalization of blue and brown staining in nuclei reflects apoptotic cells that are indicated with arrows. Scale bar, 50 μm. Proportions of TUNEL-positive renal epithelial nuclei to total nuclei in mice infused with or without miR-494 and subjected to the sham operation or I/R injury are shown. *P<0.05 (n=7 animals/group). (C) Active caspase-3 protein expression in mouse kidney with or without lenti–miR-494 infection. Kidney lysates of mice subjected to the sham operation or I/R injury were probed with specific antibody against the uncleaved, pro–caspase-3 and cleaved, active form of caspase-3. Scanning densitometry was used for semiquantitative analysis and compared with β-actin levels. Values are means ± SEM from three experiments. **P<0.01 (n=3).
Role of miR-494 in I/R-Induced Inflammatory Responses
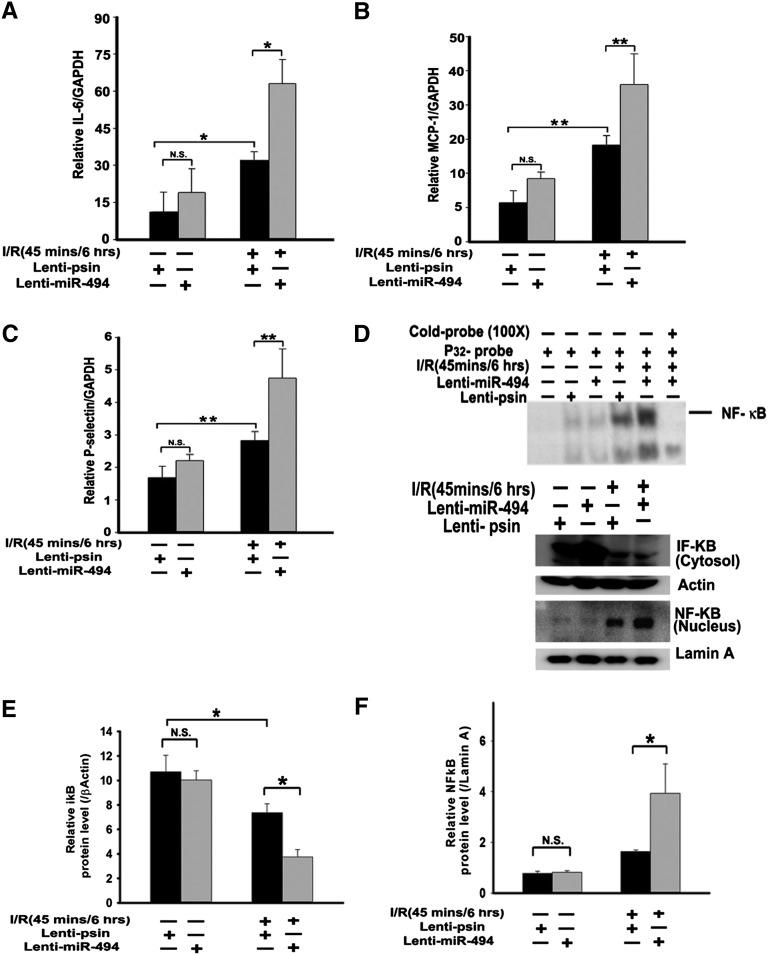
Because overexpression of sense or antisense miR-494 aggravated or attenuated, respectively, kidney damage and apoptosis, we then tested whether miR-494 also altered inflammatory cytokines and adhesion molecules, which could induce renal apoptosis as previously described.3,12,13 We found that overexpression of miR-494 elevated IL-6, P-selectin, monocyte chemotactic protein-1 (MCP-1), and polymorphonuclear leukocytes infiltration compared with the control vector after I/R (Figure 5, A–C and Supplemental Figure 3). Similarly, the renal I/R-induced inflammatory mediators release (IL-6, MCP-1, and P-selectin levels) was decreased with antisense miR-494 overexpression compared with lenti-pSin control after I/R (Supplemental Figure 4, A–C).
Figure 5.
Overexpression of miR-494 increases inflammation-related gene transcription in mouse renal tissues. Quantitative RT-PCR analysis of (A) IL-6, (B) MCP-1, and (C) P-selectin from renal cDNA derived from mice infused with or without miR-494 and then subjected to sham or I/R experiments. RT-PCR was conducted 6 hours after ischemia. Expression was normalized to the expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). *P<0.05, **P<0.01 (n=5 mice in each group). N.S., no significant difference. (D and F) NF-κB nuclear translocation and activation. (D and E) IκB degradation. (D, upper panel) Electrophoretic mobility shift assay of NF-κB expression in renal nuclear extracts of mice infused with and without infused miR-494 and subjected to a sham operation or I/R injury. (D, lower panel) Nuclear or cytosolic extracts were probed with anti–NF-κB or anti-IκB antibody, respectively, to quantify protein levels in these subcellular compartments. Anti–β-actin and lamin A served as the loading controls. *P<0.05 (n=4). N.S., no significant difference.
NF-κB plays an important role in the inflammatory response by regulating the expression of cytokines, adhesion molecules, and chemokines.14 Because IL-6, MCP-1, and P-selectin gene promoters contain functional NF-κB binding sites essential for their induction in response to inflammatory stimuli,15,16 we further assessed NF-κB activation using the electrophoretic mobility shift assay. The NF-κB DNA binding activity from nuclear extracts of mouse renal kidney cells infected with lentivirus containing miR-494 was markedly increased compared with the NF-κB DNA binding activity of control cells not expressing miR-494 (Figure 5D, upper panel, lane 5 versus lane 4). The identity of the gel shift bands was verified by competition analysis with cold NF-κB oligomers (Figure 5D, upper panel, lane 6). Furthermore, the increased NF-κB activation was accompanied by a concomitant elevation in the total amount of NF-κB protein present in the nuclei and a simultaneous decrease in cytosolic inhibitor of κBα (IκBα), an inhibitory protein that prevents translocation of NF-κβ into the nucleus (Figure 5D, lower panel). In contrast, after overexpression of antisense miR-494, the amount of NF-κB protein entering the nucleus was reduced, which was accompanied by a simultaneous elevation in cytosolic IκBα (Supplemental Figure 4, D–F). These results suggest that overexpression of miR-494 upregulates the expression of inflammatory cytokines and adhesion molecules through an NF-κB–dependent pathway after kidney I/R injury.
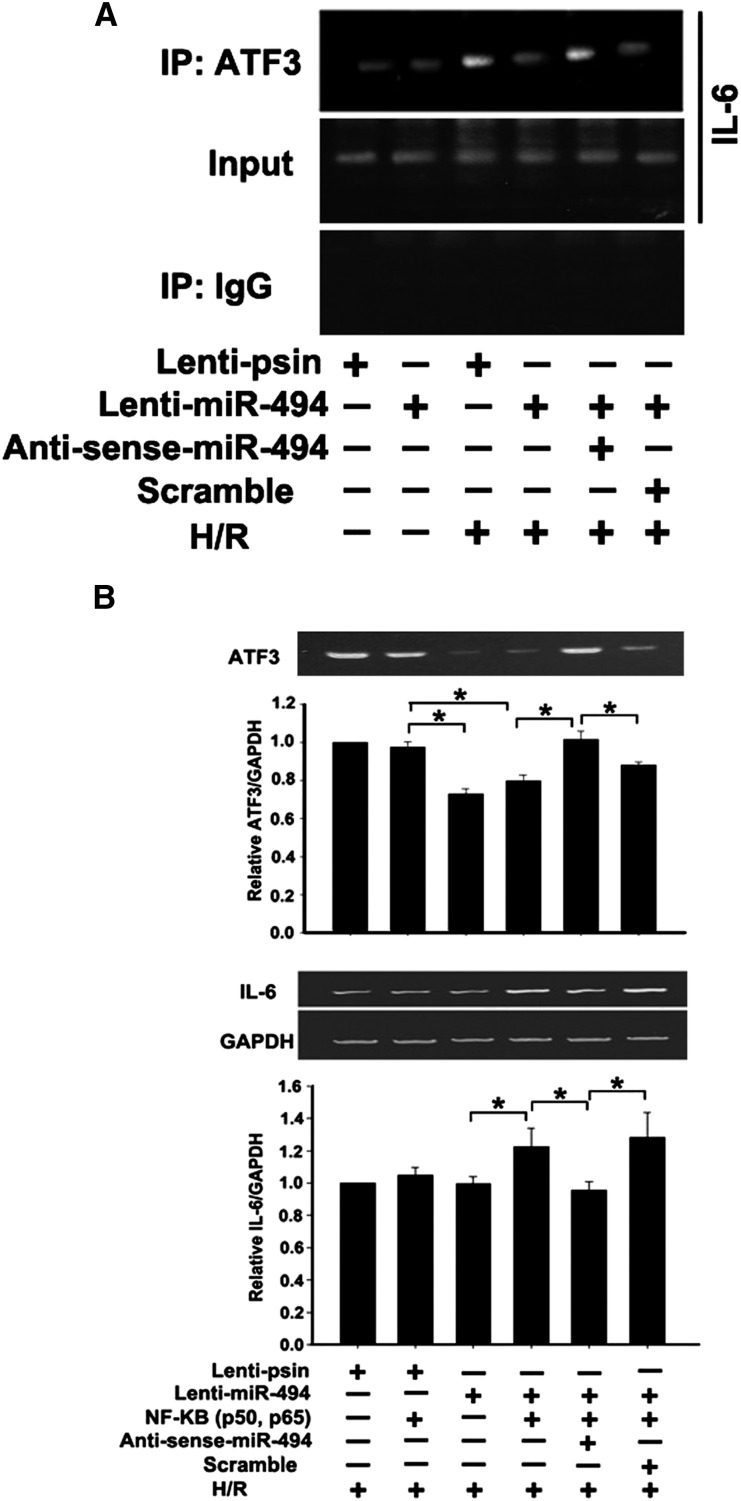
miR-494 Enhanced IL-6 Induction Associated with a Reduction in ATF3
Previous studies have identified a single ATF3 binding site close to the NF-κB binding site within the proximal region of the IL-6 promoter,17 and the maximum expression profiles of IL-6 and NF-κB occurred in hypoxia/reoxygenation with 24 hours/6 hours.3 Therefore, the chromatin immunoprecipitation (ChIP) assay was used to determine whether miR-494 could inhibit ATF3 recruited to the IL-6 gene promoter in renal tubular epithelial cells after hypoxia for 24 hours and reoxygenation for 6 hours. Overexpression of miR-494 attenuated the amount of ATF3 protein recruited to the IL-6 promoter region compared with the scrambled control, and antisense inhibition of miR-494 expression increased the ATF3 recruitment level (Figure 6A). In agreement with the ChIP assays, overexpression of miR-494 enhanced NF-κB–induced IL-6 induction, whereas antisense miR-494 abolished this effect (Figure 6B). Furthermore, the results from real-time PCR were consistent with the results from RT-PCR (Supplemental Figure 5). These results (Figures 5 and 6) suggest that miR-494 inhibits ATF3 transcription and slightly reduces the recruitment of ATF3 to the IL-6 promoter binding site, leading to enhanced NF-κB–induced IL-6 expression in renal tubular epithelial cells.
Figure 6.
Inhibition of ATF3 recruitment to the proximal promoter region of IL-6 by mir-R494 induces IL-6 inflammatory gene expression. (A) ChIP assay. NRK-52E cells were transfected with a plasmid as indicated, and cell lysates were crosslinked with formaldehyde. The ChIP assay involved the use of anti-ATF3 antibody. Immunoprecipitated DNA was amplified by PCR for the proximal promoter region of IL-6. (B) miR-494 promoted NF-κB–induced IL-6 expression. NRK-52E cells were transfected with the empty expression plasmid (vector), NF-κB (two plasmids encoding for the p50 or p65 subunit), or NF-κB together with the lenti–miR-494. Results are from RT-PCR analysis of the expression of NF-κB–induced IL-6 relative to the expression of glyceraldehyde-3-phosphate dehydrogenase 2 days after transfection. Experiments were performed three times with similar results. *P<0.05.
Patients with Acute Renal Injury Had Elevated Urinary miR-494 and Neutrophil Gelatinase-Associated Lipocalin Levels
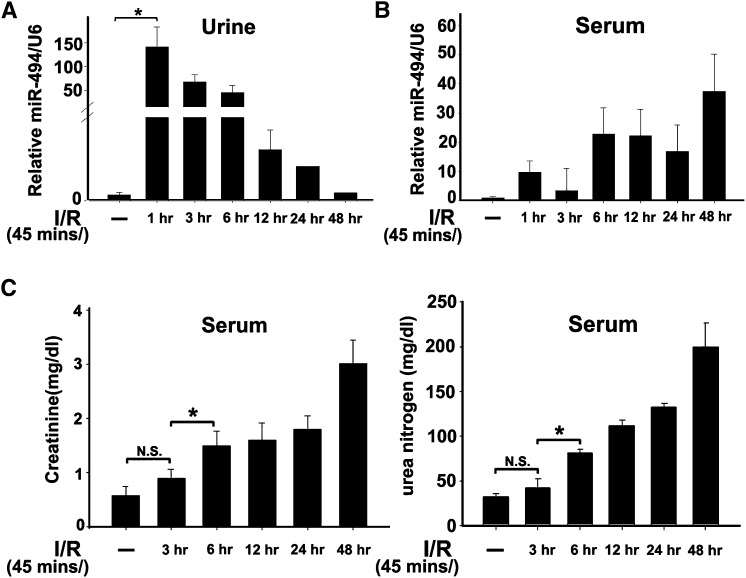
ATF3 has been reported to be a novel renal tubular cell injury biomarker for detecting early AKI.18 Therefore, we explored the possibility of miR-494, an ATF3-related miRNA, becoming an alternative indicator for AKI. We found that, in mice, urinary miR-494 levels were increased 1 hour after reperfusion, but the elevated levels were reduced dramatically 12 hours later (Figure 7A). In contrast, serum miR-494 levels did not differ until 6 hours after reperfusion (Figure 7B). Similarly, both serum creatinine and urea concentrations were elevated until 6 hours after I/R, reflecting a slower time course for creatinine than miR-494 (Figure 7C).
Figure 7.
The expression levels of miR-494 are earlier than creatinine and urea in mouse urine or serum after kidney I/R. Bilateral clamping of renal artery was 45 minutes followed by various durations of reperfusion. Levels of urinary (A) and serum (B) miR-494 were normalized to internal control U6 RNA by real time PCR assay. (C) Kidney functions assessed following I/R injury. *P<0.05 (n=5 animals/group). N.S., no significant difference.
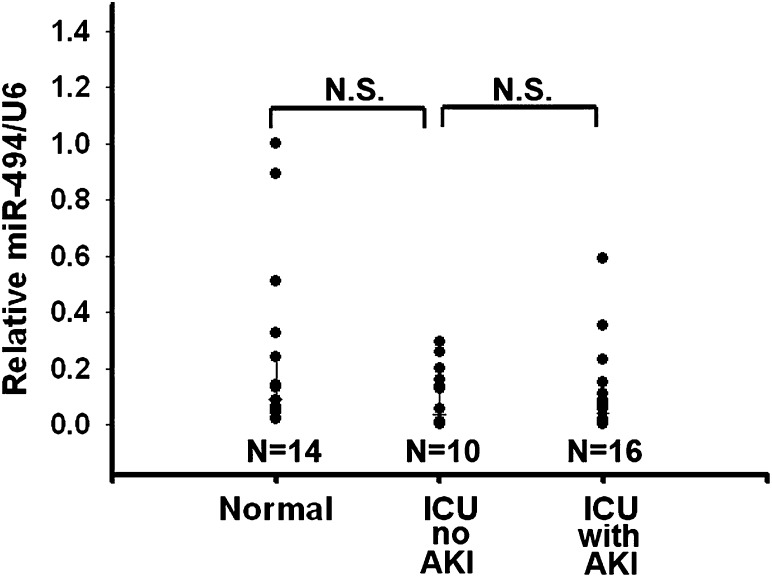
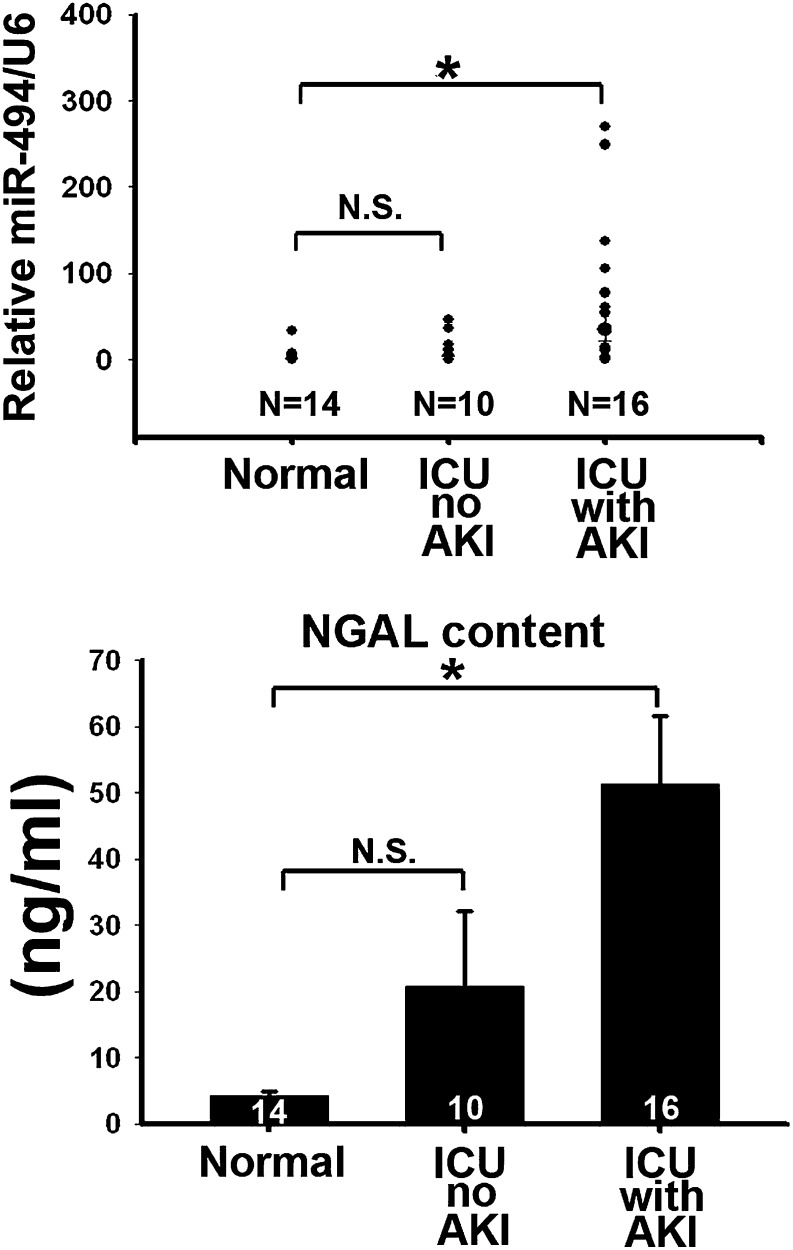
In a preliminary human study, both serum and urine samples were collected from 16 intensive care unit (ICU) patients with renal function tests that indicated AKI, 10 ICU patients without AKI syndrome, and 14 healthy volunteers. The serum miR-494 levels among the healthy volunteers (normal control), ICU patients without AKI, and ICU patients with AKI did not differ significantly (Figure 8). However, the urinary levels of miR-494 of ICU patients suffering from AKI were significantly higher compared with the normal control group (Figure 9, upper panel). These results are consistent with the known urinary AKI marker neutrophil gelatinase-associated lipocalin (NGAL) (Figure 9, lower panel). Together, these results indicate that miR-494 is expressed earlier than the traditional marker creatinine after AKI. Increased urinary miR-494 levels are reflective of AKI.
Figure 8.
Serum miR-494 levels have no difference between normal and ICU patients with and without AKI. Serum miR-494 levels were normalized to U6 RNA. N.S., no significant difference.
Figure 9.
Elevated urinary miR-494 levels are consistent with NGAL levels in AKI patients. Urinary miR-494 was measured by quantitative PCR, and urinary NGAL was measured by ELISA Kit (BioVendor) in the control patients and ICU patients without or with AKI. *P<0.05. N.S., no significant difference.
Discussion
Although ATF3 expression is believed to play an important protective role in the kidneys after I/R, little is known about the function and the regulatory mechanisms of miRNAs in I/R-induced kidney injury. In this study, we showed that miR-494 induced greater kidney injury through inhibition of ATF3 in mice. Furthermore, I/R-induced miR-494 not only increased inflammatory mediator IL-6 and adhesion molecules, such as P-selectin and MCP-1, but also, it increased the apoptosis of renal epithelial cells. We also found that the elevated expression of urinary miR-494 preceded the expression of serum creatinine after I/R injury. Additionally, a preliminary clinical analysis showed that miR-494 was expressed at higher levels in the urine of AKI patients in the ICU compared with patients without AKI or normal individuals. These data imply that miR-494 is required for the induction of renal injury during I/R, and urinary miR-494 is an earlier and noninvasive indicator compared with creatinine.
Using the human Ingenuity Pathway Analysis or TargetScan System, it was found that miR-494 not only targeted ATF3 but also many other genes, such as adiponectin receptor 2 (ADIPOR2), B-cell lymphoma 2-like 11 apoptosis facilitator, and IGF1 receptor (IGF1R) (Supplemental Figure 6). Because antiapoptotic effects of adiponectin may result from its anti-inflammatory or antioxidative effect after I/R primarily through the ADIPOR2–proliferator-activated receptor α–heme oxygenase-1 pathway,19 inhibiting ADIPOR2 by miR-494 would result in greater inflammation and hence, more damage to the kidneys. With the relationship between IGF1R and the kidneys, IGF1R has been implicated in normal mammalian glomerular integrity,20 and suppression of IGF1R by miR-494 may inhibit podocyte cell outgrowth, leading to proteinuria. Therefore, these miR-494–related targeted genes may also influence I/R-induced nephropathy. Whether other functional genes regulated by miR-494 are involved in I/R of the kidneys needs additional investigation.
Although this study revealed an aggravated, harmful role of miR-494 during renal I/R injury, miR-494 also exerted beneficial effects in the context of other stress conditions. For example, miR-494 targets both proapoptotic and antiapoptotic proteins, ultimately activating the Akt pathway and leading to cardioprotective effects against I/R-induced injury in mice.8 In addition, miR-494 has been reported to promote cancer cell death.10,21 These findings suggest that miR-494 exhibits differential effects on divergent targets to balance a common signaling pathway and eventually, determine the expression phenotype. Regardless, miR-494 is abundantly expressed in a variety of organs, such as the brain, heart, liver, and kidneys (Figure 2). Whether miR-494 is also secreted from these organs into the blood by packaging within exosomes22 to aggravate I/R-induced renal damage will need additional studies.
Clinical AKI leads to activation of innate and adaptive immune responses, resulting in numerous hallmarks of ischemic renal injury,23 such as apoptosis24 and fibrosis,25 which are regulated by miRNAs. AKI microarray data also showed that several miRNAs (miR-21, miR-20a, miR-146a, miR-199a-3p, miR-214, miR-192, miR-187, miR-805, and miR-194) are differentially expressed.26 These miRNAs are defined as the lymphocyte-independent signature of ischemic renal injury. However, in several tissues, including the kidneys, ATF3 acted as an early inducible transcriptional repressor27 and inhibited lymphocyte-dependent ischemic renal injury.3 This result may be the reason why miR-494 was not involved in the previous AKI microarray data,28 suggesting that miR-494 is expressed earlier than miR-21, miR-20a, miR-146a, miR-199a-3p, miR-214, miR-192, miR-187, miR-805, and miR-194 after kidney I/R injury. The relationship between CKD or other pathologically induced kidney diseases and miRNAs, like miR-200a, miR-200b, miR-141, miR-429, miR-205, miR-335, miR-34a and miR-192, has also been studied.28–31 These studies have not mentioned miR-494, suggesting that the majority of miR-494 expression appears in the early phase of I/R injury and not in CKD or the other pathologically induced kidney diseases.
Because miRNAs and NGAL can be detected in the urine,32,33 theoretically, they are filtered and excreted by or directly from the kidneys and/or urinary tract. In our clinical samples, there were no differences in serum miR-494 among the normal and ICU patients with or without AKI (Figure 8). However, urinary miR-494 levels of AKI patients were higher than the levels in patients without AKI and normal subjects. These results indicate that miR-494 is not filtered from the blood but released or secreted from the kidneys and/or urinary tract. Precisely which part of the kidneys, cortex, medulla, or glomerulus secretes or ruptures to release miR-494 after I/R injury will be the focus of future investigation.
Urinary miR-494 expression levels were significantly higher only in AKI patients. Besides sepsis, many of our AKI patients (Table 1) had shock or acute tubular necrosis. It is suggested that modulation of miR-494 may not be induced only by inflammatory microenvironment, which is a typical syndrome of sepsis. In addition, the conditions of our AKI patients were more serious than the conditions of patients without AKI using Acute Physiology, Age, Chronic Health Evaluation II score analysis. For patients without AKI, the death rate is about 30%, but none of the deaths were caused by kidney dysfunction (one patient each died from hepatoma, hemorrhagic stroke, or paraquat intoxication) (Table 1). Together, our study and the study by Munshi et al.33 show that consistent expression level of miR-494 and NGAL can be used as an indicator of AKI.
Table 1.
Patient demographics for AKI (−) and AKI (+) patients
| Patient Characteristics | AKI (−) Patients (n=10) | AKI (+) Patients (n=16) |
|---|---|---|
| Age in years (range) | 70.00 (42–89) | 67.25 (43–87) |
| Sex | 60% men, 40% women | 69% men, 31% women |
| Race | 100% Asian | 100% Asian |
| Comorbities | ||
| Hypertension | 6 | 6 |
| Cardiovascular accident | 1 | 2 |
| Diabetes | 5 | 7 |
| Liver cirrhosis | 1 | 0 |
| Clinical data | ||
| Admission diagnosis | ||
| Acute tubular necrosis | 0 | 3 |
| Sepsis | 2 | 6 |
| Shock | 2 | 6 |
| Acute coronary syndrome | 1 | 1 |
| Hepatoma | 1 | 0 |
| Hemorrhagic stroke | 3 | 0 |
| Paraquat intoxication | 1 | 0 |
| AKIN stage (N) | ||
| I | 0 | 7 |
| II | 0 | 5 |
| III | 0 | 4 |
| APACHE II score (average) | 12–25 (19.9) | 15–36 (21.1) |
| RRT requirement | 0 | 1 (6.25%) |
| Overall mortality | 3 (30%) | 1 (6.25%) |
| Baseline creatinine (mg/dl; average) | 0.8–2.5 (1.52) | 0.8–3.5 (1.70) |
| Peak creatinine (average) | 0.8–2.5 (1.52) | 1.8–8.3 (3.99) |
AKIN, Acute Kidney Injury Network; APACHE, Acute Physiology, Age, Chronic Health Evaluation; RRT, renal replacement therapy.
In summary, we showed that miR-494 is upregulated in I/R-induced kidney injury, which in turn, inhibits the expression of the kidney protective gene ATF3, resulting in more aggravated kidney injury. Our results indicate that blockade of endogenous miR-494 by antisense administration may reduce I/R-induced kidney injury. Finally, urinary miR-494 is expressed earlier than creatinine, which makes it a useful indicator of AKI and provides information complementary to the information from NGAL analysis.
Concise Methods
Animal Model for Renal I/R
The C57BL/6 male mice, 8–10 weeks, underwent bilateral renal artery occlusion for 45 minutes and reperfusion for the indicated time. Sham operation was identical to the treatment surgery, except for pedicle clamping. All surgical procedures were approved by the Institutional Animal Care and Use Committee, Academia Sinica, Taipei, Taiwan.
Patients and Urine Collection
A total of 40 human serum and urine samples were obtained immediately when patients were admitted to the Tzu Chi General Hospital (Hualien, Taiwan). These samples were collected from October of 2011 to August of 2012. This research project was approved by the Institutional Review Board of the Tzu Chi General Hospital, and informed consents were obtained from all patients. The samples were collected from 16 critical patients who developed AKI, defined as ≧1.5-fold increase in serum creatinine in compliance with RIFLE-Acute Kidney Injury Network criteria. ICU control samples were collected from 10 critical patients who did not develop AKI. The relevant information of these patients is listed in Table 2. In addition, serum and urine samples were obtained from 14 healthy volunteers. Urine samples of 25–35 ml were collected from the participating subjects. All samples were frozen at −80°C until use.
Table 2.
Primers used
| Gene | Forward | Reverse | Assay |
|---|---|---|---|
| miR-494 | TGAAACATACACGGGAAACCTC | Universal qPCR Primer (Invitrogen) | qPCR |
| HsaU6 | CGCAAGGATGACACGCAAATTC | Universal qPCR Primer (Invitrogen) | qPCR |
| mU6 | TGGCCCCTGCGCAAGGATG | Universal qPCR Primer (Invitrogen) | qPCR |
| mATF3 | AAGGAACTTGCAGAGCTAAGCA | GGGTGGAAAAGGAGGATTCAGTA | qPCR |
| mIL-6 | TCCAGTTGCCTTCTTGGGAC | GTGTAATTAAGCCTCCGACTTG | qPCR |
| Rat IL-6 | CCACCAGGAACGAAAGTCAAC | AAGGCAACTGGCTGGAAGTCT | qPCR |
| Rat ATF3 | AAGGAACATTGCAGAGCTAAGCA | GGGTGGAAAAGGAGGATTCAGTA | qPCR |
| Rat IL-6 | CTCTCCGCAAGAGACTTCCAG | GCCGAGTAGACCTCATAGTGA | RT-PCR |
| ATF3 | TTGGATCCATGATGCTTCAACAC | CCCAAGCTTTTACTTGTCATCGTC | RT-PCR |
| IL-6 | TGCTCAAGTGCTGAGTCACT | AGACTCATGGGAAAATCCCA | ChIP |
qPCR, quantitative PCR.
Western Blot Analysis
Kidney and cell extracts were separated by conventional SDS-PAGE and subjected to Western blot analysis using an enhanced chemiluminescence kit (Pierce). Antibodies used were anti-ATF3 (1:500; Santa Cruz Biotechnology), anti–NF-κB (1:1000), anti-IκB (1:1000; Santa Cruz Biotechnology), antiglyceraldehyde-3-phosphate dehydrogenase (1:10,000; BD Pharmingen), anticaspase-3 (1:500; Cell Signal Technology), antiaquaporin 1 (1:100; Santa Cruz Biotechnology), anti–β-actin (1:10,000; Millipore, Darmstadt, Germany), and antilamin A (1:1000; GeneTex).
Histopathology
Mouse kidneys were fixed in 10% buffered formalin overnight at 4°C and processed with paraffin fixation. Sections were stained with hematoxylin and eosin. Apoptosis in renal tissues was identified using the TUNEL assay with an ApopTag In Situ Apoptosis Detection Kit (S7160; Chemicon, Darmstadt, Germany) and counterstained with 4',6-diamidino-2-phenylindole (SouthernBiotech) following the manufacturer’s instructions. Identification of renal tubule cell types was identified using aquaporin 1 antibody (SC-25287; Santa Cruz) and sodium-potassium-chloride cotransporter isoform 2 antibody (GTX47166; GeneTex).
Measurement of Biochemical Parameters
At the end of reperfusion, 500-µl blood samples were collected through the tail vein. Samples were centrifuged at 6000×g for 3 minutes to separate the serum from the cells. Biochemical parameters were measured in serum within 24 hours.
Lentiviral Production
All lentiviral vector stocks were generated by lipofectamine-mediated transfection of 293T cells (American Type Culture Collection, Manassas, VA). The cells were cultured in DMEM (GIBCO) with 10% heat-inactivated FBS (Omega, Tarzana, CA); 293T cells (4×106) were seeded into 10-cm2 culture dishes in 5.5 ml medium and transfected the next day with 2 μg pMD-G plasmid, 8 µg pCMV8.9 plasmid, and 12 μg lenti–miR-494 vector plasmid (GeneCopoeia) or antisense miR-494 (SBI CA). The medium was collected on days 2 and 3 posttransfection and concentrated using the Vivapure LentiSELECT40 Kit (Sartorius Stedim Biotech, Aubagne Cedex, France).
Intrarenal Pelvic Injection of Lentivirus
The procedure used was described previously.3 Mice were anesthetized with intraperitoneal pentobarbital (50 mg/kg). The renal artery, renal vein, and ureter were clamped at the same time just below the renal pelvis before transfection. Recombinant lentivirus or PBS was slowly injected into the left renal artery with the use of a 30-gauge needle; subsequently, the needle was removed, and the ureter was declamped. After 2 weeks, mice were euthanized, and the kidneys were removed and homogenized for designated experiments.
Assay for Reporter Activity
miRNA plasmid was constructed using an 807-bp DNA fragment containing murine primary miR-494 DNA (NC_000078.6) under the pEGFP plasmid. ATF3 3′UTR was constructed under the pRFP plasmid (from Chien-Chang Chen, Academia Sinica, Taipei, Taiwan). Briefly, 293T cells (5×105) were seeded into six-well plates and transfected with pEGFP and pEGFP–miR-494 plasmids, respectively. After 24 hours, the cells were divided into four groups, and each group was transfected separately with the following four vectors, including pRFP, pRFP-ATF3 3′UTR, pRFP-ATF3 3′UTR (with miR-494 inhibitor; AM12409; Ambion), and pRFP-ATF3 3′UTR (with miRNA negative control II; AM17003; Ambion). Finally, the RFP/pEGFP fluorescent ratios were measured 48 hours after transfection using a fluorescence microplate reader (Molecular Devices).
RNA Isolation
Total tissue and cell RNA were extracted by Trizol (Invitrogen). Furthermore, the blood and urinary RNA of mice were extracted using the mirVana isolation kit (ABI), whereas the human urine RNA was extracted using the QiAMP circulating nucleic acid kit (Qiagen). The protocols for RNA isolation were conducted according to the manufacturer’s instructions.
Real-Time Quantitative PCR and RT-PCR
The ABI PRISM 7700 Sequence Detection System (ABI) was used for real-time quantitative PCR analysis. For the detection of miR-494 expression, stem-loop RT-PCR was performed using the NCode VILO miRNA cDNA Synthesis Kit (Invitrogen) according to the manufacturer’s instructions. Briefly, the extracted RNA was reverse-transcribed in the presence of a poly-A polymerase with an oligo-dT adaptor. Real-time PCR was conducted using SYBR Green enzyme detection, with a forward primer for the mature miRNA sequence and a universal adaptor reverse primer. Relative expression was evaluated by the comparative threshold cycle method and normalized to the expression of U6 spliceosomal RNA, which is commonly used as a reference gene in miRNA quantification. The primers used are listed in Table 2. For RT-PCR, cDNA was prepared with the Super Script Kit (Invitrogen) from 3 µg total RNA. The primers used are listed in Table 2.
Whole-Mount In Situ Hybridization
Locked nucleic acid-modified miR-494 oligonucleotide probe (Exiqon, Vedbaek, Denmark) was labeled with digoxigenin. The IsHyb In Situ Hybridization Kit (BioChain) was used according to the manufacturer’s protocol, and nuclei were stained with Contrast green (KPL) according to the manufacturer’s protocol.
In Vitro Hypoxia/Reoxygenation Experiment
Twenty-four hours after lenti–miR-494 or lenti-pSin infection, NRK-52E cells were transfected separately with the vector only, NF-κB (p50, p65) plasmids, NF-κB (p50, p65) plasmids with miR-494 inhibitor (AM12409, Ambion), and NF-κB (p50, p65) plasmids with miRNA negative control II (AM17003, Ambion). Then, the cells were incubated under conditions of normoxia or hypoxia (1% O2) for 24 hours followed by 6 hours of reoxygenation.
Cytoplasmic and Nuclear Protein Extraction
Cells and kidney tissues were processed for extraction of nuclear and cytoplasmic protein fractions according to the manufacturer’s protocols (Fermentas).
Nuclear Extracts and Electrophoretic Mobility-Shift Assays
We purchased NF-κB DNA probes containing a consensus NF-κB enhancer element (5′-AGT TGA GGG GAC TTT CCC AGG C-3′) from Santa Cruz Biotechnology. Electrophoretic mobility-shift assay analysis of nuclear NF-κB was performed as described.34 Briefly, nuclear extracts were prepared, and binding reactions were performed in 20-μl reaction mixtures with 3 μg nuclear extracts. Aliquots of the reaction mixture were loaded on a 5% polyacrylamide gel and run at 100 V at 4°C in 0.5× Tris-borate-EDTA buffer.
ChIP Assay
One day after lenti–miR-494 or lenti-pSin infection, NRK-52E cells were incubated under conditions of normoxia or hypoxia (1% O2) for 24 hours followed by 6 hours of reoxygenation. The cells were fixed in 1% formaldehyde, and the ChIP assay was conducted using the Upstate protocol (Millipore). Chromatin was immunoprecipitated with anti-ATF3 antibody (Santa Cruz Biotechnology). The purified DNA was detected by standard PCR. Primers used are listed in Table 2.
Statistical Analyses
Values are expressed as means ± SEM from at least three experiments. The statistical significance was analyzed using ANOVA followed by the Tukey test for the in vivo experiments. Samples from the patients were analyzed using the rank sum test. A value of P<0.05 was considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by National Science Council of Taiwan Grant 99-2314-B-038-036-MY3 and Department of Health Executive Yuan, ROC Taiwan Grant DOH101-TD-PB-111-NSC013.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012050438/-/DCSupplemental.
References
- 1.Padanilam BJ: Cell death induced by acute renal injury: A perspective on the contributions of apoptosis and necrosis. Am J Physiol Renal Physiol 284: F608–F627, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Schiffl H: Daily haemodialysis for acute renal failure. Curr Opin Nephrol Hypertens 11: 589–592, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Li HF, Cheng CF, Liao WJ, Lin H, Yang RB: ATF3-mediated epigenetic regulation protects against acute kidney injury. J Am Soc Nephrol 21: 1003–1013, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lovat F, Valeri N, Croce CM: MicroRNAs in the pathogenesis of cancer. Semin Oncol 38: 724–733, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Krützfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M: Silencing of microRNAs in vivo with ‘antagomirs’. Nature 438: 685–689, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Krützfeldt J, Kuwajima S, Braich R, Rajeev KG, Pena J, Tuschl T, Manoharan M, Stoffel M: Specificity, duplex degradation and subcellular localization of antagomirs. Nucleic Acids Res 35: 2885–2892, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang XH, Qian RZ, Zhang W, Chen SF, Jin HM, Hu RM: MicroRNA-320 expression in myocardial microvascular endothelial cells and its relationship with insulin-like growth factor-1 in type 2 diabetic rats. Clin Exp Pharmacol Physiol 36: 181–188, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Zhang X, Ren XP, Chen J, Liu H, Yang J, Medvedovic M, Hu Z, Fan GC: MicroRNA-494 targeting both proapoptotic and antiapoptotic proteins protects against ischemia/reperfusion-induced cardiac injury. Circulation 122: 1308–1318, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang SS, Jiang WW, Smith I, Poeta LM, Begum S, Glazer C, Shan S, Westra W, Sidransky D, Califano JA: MicroRNA alterations in head and neck squamous cell carcinoma. Int J Cancer 123: 2791–2797, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim WK, Park M, Kim YK, Tae YK, Yang HK, Lee JM, Kim H: MicroRNA-494 downregulates KIT and inhibits gastrointestinal stromal tumor cell proliferation. Clin Cancer Res 17: 7584–7594, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Olaru AV, Ghiaur G, Yamanaka S, Luvsanjav D, An F, Popescu I, Alexandrescu S, Allen S, Pawlik TM, Torbenson M, Georgiades C, Roberts LR, Gores GJ, Ferguson-Smith A, Almeida MI, Calin GA, Mezey E, Selaru FM: MicroRNA down-regulated in human cholangiocarcinoma control cell cycle through multiple targets involved in the G1/S checkpoint. Hepatology 54: 2089–2098, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kielar ML, John R, Bennett M, Richardson JA, Shelton JM, Chen L, Jeyarajah DR, Zhou XJ, Zhou H, Chiquett B, Nagami GT, Lu CY: Maladaptive role of IL-6 in ischemic acute renal failure. J Am Soc Nephrol 16: 3315–3325, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Singbartl K, Green SA, Ley K: Blocking P-selectin protects from ischemia/reperfusion-induced acute renal failure. FASEB J 14: 48–54, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Barnes PJ: Nuclear factor-kappa B. Int J Biochem Cell Biol 29: 867–870, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Pan J, Xia L, Yao L, McEver RP: Tumor necrosis factor-alpha- or lipopolysaccharide-induced expression of the murine P-selectin gene in endothelial cells involves novel kappaB sites and a variant activating transcription factor/cAMP response element. J Biol Chem 273: 10068–10077, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Plaisance S, Vanden Berghe W, Boone E, Fiers W, Haegeman G: Recombination signal sequence binding protein Jkappa is constitutively bound to the NF-kappaB site of the interleukin-6 promoter and acts as a negative regulatory factor. Mol Cell Biol 17: 3733–3743, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilchrist M, Thorsson V, Li B, Rust AG, Korb M, Roach JC, Kennedy K, Hai T, Bolouri H, Aderem A: Systems biology approaches identify ATF3 as a negative regulator of Toll-like receptor 4. Nature 441: 173–178, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Zhou H, Cheruvanky A, Hu X, Matsumoto T, Hiramatsu N, Cho ME, Berger A, Leelahavanichkul A, Doi K, Chawla LS, Illei GG, Kopp JB, Balow JE, Austin HA, 3rd, Yuen PS, Star RA: Urinary exosomal transcription factors, a new class of biomarkers for renal disease. Kidney Int 74: 613–621, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng CF, Lian WS, Chen SH, Lai PF, Li HF, Lan YF, Cheng WT, Lin H: Protective effects of adiponectin against renal ischemia-reperfusion injury via prostacyclin-PPARα-heme oxygenase-1 signaling pathway. J Cell Physiol 227: 239–249, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Bridgewater DJ, Dionne JM, Butt MJ, Pin CL, Matsell DG: The role of the type I insulin-like growth factor receptor (IGF-IR) in glomerular integrity. Growth Horm IGF Res 18: 26–37, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Ohdaira H, Sekiguchi M, Miyata K, Yoshida K: MicroRNA-494 suppresses cell proliferation and induces senescence in A549 lung cancer cells. Cell Prolif 45: 32–38, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O’Briant KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stirewalt DL, Gentleman R, Vessella RL, Nelson PS, Martin DB, Tewari M: Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A 105: 10513–10518, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tilney NL, Guttmann RD: Effects of initial ischemia/reperfusion injury on the transplanted kidney. Transplantation 64: 945–947, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Ren XP, Wu J, Wang X, Sartor MA, Qian J, Jones K, Nicolaou P, Pritchard TJ, Fan GC: MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 20. Circulation 119: 2357–2366, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JT, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engelhardt S: MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 456: 980–984, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Godwin JG, Ge X, Stephan K, Jurisch A, Tullius SG, Iacomini J: Identification of a microRNA signature of renal ischemia reperfusion injury. Proc Natl Acad Sci U S A 107: 14339–14344, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li D, Yin X, Zmuda EJ, Wolford CC, Dong X, White MF, Hai T: The repression of IRS2 gene by ATF3, a stress-inducible gene, contributes to pancreatic beta-cell apoptosis. Diabetes 57: 635–644, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Shapiro MD, Bagley J, Latz J, Godwin JG, Ge X, Tullius SG, Iacomini J: MicroRNA expression data reveals a signature of kidney damage following ischemia reperfusion injury. PLoS ONE 6: e23011, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiong M, Jiang L, Zhou Y, Qiu W, Fang L, Tan R, Wen P, Yang J: The miR-200 family regulates TGF-β1-induced renal tubular epithelial to mesenchymal transition through Smad pathway by targeting ZEB1 and ZEB2 expression. Am J Physiol Renal Physiol 302: F369–F379, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Kato M, Zhang J, Wang M, Lanting L, Yuan H, Rossi JJ, Natarajan R: MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-beta-induced collagen expression via inhibition of E-box repressors. Proc Natl Acad Sci U S A 104: 3432–3437, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ: The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol 10: 593–601, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Melkonyan HS, Feaver WJ, Meyer E, Scheinker V, Shekhtman EM, Xin Z, Umansky SR: Transrenal nucleic acids: From proof of principle to clinical tests. Ann N Y Acad Sci 1137: 73–81, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Munshi R, Johnson A, Siew ED, Ikizler TA, Ware LB, Wurfel MM, Himmelfarb J, Zager RA: MCP-1 gene activation marks acute kidney injury. J Am Soc Nephrol 22: 165–175, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin H, Cheng CF, Hou HH, Lian WS, Chao YC, Ciou YY, Djoko B, Tsai MT, Cheng CJ, Yang RB: Disruption of guanylyl cyclase-G protects against acute renal injury. J Am Soc Nephrol 19: 339–348, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.