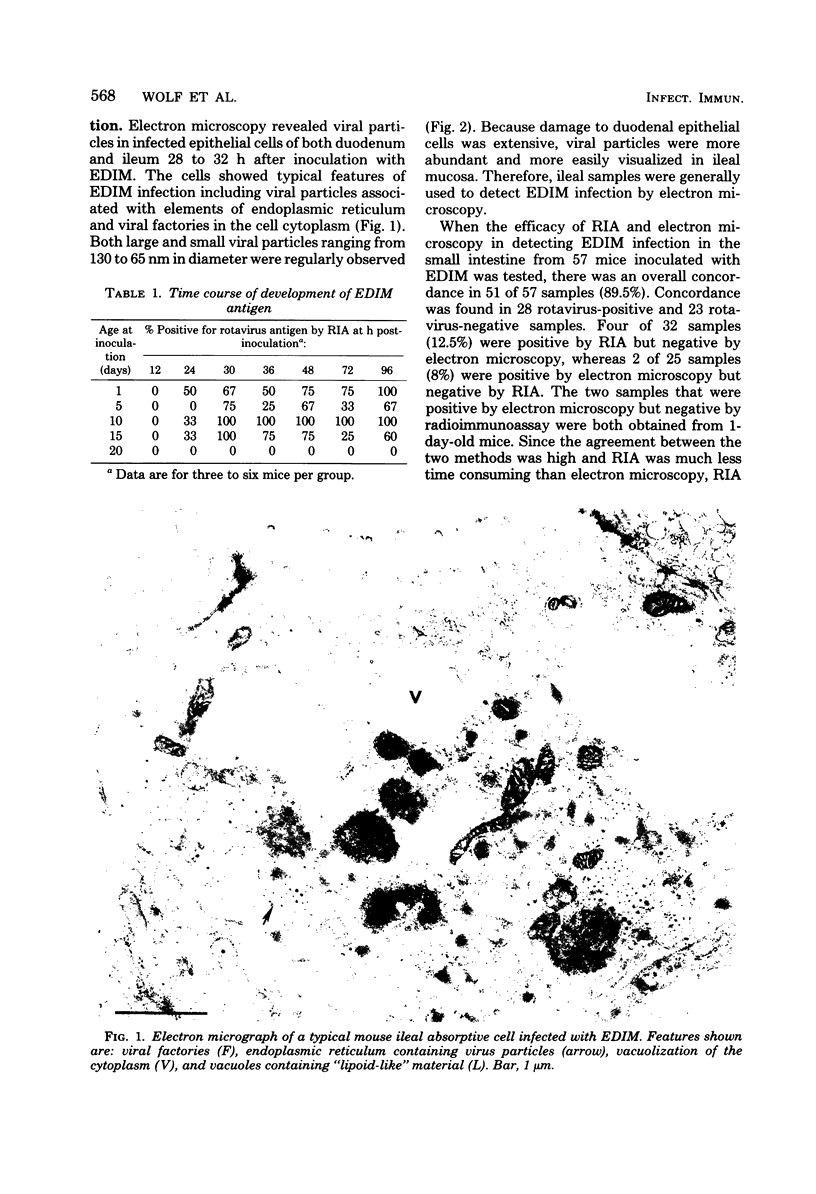
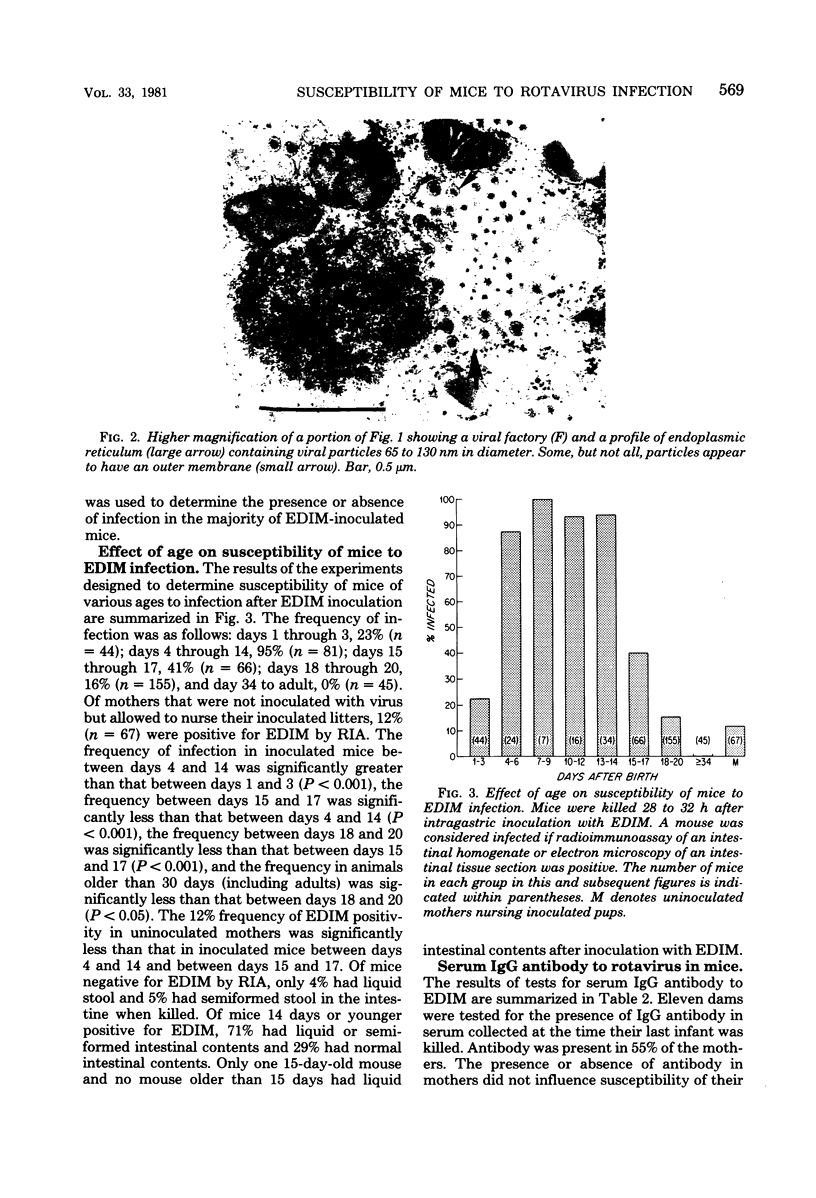
Abstract
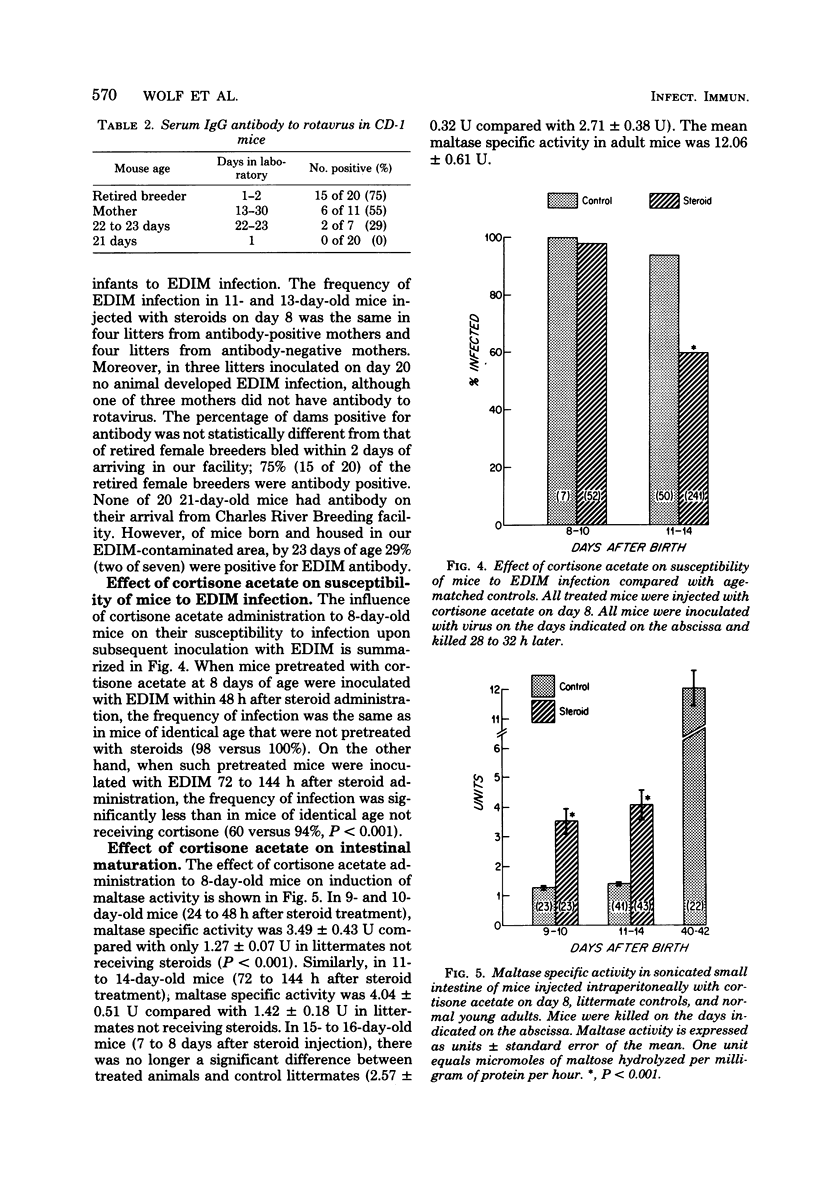
We examined susceptibility to the murine rotavirus, epizootic diarrhea of infant mice virus (EDIM), in normal suckling and weaned mice and in suckling mice treated with glucocorticoids. Normal mice 1 to 40 days old were inoculated by gastrin intubation with high doses of EDIM and subsequently evaluated for rotavirus infection by solid-phase radioimmunoassay, by electron microscopy of intestinal tissue sections or by both. Radioimmunoassay and electron microscopy showed a concordance of 89.5% in the detection of rotavirus infection. After a period of low susceptibility to EDIM infection during the first 3 days after birth (23%), susceptibility was high for the next 11 days (95%), but decreased abruptly as mice approached weaning (41% on days 15 through 17). Mice 34 days or older did not develop EDIM infection after inoculation, but rotavirus antigen was detected in 12% of uninoculated mothers nursing inoculated litters. Administration of cortisone acetate to 8-day-old mice induced partial intestinal maturation prematurely. At 3 to 6 days after cortisone acetate treatment, susceptibility to EDIM infection decreased to 60% compared with 94% in age-matched controls. Our data suggest (i) that susceptibility of mice to EDIM infection is age dependent, decreasing in concert with intestinal maturation, and (ii) that glucocorticoids, which induce premature partial intestinal maturation, modulate susceptibility of mice to EDIM.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams W. R., Kraft L. M. Electron-Microscopic Study of the Intestinal Epithelium of Mice Infected with the Agent of Epizootic Diarrhea of Infant Mice (EDIM Virus). Am J Pathol. 1967 Jul;51(1):39–60. [PMC free article] [PubMed] [Google Scholar]
- Banfield W. G., Kasnic G., Blackwell J. H. Further observations on the virus of epizootic diarrhea of infant mice. An electron microscopic study. Virology. 1968 Nov;36(3):411–421. doi: 10.1016/0042-6822(68)90166-9. [DOI] [PubMed] [Google Scholar]
- Barnett B. B., Spendlove R. S., Clark M. L. Effect of enzymes on rotavirus infectivity. J Clin Microbiol. 1979 Jul;10(1):111–113. doi: 10.1128/jcm.10.1.111-113.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK S. L., Jr The ingestion of proteins and colloidal materials by columnar absorptive cells of the small intestine in suckling rats and mice. J Biophys Biochem Cytol. 1959 Jan 25;5(1):41–50. doi: 10.1083/jcb.5.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukor G., Berry M. K., Blacklow N. R. Simplified radioimmunoassay for detection of human rotavirus in stools. J Infect Dis. 1978 Dec;138(6):906–910. doi: 10.1093/infdis/138.6.906. [DOI] [PubMed] [Google Scholar]
- Cukor G., Blacklow N. R., Capozza F. E., Panjvani Z. F., Bednarek F. Persistence of antibodies to rotavirus in human milk. J Clin Microbiol. 1979 Jan;9(1):93–96. doi: 10.1128/jcm.9.1.93-96.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels V. G., Hardy R. N., Malinowska K. W., Nathanielsz P. W. The influence of exogenous steroids on macromolecule uptake by the small intestine of the new-born rat. J Physiol. 1973 Mar;229(3):681–695. doi: 10.1113/jphysiol.1973.sp010160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Graham D. Y., Smith E. M., Gerba C. P. Rotavirus stability and inactivation. J Gen Virol. 1979 May;43(2):403–409. doi: 10.1099/0022-1317-43-2-403. [DOI] [PubMed] [Google Scholar]
- Flewett T. H., Woode G. N. The rotaviruses. Arch Virol. 1978;57(1):1–23. doi: 10.1007/BF01315633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furihata C., Kawachi T., Sugimura T. Premature induction of pepsinogen in developing rat gastric mucosa by hormones. Biochem Biophys Res Commun. 1972 May 26;47(4):705–711. doi: 10.1016/0006-291x(72)90549-9. [DOI] [PubMed] [Google Scholar]
- Goldstein R., Klein T., Freier S., Menczel J. Alkaline phosphatase and disaccharidase activities in the rat intestine from birth to weaning. I. Effect of diet on enzyme development. Am J Clin Nutr. 1971 Oct;24(10):1224–1231. doi: 10.1093/ajcn/24.10.1224. [DOI] [PubMed] [Google Scholar]
- Graham D. Y., Estes M. K. Proteolytic enhancement of rotavirus infectivity: biology mechanism. Virology. 1980 Mar;101(2):432–439. doi: 10.1016/0042-6822(80)90456-0. [DOI] [PubMed] [Google Scholar]
- Grand R. J., Chong D. A., Isselbacher K. J. Intracellular processing of disaccharidases: the effect of actinomycin D. Biochim Biophys Acta. 1971 Feb 28;261(2):341–352. doi: 10.1016/0304-4165(72)90058-x. [DOI] [PubMed] [Google Scholar]
- HALLIDAY R. The effect of steroid hormones on the absorption of antibody by the young rat. J Endocrinol. 1959 Jan;18(1):56–66. doi: 10.1677/joe.0.0180056. [DOI] [PubMed] [Google Scholar]
- Holmes I. H., Rodger S. M., Schnagl R. D., Ruck B. J., Gust I. D., Bishop R. F., Barnes G. L. Is lactase the receptor and uncoating enzyme for infantile enteritis (rota) viruses? Lancet. 1976 Jun 26;1(7974):1387–1388. doi: 10.1016/s0140-6736(76)93032-4. [DOI] [PubMed] [Google Scholar]
- KOLDOVSKY O., CHYTIL F. POSTNATAL DEVELOPMENT OF BETA-GALACTOSIDASE ACTIVITY IN THE SMALL INTESTINE OF THE RAT. EFFECT OF ADRENALECTOMY AND DIET. Biochem J. 1965 Jan;94:266–270. doi: 10.1042/bj0940266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRAFT L. M. Studies on the etiology and transmission of epidemic diarrhea of infant mice. J Exp Med. 1957 Nov 1;106(5):743–755. doi: 10.1084/jem.106.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapikian A. Z., Cline W. L., Kim H. W., Kalica A. R., Wyatt R. G., Vankirk D. H., Chanock R. M., James H. D., Jr, Vaughn A. L. Antigenic relationships among five reovirus-like (RVL) agents by complement fixation (CF) and development of new substitute CF antigens for the human RVL agent of infantile gastroenteritis. Proc Soc Exp Biol Med. 1976 Sep;152(4):535–539. doi: 10.3181/00379727-152-39434. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Malo C., Ménard D. Opposite effects of one and three injections of cortisone or thyroxine on intestinal lactase activity in suckling mice. Experientia. 1979 Apr 15;35(4):493–494. doi: 10.1007/BF01922726. [DOI] [PubMed] [Google Scholar]
- McNulty M. S. Rotaviruses. J Gen Virol. 1978 Jul;40(1):1–18. doi: 10.1099/0022-1317-40-1-1. [DOI] [PubMed] [Google Scholar]
- Messer M., Dahlqvist A. A one-step ultramicro method for the assay of intestinal disaccharidases. Anal Biochem. 1966 Mar;14(3):376–392. doi: 10.1016/0003-2697(66)90280-6. [DOI] [PubMed] [Google Scholar]
- Moog F., Denes A. E., Powell P. M. Disaccharidases in the small intestine of the mouse: normal development and influence of cortisone, actinomycin D, and cycloheximide. Dev Biol. 1973 Nov;35(1):143–159. doi: 10.1016/0012-1606(73)90012-2. [DOI] [PubMed] [Google Scholar]
- Moog F., Etzler M. E., Grey R. D. The differentiation of alkaline phosphatase in the small intestine. Ann N Y Acad Sci. 1969 Oct 14;166(2):447–465. doi: 10.1111/j.1749-6632.1969.tb46414.x. [DOI] [PubMed] [Google Scholar]
- Morris B., Morris R. The effects of cortisone acetate on stomach evacuation and the absorption of 125I-labelled globulins in young rats. J Physiol. 1974 Jul;240(1):79–89. doi: 10.1113/jphysiol.1974.sp010600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappenheimer A. M., Enders J. F. AN EPIDEMIC DIARRHEAL DISEASE OF SUCKLING MICE : II. INCLUSIONS IN THE INTESTINAL EPITHELIAL CELLS. J Exp Med. 1947 Mar 31;85(4):417–422. doi: 10.1084/jem.85.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robberecht P., Deschodt-Lanckman M., Camus J., Bruylands J., Christophe J. Rat pancreatic hydrolases from birth to weaning and dietary adaptation after weaning. Am J Physiol. 1971 Jul;221(1):376–381. doi: 10.1152/ajplegacy.1971.221.1.376. [DOI] [PubMed] [Google Scholar]
- Rodewald R. Selective antibody transport in the proximal small intestine of the neonatal rat. J Cell Biol. 1970 Jun;45(3):635–640. doi: 10.1083/jcb.45.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin D. H., Fields B. N. Molecular basis of reovirus virulence. Role of the M2 gene. J Exp Med. 1980 Oct 1;152(4):853–868. doi: 10.1084/jem.152.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass D. R., Wells P. W. Rotavirus infection in lambs: studies on passive protection. Arch Virol. 1976;52(3):201–205. doi: 10.1007/BF01348017. [DOI] [PubMed] [Google Scholar]
- Snodgrass D. R., Wells P. W. The immunoprophylaxis of of rotavirus infections in lambs. Vet Rec. 1978 Feb 18;102(7):146–148. doi: 10.1136/vr.102.7.146. [DOI] [PubMed] [Google Scholar]
- Theil K. W., Bohl E. H. Porcine rotaviral infection of cell culture: effects of certain enzymes. Am J Vet Res. 1980 Jan;41(1):140–143. [PubMed] [Google Scholar]
- Wells P. W., Snodgrass D. R., Herring J. A., Dawson A. M. Antibody titres to lamb rotavirus in colostrum and milk of vaccinated ewes. Vet Rec. 1978 Jul 15;103(3):46–48. doi: 10.1136/vr.103.3.46. [DOI] [PubMed] [Google Scholar]
- Wilsnack R. E., Blackwell J. H., Parker J. C. Identification of an agent of epizootic diarrhea of infant mice by immunofluorescent and complement-fixation tests. Am J Vet Res. 1969 Jul;30(7):1195–1204. [PubMed] [Google Scholar]
- Woode G. N., Bridger J. C., Jones J. M., Flewett T. H., Davies H. A., Davis H. A., White G. B. Morphological and antigenic relationships between viruses (rotaviruses) from acute gastroenteritis of children, calves, piglets, mice, and foals. Infect Immun. 1976 Sep;14(3):804–810. doi: 10.1128/iai.14.3.804-810.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woode G. N., Jones J., Bridger J. Levels of colostral antibodies against neonatal calf diaahoea virus. Vet Rec. 1975 Aug 23;97(8):148–149. doi: 10.1136/vr.97.8.148. [DOI] [PubMed] [Google Scholar]
- Yolken R. H., Wyatt R. G., Mata L., Urrutia J. J., Garciá B., Chanock R. M., Kapikian A. Z. Secretory antibody directed against rotavirus in human milk--measurement by means of enzyme-linked immunosorbent assay. J Pediatr. 1978 Dec;93(6):916–921. doi: 10.1016/s0022-3476(78)81211-6. [DOI] [PubMed] [Google Scholar]