Abstract
Mast cells contribute to the modulation of the immune response, but their role in autoimmune renal disease is not well understood. Here, we induced autoimmunity resulting in focal necrotizing GN by immunizing wild-type or mast cell-deficient (KitW-sh/W-sh) mice with myeloperoxidase. Mast cell-deficient mice exhibited more antimyeloperoxidase CD4+ T cells, enhanced dermal delayed-type hypersensitivity responses to myeloperoxidase, and more severe focal necrotizing GN. Furthermore, the lymph nodes draining the sites of immunization had fewer Tregs and reduced production of IL-10 in mice lacking mast cells. Reconstituting these mice with mast cells significantly increased the numbers of Tregs in the lymph nodes and attenuated both autoimmunity and severity of disease. After immunization with myeloperoxidase, mast cells migrated from the skin to the lymph nodes to contact Tregs. In an ex vivo assay, mast cells enhanced Treg suppression through IL-10. Reconstitution of mast cell-deficient mice with IL-10–deficient mast cells led to enhanced autoimmunity to myeloperoxidase and greater disease severity compared with reconstitution with IL-10–intact mast cells. Taken together, these studies establish a role for mast cells in mediating peripheral tolerance to myeloperoxidase, protecting them from the development of focal necrotizing GN in ANCA-associated vasculitis.
Mast cells (MCs) have the potential to participate in diverse roles in the immune system. In addition to their well characterized effector role in IgE-mediated allergic inflammation, MCs are now recognized as mediators of host antimicrobial defense, and they play injurious roles in many models of chronic human diseases. In innate immunity, MCs, by virtue of their tissue distribution in mucosal and dermal surfaces together with their array of preformed and synthesized mediators (including TNF, IL-1α, IL-1β, IL-6, and leukotrienes), act as sentinels and first responders in host defense.1 Additionally, MCs can participate in the generation of adaptive immunity.2 Several MC products, such as histamine and the cytokines IFNγ, IL-4, IL-6, and TGF-β1, can activate T cells and influence the polarization of T helper cell subset differentiation.3
Emerging evidence also confirms a role for MC in inducing immunoregulation. MCs have amongst their capabilities the capacity to express cell surface immunomodulatory molecules, including both TNF receptor (OX40L and 4–1BB) and B7 family members (PD-L1),4,5 and synthesize anti-inflammatory mediators, such as IL-10.6 Studies in ultraviolet B-induced immunosuppression of contact hypersensitivity and mosquito bite-induced immunosuppression of delayed type hypersensitivity (DTH) have highlighted the importance of MC-derived IL-10 in mediating immune regulation.7–9
MCs have been shown to participate, together with T regulatory cells (Tregs), in the maintenance of skin graft tolerance and in a model of glomerular inflammation induced by immunity to a foreign protein planted in glomeruli.10,11 Both studies highlight the importance of IL-9 (a known MC growth factor potentially made by Tregs) in facilitating MC immunomodulation.
The presence of MCs in diseased organs in many human autoimmune diseases has suggested that these cells may participate in the associated immunopathology. The role of MCs in autoimmunity has been assessed in several well characterized animal models of human disease.12 These studies use the availability of MC-deficient KitW/Wv or KitW-sh/W-sh mice. This MC deficiency phenotype results from a number of different mutations in c-Kit or its signaling pathway associated with the failure of MC lineage development.13 Although the first strain to be widely used was the KitW/Wv strain, these mice have important phenotypic abnormalities beyond the absence of MCs, and therefore, this strain may not be optimal for determining the functional role of MCs.14 However, other strains, including KitW-sh/W-sh mice used in the current experiments, do not have major extra MC phenotypic abnormalities other than the deficiency of MC, and they are the preferred strain to observe the functional role of MCs. The standard proof in defining a role for MCs is to show a change in disease severity in MC-deficient mice and then confirm that the change in disease outcome is dependent specifically on MCs by showing that MC reconstitution restores disease severity.1 As summarized in a recent comprehensive review, experimental models of autoimmunity almost entirely show proinflammatory roles for MCs in diseases models (including bullous pemphigus, systemic sclerosis, type 1 diabetes mellitus, and rheumatoid arthritis).12 In experimental autoimmune encephalomyelitis, a widely studied model, there is no consensus as to the role of MCs.15–17
GN is one of the major causes of end stage renal failure worldwide, and thus, it constitutes a major burden of human disease. The most common form of rapidly progressive GN is caused by antineutrophil cytoplasmic antibody (ANCA) -associated vasculitis (AAV). AAV has an autoimmune basis, involving T and B cell autoreactivity to neutrophil lysosomal enzymes proteinase-3 and myeloperoxidase (MPO), resulting in the development of focal necrotizing GN. Proof that immunity to MPO can induce GN comes from elegant studies generating immunity to MPO in MPO-deficient (−/−) mice and inducing GN in Rag−/− mice by splenocyte transfer.18 MPO autoimmunity can also be induced in susceptible mouse strains by immunizing C57BL/6 mice with murine MPO. Planting MPO in the glomeruli of these mice with established systemic autoimmunity induces GN through autoreactive MPO-specific CD4+ effectors.19
MCs have been found in increased numbers in affected kidneys correlating with disease severity, suggesting their potential role in AAV,20 although to date, no functional data have been provided to test this hypothesis. In these studies, we used MC-deficient and MC-reconstituted KitW-sh/W-sh mice to demonstrate that, in the induction of autoimmunity to MPO, MCs migrate to draining lymph nodes (LNs), where they interact with Tregs. These interactions regulate autoimmunity and diminish the severity of subsequently induced anti–MPO-mediated GN. Studies with IL-10−/− MC ex vivo and in vivo show that MC production of IL-10 is responsible for this immunomodulation.
Results
Renal Injury and Systemic Autoimmunity to MPO in MC Deficient KitWsh/Wsh Mice Developing Anti–MPO-Induced Glomerulonephritis
To induce experimental anti-MPO GN in C57BL/6 mice, autoimmunity to MPO was established; then, GN was triggered by injecting a subnephritogenic dose of antiglomerular basement membrane (GBM) antibody to recruit and degranulate neutrophils, thereby depositing MPO in the glomerular capillary bed. This process results in the accumulation of MPO-specific CD4+ cells that direct DTH type effectors. Over 4 days, GN develops and resembles glomerular lesions observed in human AAV. In this model, the subnephritogenic dose of anti-GBM antibody does not contribute directly to injury except by facilitating neutrophil influx and MPO release. Previously published controls involving this anti-GBM antibody and this dose included administration to ovalbumin-immunized wild-type mice as well as MPO-immunized MPO−/− mice. These mice did not develop GN.19,21
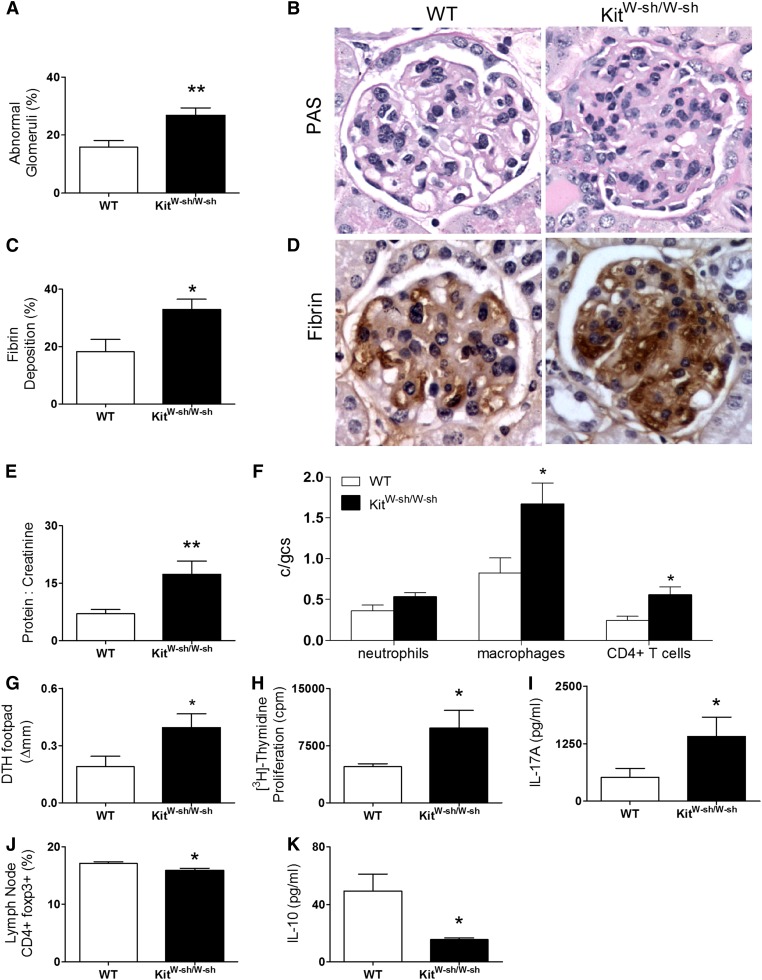
MPO-immunized KitW-sh/W-sh mice induced with anti-MPO GN showed enhanced renal injury with increases in focal glomerular necrosis (Figure 1, A and B), fibrin deposition (Figure 1, C and D), and proteinuria (Figure 1E) compared with MC-intact C57BL/6 mice. Analyses of cellular effectors of renal damage revealed greater numbers of glomerular CD4+ T cells and macrophages in KitW-sh/W-sh mice. Although glomerular neutrophil accumulation was not significant, there was a trend to increased neutrophil numbers in KitW-sh/W-sh mice (Figure 1F).
Figure 1.
MC-deficient KitWsh/Wsh mice develop augmented anti-MPO GN. (A and B) MPO-immunized KitW-sh/W-sh mice (8–12 weeks old; n=8) compared with age- and sex-matched wild-type mice (n=8) developed augmented anti-MPO focal necrotizing GN showed by quantitating percentage abnormal glomeruli (C and D) and glomerular fibrin deposition. (E) Proteinuria was elevated in KitW-sh/W-sh mice compared with wild-type mice. (F) Increased glomerular leukocyte accumulation of macrophages and CD4+ T cells correlated with disease severity. There was a trend to increase glomerular neutrophil numbers in KitW-sh/W-sh mice. (G) MPO-specific dermal footpad DTH was enhanced in KitW-sh/W-sh mice associated with (H) increased systemic CD4+ anti-MPO T cell responses assessed by in vitro MPO recall thymidine proliferation and (I) elevated production of the proinflammatory cytokine IL-17A. (J) Decreased proportion of CD4+foxp3+ Tregs and (K) reduced production of the anti-inflammatory cytokine, IL-10, was seen in LNs draining MPO immunization sites from KitW-sh/W-sh mice compared with wild-type mice. Data are representative of three independent experiments. Error bars denote mean ± SEM, and statistical analysis is by unpaired t test. *P<0.05, **P<0.01.
Systemic autoimmunity was assessed by measuring dermal DTH to MPO, MPO cytokine recall responses, and proliferation of cells isolated from LNs draining the site of MPO immunization. Development of MPO autoimmunity was observed in both C57BL/6 and C57BL/6 KitW-sh/W-sh mice. T cell-mediated immune responses were significantly enhanced in KitW-sh/W-sh mice, and they were confirmed by increased MPO-specific dermal DTH swelling (Figure 1G) and increased proliferation by in vitro thymidine incorporation (Figure 1H) and IL-17A production (Figure 1I) compared with wild-type mice. Conversely, the percentage of foxp3+CD4+ cells and the production of IL-10 by draining LN cells was reduced in KitW-sh/W-sh mice (Figure 1, J and K). Humoral immunity measured by ELISA for anti-MPO antibody (ANCA) was similar in both groups (Supplemental Figure 1A).
Reconstitution of KitWsh/Wsh Mice with MCs
To confirm that the increased autoimmunity observed in KitW-sh/W-sh mice was caused by their MC deficiency, in vitro-cultured MCs were adoptively transferred to KitW-sh/W-sh mice.22 The development of anti-MPO GN in MC-reconstituted KitW-sh/W-sh mice was then compared with age-matched nonreconstituted KitW-sh/W-sh mice.
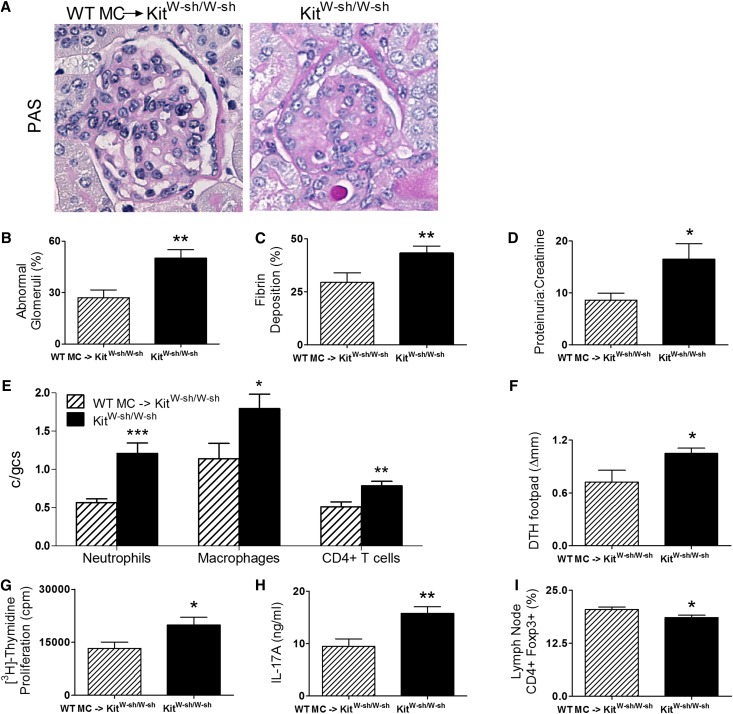
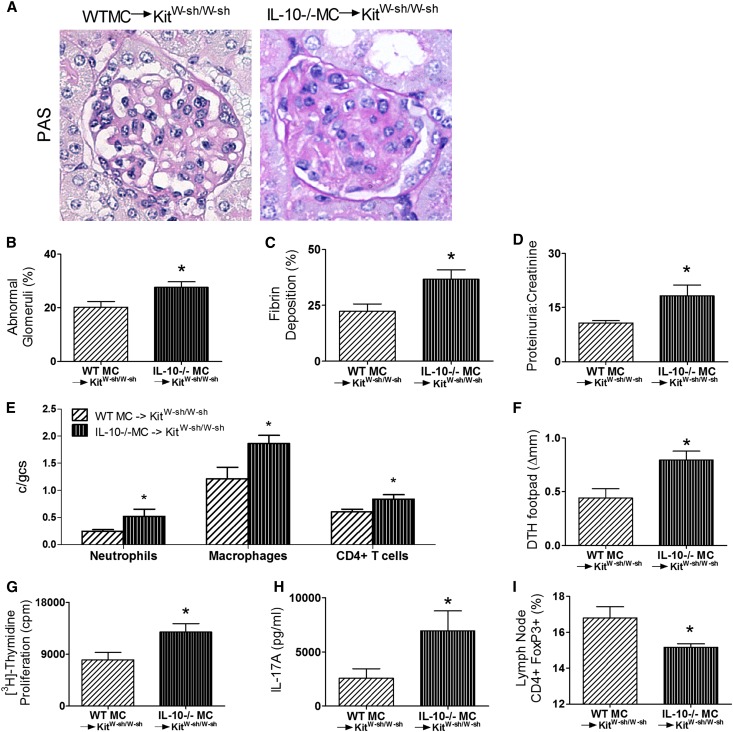
MC reconstitution resulted in attenuated glomerular injury with fewer abnormal glomeruli (Figure 2, A and B and Supplemental Figure 2) and less fibrin deposition (Figure 2C) and proteinuria (Figure 2D). Infiltrating glomerular DTH effector leukocytes, neutrophils, macrophages, and CD4+ cells were also reduced in MC-reconstituted KitW-sh/W-sh mice (Figure 2E). Systemic autoimmunity, assessed by dermal DTH swelling to MPO, was significantly reduced by MC reconstitution (Figure 2F). Similarly, MPO challenge of LN cells from draining immunization sites showed less MPO-induced cell proliferation and reduced IL-17A production in MC-reconstituted KitW-sh/W-sh mice (Figure 2, G and H). The percentage of CD4+ LN cells that expressed foxp3 was also elevated by MC reconstitution (Figure 2I). Similar to a previous experiment, ANCA titers were similar between reconstituted and nonreconstituted KitW-sh/W-sh mice (Supplemental Figure 1B).
Figure 2.
MC reconstitution of KitW-sh/W-sh mice limits anti-MPO renal injury and systemic autoimmune anti-MPO responses. (A–C) Structural renal injury, assessed by quantifying percentage abnormal glomeruli and glomerular fibrin deposition, was significantly attenuated in MPO-immunized KitW-sh/W-sh mice reconstituted with bone marrow-derived wild-type MCs (n=9–10) compared with immunized nonreconstituted KitW-sh/W-sh mice (n=10). (D) Functional injury assessed by the development of proteinuria and quantitated by creatinine/protein ratio was also significantly reduced by MC reconstitution. (E) Concordantly, glomerular influx of neutrophils, macrophages, and CD4+ T cells was decreased in wild-type MC-reconstituted KitW-sh/W-sh mice compared with KitW-sh/W-sh mice. (F–H) Systemic anti-MPO autoimmunity was also reduced in wild-type MC-reconstituted KitW-sh/W-sh mice assessed by dermal footpad MPO DTH response, MPO recall T cell proliferation, and IL-17A production compared with KitW-sh/W-sh mice. (I) The percentage of CD4+foxp3+ Tregs were restored after wild-type MCs had repopulated in KitW-sh/W-sh mice. Results are representative of two independent experiments, with error bars representing mean ± SEM. Statistical analysis is by unpaired t test. *P<0.05, **P<0.01, and ***P<0.001.
MCs Migrate from Sites of MPO Presentation to Draining LN
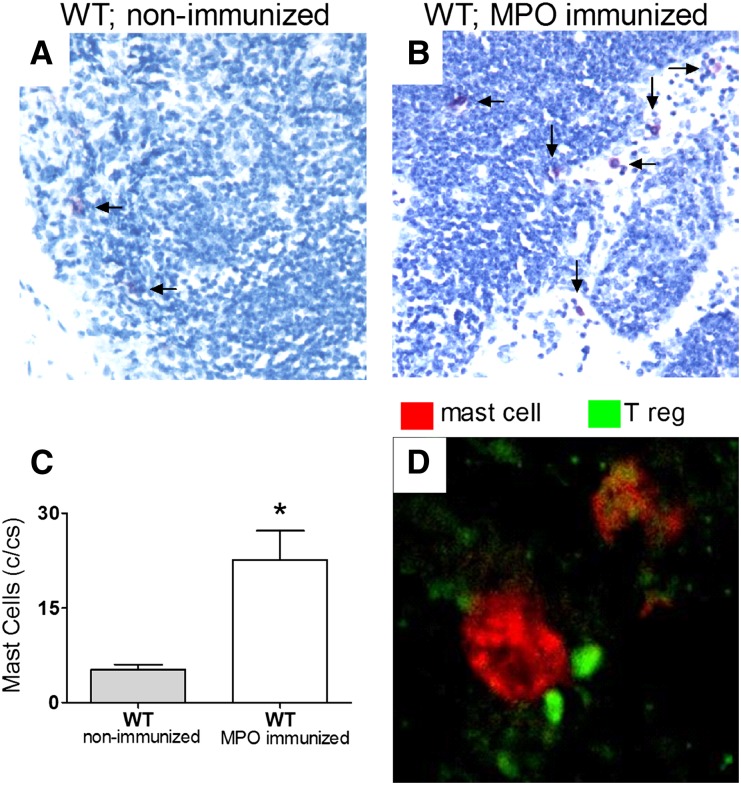
Six days post-MPO immunization, draining LNs from MC-intact WT mice were examined to explore the involvement of MC participation in the cellular events inducing and regulating autoimmunity. There was a fourfold increase in MC numbers at this time in LN draining sites of MPO immunization compared with nonimmunized WT mice (Figure 3, A–C). We then used immunostaining of Tregs using the Treg-specific transcription factor, foxp3, and MCs using cell surface marker c-Kit and confocal microscopy to assess potential MC and Treg interaction. Frequent intimate contact between MCs and Tregs, particularly in the subcapsular region of LNs, was observed (Figure 3D).
Figure 3.
MC and Treg cell interaction in draining LNs 6 days after MPO immune challenge. (A and C) MCs normally reside at low frequency within the draining LNs of naive C57BL/6 (wild-type) mice (n=4). (B and C) Six days after MPO immunization, there is a significant increase in the number of MCs migrating to draining LNs (MPO-immunized wild-type mice; n=8). Representative toluidine blue-stained LN sections of wild-type nonimmunized and wild-type MPO-immunized mice, in which MCs are stained purple (arrows), are shown. (D) Confocal immunofluorescence microscopy of draining LNs of immunized mice reveals that MCs (red) and Tregs (green) reside in close proximity to each other. Data are representative of two independent experiments, and error bars indicate mean ± SEM. *P<0.05.
MC Immunomodulation of MPO Autoimmunity Is Dependent on MC IL-10
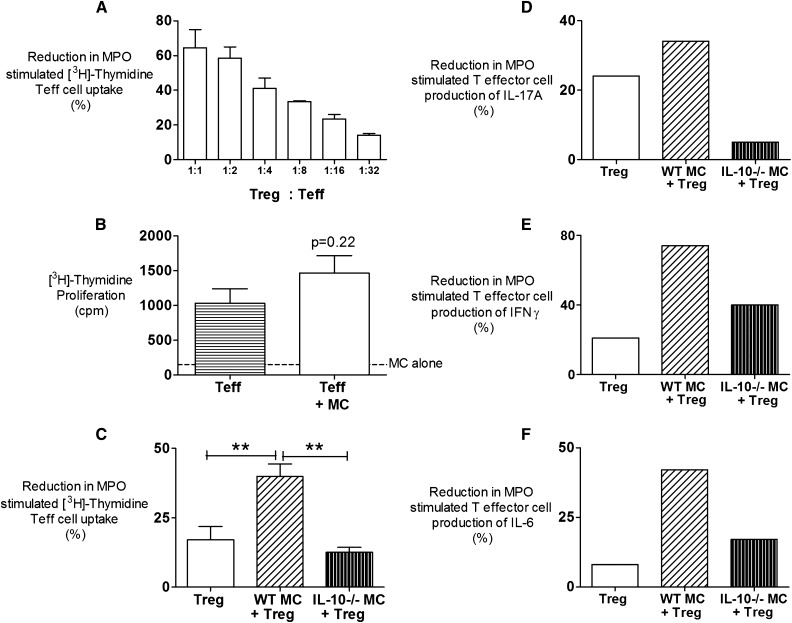
To explore the mechanisms of MC-dependent immunoregulation, we established an ex vivo cell coculture system using foxp3-GFP (green fluorescent protein expressing) mice. Anti-MPO autoimmunity was induced in foxp3-GFP mice by MPO immunization; after 10 days, draining LNs were isolated, and populations of effector T cells (Teffs; CD4+foxp3−) and Tregs (CD4+foxp3+) were separated. By varying the ratios of these cells in an MPO recall assay, we showed a dose–response effect of Tregs on MPO recall responses by Teffs (Figure 4A). We found that culturing Teffs with wild-type MCs did not diminish recall responses to MPO (Figure 4B), whereas the addition of wild-type MCs to Treg enhanced their capacity to inhibit Teff proliferative responses to MPO in an IL-10–dependent manner (Figure 4C). Furthermore, Tregs in the presence of wild-type MCs decrease MPO Teff capacity to produce proinflammatory cytokines (IFNγ, IL-17A, and IL-6), and this production is also dependent on MC IL-10 (Figure 4, D–F).
Figure 4.
CD4+ Teff cell suppression assay using Tregs cells and MCs. (A) A standard coculture system was designed using varying ratios of CD4 Teffs and CD4+ Tregs derived from LNs of mice draining sites of MPO immunization. Coculture of CD4+foxp3− LN cells from MPO-immunized mice with CD4+foxp3+ cells of a ratio of 32:1 reduced Teff cell proliferation responses to MPO by 18%. All cells were cultured in the presence of antigen-presenting cells, and results are representative of two independent ex vivo experiments. (B) Coculturing Teffs with MCs does not increase MPO proliferative responses. Minimal baseline proliferation from MCs cultured with MPO was observed (dotted line). Results are expressed as an average from four independent experiments. (C) The capacity of MCs to enhance Treg immunosuppression was assessed in the presence or absence of either wild-type or IL-10−/− MCs in a ratio of 32 Teffs to 1 Treg to 1 MC. Wild-type MCs significantly enhanced MPO-specific Treg suppression shown by a 40% reduction in T effector cell proliferation, whereas the addition of IL-10−/− MCs had no effect. Results are expressed as an average from four individual experiments. (D–F) There is a decrease in production of proinflammatory cytokines IFNγ, IL-17A, and IL-6 by Teffs when cultured in the presence of Tregs and wild-type MCs. Results are representative of three independent experiments. Error bars represent mean ± SEM with statistical analysis by one-way ANOVA. **P<0.01.
In Vivo MC Immunomodulation of MPO Autoimmunity and Renal Injury Is Dependent on MC IL-10
We hypothesized that IL-10 was a likely effector of MC immunomodulation for two reasons. Previous studies, albeit in nonautoimmune models, have shown that MC IL-10 can account for immunomodulation.7,8 Our study shows that IL-10 levels were reduced in draining LNs of MC-deficient mice, showing enhanced MPO autoimmunity (Figure 1J). We, therefore, reconstituted KitW-sh/W-sh mice with either IL-10−/− or IL-10+/+ (wild-type) MCs and compared the development of anti-MPO autoimmunity and renal injury in experimental autoimmune anti-MPO GN. Successful reconstitution of KitW-sh/W-sh mice was verified after completion of the experiment by immunohistological staining LN MCs and genotyping splenic MCs. We found that wild-type MC-reconstituted KitW-sh/W-sh mice were protected from glomerular injury compared with IL-10−/− MC-reconstituted mice. The extent of focal glomerular necrosis (Figure 5, A and B), fibrin deposition (Figure 5C), and proteinuria (Figure 5D) was significantly less. Glomerular infiltration of leukocytes was also reduced (Figure 5E). Systemic autoimmunity to MPO was attenuated, which was shown by the reduction in MPO-induced dermal DTH (Figure 5F), a reduction in the extent of MPO recall-induced draining LN cell proliferation (Figure 5G), and decreased frequency of IL-17A producing CD4+ T cells (Figure 5H). The percentage of CD4+foxp3+ Tregs in the draining nodes was significantly elevated compared with the percentage observed in KitW-sh/W-sh mice reconstituted with IL-10−/− MCs (Figure 5I).
Figure 5.
MC-derived IL-10 attenuates MPO autoimmunity and protection against renal injury. (A–D) Reconstituting KitW-sh/W-sh mice with IL-10−/− MCs (n=10) did not modulate anti-MPO GN with increased percentage of abnormal glomeruli, glomerular fibrin deposition, and elevated urinary protein compared with wild-type IL-10+/+ MC-reconstituted KitW-sh/W-sh mice (n=10). (E) Accumulation of glomerular leukocytes correlated with disease severity, with increased numbers of neutrophils, macrophages, and CD4+ T cells in IL-10−/− MC-reconstituted KitW-sh/W-sh mice. (F–H) Systemic cell-mediated MPO-specific DTH footpad responses were enhanced in IL-10−/− MC-reconstituted KitW-sh/W-sh mice, resulting from increased T cell proliferation and IL-17A production compared with wild-type MC-reconstituted KitW-sh/W-sh mice. (I) Decrease in the proportion of CD4+foxp3+ Tregs in the LNs draining MPO immunization sites was apparent in IL-10−/− MC-reconstituted mice compared with wild-type MC-reconstituted KitW-sh/W-sh mice. Data representative of two independent experiments. Error bars depict mean ± SEM. *P<0.05.
Discussion
MCs have been observed to play proinflammatory roles in most of the experimental immune disease models in which they have been studied.12,23 However, several studies suggest that MCs can also play a role in immune regulation. These studies include UV-induced skin damage,7,24 mosquito bite-induced DTH immune responses,8 and graft tolerance.10,25 These models also highlight the ability of MCs to produce molecules that may attenuate injurious adaptive immune responses, including TGF-β, IL-10, IL-4, granzyme B, and perforin, and they may express surface receptors (OX40L) that facilitate immunoregulation after direct contact with regulatory cells.1,26 The role of MCs in autoimmunity has been assessed in a number of animal models of human diseases using MC-deficient mice. The overwhelming outcome has been to suggest that MCs play a proinflammatory injurious role.12 Unexpectedly, we found that MPO-immunized KitW-sh/W-sh mice developed enhanced functional (proteinuria) and structural (glomerular; necrosis, fibrin deposition, and leukocyte accumulation) renal injury compared with MPO-immunized wild-type mice. MPO-immunized KitW-sh/W-sh mice developed enhanced MPO autoimmunity (measured by dermal DTH responses to MPO) as well as elevated systemic CD4+ anti-MPO responses of MPO-specific cell proliferation and increased production of IL-17A from MPO-stimulated lymphocytes isolated from draining LNs compared with wild-type controls. No difference in the IFNγ levels was observed between groups (data not shown). We have previously shown that, in autoimmune anti-MPO GN, CD4+ Th17 cells are important in inducing these injurious cell-mediated effector responses.21,27 Taken together, the increased autoimmunity to MPO and intensity of MPO-triggered disease suggest a cause and effect relationship. Development of humoral immunity was unaltered between groups. The current model used is T cell-dependent and does not allow for an extensive development of the B cell response, in which MPO-immunized µchain−/− mice still develop GN.19 On day 16, a difference in ANCA titers was not detected (Supplemental Figure 1). Differences in titers are more likely to be detected at later stages in the development of autoimmunity. We believe that one of the roles of ANCA is to facilitate glomerular MPO deposition by inducing neutrophil recruitment. However, in this model, disease is triggered by passive deposition of neutrophils using a subnephritogenic dose of anti-GBM antibody. Furthermore, to confirm that the subnephritogenic dose of anti-GBM antibody does not elicit overt injury beyond its role in neutrophil recruitment and MPO deposition in KitW-sh/W-sh mice, we administered the standard triggering doses of anti-GBM antibody to wild-type and KitW-sh/W-sh mice immunized with an irrelevant antigen, ovalbumin. KitW-sh/W-sh mice did not show increased injury. Levels of proteinuria were not different and were both similar to protein excretion in wild-type mice (not given anti-GBM antibodies) (Supplemental Figure 3).
To verify that the protective effects observed in KitW-sh/W-sh mice are a result of its MC deficiency and not related to other potential c-Kit abnormalities, MC reconstitution of C57BL/6 KitW-sh/W-sh mice was subjected to autoimmune anti-MPO GN. Reconstitution of KitW-sh/W-sh mice resulted in attenuated systemic anti-MPO autoimmunity associated with significant reduction in consequent functional and morphologic glomerular injury, and it was similar to disease outcome in MPO-immunized wild-type mice. The observed enhanced autoimmunity and increased glomerular injury in MC-deficient mice, together with the capacity for MC reconstitution to diminish anti-MPO autoimmunity and the severity of GN in KitW-sh/W-sh mice, strongly suggest that MCs are critical in protecting mice from the development of anti-MPO autoimmunity and consequently, the severity of GN. Furthermore, the ability of purified MCs to reduce the intensity of autoimmunity and renal injury in KitW-sh/W-sh mice strongly supports the role of MCs in immunomodulation in this disease model and not other potential MC-independent c-Kit dependent factors. This line of proof of MC function is the accepted standard when using these mice to confirm that the biologic abnormalities observed in KitW-sh/W-sh mice are induced by MCs.1
Our attention was focused on the potential involvement of MCs in the LN draining the site of MPO immunization was used to find evidence consistent with MC participation in the cellular events inducing and regulating autoimmunity. Previous studies have shown that MCs can migrate to nodes at the time of antigen presentation and that the migration is directed by the CXCR4/CXCL12 chemokine pathway.9,28,29 From this information, we examined the migratory pattern of MCs in MPO-immunized and nonimmunized MC intact wild-type mice. There was a fourfold increase in the number of MCs observed in the draining LNs of MPO-immunized mice compared with nonimmunized wild-type mice, and confocal immunofluorescence imaging of these nodes revealed that MCs reside in close proximity with foxp3+ Tregs. These observations provide evidence consistent with a role for MCs in Treg immunomodulation of developing MPO autoimmunity. Such contact between Tregs and MCs has been shown in the draining LN of experimental skin allografts, where MC and Treg interaction functionally underpins immunoregulation in allogeneic responses, promoting graft tolerance.10,25
To date, the most clearly shown mechanism of MC immunomodulation is MC production of IL-10.6,30 In the current study, we found significantly lower levels of IL-10 in LN cells from MC-deficient KitW-sh/W-sh mice associated with enhanced anti-MPO autoimmunity compared with wild-type mice (Figure 1J). This finding raised the possibility that MC IL-10 could mediate the observed immunosuppression. The observed interaction between MCs and Tregs in LNs was also consistent with local MC-produced IL-10 reducing recall MPO-induced proliferation of autoimmune anti-MPO CD4+ cells. This hypothesis was supported by the ex vivo studies, where MC coculture with Tregs attenuated both the proliferation of anti-MPO CD4+ cells and the production of proinflammatory cytokines. Both these effects were dependent of MC IL-10 production. These results support the findings that MCs in vivo act as immunomodulators of anti-MPO autoimmunity.
Proof of the functional capacity of MC-derived IL-10 in attenuating the development of autoimmune anti-MPO GN was achieved by reconstituting KitW-sh/W-sh mice with either IL-10−/− or wild-type MCs. IL-10–competent MC reconstitution resulted in attenuation of anti-MPO autoimmunity and severity of GN compared with IL-10−/− MC-reconstituted KitW-sh/W-sh mice. IL-10−/− MC-reconstituted KitW-sh/W-sh mice did not have the ability to modulate GN and developed proteinuria, fibrinoid necrosis, and increased accumulation of glomerular leukocyte effectors, which was not observed in KitW-sh/W-sh mice reconstituted with wild-type MCs. IL-10−/− MC-reconstituted KitW-sh/W-sh mice also developed increased proinflammatory anti-MPO CD4+ responses (MPO recall cell proliferation and IL-17A) and a decrease in the proportion of draining LN CD4+ Tregs. Taken together with the ex vivo coculture experiment, these results show that immunomodulation of autoimmune anti-MPO GN occurs through MC-derived IL-10.
MCs are characterized by the pleiotropic functional capacities and their ability to become variably involved in potentially paradoxical outcomes by minor differences in activation brought about by differences in the balance of local environmental signals that they receive.31 Thus, as well as immunomodulation, MCs can enhance immune development, facilitate leukocyte recruitment, and mediate injury by assisting the development of immune effector responses.22,32 It is possible that MCs may be making such contributions in the current experiments. However, the power of whole-animal studies is the capacity to assess the net effect of all the contributions that MCs may be making. In autoimmune anti-MPO vasculitis and GN, the overall effect of MCs is significant enhancement of peripheral tolerance and protection from the development and severity of autoimmunity and disease.
In conclusion, this study supports a role for MCs in the immunoregulation of induced autoimmunity to MPO, thereby limiting the severity of GN directed by autoimmune anti-MPO effector cells. This study provides evidence that MCs are involved in the closely orchestrated network of events that maintains peripheral tolerance, thus protecting from the development of autoimmunity. The suggestion that MCs may play such an immunoregulatory role has led to the hypothesis that MC immunosuppressor cells may exist.26 Distinct subpopulations of such regulatory subsets of other leukocyte populations have been phenotypically shown (M2 macrophages and N2 neutrophils).33,34 MC biology emphasizes the plasticity of MCs responding in different ways (pro- or anti-inflammatory) according to the net effect of signals that they detect from their environment. Thus, acquisition of immunoregulatory phenotypes of MCs could be considered as the development of a new MC regulatory subpopulation.
Concise Methods
Mice
C57BL/6 (wild-type) male mice (n=8–10 per group) were purchased from Monash University Animal Services (Melbourne, Australia), and age- and sex-matched MC-deficient C57BL/6 KitW-sh/W-sh mice (n=8–10 per group) were purchased from Jackson Laboratories (Bar Harbor, Connecticut, MA). IL-10−/− C57BL/6 male mice, purchased from the University of Adelaide (South Australia, Australia,) and originally from Jackson Laboratories, were used as donors to obtain bone marrow MCs. Foxp3-GFP mice35 (n=4) were bred at Monash Medical Centre Animal Facility. All mice were housed at Monash Medical Centre, and studies were approved by Monash University Animal Ethics Committee in accordance with the Australia National Health and Medical Research Council animal experimentation guidelines.
Experimental Design
Experimental mouse groups consisted of 8- to 10-week-old wild-type and KitW-sh/W-sh mice or 20- to 22-week-old MC reconstituted KitW-sh/W-sh mice with age-matched control nonreconstituted KitW-sh/W-sh mice. To induce autoimmune anti-MPO focal necrotizing GN, mice were immunized intraperitoneally with 20 µg murine MPO in Freund’s Complete Adjuvant (Sigma-Aldrich, St. Louis, MO) on day 0. On day 7, mice received another injection of 20 µg murine MPO in Freund’s Incomplete Adjuvant (Sigma-Aldrich). MPO was purified from differentiated 32Dcl3 cells.36 On days 16 and 17, mice were administered an intravenous injection of 1.5 mg sheep anti-mouse GBM globulin and humanely killed after an additional 4 days for assessment of systemic autoimmunity and GN. For studies assessing the migration of MCs to the draining LNs, wild-type mice (n=8) were immunized with 15 µg murine MPO in Freund’s Complete Adjuvant subcutaneously and killed on day 6. Draining LNs were harvested for histologic assessment of MC numbers.
MC Reconstitution
KitW-sh/W-sh mice were reconstituted with MCs using a standard in vitro differentiation of MCs technique.13,22 Briefly, bone marrow cells were harvested from 6 to 8 week old wild-type or IL-10−/− mice. Cells were cultured in RPMI supplemented with 15% FCS, 1% Pen/Strep, 2 mM l-glutamine, 1 mM sodium pyruvate (Invitrogen, Melbourne, Australia), 50 µm 2-mercaptoethanol (Sigma-Aldrich), IL-3 (obtained from WEHI3 cell culture supernatants), and 12.5 ng/ml recombinant mouse stem cell factor (R&D Systems, Minneapolis, MN). Nonadherent cells were transferred into fresh culture media every 3 days for 6–8 weeks. This technique results in cell culture with >95% cells showing the expression of c-Kit and IgE Fc receptors characteristic of MCs as well as characteristic granularity by toluidine blue stain (1% toluidine blue [Sigma] in 50% ethanol, pH 2.3) (Supplemental Figure 4). MC reconstitution was induced by injecting 5×106 cells intravenously into KitW-sh/W-sh mice. By 12 weeks, full reconstitution of MCs was confirmed by toluidine blue staining in peripheral tissues.
Assessment of Renal Disease
Glomerular pathology was assessed on 3-µm-thick buffered formalin-fixed paraffin sections and stained with periodic acid-Schiff reagent (HDS Supplies, New South Wales, Australia). A minimum of 40 consecutive glomeruli per mouse was assessed for abnormalities.37 Abnormalities included necrosis, segmental proliferation, hypercellularity, and capillary wall thickening. Results are expressed as percentage of abnormal glomeruli.
Fibrin staining was performed on 3-µm-thick paraffin-cut sections using rabbit anti-mouse fibrinogen (R-4025; a gift from Dr. J. Degen, Childrens Research Foundation, Cincinnati, OH) with an ABC kit (Dako-Cytomation, Glostrup, Denmark) using 3,3′-diaminobenzidine (Sigma-Aldrich) as a substrate.38 A minimum of 40 glomeruli per mouse was scored, and results were expressed as the percentage of glomeruli with fibrin deposition.
Proteinuria was assessed by housing mice in individual cages to collect urine over the final 24 hours of each experiment. Urinary protein was measured by modified Bradford’s method using Bradford’s reagent (Page Blue G-90 [Sigma-Aldrich)], 85% orthophosphoric acid [Ajax Finechem, Australia] in distilled water) and calculated from the 24-hour urine volume and the urinary protein concentration (milligrams per 24 hours).39 Urine creatinine concentrations were measured by an enzymatic creatininase assay using standard laboratory methods at Monash Medical Centre. Both protein concentration and creatinine units were converted to milligrams per milliliter, and results are expressed as a ratio of protein to creatinine.
Assessment of Immune Cells in Kidney and LNs
Glomerular leukocytes (neutrophils, macrophages, and CD4+ T cells) were assessed by an immunoperoxidase-staining technique. Periodate lysine paraformaldehyde frozen 6-µm cryostat-cut kidney sections were stained using a three-layer immunoperoxidase technique as previously described.40 The primary antibodies used were RB6–85C for neutrophils (anti-GR-1; DNAX, Palo Alto, CA), FA/11 for macrophages (anti-mouse CD68; provided by Dr. G. Koch, MRC, Cambridge, England), and GK1.5 for CD4+ T cells (anti-CD4; American Type Culture Collection, Manassas, VA). The secondary antibody used was rabbit anti-rat biotin (BD Bioscience, North Ryde, Australia) followed by tertiary antibody swine anti-rabbit horseradish peroxidase (DAKO-Cytomation). A minimum of 40 glomeruli per mouse was scored, and results were expressed as positive cells per glomerular cross-section (c/gcs).
LN draining sites of MPO immunization were fixed in Carnoy’s solution (60% ethanol, 30% chloroform; Merck, Darmstadt, Germany) and 10% glacial acetic acid (Thermo Fisher Scientific, Waltham, MA) for 18 hours and embedded in paraffin (BDH Laboratory Supplies, Poole, England). For assessment of MC numbers, 3-µm cut sections were stained using toluidine blue. For MC/Treg interactions, sections of paraffin-embedded Carnoy’s fixed tissue were treated with antigen retrieval (microwave oven heating in 0.1 M sodium citrate, pH 6, for 20 minutes) followed by immunofluorescence staining for foxp3 and c-Kit using a Vectastatin Elite ABC kit (Vector Burlingame, CA). Sections were blocked (10% rabbit serum, 10% FCS, 5% BSA/PBS) and then incubated with 4 µg/ml anti-mouse foxp3 (FJK-16s; eBiosciences, San Diego, CA) in 5% rabbit and mouse serum. Endogenous peroxidase activity was ablated by incubation with 0.3% hydrogen peroxidize (Thermo Fisher Scientific) in methanol for 20 minutes and then treated with avidin/biotin blocking solution. Secondary antibody (biotin-conjugated rabbit anti-rat Ig [DAKO-Cytomation] with 5% sheep and mouse serum) was then incubated with the ABC kit. Sections were then incubated with 4 µg/ml c-Kit conjugated to PE fluorochrome (2B8; BD Biosciences PharMingen), and Sudan black was used to minimize autofluorescence. Images were acquired using a Nikon C1 confocal laser scan head attached to a Nikon Ti-E inverted microscope; 488- and 561-nm lasers were used to specifically excite Alexa488 and Alexa568 flurophores, respectively. Single-plane 512×512×12-bit images were captured in a line sequential manner (three-line average) using a 20×0.75 NA objective with the pinhole set at 1.0 AU.
Systemic Immune Responses to MPO
To assess DTH responses to MPO, mice were challenged with 10 µg murine MPO in 40 µL saline (Baxter, New South Wales, Australia) in the right hind footpad, whereas the left hind footpad received saline 24 hours before euthanasia. The difference in the swelling between the right and left footpad 24 hours later was measured using a micrometer and represented the degree of DTH to MPO (∆mm). Measurement of circulating anti-mouse MPO IgG titers in sera was measured by ELISA as previously described.21
For in vitro measurement of MPO-specific cell proliferation, inguinal and para-aortic draining LNs were harvested aseptically from experimental mice and single-cell suspensions prepared in RPMI/10% FCS (JRH, KS). Cells were seeded at 5×105 cells/well in 96-well flat-bottom plates (Sarstedt, Newton, NC), restimulated with 10 µg/ml MPO, and incubated for 72 hours. During the last 16 hours of culture, 0.5 µCi of [3H]-Thymidine (Perkin Elmer, New York, NY) was added per well. Cells were harvested using a 96-well harvester (Filtermate Harvester; Packard Bioscience, South Africa) onto a glass-fiber filter membrane (Packard, Canberra, Australia). Membrane filters were air dried, and scintillation fluid (Perkin Elmer, Waltham, MA) was added. Incorporated radioactivity was measured using the Wallac 1940 liquid scintillation β-counter (Cambridge Scientific, Cambridge, MA). [3H]-Thymidine incorporation was expressed as counts per minute (cpm).
For measurement of cytokine production, 2×106 LN cells/well were cultured with 10 µg/ml MPO in 48-well plates for 72 hours. IL-10 and IL-17A in supernatants were measured by ELISA. Concentrations of IL-10 were measured using rat anti-mouse IL-10 capture antibody (18141D; BD Pharmingen, San Diego, CA), recombinant mouse IL-10 standards (18141D; BD Pharmingen), and biotinylated rat anti-mouse IL-10 detection antibody (BD Pharmingen). IL-17A was measured using paired antibodies (DuoSet; eBioscience).
Assessment of MC Capacity to Enhance Treg Cell Modulation of Anti-MPO CD4+ T Effector Recall Responses
Foxp3-GFP mice (n=5) were immunized with MPO in Freund’s adjuvant, and after 10 days, draining LNs were harvested. CD4+ cells were isolated by magnetic cell sorting according to the manufacturer’s instructions (MACS CD4+ T Cell Isolation Kit; Miltenyi Biotec, North Ryde, Australia). CD4+ cells were then sorted by fluorescence-activated cell sorter (Beckman Coulter, Gladesville, Australia) based on GFP expression. For Treg inhibition assay, CD4+foxp3− Teffs (10×104 cells) were cocultured with increasing amounts of CD4+foxp3+ Tregs (0.3×104 to 10×104 cells) together with erythrocyte-lysed, MACS CD4+-depleted, mitomycin C-treated (50 µg/ml for 30 minutes and 37°C and then washed three times) splenocytes (2×105 cells). For assessment of MC capacity to influence CD4+ T cells, bone marrow cultured MCs (0.3×104 cells) were added to the coculture with a ratio of 32 Teff to 1 Treg to 1 MC. Cells were added in a 96-well round-bottom plate with 2 µg/ml MPO for 72 hours, and proliferation was measured by adding [3H]-thymidine for the last 16 hours of culture. Results were expressed as percentage reduction in anti-MPO Teff proliferation. Cytokines were measured in supernatants using a BD Cytometric Bead Array Mouse Th1/Th2/Th17 Cytokine Kit according to the manufacturer’s instructions (BD Biosciences).
Disclosures
None.
Supplementary Material
Acknowledgments
These studies were supported by grants from the National Health and Medical Research Council of Australia.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012060572/-/DCSupplemental.
See related editorial, “Mast Cells: Subordinates or Masterminds in Autoimmunity?,” on pages 1913–1914.
References
- 1.Sayed BA, Christy A, Quirion MR, Brown MA: The master switch: The role of mast cells in autoimmunity and tolerance. Annu Rev Immunol 26: 705–739, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Galli SJ, Nakae S, Tsai M: Mast cells in the development of adaptive immune responses. Nat Immunol 6: 135–142, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Sayed BA, Brown MA: Mast cells as modulators of T-cell responses. Immunol Rev 217: 53–64, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Gri G, Piconese S, Frossi B, Manfroi V, Merluzzi S, Tripodo C, Viola A, Odom S, Rivera J, Colombo MP, Pucillo CE: CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. Immunity 29: 771–781, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakae S, Suto H, Iikura M, Kakurai M, Sedgwick JD, Tsai M, Galli SJ: Mast cells enhance T cell activation: Importance of mast cell costimulatory molecules and secreted TNF. J Immunol 176: 2238–2248, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Galli SJ, Grimbaldeston M, Tsai M: Immunomodulatory mast cells: Negative, as well as positive, regulators of immunity. Nat Rev Immunol 8: 478–486, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ: Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol 8: 1095–1104, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Depinay N, Hacini F, Beghdadi W, Peronet R, Mécheri S: Mast cell-dependent down-regulation of antigen-specific immune responses by mosquito bites. J Immunol 176: 4141–4146, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Limón-Flores AY, Chacón-Salinas R, Ramos G, Ullrich SE: Mast cells mediate the immune suppression induced by dermal exposure to JP-8 jet fuel. Toxicol Sci 112: 144–152, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, Scott ZA, Coyle AJ, Reed JL, Van Snick J, Strom TB, Zheng XX, Noelle RJ: Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature 442: 997–1002, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Eller K, Wolf D, Huber JM, Metz M, Mayer G, McKenzie AN, Maurer M, Rosenkranz AR, Wolf AM: IL-9 production by regulatory T cells recruits mast cells that are essential for regulatory T cell-induced immune suppression. J Immunol 186: 83–91, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker ME, Hatfield JK, Brown MA: New insights into the role of mast cells in autoimmunity: Evidence for a common mechanism of action? Biochim Biophys Acta 1822: 57–65,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ: Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol 167: 835–848, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feyerabend TB, Weiser A, Tietz A, Stassen M, Harris N, Kopf M, Radermacher P, Möller P, Benoist C, Mathis D, Fehling HJ, Rodewald HR: Cre-mediated cell ablation contests mast cell contribution in models of antibody- and T cell-mediated autoimmunity. Immunity 35: 832–844, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Li H, Nourbakhsh B, Safavi F, Li K, Xu H, Cullimore M, Zhou F, Zhang G, Rostami A: Kit (W-sh) mice develop earlier and more severe experimental autoimmune encephalomyelitis due to absence of immune suppression. J Immunol 187: 274–282, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett JL, Blanchet MR, Zhao L, Zbytnuik L, Antignano F, Gold M, Kubes P, McNagny KM: Bone marrow-derived mast cells accumulate in the central nervous system during inflammation but are dispensable for experimental autoimmune encephalomyelitis pathogenesis. J Immunol 182: 5507–5514, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Secor VH, Secor WE, Gutekunst CA, Brown MA: Mast cells are essential for early onset and severe disease in a murine model of multiple sclerosis. J Exp Med 191: 813–822, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao H, Heeringa P, Hu P, Liu Z, Zhao M, Aratani Y, Maeda N, Falk RJ, Jennette JC: Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest 110: 955–963, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruth AJ, Kitching AR, Kwan RY, Odobasic D, Ooi JD, Timoshanko JR, Hickey MJ, Holdsworth SR: Anti-neutrophil cytoplasmic antibodies and effector CD4+ cells play nonredundant roles in anti-myeloperoxidase crescentic glomerulonephritis. J Am Soc Nephrol 17: 1940–1949, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Holdsworth SR, Summers SA: Role of mast cells in progressive renal diseases. J Am Soc Nephrol 19: 2254–2261, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Gan PY, Steinmetz OM, Tan DS, O’Sullivan KM, Ooi JD, Iwakura Y, Kitching AR, Holdsworth SR: Th17 cells promote autoimmune anti-myeloperoxidase glomerulonephritis. J Am Soc Nephrol 21: 925–931, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Summers SA, Chan J, Gan PY, Dewage L, Nozaki Y, Steinmetz OM, Nikolic-Paterson DJ, Kitching AR, Holdsworth SR: Mast cells mediate acute kidney injury through the production of TNF. J Am Soc Nephrol 22: 2226–2236, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benoist C, Mathis D: Mast cells in autoimmune disease. Nature 420: 875–878, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Hart PH, Grimbaldeston MA, Swift GJ, Jaksic A, Noonan FP, Finlay-Jones JJ: Dermal mast cells determine susceptibility to ultraviolet B-induced systemic suppression of contact hypersensitivity responses in mice. J Exp Med 187: 2045–2053, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Vries VC, Wasiuk A, Bennett KA, Benson MJ, Elgueta R, Waldschmidt TJ, Noelle RJ: Mast cell degranulation breaks peripheral tolerance. Am J Transplant 9: 2270–2280, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frossi B, Gri G, Tripodo C, Pucillo C: Exploring a regulatory role for mast cells: ‘MCregs’? Trends Immunol 31: 97–102, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Summers SA, Steinmetz OM, Gan PY, Ooi JD, Odobasic D, Kitching AR, Holdsworth SR: Toll-like receptor 2 induces Th17 myeloperoxidase autoimmunity while Toll-like receptor 9 drives Th1 autoimmunity in murine vasculitis. Arthritis Rheum 63: 1124–1135, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Byrne SN, Limón-Flores AY, Ullrich SE: Mast cell migration from the skin to the draining lymph nodes upon ultraviolet irradiation represents a key step in the induction of immune suppression. J Immunol 180: 4648–4655, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang HW, Tedla N, Lloyd AR, Wakefield D, McNeil PH: Mast cell activation and migration to lymph nodes during induction of an immune response in mice. J Clin Invest 102: 1617–1626, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hershko AY, Rivera J: Mast cell and T cell communication; amplification and control of adaptive immunity. Immunol Lett 128: 98–104, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galli SJ, Tsai M: Mast cells: Versatile regulators of inflammation, tissue remodeling, host defense and homeostasis. J Dermatol Sci 49: 7–19, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Timoshanko JR, Kitching AR, Semple TJ, Tipping PG, Holdsworth SR: A pathogenetic role for mast cells in experimental crescentic glomerulonephritis. J Am Soc Nephrol 17: 150–159, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Cao Q, Wang C, Zheng D, Wang Y, Lee VW, Wang YM, Zheng G, Tan TK, Yu D, Alexander SI, Harris DC, Wang Y: IL-25 induces M2 macrophages and reduces renal injury in proteinuric kidney disease. J Am Soc Nephrol 22: 1229–1239, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mantovani A: The yin-yang of tumor-associated neutrophils. Cancer Cell 16: 173–174, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY: Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity 22: 329–341, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Apostolopoulos J, Ooi JD, Odobasic D, Holdsworth SR, Kitching AR: The isolation and purification of biologically active recombinant and native autoantigens for the study of autoimmune disease. J Immunol Methods 308: 167–178, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Dean EG, Wilson GR, Li M, Edgtton KL, O’Sullivan KM, Hudson BG, Holdsworth SR, Kitching AR: Experimental autoimmune Goodpasture’s disease: A pathogenetic role for both effector cells and antibody in injury. Kidney Int 67: 566–575, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Drew AF, Tucker HL, Liu H, Witte DP, Degen JL, Tipping PG: Crescentic glomerulonephritis is diminished in fibrinogen-deficient mice. Am J Physiol Renal Physiol 281: F1157–F1163, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Huang XR, Tipping PG, Apostolopoulos J, Oettinger C, D’Souza M, Milton G, Holdsworth SR: Mechanisms of T cell-induced glomerular injury in anti-glomerular basement membrane (GBM) glomerulonephritis in rats. Clin Exp Immunol 109: 134–142, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tipping PG, Huang XR, Berndt MC, Holdsworth SR: P-selectin directs T lymphocyte-mediated injury in delayed-type hypersensitivity responses: studies in glomerulonephritis and cutaneous delayed-type hypersensitivity. Eur J Immunol 26: 454–460, 1996 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.