Abstract
Thirty to sixty percent of patients with ESRD on dialysis have coronary heart disease, but the optimal strategy for coronary revascularization is unknown. We used data from the United States Renal Data System to define a cohort of 21,981 patients on maintenance dialysis who received initial coronary revascularization with either coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI) between 1997 and 2009 and had at least 6 months of prior Medicare coverage as their primary payer. The primary outcome was death from any cause, and the secondary outcome was a composite of death or myocardial infarction. Overall survival rates were consistently poor during the study period, with unadjusted 5-year survival rates of 22%–25% irrespective of revascularization strategy. Using multivariable-adjusted proportional hazards regression, we found that CABG compared with PCI associated with significantly lower risks for both death (HR=0.87, 95% CI=0.84–0.90) and the composite of death or myocardial infarction (HR=0.88, 95% CI=0.86–0.91). Results were similar in analyses using a propensity score-matched cohort. In the absence of data from randomized trials, these results suggest that CABG may be preferred over PCI for multivessel coronary revascularization in appropriately selected patients on maintenance dialysis.
Cardiovascular disease is the leading cause of death in patients with ESRD.1 Coronary heart disease affects 30%–60% of patients with ESRD, and it usually involves multiple vessels, proximal lesions, heavy calcifications, or diffuse disease.2–4 Because of the high burden and poor prognosis of coronary disease in this patient population, optimal management of coronary heart disease—particularly the choice of revascularization modality—is a critical clinical issue.
Although there have been several randomized trials comparing multivessel coronary artery bypass grafting (CABG) with multivessel percutaneous coronary intervention (PCI),5,6 none of these trials included patients with ESRD. Evidence from previous observational studies is mixed; some studies indicate a long-term survival benefit associated with CABG versus PCI,7–10 whereas other studies show no significant differences in survival.11–15 These discrepant results may have stemmed, at least in part, from the heterogeneity of the studied populations (e.g., inclusion of patients with single- and multivessel coronary disease and small sample sizes from single institutions). Moreover, most of these studies were performed between the 1970s and early 2000s, and therefore, they do not reflect contemporary practice patterns, such as the use of drug-eluting stents.
To address these issues, we used data from the US Renal Data System (USRDS), which collects extensive information for over 95% of patients with ESRD in the United States.1 We examined the comparative effectiveness of CABG versus PCI between 1997 and 2009 in patients with ESRD on maintenance dialysis. We restricted our analysis to patients undergoing multivessel coronary revascularization to minimize indication bias, because they have the most similar likelihood of receiving either CABG or PCI. We hypothesized that an initial strategy of CABG would be associated with lower risks of mortality and cardiovascular morbidity compared with PCI.
Results
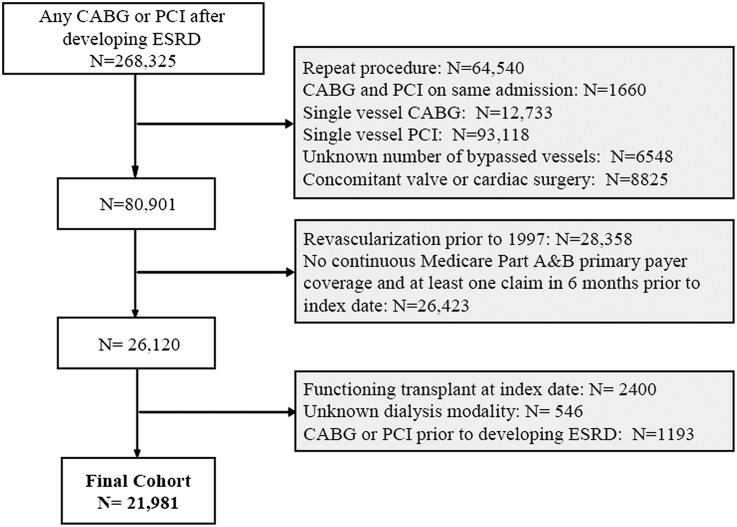
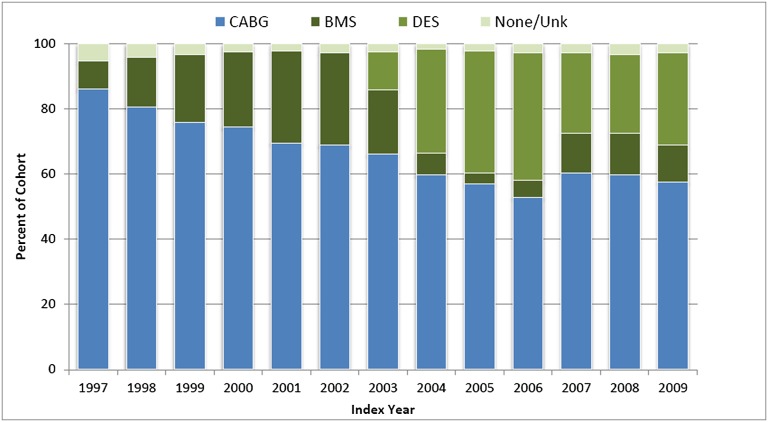
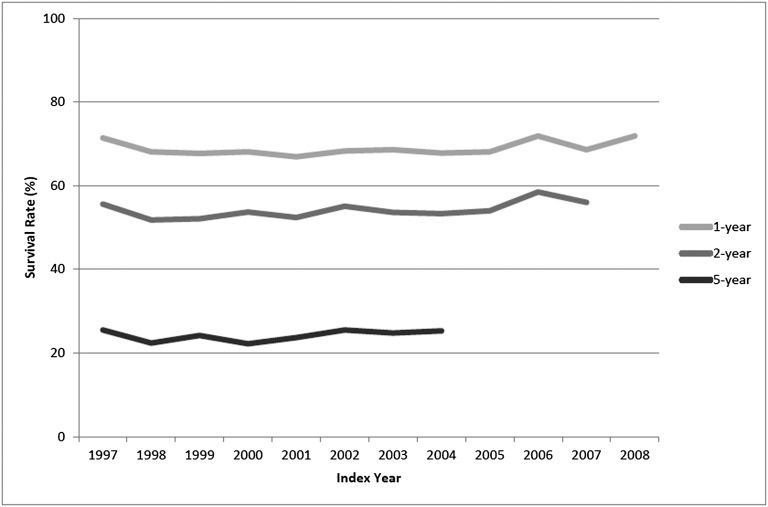
We identified 21,981 patients who received initial coronary revascularization with multivessel CABG or multivessel PCI between 1997 and 2009 and met the inclusion and exclusion criteria (Figure 1). Among patients undergoing revascularization, the proportion of patients undergoing multivessel CABG fell from 86% in 1997 to 53% in 2006 (Figure 2). After the introduction of drug-eluting stents in April of 2003, the proportion of patients undergoing PCI who received a drug-eluting stent peaked at 87% in 2005 and fell to 61% of all PCIs in 2008. Overall survival rates remained relatively constant over the study period (Figure 3). For example, for patients undergoing multivessel coronary revascularization in 1997, the 1-year survival rate was 71% (95% confidence interval [CI]=69%–74%); in 2008, the 1-year survival rate was 72% (CI=70%–74%).
Figure 1.
Cohort assembly of 21,981 patients on maintenance dialysis undergoing initial coronary revascularization between 1997 and 2009 in the USRDS.
Figure 2.
Distribution of initial multivessel revascularization type by index year among patients on maintenance dialysis in the USRDS. The proportion of patients undergoing multivessel CABG reached a nadir in 2006. After the introduction of drug-eluting stents in April 2003, the proportion of patients undergoing PCI who received a drug-eluting stent peaked at 87% in 2005. CABG, coronary artery bypass grafting; BMS, bare metal stent; DES, drug-eluting stent; Unk, unknown.
Figure 3.
Survival rates at 1, 2, and 5 years after initial multivessel coronary revascularization for patients on maintenance dialysis in the USRDS by index year.
In the full cohort, patients receiving multivessel CABG were generally younger and less often presented with a myocardial infarction (MI) on the index hospitalization, but they more often had a history of prior MI and angina compared with patients receiving multivessel PCI (Table 1 and Supplemental Table 1). The strongest predictors for receipt of CABG rather than PCI were earlier year of the index revascularization, cerebral atherosclerotic disease, and younger age (Supplemental Table 2). The c statistic for our propensity score model was 0.66, indicating modest predictability of coronary intervention. We matched 92% of patients (n=7049) who received a multivessel PCI to otherwise similar patients who received a multivessel CABG in a 1:1 ratio. Baseline characteristics were well balanced among the CABG and PCI recipients after propensity score matching (Table 1 and Supplemental Table 1).
Table 1.
Selected baseline characteristics of the unmatched and propensity score-matched cohorts
| Full Cohort | Matched Cohort | |||||
|---|---|---|---|---|---|---|
| CABG (n=14,316) | PCI (n=7665) | P | CABG (n=7049) | PCI (n=7049) | P | |
| Demographics | ||||||
| Mean age (SD; yr) | 63.1 (10.8) | 64.9 (12.0) | <0.001 | 64.2 (10.6) | 64.4 (12.0) | 0.50 |
| Male | 62.9 | 56.4 | <0.001 | 57.2 | 57.9 | 0.40 |
| Race | 0.03 | 0.80 | ||||
| White | 66.2 | 66.6 | 66.8 | 66.3 | ||
| Black | 26.6 | 27.1 | 27.7 | 27.2 | ||
| Other/unknown | 7.3 | 6.3 | 6.5 | 6.5 | ||
| Dialysis modality | ||||||
| Hemodialysis | 90.9 | 92.1 | 0.004 | 91.7 | 91.7 | >0.90 |
| Peritoneal dialysis | 9.1 | 7.9 | 8.3 | 8.3 | ||
| Prior failed kidney transplant | 4.9 | 5.5 | 0.05 | 5.5 | 5.4 | 0.80 |
| Duration of ESRD median (IQR; yr) | 2.7 (1.4–4.7) | 2.6 (1.4–4.7) | 0.50 | 2.7 (1.4–4.8) | 2.7 (1.4–4.7) | 0.10 |
| <1 | 14.7 | 15.2 | 0.40 | 15.1 | 15.0 | >0.90 |
| 1–3 | 39.7 | 40.0 | 39.6 | 39.8 | ||
| >3 | 45.6 | 44.8 | 45.3 | 45.2 | ||
| Cause of ESRD | 0.05 | 0.80 | ||||
| Diabetes | 56.1 | 58.0 | 58.5 | 57.7 | ||
| Hypertension | 24.5 | 23.5 | 23.5 | 23.8 | ||
| Glomerulonephritis | 8.3 | 8.1 | 7.9 | 8.1 | ||
| Other/unknown | 11.1 | 10.4 | 10.1 | 10.4 | ||
| Non-nephrology outpatient visits median (IQR) | 13 (8–20) | 14 (9–23) | <0.001 | 14 (8–22) | 14 (8–22) | 0.40 |
| Hospital days before index date median (IQR) | 5 (2–10) | 5 (2–12) | <0.001 | 5 (2–11) | 5 (2–12) | 0.70 |
| Used skilled nursing facility | 4.9 | 10.2 | <0.001 | 7.4 | 7.2 | 0.70 |
| MI on index presentation | 23.9 | 27.6 | <0.001 | 26.7 | 26.9 | 0.80 |
| Cardiovascular history | ||||||
| Prior myocardial infarction | 20.0 | 17.5 | <0.001 | 17.4 | 17.6 | 0.70 |
| Angina | 29.1 | 22.4 | <0.001 | 22.4 | 23.1 | 0.30 |
| Heart failure | 39.7 | 44.1 | <0.001 | 42.1 | 42.0 | >0.90 |
| Hypertension | 79.6 | 80.9 | 0.02 | 80.0 | 80.1 | 0.80 |
| Atrial fibrillation | 9.3 | 13.1 | <0.001 | 10.8 | 11.4 | 0.30 |
| Arrhythmia | 7.1 | 8.0 | 0.01 | 7.4 | 7.4 | >0.90 |
| Pacemaker | 0.8 | 1.0 | 0.05 | 0.8 | 0.9 | 0.40 |
| Ventricular fibrillation | 2.4 | 3.2 | <0.001 | 3.0 | 3.0 | 0.90 |
| Implantable cardiac defibrillator | 0.2 | 0.2 | 0.09 | 0.2 | 0.6 | 0.90 |
| Valvular disease | 16.0 | 18.0 | <0.001 | 17.0 | 17.2 | 0.80 |
| Stroke | 5.9 | 7.7 | <0.001 | 7.0 | 7.0 | 0.90 |
| Transient ischemic attack | 2.6 | 3.2 | 0.02 | 2.9 | 3.0 | 0.50 |
| Peripheral vascular disease | 24.0 | 28.0 | <0.001 | 26.4 | 26.7 | 0.70 |
| Other medical conditions | ||||||
| Diabetes mellitus | 63.6 | 67.5 | <0.001 | 66.6 | 66.5 | 0.90 |
| Hyperlipidemia | 25.2 | 25.7 | 0.40 | 25.4 | 25.7 | 0.70 |
| Chronic liver disease | 6.6 | 6.0 | 0.08 | 6.1 | 6.0 | 0.80 |
| Chronic lung disease | 16.6 | 20.9 | <0.001 | 29.6 | 19.6 | >0.90 |
| Systemic cancer | 4.1 | 5.6 | <0.001 | 5.2 | 4.9 | 0.40 |
| Dementia | 1.6 | 3.3 | <0.001 | 2.2 | 2.5 | 0.30 |
| Depression | 5.0 | 7.1 | <0.001 | 6.2 | 6.0 | 0.70 |
| Tobacco use | 5.0 | 4.3 | 0.02 | 4.4 | 4.3 | 0.80 |
| Obesity | 3.1 | 3.9 | 0.004 | 3.4 | 3.6 | 0.50 |
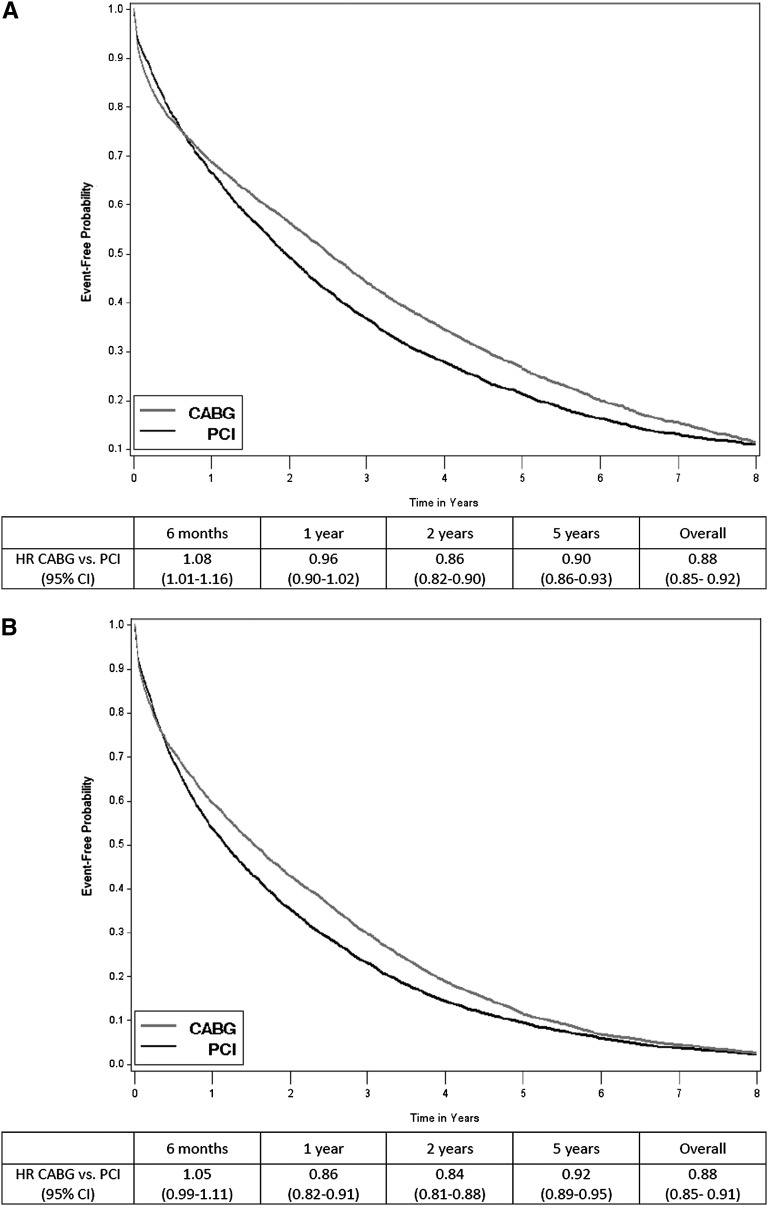
The median follow-up time in the full cohort was 1.7 years (interquartile range [IQR]=0.5–3.6): 1.9 years (IQR=0.5–3.9) for patients receiving CABG and 1.4 years (IQR=0.5–3.0) for patients receiving PCI. During this time, 70.9% (n=10,154) of CABG recipients died compared with 70.2% (n=5378) of PCI recipients. Over one-half of all deaths were categorized as cardiovascular, and 11% of deaths were caused by infection; these proportions remained similar in CABG and PCI recipients. There was no significant difference in relative survival with CABG compared with PCI at 1 year (hazard ratio [HR]=0.96, CI=0.91–1.01), but when the entire follow-up period was considered, CABG was associated with a significantly lower risk of death compared with PCI (HR=0.87, CI=0.84–0.90). Results were not materially changed in analyses using the propensity score-matched cohort (Figure 4A). During the follow-up period, 7.8% of CABG recipients and 5.9% of PCI recipients received a kidney transplant, and analyses that censored patients at the time of kidney transplant did not materially change the findings (data not shown).
Figure 4.
Kaplan-Meier curves showing unadjusted event-free probabilities and hazard ratios after multivessel CABG versus PCI in the propensity score-matched cohort of patients on maintenance dialysis. (A) Death. (B) Death or myocardial infarction.
In the full cohort, 18.3% (n=2624) of patients receiving CABG had an MI after the index revascularization compared with 26.3% (n=2017) of patients receiving PCI. Over the entire follow-up period, CABG was associated with a lower risk of MI or death in adjusted analyses using the full cohort (HR=0.88, CI=0.86–0.91). Results were not materially changed when we repeated the analyses with the propensity-matched cohort (Figure 4B) or censored patients at the time of kidney transplant (data not shown).
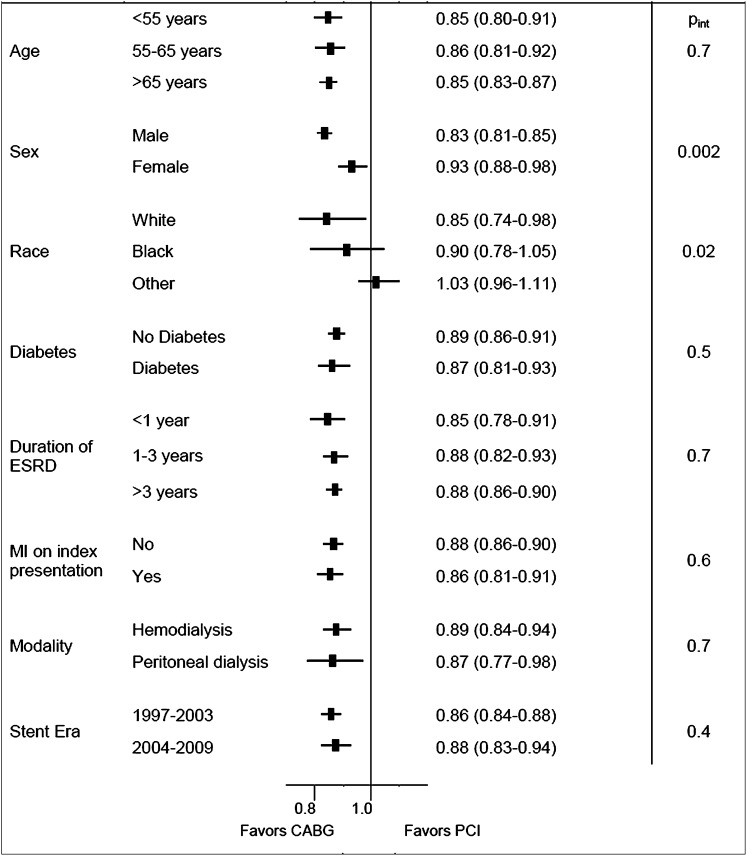
The beneficial association of CABG on death was significantly larger for men than women (Figure 5). Our results suggest that CABG may not be associated with a significant benefit in patients of nonwhite or nonblack race, but the formal test for interaction was not statistically significant after accounting for multiple comparisons (Pint=0.02). We found no evidence that age, race, diabetes, duration of ESRD, MI on index presentation, dialysis modality, stent era, or index year significantly modified the association of CABG and PCI on death (Figure 5) (all Pint>0.4).
Figure 5.
Forest plot showing the adjusted hazard ratios (95% confidence interval) for multivessel CABG versus PCI in specified subgroups of patients on maintenance dialysis in the USRDS. Pint, P value for interaction term.
Discussion
Our analysis suggests that, among patients with ESRD undergoing multivessel coronary revascularization, CABG is associated with a significant reduction in all-cause mortality relative to PCI. We found a 13% reduction in hazard of death from any cause and a 12% reduction in hazard of the composite outcome of MI or death associated with CABG compared with PCI for initial multivessel coronary revascularization. In light of the absence of any randomized trials or recent nationally representative observational studies, these results help inform patients with ESRD and their physicians about the clinical outcomes after coronary revascularization in this high-risk population.
Our analysis helps to fill an important gap in the currently available evidence regarding CABG compared with PCI in patients with ESRD on dialysis. Most previous studies were from a single center or had a limited sample size, and therefore, the resultant point estimates, although generally favoring CABG over PCI, often had wide confidence limits that were not statistically significant.11–15 In contrast, other studies that did show statistically significant results often had risk reductions that were much lower than those results reported for the non-ESRD population, raising concerns about potential confounding. For example, the work by Szczech et al.9 examined 59,576 patients undergoing coronary revascularization between 1993 and 1995 in New York state, but only 407 patients had ESRD and met the study criteria. That study reported a 61% lower risk of death with CABG compared with PCI, but the small sample size limited their ability to adjust for important confounders that may have biased the results. Although most previous studies were conducted before the introduction of drug-eluting stents in 2003, more recently, the study by Sunagawa et al.10 examined CABG versus PCI with drug-eluting stents in a cohort of 104 patients from Kurashiki Central Hospital on maintenance hemodialysis and showed a 50% lower risk of death at 2 years after CABG compared with PCI. No adjustments for baseline characteristics were performed in that analysis, again raising the possibility of residual confounding and biased estimates. Moreover, the relatively low 2-year mortality rates (15% and 32.4% for CABG and PCI recipients, respectively) in that Japanese cohort underscore the difficulty in extrapolating results from other countries to patients in the United States.
Our analysis complements and extends two previous studies by Herzog et al.7 using data from the USRDS. The first study examined patients on dialysis undergoing initial coronary revascularization between 1990 and 1995 and showed an 8% lower risk of death (CI=3%–14%) associated with CABG compared with PCI. The second study examined revascularizations between 1995 and 19988 and found a 20% lower risk of death with CABG (CI=16%–26%).8 Our analysis covers the time period between 1997 and 2009, and the 13% lower risk of death associated with CABG compared with PCI is consistent with these two previous studies. Although the studies by Herzog et al.7,8 included patients undergoing single- and multivessel coronary revascularization, we limited our analysis to patients with multivessel revascularization to maximize the comparability of patients undergoing CABG and PCI and minimize potential indication bias.
We explored several potential modifiers of the association of CABG and PCI with outcomes. We found that sex modifies the relative effectiveness of CABG and PCI on survival, which is in contrast to studies in the general population that did not show significant differences between men and women.6 However, it should be emphasized that, in our study, both men and women still derived a benefit associated with CABG compared with PCI. In addition, we saw a trend that patients of nonwhite or nonblack race seemed to have no significant advantage associated with CABG compared with PCI. Prior studies in non-ESRD populations have shown that South Asians and Native Americans have higher prevalence of hyperlipidemia and glucose intolerance, more carotid atherosclerosis, and higher rates of cardiovascular disease compared with whites or Chinese.16,17 Whether these factors influenced our results is not known.18 Moreover, in our study, the nonwhite and nonblack patients were a relatively small proportion of the overall cohort (6.9%), and we were unable to identify race at a more granular level (e.g., South Asian versus Chinese versus Southeast Asian). Additional studies are needed to clarify the associations and mechanisms underlying potential racial differences in outcomes after coronary revascularization.
Although the 5-year survival of patients without kidney disease after multivessel coronary revascularization is over 90%,6 survival after coronary revascularization in dialysis patients remains dismal, with little improvement over the past two decades. In a study of patients on maintenance dialysis revascularized between 1978 and 1995, 5-year survival ranged between 23% and 27%,7 nearly identical to the 22%–25% 5-year survival rates found in our study. These survival rates are considerably worse than the 5-year survival rates of 60%–70% reported for octogenarians undergoing multivessel coronary revascularization.19 Our results underscore the extremely high risk of adverse events faced by patients on dialysis and highlight the need to improve outcomes of coronary heart disease in this vulnerable population.
Although our analysis has several strengths, it also has several limitations. First, as with any observational study in which the treatments were not randomly assigned, there may still be residual selection bias and confounding, despite adjustment for a large number of covariates. Second, we used Medicare claims data and International Classification of Diseases, Ninth Revision (ICD-9) codes to ascertain comorbid conditions, which can underestimate their true prevalence. In addition, the USRDS data do not include details of the coronary anatomy (e.g., bifurcation disease, small caliber vessels, or diffuse disease pattern) or left ventricular systolic function, which may influence the choice of revascularization procedures20,21 and postrevascularization outcomes. We used Cox proportional hazards analysis, despite the data exhibiting obvious divergences from the proportionality assumption. We opted to present results from Cox regression representing the average relative hazard over a given observation period, which is the parameter of interest to patients and providers when making clinical decisions between two interventions that are time-invariant. To further increase the use of our information for the medical decision-making, we provided HRs for different durations of follow-up. Third, we did not have information on medication use either during the procedure (such as glycoprotein IIb/IIIa platelet inhibitors) or after hospital discharge (such as antiplatelet agents, β-blockers, or statins), which is known to affect survival in the non-ESRD population.22–24
In conclusion, we show that, in a large, contemporary cohort of patients on maintenance dialysis in the United States, CABG compared with PCI is associated with a significantly lower risk of death. Because organizing a randomized trial of CABG and PCI in patients on maintenance dialysis would be quite difficult, observational studies such as our study may be the best source of evidence for optimal management strategies. Our results suggest that CABG may be preferred over PCI for multivessel coronary revascularization in appropriately selected patients with ESRD on maintenance dialysis.
Concise Methods
Study Population
We included all patients in the USRDS who received a first documented CABG (ICD-9 procedure codes 36.1x) or PCI (ICD-9 procedure codes 36.00, 36.01, 36.02, 36.05, 36.06, 36.07, 36.09, and 00.66) after developing ESRD (Figure 1). The hospital admission date was defined as the index date. We excluded patients receiving CABG and PCI during the same hospitalization and restricted the cohort to patients undergoing documented multivessel procedures (identified using ICD-9 procedure code 36.12, 36.13, 36.14, or 36.16 for multivessel CABG, 36.05 before October of 2005, and 00.66 and either 00.41, 00.42, or 00.43 or current procedural terminology code 92981, 92984, or 92996 after October of 2005 for multivessel PCI). Among patients undergoing CABG, we excluded patients undergoing concomitant ventricular reconstruction or pericardial or valve surgery (ICD-9: 35.xx, 37.31, 37.32, 37.35, 37.4, or 37.5).
We had complete data starting from January 1, 1996, and therefore, we excluded patients receiving revascularization before January 1, 1997 to allow at least 6 months in which to assess for comorbid conditions (detailed below). We required patients to have continuous Medicare Part A and B coverage as the primary payer for 6 months before the index date to ensure a uniform period in which to ascertain comorbid conditions and past health care use. Patients younger than 65 years generally became eligible for Medicare benefits between 91 days (end of waiting period) and 36 months after initiation of dialysis, whereas patients older than age 65 years were eligible for Medicare benefits based on their age. We also required patients to be on either hemodialysis or peritoneal dialysis at the index date, and we excluded patients with a functioning renal transplant or unknown mode of renal replacement therapy. Finally, we excluded patients who had CABG or PCI before developing ESRD using ICD-9 diagnosis code V45.81 or V45.82.
Follow-Up and Outcomes
The primary outcome of interest was death from any cause that was ascertained from the USRDS patient file, which derives information on patient deaths, irrespective of Medicare coverage status.
The secondary outcome was a composite of first MI or death from any cause. We defined MI as an ICD-9 diagnosis code of 410.xx as a primary hospitalization diagnosis code or 410.x1 in any secondary diagnosis code position. An MI occurring during the index hospitalization was not considered an outcome, because it may have occurred before the revascularization.
Covariates
We obtained data on age, sex, race (white, black, and other), dialysis modality (hemodialysis or peritoneal dialysis), duration of ESRD, cause of ESRD, and history of failed kidney transplant from the USRDS patient and treatment history files.
We defined comorbid conditions using ICD-9 codes and procedure codes from more than or equal to one inpatient or more than or equal to two outpatient encounters separated by at least 1 day in the 6 months before (but not including) the index date (Supplemental Table 3). We identified the following cardiovascular comorbid conditions (atrial fibrillation, prior MI, arrhythmias, heart failure, hypertension, pacemaker, stroke, transient ischemic attack, angina, valvular disease, ventricular fibrillation, implantable cardiac defibrillator, or peripheral vascular disease), other medical comorbid conditions (diabetes mellitus, hyperlipidemia, chronic liver disease, chronic lung disease, systemic cancer, dementia, depression, tobacco use, drug abuse, alcohol abuse, obesity, central nervous system bleed and other vascular lesions, gastrointestinal bleeding, peptic ulcer disease, psychosis, human immunodeficiency virus, pulmonary hypertension, rheumatological disease), hyperparathyroidism, and electrolyte or metabolic derangements (hyper- and hyponatremia, hyper- and hypokalemia, hyper- and hypocalcemia, phosphorus disorders, magnesium disorders, acidosis, alkalosis, and mixed acid/base disorders). We identified an MI on index presentation by presence of an ICD-9 code of 410.xx in the primary diagnosis code position or 410.x1 in any secondary diagnosis code position.
To adjust for differences in prior health care use, we identified the number of non-nephrology outpatient visits, number of hospitalized days, and patients who had any nursing home stay in the 6 months before the index date. We also categorized patients into one of nine US census regions based on the zip code in which they received ESRD treatment.
Statistical Analyses
Differences in baseline characteristics among patients undergoing multivessel CABG were compared with those characteristics of patients undergoing multivessel PCI using chi-squared, t, and nonparametric tests as appropriate.
Follow-up for the primary outcome was until January 1, 2010. Because ascertainment of MI required hospitalization information, for the composite outcome of death or MI, we followed patients until death, the first time that Medicare Part A and B coverage ended, or January 1, 2010. Because receipt of kidney transplantation can change survival rates in patients with ESRD, we repeated our analyses censoring at the time of kidney transplantation.
We expected a higher mortality risk associated with CABG in the postoperative period, but because the clinically relevant outcome for clinicians and patients is the long-term average survival benefit conferred by one revascularization strategy over another, we used Cox regression to estimate any beneficial association of CABG versus PCI on outcomes. Multivariable-adjusted models included all of the comorbid conditions listed above and stratification by region.
Given the baseline differences in patient characteristics between CABG and PCI recipients, we also conducted a companion analysis using a propensity score-matched cohort.25 We used all of the baseline variables included in our multivariable-adjusted models as predictors of CABG or PCI. We used a greedy matching algorithm26 to match each patient who received a multivessel PCI with a patient who received a multivessel CABG with a difference in propensity scores of no greater than 0.01. We also required that the patients match by index year. We calculated Kaplan–Meier survival rates using the matched cohort and repeated the Cox regression models for our outcomes of interest.
We hypothesized that there may be significant interaction by the following variables: patient age, sex, race, diabetes status, duration of ESRD requiring renal replacement therapy (<1, 1–3, and >3 years), having an MI during the index presentation, dialysis modality, and index year. Also, given the rapid adoption of drug-eluting stents after their introduction to the US market in April of 2003, we tested for interaction by stent era: 1997–2003 (bare metal stent era) and 2004–2009 (drug-eluting stent era). We included a multiplicative interaction term in the models and report the interaction P value (Pint). We considered an interaction term to be significant by Bonferroni corrected α-levels of 0.002 (0.05/23).
The institutional review board of Stanford University approved the study. A waiver of informed consent was obtained because of the nature of the study. All analyses were conducted using SAS Enterprise Guide 4.3 (Cary, NC).
Disclosures
None.
Supplementary Material
Acknowledgments
T.I.C., D.S., and D.S.K. were supported by Grant 0875162N from the American Heart Association, which had no role in design, conduct, analysis, interpretation, and presentation of the data or the decision to submit the manuscript for publication. T.I.C. is also supported by American Heart Association National Scientist Development Grant 12SDG11670032. W.C.W. was supported by Grant 1R21DK089368 (entitled “Coronary Artery Bypass Grafting and Percutaneous Coronary Intervention for the Treatment of Coronary Artery Disease in Individuals with Chronic Kidney Disease”) as well as Grants 1R21DK077336, 1R01DK090181, 1R01AR057327, 1R01DK090008, and 1R01DK095024 (all from the National Institutes of Health), and contracts HHSAA2900200500401 and HHSN268201100003C.
This work was conducted under a data use agreement between W.C.W. and the National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK). An NIDDK officer reviewed this manuscript for research compliance and approved of its submission for publication. Data reported herein were supplied by the United States Renal Data System (USRDS). Interpretation and reporting of these data are the responsibility of the authors and should in no way be seen as official policy or interpretation of the US government.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012060554/-/DCSupplemental.
References
- 1.US Renal Data System: USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, NIDDK, National Institutes of Health, 2011 [Google Scholar]
- 2.Charytan D, Kuntz RE, Mauri L, DeFilippi C: Distribution of coronary artery disease and relation to mortality in asymptomatic hemodialysis patients. Am J Kidney Dis 49: 409–416, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Kumar N, Baker CSR, Chan K, Duncan N, Malik I, Frankel A, Ashby DR, McLean A, Palmer A, Cairns TD, Taube D: Cardiac survival after pre-emptive coronary angiography in transplant patients and those awaiting transplantation. Clin J Am Soc Nephrol 6: 1912–1919, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joki N, Hase H, Takahashi Y, Ishikawa H, Nakamura R, Imamura Y, Tanaka Y, Saijyo T, Fukazawa M, Inishi Y, Nakamura M, Yamaguchi T: Angiographical severity of coronary atherosclerosis predicts death in the first year of hemodialysis. Int Urol Nephrol 35: 289–297, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Coca SG, Krumholz HM, Garg AX, Parikh CR: Underrepresentation of renal disease in randomized controlled trials of cardiovascular disease. JAMA 296: 1377–1384, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Hlatky MA, Boothroyd DB, Bravata DM, Boersma E, Booth J, Brooks MM, Carrié D, Clayton TC, Danchin N, Flather M, Hamm CW, Hueb WA, Kähler J, Kelsey SF, King SB, Kosinski AS, Lopes N, McDonald KM, Rodriguez A, Serruys P, Sigwart U, Stables RH, Owens DK, Pocock SJ: Coronary artery bypass surgery compared with percutaneous coronary interventions for multivessel disease: A collaborative analysis of individual patient data from ten randomised trials. Lancet 373: 1190–1197, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Herzog CA, Ma JZ, Collins AJ: Long-term outcome of dialysis patients in the United States with coronary revascularization procedures. Kidney Int 56: 324–332, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Herzog CA, Ma JZ, Collins AJ: Comparative survival of dialysis patients in the United States after coronary angioplasty, coronary artery stenting, and coronary artery bypass surgery and impact of diabetes. Circulation 106: 2207–2211, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Szczech LA, Reddan DN, Owen WF, Califf R, Racz M, Jones RH, Hannan EL: Differential survival after coronary revascularization procedures among patients with renal insufficiency. Kidney Int 60: 292–299, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Sunagawa G, Komiya T, Tamura N, Sakaguchi G, Kobayashi T, Murashita T: Coronary artery bypass surgery is superior to percutaneous coronary intervention with drug-eluting stents for patients with chronic renal failure on hemodialysis. Ann Thorac Surg 89: 1896–1900, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Chertow GM, Normand S-LT, Silva LR, McNeil BJ: Survival after acute myocardial infarction in patients with end-stage renal disease: Results from the cooperative cardiovascular project. Am J Kidney Dis 35: 1044–1051, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Hemmelgarn BR, Southern D, Culleton BF, Mitchell LB, Knudtson ML, Ghali WA, Alberta Provincial Project for Outcomes Assessment in Coronary Heart Disease (APPROACH) Investigators : Survival after coronary revascularization among patients with kidney disease. Circulation 110: 1890–1895, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Rinehart AL, Herzog CA, Collins AJ, Flack JM, Ma JZ, Opsahl JA: A comparison of coronary angioplasty and coronary artery bypass grafting outcomes in chronic dialysis patients. Am J Kidney Dis 25: 281–290, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Ivens K, Gradaus F, Heering P, Schoebel FC, Klein M, Schulte HD, Strauer BE, Grabensee B: Myocardial revascularization in patients with end-stage renal disease: Comparison of percutaneous transluminal coronary angioplasty and coronary artery bypass grafting. Int Urol Nephrol 32: 717–723, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Koyanagi T, Nishida H, Kitamura M, Endo M, Koyanagi H, Kawaguchi M, Magosaki N, Sumiyoshi T, Hosoda S: Comparison of clinical outcomes of coronary artery bypass grafting and percutaneous transluminal coronary angioplasty in renal dialysis patients. Ann Thorac Surg 61: 1793–1796, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Anand SS, Yusuf S, Jacobs R, Davis AD, Yi Q, Gerstein H, Montague PA, Lonn E: Risk factors, atherosclerosis, and cardiovascular disease among Aboriginal people in Canada: The Study of Health Assessment and Risk Evaluation in Aboriginal Peoples (SHARE-AP). Lancet 358: 1147–1153, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Anand SS, Yusuf S, Vuksan V, Devanesen S, Teo KK, Montague PA, Kelemen L, Yi C, Lonn E, Gerstein H, Hegele RA, McQueen M: Differences in risk factors, atherosclerosis, and cardiovascular disease between ethnic groups in Canada: The Study of Health Assessment and Risk in Ethnic groups (SHARE). Lancet 356: 279–284, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Brister SJ, Hamdulay Z, Verma S, Maganti M, Buchanan MR: Ethnic diversity: South Asian ethnicity is associated with increased coronary artery bypass grafting mortality. J Thorac Cardiovasc Surg 133: 150–154, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Dacey LJ, Likosky DS, Ryan TJ, Jr, Robb JF, Quinn RD, DeVries JT, Hearne MJ, Leavitt BJ, Dunton RF, Clough RA, Sisto D, Ross CS, Olmstead EM, O’Connor GT, Malenka DJ, Northern New England Cardiovascular Disease Study Group : Long-term survival after surgery versus percutaneous intervention in octogenarians with multivessel coronary disease. Ann Thorac Surg 84: 1904–1911, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH: 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 124: 2574–2609, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG, Cigarroa JE, Disesa VJ, Hiratzka LF, Hutter AM, Jr, Jessen ME, Keeley EC, Lahey SJ, Lange RA, London MJ, Mack MJ, Patel MR, Puskas JD, Sabik JF, Selnes O, Shahian DM, Trost JC, Winniford MD: 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 124: 2610–2642, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Scandinavian Simvastatin Survival Study G : Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S). Lancet 344: 1383–1389, 1994 [PubMed] [Google Scholar]
- 23.Yusuf S, Peto R, Lewis J, Collins R, Sleight P: Beta blockade during and after myocardial infarction: An overview of the randomized trials. Prog Cardiovasc Dis 27: 335–371, 1985 [DOI] [PubMed] [Google Scholar]
- 24.Antithrombotic Trialists’ Collaboration : Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 324: 71–86, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenbaum PR, Rubin DB: The central role of the propensity score in observational studies for causal effects. Biometrika 70: 41–55, 1983 [Google Scholar]
- 26.Kosanke J, Bergstralh E: Gmatch Macro: Match 1 or More Controls to Cases Using the Greedy Algorithm, 2004. Available at: http://mayoresearch.mayo.edu/mayo/research/biostat/sasmacros.cfm Accessed on October 1, 2010
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.