Abstract
Up to 80% of patients with idiopathic membranous nephropathy have non–complement-fixing IgG4 autoantibodies to the phospholipase A2 receptor (PLA2R). Membranous nephropathy recurs in approximately 40% of patients after kidney transplantation, but the mechanism is unknown. Here, we describe a patient with recurrent membranous nephropathy 13 days after kidney transplantation whose graft biopsy specimen showed granular staining for C3, C5b-9, C1q, and IgG3κ; electron microscopy revealed subepithelial nonorganized deposits. A search for hematologic disorders was negative. Retrospective evaluation of a biopsy sample from the native kidney revealed a similar pattern: monotypic IgG3κ deposits together with C3, C1q, and C5b-9. Glomerular deposits contained PLA2R in both the graft and the native kidney, suggesting that the recurrence was the result of circulating anti-PLA2R antibodies binding to PLA2R antigen expressed on donor podocytes. Confocal analysis of anti-PLA2R and antihuman IgG3 showed co-localization, and the patient had IgG3κ-restricted circulating anti-PLA2R antibodies. Treatment with rituximab stabilized both proteinuria and serum creatinine, and circulating anti-PLA2R became undetectable. In summary, this case of recurrent membranous nephropathy in a graft suggests that circulating monoclonal anti-PLA2R IgG3κ caused the disease and activated complement by the classic pathway.
Membranous nephropathy (MN) is one of the more common causes of nephrotic syndrome in the adult population, accounting for about 20% of cases. It can be idiopathic, without identified cause (70%–80%), or secondary to various clinical conditions, including infections (hepatitis B, syphilis), systemic lupus erythematosus, cancers, and drug intoxications.1
MN is an immunologically mediated disease defined by immune complex deposition in the subepithelial space that causes a membrane-like thickening. The immune deposits consist of IgG, antigens that have long eluded identification, and the membrane attack complex of complement C5b-9. IgG4 is the most prominent deposited subclass in idiopathic MN, although variable amounts of IgG1 are usually associated; in secondary MN, IgG1, IgG2, and IgG3 exceed IgG4.2,3 The formation of subepithelial immune deposits and complement activation are presumably responsible for functional impairment of the glomerular capillary wall, causing proteinuria. Evidence now suggests that MN is triggered by antibodies directed against podocyte proteins. Two major antigens, both membrane glycoproteins, have been identified. The first is neutral endopeptidase, the alloantigen involved in rare neonatal cases of MN that occur in newborns from neutral endopeptidase–deficient mothers.4 The disease could be transferred to rabbits injected with immunoglobulin purified from the infant’s mother’s serum but not from the father’s serum.5 The second antigen is the M-type phospholipase A2 receptor (PLA2R), the first antigen identified in idiopathic MN in adults, which is considered an autoimmune disease.6
Although anti-PLA2R antibodies are found in about 70% of patients with idiopathic MN6–9 and seem to correlate with disease activity and proteinuria,6,10,11 there is no definitive proof that these antibodies are pathogenic. First, PLA2R-related MN could not be induced by transfer of patients’ serum or IgG to mouse, rat, or rabbit because these species do not express PLA2R antigen in glomeruli. Second, as yet there is no animal model of PLA2R-related MN that could phenocopy Heymann nephritis, a reliable form of MN in the rat in which the target antigen, megalin, is also located at the podocyte surface.12,13 Third, anti-PLA2R antibodies can occasionally be found in patients with idiopathic MN but without PLA2R antigen in subepithelial immune deposits, a finding suggesting that at least some anti-PLA2R antibodies might not be pathogenic.14 Fourth, although PLA2R-related MN can recur in the kidney graft, sometimes after only a few days,15–17 some patients with high-titer anti-PLA2R antibodies at the time of transplantation will not have clinical or histologic recurrence.16 In those cases, however, differences between donor and recipient PLA2R sequence variants might account for the lack of recurrence.
Here we report an exceptional case of recurrent PLA2R-related MN with monotypic IgG3κ deposits and circulating anti-PLA2R antibodies restricted to IgG3κ, which provides an argument favoring the pathogenicity of anti-PLA2R antibodies, at least in this particular situation.
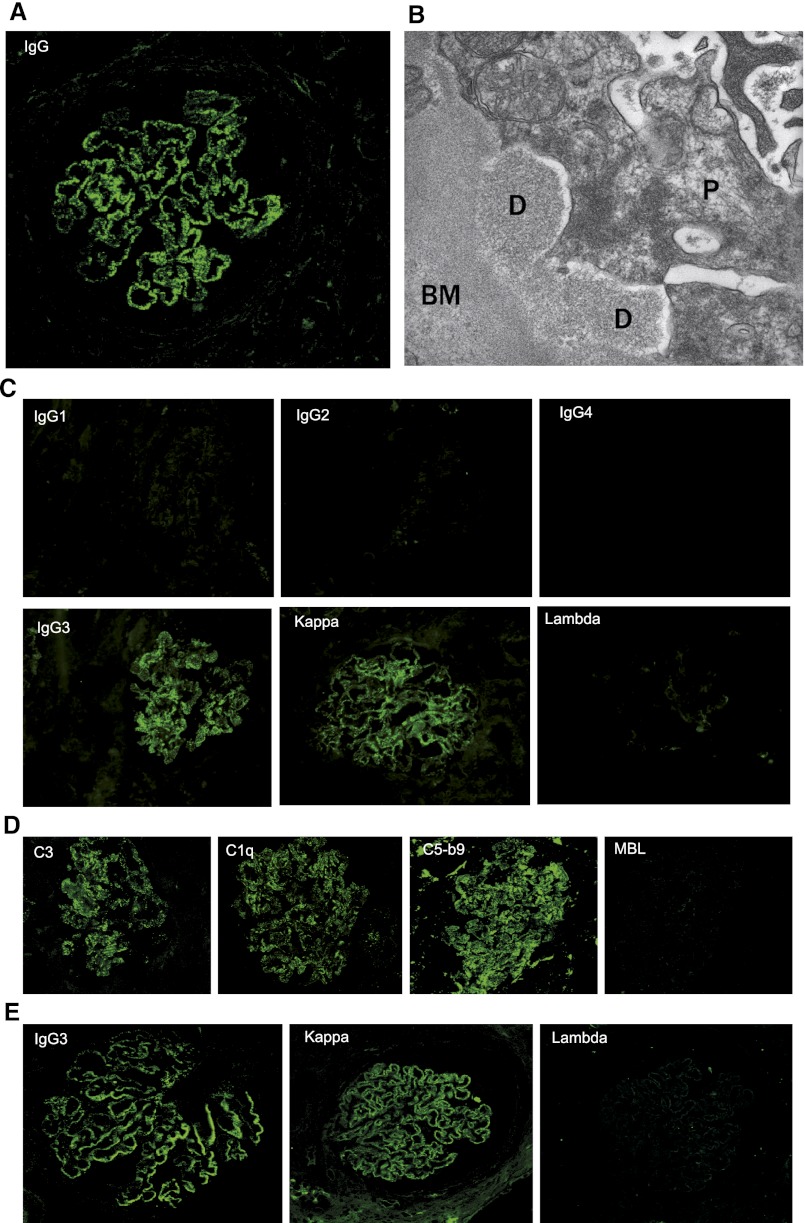
A kidney allograft biopsy was performed 13 days after transplantation because of delayed graft function (plasma creatinine, 2.82 mg/dl) and proteinuria (1.85 g/d) in a 52-year-old man in whom MN had been diagnosed 13 years earlier and who has been receiving hemodialysis for the last 6 years. Pretransplantation assessment of the glomerulopathy failed to identify a cause, thereby suggesting idiopathic MN. The biopsy revealed early recurrence of MN, characterized by abundant granular deposits of IgG on the outer aspect of the glomerular basement membrane (Figure 1A). These deposits did not show any organization by electron microscopy (Figure 1B). We performed a subclass and light-chain isotype analysis of deposited IgG, which exclusively stained for IgG3κ (Figure 1C). Biopsy specimen also contained C3, C1q, and C5b-9 in deposits but no mannose-binding lectin (MBL) (Figure 1D). The positive control for MBL staining is shown in Supplemental Figure 1.
Figure 1.
Characterization of immune deposits in kidney biopsy specimens from grafted (A–D) and native (E) kidneys. (A) Immunofluorescence study showing early recurrence of the MN (day 13) characterized by granular deposits of IgG. (B) Representative segment of the capillary wall analyzed by electron microscopy. Electron-dense deposits seen on the outer aspect of the glomerular basement membrane do not show any organization. (C) Immunostaining for IgG subclasses and light-chain isotypes showing the presence of monotypic IgG3κ. (D) Complement components, including C3, C1q and C5b-9, detected in the absence of MBL. (E) Kidney biopsy specimen from native kidney stained for IgG3 and light-chain isotype. The specimens shown in E are paraffin sections, whereas those shown in A, C, and D are cryostat sections. Original magnification for A, C, D, and E x400; for B x40,000.
The finding of monotypic IgG3κ deposits prompted an extensive search for a hematologic disorder. Electrophoresis and immunofixation of serum proteins did not disclose any qualitative anomaly. Serum κ and λ free light-chain levels and immunoglobulin classes were normal, except for IgG, which was moderately decreased. Blood lymphocyte immunophenotyping was unremarkable. Positron emission tomography did not reveal hyperfixation, and the bone marrow biopsy specimen was normal, revealing rare, polytypic plasma cells.
We then asked whether deposits in the native kidney biopsy specimen were also monotypic. The pathologic report described granular deposits of IgG, C3, and C1q, but there was no information on light-chain isotype. Because of the lack of frozen kidney biopsy specimen, we developed a new technique to perform subclass and isotype analysis in paraffin-embedded sections. The deposits stained for IgG3 and κ but did not stain for the λ isotype (Figure 1E).
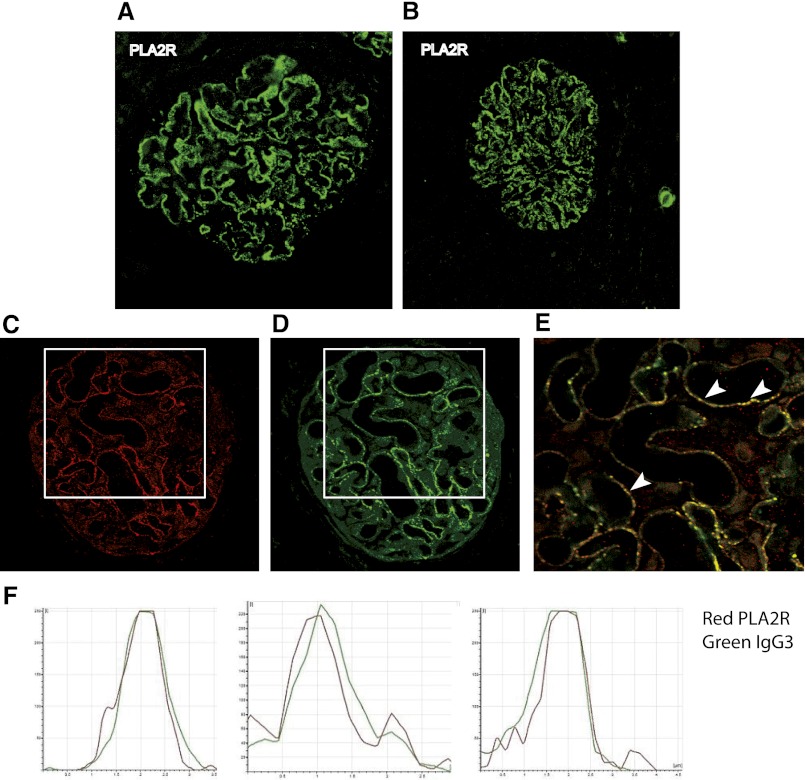
PLA2R antigen was detected in a granular pattern typical of subepithelial immune deposits in the native and kidney graft biopsy specimens (Figure 2, A and B). Co-localization with IgG3 was established by confocal microscopy (Figure 2, C–F).
Figure 2.
Detection of PLA2R in glomerular immune deposits. Immunofluorescence analysis of paraffin kidney biopsy specimens shows PLA2R in (A) native and (B) grafted kidney. (C–E) Positively stained glomeruli in a biopsy specimen from grafted kidney that has been double-labeled with anti-PLA2R (C) and anti-human IgG3 antibodies (D); E shows the merged image of boxed areas in C and D. (F) Quantitative analysis of the fluorescence recorded across sections of a representative capillary loop (arrowheads). Note superimposition of the two signals, which indicates that subepithelial immune deposits are composed of PLA2R (red) and IgG3 (green). Original magnification for A, B, C, D, and E x400.
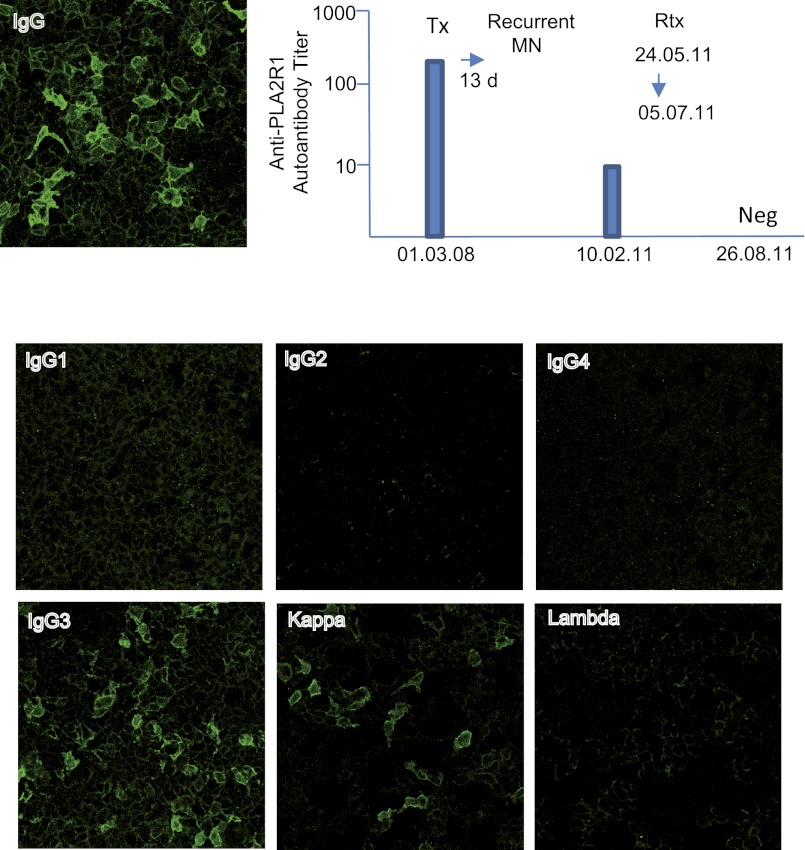
Because both the native and kidney graft biopsy specimens featured monotypic IgG3κ deposits in the absence of IgG4, we reasoned that the circulating anti-PLA2R antibodies could also be monotypic. Using the indirect immunofluorescence test (Euroimmun AG, Lübeck, Germany), we first showed that in patient’s serum anti-PLA2R antibodies were present at the time of transplantation (Figure 3). Next, using subclass- and light-chain isotype–specific revealing antibodies, we found that anti-PLA2R antibodies were restricted to IgG3κ, which is the immunoglobulin isotype detected in glomerular deposits (Figure 2).
Figure 3.
Detection and isotyping of circulating anti-PLA2R antibodies in patient’s serum using immunofluorescence test (Euroimmun). The serum sample after transplantation before rituximab treatment contains anti-PLA2R antibodies that are restricted to IgG3κ. Neg, negative; Rtx, rituximab; TX, transplantation.
On the basis of these findings, the patient received four injections of rituximab (375 mg/m2) at 2-week intervals. Six months later, proteinuria had decreased dramatically (from 5.1 g/d before rituximab to 0.4 g/d), kidney graft function had stabilized (serum creatinine, 2.72 mg/dl before rituximab versus 2.56 mg/dl), and anti-PLA2R antibodies had disappeared (Figure 3).
Discussion
To our knowledge, this is the first report of monotypic anti-PLA2R IgG3κ associated with MN, activation of the classic complement pathway, and very early recurrence of the disease on the kidney graft. The finding of IgG3κ being co-localized with PLA2R in both the native and the kidney graft biopsy specimens, and of circulating anti-PLA2R IgG3κ, strongly suggests that this antibody was responsible for the glomerular disease and its recurrence in this patient.
Circulating monoclonal immunoglobulin may recognize self-antigens as shown for IgM anti-IgG in type 2 cryoglobulins, perinuclear ANCA,18,19 and anti–glomerular basement membrane antibodies.20,21 In this patient, the rate of synthesis of the antibody was probably low because no monoclonal component could be detected in the blood, even by sensitive methods. A similar situation is observed in patients with proliferative GN with monoclonal IgG deposits, which may also recur in the allograft; however, no target antigen has yet been identified in this entity.22,23 It is noteworthy that in the four patients with recurrence, no serum monoclonal component could be detected at the time of first biopsy or at the time of recurrence, although all four patients featured IgG3 deposits (three κ, one λ). IgG3 is the isoform identified in most cases of GN with monoclonal IgG deposits.22,24 This subclass, which accounts for only 2%–8% of serum IgG in normal individuals, is especially prone to self-aggregability via Fc-Fc interactions.25 In addition, compared with other IgG subclasses, it has a slightly higher molecular weight, the greatest complement-fixing ability, and globally more cationic charge, characteristics that may make it intrinsically “nephritogenic.”25,26 Although some patients with GN with monoclonal IgG deposits have a history of autoimmune disease, none had MN. In our patient, development of MN was probably caused by the anti-PLA2R activity of the IgG3, although deposition of anti-PLA2R antibody might also be enhanced by the physicochemical properties of IgG3.
Our case is unusual among idiopathic MN cases because of signs of activation of the classic complement pathway, such as C1q deposits, associated with C3 and C5b-9 in both native and graft biopsy specimens. There was no evidence of lupus disease. MBL was not found in immune deposits, suggesting that in this case, the MBL pathway was probably not activated, in contrast with recent findings which indicate that anti-PLA2R IgG4 can activate the MBL pathway.27 In addition, MBL was identified in the glomeruli of patients with idiopathic MN.3,28 The high C1q-binding ability of IgG3 probably explains the discrepancy with most common forms of idiopathic MN, in which IgG4 is the prevailing subclass.
Few reports have described very early recurrence of PLA2R-related MN, and all such cases were associated with polyclonal anti-PLA2R antibodies.15–17 Recurrence occurred as early as 6–8 days after transplantation,16, 17 suggesting that anti-PLA2R antibodies brought by the recipient’s serum rapidly induced formation of subepithelial deposits in the graft glomeruli, thus reproducing the passive Heymann nephritis model.29,30
Because of the monoclonal nature of the antibody, our observation marks a further step in the demonstration of pathogenicity of anti-PLA2R antibodies.
Concise Methods
Patient
The patient is a 56-year-old man in whom nephrotic syndrome of rapid onset was diagnosed in 1993. Antinuclear antibodies were absent, and serum complement C3 and C4 concentrations were normal. Diagnosis of MN was established on a first kidney biopsy specimen. Light-chain isotype was not determined. At that time, no evidence indicated a secondary cause, although the patient was a heavy smoker (30 pack-years). His medical history included childhood asthma, an acute non-A, non-B, non-C viral hepatitis at age 10 years, and tonsillectomy. Family medical history was unremarkable. Because the serum creatinine level increased to 1.36 mg/dl in July 1994, the patient was treated with steroids and cyclophosphamide according to a revised Ponticelli protocol.31 In May 1995, a second kidney biopsy showed diffuse interstitial fibrosis. Immunosuppressive treatment was stopped. The patient was then lost to follow-up until February 2002, when he was seen with a major episode of volume expansion and dilated cardiomyopathy. Hemodialysis was started. Between 2002 and 2008, no additional immunologic or hematologic disorder was diagnosed. The patient received a kidney transplant in February 2008. Anti-HLA antibodies were negative. Induction was achieved with anti-IL2 receptor antibodies, and the immunosuppressive regimen included prednisone, tacrolimus, and mycophenolate mofetil. A biopsy of the graft was performed on day 13 because of delayed improvement of renal function (serum creatinine, 2.71 mg/dl) and proteinuria (1.85 g/d) in the absence of urologic complication or off-target tacrolimus concentrations. The donor presented no significant medical history and had normal kidney function and proteinuria. Informed consent was obtained for further immunopathologic studies.
Analysis of Renal Biopsy Specimen
The patient’s biopsy specimen was prepared for light, immunofluorescence, and electron microscopy by standard techniques. For detection of IgG subclasses, light-chain isotypes, and complement components, cryosections of the biopsy specimen were incubated with the following antibodies: FITC-conjugated, monoclonal antihuman IgG1 (Sigma-Aldrich), IgG2 (Sigma-Aldrich), IgG3 (Sigma-Aldrich), and IgG4 (Sigma-Aldrich) antibodies; rabbit polyclonal antihuman κ or λ light chains (Dako); antihuman C3 complement (Dako); antihuman C1q complement (Dako); nonconjugated monoclonal antihuman C5b-9 (Dako); and anti–mannan-binding lectin antibody 3B6 (abcam).
Detection of PLA2R Antigen, IgG3, and Light-Chain Isotypes in Paraffin-Embedded Kidney Biopsy Specimens
PLA2R was assessed in glomerular deposits by confocal microscopy with rabbit affinity purified specific anti-PLA2R antibodies (Atlas Antibodies), followed by goat Alexa 488 conjugated antirabbit Fab IgG (Molecular Probes). Co-localization of PLA2R and IgG3 was analyzed by confocal microscopy. The biopsy specimens were first incubated with anti-PLA2R antibodies, then with goat Alexa 568–conjugated antirabbit Fab IgG antibodies (Molecular Probes) and FITC-conjugated monoclonal antihuman IgG3 from a mouse (Sigma-Aldrich). Sections were examined under a confocal microscope (Leica TCS-SP2) and analyzed with Leica Confocal software, version 2.61. For PLA2R and IgG3 staining, the antibodies were deposited on deparaffinized sections retrieved by heating in citric acid (pH, 6.0), followed by HistoReveal (abcam) treatment. For detection of light-chain isotype, FITC-conjugated rabbit polyclonal antihuman κ or λ light-chain antibodies (Dako) were deposited on the deparaffinized sections pretreated with HistoReveal (abcam).
Detection and Subclass Analysis of Circulating Anti-PLA2R Antibodies
Circulating antibodies against PLA2R were assessed by commercially available indirect immunofluorescence test (Euroimmun). For detection of IgG subclass-specific antibodies, FITC-conjugated monoclonal antihuman IgG1 (Sigma-Aldrich),d IgG2 (Sigma-Aldrich), IgG3 (Sigma-Aldrich), and IgG4 (Sigma-Aldrich) antibodies from mouse were used. For detection of light-chain isotypes, FITC-conjugated rabbit polyclonal antihuman κ or λ light chains were used (Dako).
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Philippe Fontanges for assistance with the confocal microscope.
The work was supported by grants from Fondation pour la Recherche Médicale (Equipe FRM 2012) and by grant from Agence Nationale pour la Recherche (ANR-07-Physio-016-01).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012060577/-/DCSupplemental.
See related editorial, “Monoclonal Anti-PLA2R and Recurrent Membranous Nephropathy: Another Piece of the Puzzle,” on pages 1911–1913.
References
- 1.Glassock RJ: The pathogenesis of idiopathic membranous nephropathy: A 50-year odyssey. Am J Kidney Dis 56: 157–167, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Ohtani H, Wakui H, Komatsuda A, Okuyama S, Masai R, Maki N, Kigawa A, Sawada K, Imai H: Distribution of glomerular IgG subclass deposits in malignancy-associated membranous nephropathy. Nephrol Dial Transplant 19: 574–579, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Segawa Y, Hisano S, Matsushita M, Fujita T, Hirose S, Takeshita M, Iwasaki H: IgG subclasses and complement pathway in segmental and global membranous nephropathy. Pediatr Nephrol 25: 1091–1099, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Debiec H, Guigonis V, Mougenot B, Decobert F, Haymann JP, Bensman A, Deschênes G, Ronco PM: Antenatal membranous glomerulonephritis due to anti-neutral endopeptidase antibodies. N Engl J Med 346: 2053–2060, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Ronco P, Debiec H: Molecular pathomechanisms of membranous nephropathy: From Heymann nephritis to alloimmunization. J Am Soc Nephrol 16: 1205–1213, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Beck LH, Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ: M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361: 11–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoxha E, Harendza S, Zahner G, Panzer U, Steinmetz O, Fechner K, Helmchen U, Stahl RA: An immunofluorescence test for phospholipase-A₂-receptor antibodies and its clinical usefulness in patients with membranous glomerulonephritis. Nephrol Dial Transplant 26: 2526–2532, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Qin W, Beck LH, Jr, Zeng C, Chen Z, Li S, Zuo K, Salant DJ, Liu Z: Anti-phospholipase A2 receptor antibody in membranous nephropathy. J Am Soc Nephrol 22: 1137–1143, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ronco P, Debiec H: Pathogenesis of membranous nephropathy: Recent advances and future challenges. Nat Rev Nephrol 8: 203–213, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Hofstra JM, Beck LH, Jr, Beck DM, Wetzels JF, Salant DJ: Anti-phospholipase A₂ receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. Clin J Am Soc Nephrol 6: 1286–1291, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beck LH, Jr, Fervenza FC, Beck DM, Bonegio RG, Malik FA, Erickson SB, Cosio FG, Cattran DC, Salant DJ: Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J Am Soc Nephrol 22: 1543–1550, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heymann W, Hackel DB, Harwood S, Wilson SGF, Hunter JL: Production of nephrotic syndrome in rats by Freund’s adjuvants and rat kidney suspensions. Proc Soc Exp Biol Med 100: 660–664, 1959 [DOI] [PubMed] [Google Scholar]
- 13.Ronco P, Debiec H: Advances in membranous nephropathy: Success stories of a long journey. Clin Exp Pharmacol Physiol 38: 460–6, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Debiec H, Ronco P: PLA2R autoantibodies and PLA2R glomerular deposits in membranous nephropathy. N Engl J Med 364: 689–690, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Stahl R, Hoxha E, Fechner K: PLA2R autoantibodies and recurrent membranous nephropathy after transplantation. N Engl J Med 363: 496–498, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Debiec H, Martin L, Jouanneau C, Dautin G, Mesnard L, Rondeau E, Mousson C, Ronco P: Autoantibodies specific for the phospholipase A2 receptor in recurrent and De Novo membranous nephropathy. Am J Transplant 11: 2144–2152, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Blosser CD, Ayalon R, Nair R, Thomas C, Beck LH, Jr: Very early recurrence of anti-phospholipase A2 receptor-positive membranous nephropathy after transplantation. Am J Transplant 12: 1637–1642, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Bayle P, Laplanche G, Gorguet B, Oksman F, Boulinguez S, Bazex J: Neutrophilic dermatosis: A case of overlapping syndrome with monoclonal antineutrophil cytoplasmic autoantibody activity. Dermatology 189: 69–71, 1994 [DOI] [PubMed] [Google Scholar]
- 19.Cohavy O, Harth G, Horwitz M, Eggena M, Landers C, Sutton C, Targan SR, Braun J: Identification of a novel mycobacterial histone H1 homologue (HupB) as an antigenic target of pANCA monoclonal antibody and serum immunoglobulin A from patients with Crohn’s disease. Infect Immun 67: 6510–6517, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fervenza FC, Terreros D, Boutaud A, Hudson BG, Williams RA, Jr, Donadio JV, Jr, Schwab TR: Recurrent Goodpasture’s disease due to a monoclonal IgA-kappa circulating antibody. Am J Kidney Dis 34: 549–555, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Borza DB, Chedid MF, Colon S, Lager DJ, Leung N, Fervenza FC: Recurrent Goodpasture’s disease secondary to a monoclonal IgA1-kappa antibody autoreactive with the alpha1/alpha2 chains of type IV collagen. Am J Kidney Dis 45: 397–406, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Nasr SH, Satoskar A, Markowitz GS, Valeri AM, Appel GB, Stokes MB, Nadasdy T, D’Agati VD: Proliferative glomerulonephritis with monoclonal IgG deposits. J Am Soc Nephrol 20: 2055–2064, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nasr SH, Sethi S, Cornell LD, Fidler ME, Boelkins M, Fervenza FC, Cosio FG, D’Agati VD: Proliferative glomerulonephritis with monoclonal IgG deposits recurs in the allograft. Clin J Am Soc Nephrol 6: 122–132, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guiard E, Karras A, Plaisier E, Duong Van Huyen JP, Fakhouri F, Rougier JP, Noel LH, Callard P, Delahousse M, Ronco P: Patterns of noncryoglobulinemic glomerulonephritis with monoclonal Ig deposits: correlation with IgG subclass and response to rituximab. Clin J Am Soc Nephrol 6: 1609–1616, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Capra JD, Kunkel HG: Aggregation of gamma-G3 proteins: Relevance to the hyperviscosity syndrome. J Clin Invest 49: 610–621, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdelmoula M, Spertini F, Shibata T, Gyotoku Y, Luzuy S, Lambert PH, Izui S: IgG3 is the major source of cryoglobulins in mice. J Immunol 143: 526–532, 1989 [PubMed] [Google Scholar]
- 27.Ma H, Beck LH, Salant DJ: Membranous nephropathy-associated anti-phospholipase A2 receptor IgG4 autoantibodies activate the lectin complement pathway. [abstract] J Am Soc Nephrol 22: 62A, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lhotta K, Würzner R, König P: Glomerular deposition of mannose-binding lectin in human glomerulonephritis. Nephrol Dial Transplant 14: 881–886, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Van Damme BJ, Fleuren GJ, Bakker WW, Vernier RL, Hoedemaeker PJ: Experimental glomerulonephritis in the rat induced by antibodies directed against tubular antigens. V. Fixed glomerular antigens in the pathogenesis of heterologous immune complex glomerulonephritis. Lab Invest 38: 502–510, 1978 [PubMed] [Google Scholar]
- 30.Couser WG, Steinmuller DR, Stilmant MM, Salant DJ, Lowenstein LM: Experimental glomerulonephritis in the isolated perfused rat kidney. J Clin Invest 62: 1275–1287, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ponticelli C, Altieri P, Scolari F, Passerini P, Roccatello D, Cesana B, Melis P, Valzorio B, Sasdelli M, Pasquali S, Pozzi C, Piccoli G, Lupo A, Segagni S, Antonucci F, Dugo M, Minari M, Scalia A, Pedrini L, Pisano G, Grassi C, Farina M, Bellazzi R: A randomized study comparing methylprednisolone plus chlorambucil versus methylprednisolone plus cyclophosphamide in idiopathic membranous nephropathy. J Am Soc Nephrol 9: 444–450, 1998 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.