Abstract
The effect of low titers of donor-specific antibodies (DSAs) detected only by sensitive solid-phase assays (SPAs) on renal transplant outcomes is unclear. We report the results of a systematic review and meta-analysis of rejection rates and graft outcomes for renal transplant recipients with such preformed DSAs, defined by positive results on SPA but negative complement-dependent cytotoxicity and flow cytometry crossmatch results. Our search identified seven retrospective cohort studies comprising a total of 1119 patients, including 145 with isolated DSA-SPA. Together, these studies suggest that the presence of DSA-SPA, despite a negative flow cytometry crossmatch result, nearly doubles the risk for antibody-mediated rejection (relative risk [RR], 1.98; 95% confidence interval [CI], 1.36–2.89; P<0.001) and increases the risk for graft failure by 76% (RR, 1.76; 95% CI, 1.13–2.74; P=0.01). These results suggest that donor selection should consider the presence of antibodies in the recipient, identified by the SPA, even in the presence of a negative flow cytometry crossmatch result.
In 1969, Patel and Terasaki published their landmark study that definitively established the detrimental effect of preformed donor-specific antibodies (DSAs) detected by complement-dependent cytotoxicity (CDC) crossmatch on short-term allograft survival, but they noted the limited sensitivity of the technique in the detection of preformed DSAs.1 Since that time, technological advances have led to increasingly more sensitive tests for the detection of anti-HLA DSAs, including flow cytometry crossmatch and the more recent solid-phase assays (SPAs), such as Luminex.2,3 The clinical significance of DSAs detected by sensitive SPA remains unclear, with disparate findings being reported in the literature.4 Further, the clinical utility of these tests, particularly for prognostication or modifying immunosuppressive therapy, when used individually or in some sequential manner remains unclear. Our aim was to perform a systematic review and meta-analysis of published reports of rejection rates and graft outcomes among renal transplant recipients with preformed DSA at levels detectable only by SPA but not by CDC or flow crossmatch.
Results
We ultimately identified seven studies that met our inclusion criteria for the primary quantitative analysis (group 1).5–11 All seven studies were retrospective cohort studies; three were from the United States and four from Europe. Our ability to compare certain patient characteristics and immunosuppression protocols across studies was limited by the variable reporting of these characteristics in the included reports. Demographic and transplant characteristics for included studies are summarized in Table 1. Notably, mean and median age of the patients included with DSA-SPA tended to be similar to the age of their comparative cohort without DSA in each individual study. The types of donors included in these studies varied; two studies included only deceased-donor transplants,5,10 one study included only living-donor transplants,8 three studies included both donor types,7,9,11 and one study did not describe the type of donors in the cohort.6
Table 1.
Characteristics of patient cohorts in the individual studies included in the analysis
| Study, Year | Patients (n) | Age (yr) | Women (%) | Black (%) | First Transplant (%) | Follow-up Period (mo) | Patients with Living Donors (%) |
|---|---|---|---|---|---|---|---|
| Group 1 | |||||||
| Patel et al., 20078 | 20 DSA+ | 48.6a | 45 | 35 | NA | 8.2a | 100 |
| 40 DSA− | 54.1a | 42.5 | 25 | NA | 11.1a | 100 | |
| Gupta et al., 20086 | 16 DSA+ | 50.1b | 60 | NA | 79 | NA | |
| 95 DSA− | 44 | 32d | NA | 91d | 12 and 60c | NA | |
| Vlad et al., 200910 | 13 DSA+ | 52b | 38% | NA | 80% | ||
| 265 DSA− | 48 | 0 | |||||
| Couzi et al., 20115 | 18 DSA+ | ||||||
| 39 DSA− | 46.57b | 44.40 | 0 | 34 | 32.52b | 0 | |
| Willicombe et al., 201111 | 45 DSA+ | 47.2b | 53.30 | 15.60 | 57.80 | 22.68b | 55.6 |
| 435 DSA− | 47.4b | 32.4d | 12.60 | 91.7d | 23.52b | 45.3 | |
| Verghese et al., 20109 | 10 DSA+ | 16.3b | 50 | 10 | 33.6b | 20 | |
| 72 DSA− | 15.1b | 45.80 | 5.60 | 39.6b | 43.1 | ||
| Higgins et al., 20117 | 23 DSA+ | 46.3b | 65.20 | NA | 57.00 | 39.6b | 87 |
| 28 DSA− | 44.2b | 53.60 | NA | 61 | 50 | ||
| Group 2 | |||||||
| Gibney et al., 200615 | 20 DSA+ | ||||||
| 35 DSA− | 48.9b,e | 62 Fe | 9e | 75e | 6b | 39e | |
| van den Berg-Loonen et al., 200818 | 13 DSA+ | 51a | 62 | NA | 54 | 96f | 0 |
| 14 DSA− | 51.5a | 64 | NA | 36 | 0 | ||
| Aubert et al., 200913 | 11 DSA+ | 42.8b | 73 | NA | NA | 12f | 36 |
| 102 DSA− | 46.6b | 26 | NA | NA | 40 | ||
| Amico et al., 200912 | 67 DSA+ | 47b | 57 | NA | 55 | 48 | |
| 267 DSA− | 52a | 31 | NA | 91 | 60 mof and 200 dg | 55 | |
| Mahmoud et al., 200917 | 16 DSA+ | 30.3b | 31.25 | NA | 100 | 100 | |
| 137 DSA− | 28.7b | 24.00 | NA | 100.00 | 3f | 100 | |
| Lefaucheur et al., 201016 | 76 DSA+ | NA | NA | NA | NA | 51.4b; 96f | 0 |
| 326 DSA− | NA | NA | NA | NA | 96f | 0 | |
| Dunn et al., 201119 | 46 DSA+ | 42.5b | 61 | 24i | 56 | 56a | 36.8 |
| 541 DSA− | 42.8–47.4b | 40.8 | 14.8 | 74 | 43.8–51a | 70 | |
| Caro-Orleas et al., 201214 | 103 DSA+ | 47a | 75.70 | NA | 60 | 62.5a | 0 |
| 780 DSA− | 48a | 37 | NA | 92 | 73.5a | 0 | |
| Group 3 | |||||||
| Bielmann et al., 200726 | 9 DSA+ | 48a | 67 | NA | 44 | 22 | |
| 56 DSA− | 53a | 36 | NA | 91 | 57 | ||
| Billen et al., 200927 | 32 DSA+ | 57 | 25 | NA | 78 | 6.9a | |
| 133 DSA− | 53 | 41 | NA | 82 | 7.5a | ||
| Morris et al., 201028 | 15 DSA+ | 100 | |||||
| 134 DSA− | 47.5b | 42 | 6 | 87.90 | 18h | 100 | |
| Group 4 | |||||||
| Vlad et al., 200910 | 14 DSA+ | 52b,e | 38% | NA | Majority | 0 | |
| 33 DSA− | 48 | 0 | |||||
| Higgins et al., 20117 | 44 DSA+ | 43.4b | 75 | NA | 30 | 93 | |
| 28 DSA− | 44.2b | 53.60 | NA | 61 | 39.6b | 50 |
NA, not available.
Median.
Mean.
Time intervals at which data are reported.
Estimate of percent female.
Data for entire cohort reported.
Maximum duration of follow-up for allograft survival.
Maximum duration of follow-up for AMR.
Minimum follow-up period.
Nonwhite.
Induction and maintenance regimens also varied widely across studies. Gupta et al. reported that no induction regimen was routinely used, whereas the other studies used antithymocyte globulin, alemtuzumab, or an IL-2 inhibitor (Table 2).5–11 Two of the seven studies consistently used a steroid-free maintenance regimen, including Willicombe et al., who maintained patients on tacrolimus monotherapy.10,11 Patel et al.8 and Verghese et al.9 maintained patients on a calcineurin inhibitor (CNI) and prednisone, whereas the Gupta, Couzi, and Higgins and colleagues used a triple-drug regimen that included a CNI, antimetabolite, and prednisone.5–7 Although the immunosuppression protocols used varied across studies, the cohorts positive and negative for DSA-SPA within each individual study received similar induction and maintenance immunosuppression regimens. No DSA-SPA–positive recipients received peri-transplant desensitization except for patients in the study by Higgins et al.7
Table 2.
Transplant-specific characteristics for patients included in the analysis
| Study, Year | Patients (n) | Desensitized | MFI Cutoff Positive DSA | Induction | Maintenance | Steroid-Free | No DSA Group Includes Pts with Other HLA Ab | Renal Function at Follow-up |
|---|---|---|---|---|---|---|---|---|
| Patel et al., 20078 | 20 DSA+ | No | NA | 20% none, 55% rATG, 15% CD25 monoclonal antibody, 10% alemtuzumab | 85% prednisone, 15% CSA, 85% FK | Some | ND | 1.3 |
| 40 DSA− | 1.1 | |||||||
| Gupta et al., 20086 | 16 DSA+ | No | NA | None | CSA, azathioprine, prednisone | No | Yes | 1.55 |
| 115 DSA− | 1.77 | |||||||
| Vlad et al., 200910 | 13 DSA+ | No | 1000±500 | rATG (6 mg/kg) | FK, MMF, steroids tapered on day 5 | Yes | ND | NA |
| 265 DSA− | ||||||||
| Couzi et al., 20115 | 11 DSA+ | No | >500 | rATG or daclizumab | MMF + CNI + steroids | No | ND | 1 yr eGFR, 59 |
| 34 DSA− | 1 yr eGFR, 66 | |||||||
| Willicombe et al., 201111 | 45 DSA+ | No | NA | Alemtuzumab | FK alone | Yes | ND | 3 yr Cr, 2.67 |
| 435 DSA− | 3 yr Cr, 1.41 | |||||||
| Verghese et al., 20109 | 10 DSA+ | No | NA | Daclizumab majority; alemtuzumab small minority | CNI/prednisone or some with FK + (MMF or rapamycin) | Some | ND | NA |
| 72 DSA− | ||||||||
| Higgins et al., 20117 | 23 DSA+ | Yes | >500 | basiliximab | FK + MMF + prednisone | No | ND | 3 yr eGFR, 46.9 |
| 28 DSA− | 3 yr eGFR, 56.7 | |||||||
| Gibney et al., 200615 | 20 DSA+ | No | NA | 52% rATG, 15% CD25 monoclonal antibody | 70% FK/rapamycin/prednisone, 30% CNI + (rapamycin or MMF) + prednisone entire cohort | No | ND | NA |
| 35 DSA− | ||||||||
| van den Berg-Loonen et al., 200818 | 13 DSA+ | No | NA | None | CNI + (azathioprine or MMF or rapamycin) + prednisone | No | ND | NA |
| 14 DSA− | ||||||||
| Aubert et al., 200913 | 11 DSA+ | No | >500 | Basiliximab: first kidney transplant; or rATG: retransplant or panel-reactive antibody>50% | FK + MMF + prednisone | No | Yes (44 patients) | NA |
| 102 DSA− | ||||||||
| Amico et al., 200912 | 67 DSA+ | No | >500 | 52% none, 39% basiliximab, 9% daclizumab | No | ND | NA | |
| 267 DSA− | ||||||||
| Mahmoud et al., 2009177 | 16 DSA+ | No | NA | None | Steroid + azathioprine + (CNI or rapamycin) | No | Yes | 3 yr Cr, 2.5 |
| 137 DSA− | 3 yr Cr, 1.5 | |||||||
| Lefaucheur et al., 201016 | 76 DSA+ | Yes | >300 (normalized) | rATG | CNI + MMF + steroids | No | Yes | NA |
| 326 DSA− | ||||||||
| Dunn et al., 201119 | 46 DSA+ | No | NA | rATG (4–6 mg/kg) | CNI + MPA | Yes | Yes | NA |
| 541 DSA− | >500 (baseline adjusted) | |||||||
| Caro-Orleas et al., 201214 | 103 DSA+ | No | >1500 (raw) | 1993–1996, rATG; 1997 onward: for high immunologic risk, rATG; for moderate immunologic risk, daclizumab or basiliximab | CNI + (azathioprine or MMF) + steroids | No | Yes | NA |
| 780 DSA− | ||||||||
| Bielmann et al., 200726 | 9 DSA+ | No | NA | rATG | FK + MMF + steroids | No | Yes | 1.1 |
| 56 DSA− | Basiliximab | Yes | 1.3 | |||||
| Billen et al., 200927 | 32 DSA+ | No | >1000 | No induction; 5 received basiliximab | 50% CNI (FK majority) + prednisolone; 50% receiving CNI + prednisolone + azathioprine/MMF/rapamycin | No | ND | NA |
| 133 DSA− | ||||||||
| Morris et al., 201028 | 15 DSA+ | No | NA | rATG | MMF, FK, prednisone | No | Yes | NA |
| 134 DSA− | ||||||||
| Vlad et al., 200910 | 14 DSA+ | No | 1000±500 | rATG (6 mg/kg) | FK, MMF, steroids tapered on day 5 | Yes | ND | NA |
| 265 DSA− | ||||||||
| Higgins et al., 20117 | 44 DSA+ | Yes | >500 | Basiliximab | FK + MMF + prednisone | No | ND | 3 yr eGFR, 54.2 |
| 28 DSA− | 3 yr eGFR, 56.7 |
Renal function reported as creatinine mg/dl unless otherwise stated; estimated GFR reported as ml/min. MFI, mean fluorescence intensity; NA, not applicable; CSA, cyclosporine A; ND, not discussed; rATG, rabbit antithymocyte globulin; FK, tacrolimus; MMF, mycophenolate mofetil; CNI, calcineurin inhibitor; eGFR, estimated GFR; Cr, creatinine.
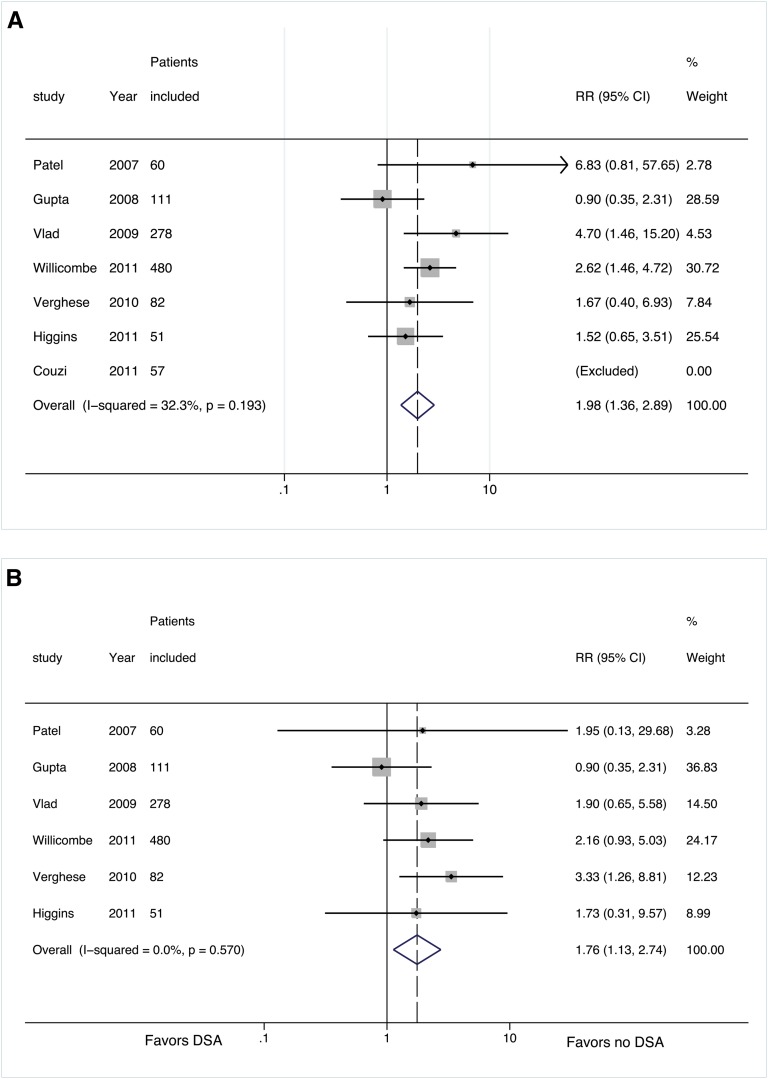
The seven retrospective cohort studies included in our primary analysis (group 1, flow crossmatch negative) represented a total of 1119 patients, of whom 145 were DSA-SPA positive.5–11 We excluded Couzi and colleagues’ study from the analysis because no rejection episodes occurred in either comparison group during the follow-up period (Figure 2). Further, Couzi et al. did not report specific graft survival data for their groups beyond stating that there was no statistical difference precluding their inclusion in the pooled analysis.5 Although the increased relative risk (RR) of developing antibody-mediated rejection (AMR) in DSA-SPA–positive patients reached statistical significance in only two of the individual studies,10,11 the pooled incidence of AMR across the six included studies was significantly higher among patients with preformed DSA-SPA (35 of 145 [25%]) than patients without DSA (110 of 974 [11.2%]) (RR, 1.98; 95% confidence interval [CI], 1.36–2.89; P<0.001) (Figure 2A, and Table 2). With the exception of a single study, individual studies did not show a statistically significant higher risk for graft failure in patients with preformed DSA-SPA.9 The incidence of graft failure across all included studies was 15% (22 of 145) in the DSA-SPA–positive group and 9.4% (92 of 974) in the DSA-negative group despite the relatively short (and variable) follow-up period; the pooled analysis demonstrates a significant decrease in graft survival in the presence of preformed DSA-SPA (RR, 1.76; 95% CI, 1.13–2.74; P=0.01) (Figure 2B and Table 2).
Figure 2.
Forest plots for risk stratified by flow crossmatch results for group 1. (A) Risk of AMR with and without DSA. (B) Risk of graft failure with and without DSA. For all forest plots, the solid line represents the null effect. The dotted line represents the summary effect for the studies included in the analysis. The shaded boxes are proportional to the relative weight of the studies used in each analysis. The center dot within the box represent the point estimate for that study and the horizontal line passing through each square represents the confidence interval of each study point estimate. The center of the diamond represents the summary effect and the lateral tips of the diamond represent the confidence intervals of the summary estimate.
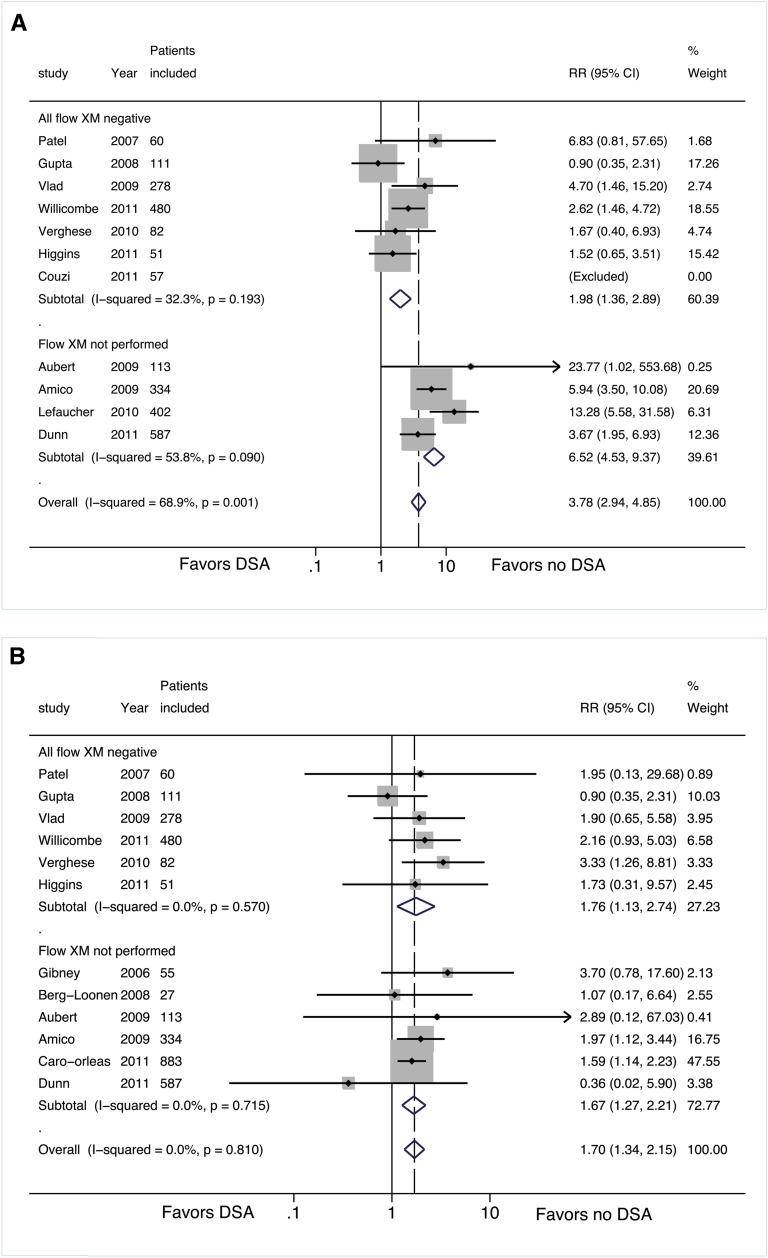
We also identified nine studies in which flow cytometry crossmatch was not performed but comparative graft outcomes data in DSA-SPA–positive versus DSA-negative patients was reported with a negative T and B cell CDC crossmatch (group 2).12–20 These studies were excluded from our primary analysis. A quantitative analysis of this cohort (group 2) was limited to eight studies because adequate data could not be extracted from one of the studies identified for inclusion in this analysis.20 As was seen in the studies included in our primary analysis (group 1, negative flow crossmatch), all eight studies in group 2 (unknown flow crossmatch) were retrospective cohort studies in which the age of patients included with DSA-SPA was similar to the age of their comparative cohort without DSA in each individual study.12–19 The types of donors included in these studies varied; three studies included only deceased-donor transplants,14,16,18 one study included only living-donor transplants,17 and four studies included both types of donors.12,13,15 Induction regimens varied considerably across studies. Two studies reported the absence of a standard induction regimen,17,18 whereas the other studies variably used antithymocyte globulin or an IL-2 receptor blocker for induction therapy at the time of transplantation (Table 2). None of the studies included in group 2 consistently used a steroid-free maintenance regimen for their cohort.12–18 The cohorts positive and negative for DSA-SPA within the individual studies received similar induction and maintenance regimens, and only a single study included patients with DSA-SPA who received peri-transplant desensitization.16
The eight retrospective cohort studies in group 2 represented 1967 patients, of whom 306 (15.6%) were DSA-SPA positive (Table 1). Only three of these studies reported data on rejection episodes, and the pooled incidence of AMR across these three studies was significantly higher in patients with preformed DSA-SPA (62 of 154 [40%]) than patients without DSA (23 of 695 [3.3%]) (RR, 7.81; 95% CI, 5.03–12.12; P<0.001) (Figure 3A and Supplemental Table 1A). Of the three studies that reported rejection episodes, two showed a significantly higher risk for graft failure among patients with preformed DSA-SPA.12,14 Among the eight studies in group 2, two13,17 were excluded from the pooled analysis for graft failure because the rate of graft failure was not reported. Graft failure across the studies in group 2 was significantly more frequent in the DSA-SPA–positive group (56 of 306 [18.3%]) than in the DSA-negative group (174 of 1661 [10.5%]) (RR, 1.74; 95% CI, 1.38–2.21, P<0.001) (Figure 3B and Supplemental Table 1B). Although no significant heterogeneity was noted when studies in group 1 and group 2 were pooled for analysis of risk for graft failure (I2 = 0%; P=0.847, Figure 3B), heterogeneity was significant in the combined pooled analysis of risk for biopsy-proven AMR (I2 = 72.2%; P<0.001, Figure 3A).
Figure 3.
Forest plots for risk stratified by flow crossmatch results for groups 1 and 2. (A) Risk of AMR with and without DSA. (B) Risk of graft failure with and without DSA. For all forest plots, the solid line represents the null effect. The dotted line represents the summary effect for the studies included in the analysis. The shaded boxes are proportional to the relative weight of the studies used in each analysis. The center dot within the box represent the point estimate for that study and the horizontal line passing through each square represents the confidence interval of each study point estimate. The center of the diamond represents the summary effect and the lateral tips of the diamond represent the confidence intervals of the summary estimate.
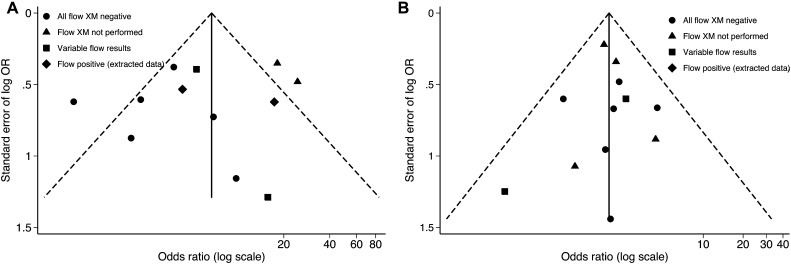
Finally, no significant publication bias was detected according to the Peters or the Begg test for studies that reported AMR (P=0.520 and 0.851, respectively, for group 1) and graft failure (P=0.937 and 0.573, respectively, for group 1). The funnel plot for publication bias is shown for studies that reported AMR and/or graft failure in the various groups (Figure 4A and 4B).
Figure 4.
Funnel plot to assess the influence of publication bias on the pooled analysis. OR, odds ratio; XM, crossmatch.
Discussion
The CDC crossmatch has definitively been shown to be a specific test for the detection of DSA and to be associated with immediate poor allograft outcomes.1 However, to date, the long-term prognostic value of the highly sensitive SPA for DSA has remained inconclusive.4 Our pooled analysis of the seven retrospective cohort studies comparing groups that were positive and negative for DSA-SPA in the setting of a negative CDC and negative flow crossmatch result demonstrates a statistically significantly increased risk for biopsy-proven AMR and allograft failure in the DSA-SPA–positive group, despite the relatively short follow-up period of most studies included in the analysis (Table 1). Our results suggest that preformed DSA-SPA are clinically significant even in the absence of a positive flow cytometery crossmatch and indicate an immunologically higher-risk transplant recipient. As a result, these patients should be monitored closely for development of early AMR and appear to be at risk for worse graft outcomes in the short to intermediate term.
The meta-analysis of eight retrospective cohort studies (group 2) comparing DSA-SPA–positive and –negative patients by SPA in the setting of a negative CDC crossmatch result but with unknown results on flow crossmatch (because it was not performed) showed an even greater significant increase in the likelihood of developing a biopsy-proven AMR episode and allograft failure in the DSA-SPA–positive group. These studies were excluded from the group 1 cohort because they probably include patients who are flow crossmatch positive (if they were to be tested) and as a result reflect a group at heterogeneous immunologic risk group compared with recipients who have a definite negative flow crossmatch result. This cohort (group 2) demonstrated a much higher risk for AMR (RR, 7.81; 95% CI, 5.029–12.122; P<0.001) consistent with our hypothesis that this group includes some higher-immunologic-risk patients. In addition, the fact that significant statistical heterogeneity was detected when we attempted to combine studies in group 1 and 2 but no significant heterogeneity was found in group 1 or group 2 individually supported our a priori stratification of patient cohorts based on the results (or absence thereof) of the flow crossmatch. The presence of DSA-SPA for studies included in group 2 was also associated with relatively early graft failure (RR, 1.737; 95% CI, 1.317–2.293; P<0.001) but appeared to be similar to the pooled analysis of group 1.
The absence of statistical heterogeneity upon pooling studies for risk of graft failure suggests that although positive flow crossmatch results are more useful in identifying patients at high risk of AMR, they do not appear to improve the risk stratification that occurs with the identification of DSA-SPA for overall graft survival in the short term. These results demonstrate the relative value of these two testing modalities in the stratification of immunologic risk; the addition of a flow cytometry crossmatch test improves the specificity for identifying patients at risk of developing AMR. However, the risk identified by flow crossmatch improves but does not appear to help refine the risk of graft failure in the short term. As a result, we suggest that an effective means of risk stratification at the time of transplant would be the following sequence of immunologic testing: CDC crossmatch followed by SPA for DSA identification and then followed by flow crossmatch testing only if further stratification for risk of AMR is desired. Unfortunately, our pooled analysis was unable to discern trends in risk associated with specific HLA antibodies because these were identified relatively infrequently in the individual studies. In addition, when mentioned, there was little uniformity in the description of the class of antigen directed against or the mean fluorescence intensity.
To our knowledge, our study represents the first attempt to systematically review the literature for the influence of preformed DSA-SPA on the risk of rejection and renal allograft outcomes. Careful risk stratification of our included studies by the reported flow cytometry crossmatch results allowed us to pool patients of similar immunologic risk into groups and demonstrates that with adequate power, the detrimental effects of DSA-SPA can be observed. This stratification also allows us to have greater confidence that the relatively similar (low) concentrations (mean fluorescence intensity) of DSA in the flow-negative studies (group 1) were similar. These results refute the assertion by some authors that low DSA levels with a negative flow cytometry crossmatch result at the time of transplantation do not suggest a high-immunologic-risk transplant recipient with a greater risk of earlier failure. Our results also support the suspicion that negative studies in the literature were underpowered or, in the case of graft outcomes, had follow-up durations inadequate for demonstrating a difference. We noted large variations in the duration of follow-up in the individual studies that reported graft outcomes, and this is likely to have a direct effect on the graft failure rates reported. This is an important limitation to note in terms of interpreting our point estimate for the relative risk of graft failure in the presence of DSAs.
Our analysis has several limitations related to the reviewed literature. Immunosuppression protocols—with respect to both induction agents and maintenance immunosuppressive protocols—varied considerably across the included studies and in some cases within individual studies (Table 1). The considerable variation noted precludes us from comparing the risk of AMR or failure with any specific protocol. Further work is needed to determine whether a characteristic or threshold level of DSA detected by SPA alone is most clinically predictive of the development of AMR and/or allograft failure. Standardization of assays, which would ensure greater uniformity in how results in the field are reported, would allow the improvement or development of an evidence base to inform clinical care. Given the widespread use of survival analysis in reporting outcomes after transplantation, uniform implementation of the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines and greater transparency in the results with the use of reporting tools for such outcomes as at-risk tables with survival curves, tabular presentation of the Kaplan-Meier estimates, and the use of life tables with standard time points should be encouraged if not required.21,22 Finally, the risk of graft failure at least partly depends on how individuals with AMR were treated; inadequate information about treatment and response to treatment in the pooled studies limits our ability to comment on this.
In conclusion, our pooled analysis demonstrates the significant elevated risk for early acute AMR and inferior graft outcomes associated with the presence of DSA-SPA despite negative flow crossmatch results. These findings suggest the need for increased vigilance, perhaps with protocol biopsies, consideration of desensitization, and augmented immunosuppression induction/maintenance regimens even in patients with DSA detected by SPA with a negative crossmatch—and perhaps more so in patients with DSAs detected by SPA at centers that have forgone flow crossmatch testing.
Concise Methods
Search Strategy
We searched PubMed using the following search phrase “(antibodies OR solid-phase assay OR Luminex® OR antibody mediated rejection) AND kidney AND crossmatch.” A similar search was performed in the Cochrane database. In addition, we hand-searched archived abstracts presented at the American Society of Nephrology and the American Transplant Congress meetings between 2005 and 2010 for reported studies that were not subsequently published as full manuscripts in the peer reviewed literature. For completeness, we also reviewed all references listed in the full-text publications included in our meta-analysis that were not identified by our search criteria.
Study Selection and Data Extraction
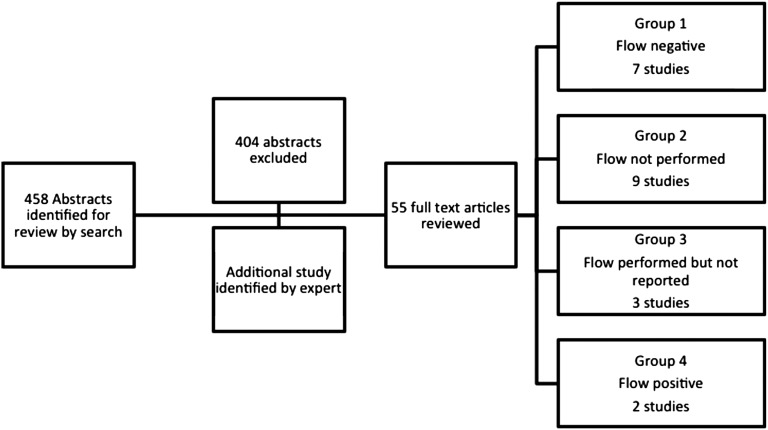
In the first step, all abstracts of the 458 potentially relevant citations identified by our search were reviewed (Figure 1). We excluded unrelated articles. We reviewed the full text of the 54 remaining citations that possibly met our inclusion criteria. Any study that compared data between DSA-positive and -negative groups according to the Luminex solid-phase assay (DSA-SPA) on biopsy-proven (antibody-mediated) rejection episodes and/or allograft survival rates among adult renal transplant patients was included in our analysis. Studies that did not explicitly report a negative current T and B cell complement–dependent cytotoxicity and T cell flow cytometry crossmatch (n=47) for their cohort were excluded from our primary cohort (group 1) for analysis, resulting in a final group of seven studies (Figure 2, A and B, and Table 3). For reports that did not include the requisite data in the published manuscript, we attempted to contact corresponding authors to obtain additional information (Figure 1). An additional 14 studies reporting comparative graft outcomes data in DSA-SPA–positive versus DSA-negative patients with negative current T and B cell CDC crossmatch were identified. Although these were not part of our group 1 analysis, they were included for additional analyses. An additional study was brought to our attention by an expert.19 These additional studies were grouped into one of the following categories: (1) flow cytometry crossmatch not performed (group 2); (2) flow crossmatch performed, with reported results that did not differentiate patients by the results of the flow crossmatch (group 3); and (3) flow performed selectively in high-risk patients, with negative results (group 4). We have included pooled analyses of cohorts that were included in group 1 and group 2 for AMR and graft loss (Figure 3, A and B, and Supplemental Table 1, A and B). Analysis of data extracted from studies included in groups 2 through 4 are provided in the supplemental material. Data extracted from the studies, including patient demographic characteristics, allograft characteristics, induction and maintenance regimens, rates of AMR episodes, and graft survival when available, are shown in Tables 1 and 2.
Figure 1.
Flow diagram of search results and identification of studies included in the analysis.
Table 3.
RRs and tests of heterogeneity for the pooled analysis for AMR and graft failure rates for group 1
| Study, Year | RR (95% CI) | Weight (%) | z Statistic | P Value | I2 Statistic (%) | P Value |
|---|---|---|---|---|---|---|
| AMR | ||||||
| Patel et al., 20078 | 6.833 (0.81–57.652) | 2.78 | ||||
| Gupta et al., 20086 | 0.904 (0.354–2.308) | 28.59 | ||||
| Vlad et al., 200910 | 4.705 (1.456–15.202) | 4.53 | ||||
| Willicombe et al., 201111 | 2.620 (1.456–4.718) | 30.72 | ||||
| Verghese et al., 20109 | 1.667 (0.401–6.935) | 7.84 | ||||
| Higgins et al., 20117 | 1.515 (0.654–3.512) | 25.54 | ||||
| Couzi et al., 20115 | Excluded | |||||
| Group 1 pooled RR | 1.984 (1.364–2.885) | 100 | 3.58 | <0.001 | 32.3 | 0.19 |
| Graft failure rates | ||||||
| Patel et al., 20078 | 1.952 (0.81–57.652) | 3.28 | ||||
| Gupta et al., 20086 | 0.904 (0.354–2.308) | 36.83 | ||||
| Vlad et al., 200910 | 1.901 (1.456–15.202) | 14.50 | ||||
| Willicombe et al., 201111 | 2.165 (1.456–4.718) | 24.17 | ||||
| Verghese et al., 20109 | 3.333 (0.401–6.935) | 12.23 | ||||
| Higgins et al., 20117 | 1.731 (0.654–3.512) | 8.99 | ||||
| Group 1 pooled RR | 1.759 (1.128–2.743) | 100 | 2.49 | 0.013 | 0 | 0.57 |
Bold indicates pooled estimates.
Outcome Measures
We identified two primary outcomes a priori for our analysis: the RR of developing (1) an AMR episode and (2) graft failure during the follow-up period.
Quantitative Data Synthesis
Treatment effects were summarized as RRs with 95% CIs using the metan function in Stata software, version 11.2 (Stata Corp., College Station, TX). The heterogeneity statistic (Q) and I2 values were also estimated by the metan function on the basis of the work of Higgins and Thompson, and, when appropriate, stratification of data was used to try to assess the cause for heterogeneity. For the purposes of our analysis, we used both the Mantel-Haenszel fixed-effects method as well as the DerSimonian and Laird random-effects method (1986), which incorporates an estimate of between-study variation (i.e., heterogeneity). Given the very similar results, we have reported the results of the Mantel-Haenszel fixed-effects method that resulted in slightly more conservative pooled ratios than the random-effects model (1.984 versus 2.073 for risk of AMR and 1.759 versus 1.864 for risk of graft failure) for our primary analysis. Publication bias was tested separately for reports of AMR and graft overall graft outcomes according to use of both the Begg adjusted rank correlation test and the Peter test; in addition, these were depicted graphically using a funnel plot (Figure 4).23–25 Given the variability of the study sizes, a sensitivity analysis was also performed to ensure that the results of our analysis were not being influenced by any one trial (data not shown).
Disclosures
None.
Supplementary Material
Acknowledgments
Parts of this analysis were presented at the 2011 meeting of the American Society of Nephrology.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012070664/-/DCSupplemental.
References
- 1.Patel R, Terasaki PI: Significance of the positive crossmatch test in kidney transplantation. N Engl J Med 280: 735–739, 1969 [DOI] [PubMed] [Google Scholar]
- 2.Karpinski M, Rush D, Jeffery J, Exner M, Regele H, Dancea S, Pochinco D, Birk P, Nickerson P: Flow cytometric crossmatching in primary renal transplant recipients with a negative anti-human globulin enhanced cytotoxicity crossmatch. J Am Soc Nephrol 12: 2807–2814, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Vaidya S, Partlow D, Susskind B, Noor M, Barnes T, Gugliuzza K: Prediction of crossmatch outcome of highly sensitized patients by single and/or multiple antigen bead luminex assay. Transplantation 82: 1524–1528, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Segev D, Wang G, Gloor J, Stegall M, Kapur S, Dunn T, Pelletier R, Singh P, Posner M, Shapiro RE-A: JM, Light, J, Marsh, C, Melancon, J, Lipkowitz, G, Wellen, J, Oberholzer, J, Montgomery, R: Risks of HLA incompatible kidney transplants by antibody strength: Initial results from a National study of 603 patients [Abstract]. American Transplant Congress, Philadelphia, PA, April 30–May 4, 2011 [Google Scholar]
- 5.Couzi L, Araujo C, Guidicelli G, Bachelet T, Moreau K, Morel D, Robert G, Wallerand H, Moreau JF, Taupin JL, Merville P: Interpretation of positive flow cytometric crossmatch in the era of the single-antigen bead assay. Transplantation 91: 527–535, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Gupta A, Iveson V, Varagunam M, Bodger S, Sinnott P, Thuraisingham RC: Pretransplant donor-specific antibodies in cytotoxic negative crossmatch kidney transplants: Are they relevant? Transplantation 85: 1200–1204, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Higgins R, Lowe D, Hathaway M, Williams C, Lam FT, Kashi H, Tan LC, Imray C, Fletcher S, Chen K, Krishnan N, Hamer R, Daga S, Edey M, Zehnder D, Briggs D: Human leukocyte antigen antibody-incompatible renal transplantation: Excellent medium-term outcomes with negative cytotoxic crossmatch. Transplantation 92: 900–906, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Patel AM, Pancoska C, Mulgaonkar S, Weng FL: Renal transplantation in patients with pre-transplant donor-specific antibodies and negative flow cytometry crossmatches. Am J Transplant 7: 2371–2377, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Verghese PS, Smith JM, McDonald RA, Schwartz SM, Nelson KA, Warner PR: Impaired graft survival in pediatric renal transplant recipients with donor-specific antibodies detected by solid-phase assays. Pediatr Transplant 14: 730–734, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Vlad G, Ho EK, Vasilescu ER, Colovai AI, Stokes MB, Markowitz GS, D’Agati VD, Cohen DJ, Ratner LE, Suciu-Foca N: Relevance of different antibody detection methods for the prediction of antibody-mediated rejection and deceased-donor kidney allograft survival. Hum Immunol 70: 589–594, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Willicombe M, Brookes P, Santos-Nunez E, Galliford J, Ballow A, Mclean A, Roufosse C, Cook HT, Dorling A, Warrens AN, Cairns T, Taube D: Outcome of patients with preformed donor-specific antibodies following alemtuzumab induction and tacrolimus monotherapy. Am J Transplant 11: 470–477, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Amico P, Hönger G, Mayr M, Steiger J, Hopfer H, Schaub S: Clinical relevance of pretransplant donor-specific HLA antibodies detected by single-antigen flow-beads. Transplantation 87: 1681–1688, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Aubert V, Venetz JP, Pantaleo G, Pascual M: Low levels of human leukocyte antigen donor-specific antibodies detected by solid phase assay before transplantation are frequently clinically irrelevant. Hum Immunol 70: 580–583, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Caro-Oleas JL, González-Escribano MF, González-Roncero FM, Acevedo-Calado MJ, Cabello-Chaves V, Gentil-Govantes MA, Núñez-Roldán A: Clinical relevance of HLA donor-specific antibodies detected by single antigen assay in kidney transplantation. Nephrol Dial Transplant 27: 1231–1238, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Gibney EM, Cagle LR, Freed B, Warnell SE, Chan L, Wiseman AC: Detection of donor-specific antibodies using HLA-coated microspheres: Another tool for kidney transplant risk stratification. Nephrol Dial Transplant 21: 2625–2629, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Lefaucheur C, Loupy A, Hill GS, Andrade J, Nochy D, Antoine C, Gautreau C, Charron D, Glotz D, Suberbielle-Boissel C: Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J Am Soc Nephrol 21: 1398–1406, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahmoud KM, Ismail AM, Sheashaa HA, Gheith OA, Kamal MM, Ghoneim MA: Value of donor-specific antibody detection in first-graft renal transplant recipients with a negative complement-dependent cytotoxic crossmatch. Exp Clin Transplant. 7: 124–128, 2009 [PubMed] [Google Scholar]
- 18.van den Berg-Loonen EM, Billen EV, Voorter CE, van Heurn LW, Claas FH, van Hooff JP, Christiaans MH: Clinical relevance of pretransplant donor-directed antibodies detected by single antigen beads in highly sensitized renal transplant patients. Transplantation 85: 1086–1090, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Dunn TB, Noreen H, Gillingham K, Maurer D, Ozturk OG, Pruett TL, Bray RA, Gebel HM, Matas AJ: Revisiting traditional risk factors for rejection and graft loss after kidney transplantation. Am J Transplant 11: 2132–2143, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otten HG, Verhaar MC, Borst HP, Hené RJ, van Zuilen AD: Pretransplant donor-specific HLA class-I and -II antibodies are associated with an increased risk for kidney graft failure. Am J Transplant 12: 1618–1623, 2012 [DOI] [PubMed] [Google Scholar]
- 21.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative : The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. PLoS Med 4: e296, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lang TA, Secic M: Assessing time-to-event as an endpoint. Reporting survival analyses. In: How to Report Statistics in Medicine: Annotated Guidelines for Authors, Editors, and Reviewers, 2nd Ed, Philadelphia, ACP Press, 2006, pp 490. [Google Scholar]
- 23.Begg CB, Mazumdar M: Operating characteristics of a rank correlation test for publication bias. Biometrics 50: 1088–1101, 1994 [PubMed] [Google Scholar]
- 24.Harbord RM, Egger M, Sterne JA: A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med 25: 3443–3457, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Sterne JA, Harbord RM: Funnel plots in meta-analysis. Stata J 4: 127–141, 2004 [Google Scholar]
- 26.Bielmann D, Hönger G, Lutz D, Mihatsch MJ, Steiger J, Schaub S: Pretransplant risk assessment in renal allograft recipients using virtual crossmatching. Am J Transplant 7: 626–632, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Billen EV, Christiaans MH, van den Berg-Loonen EM: Clinical relevance of Luminex donor-specific crossmatches: Data from 165 renal transplants. Tissue Antigens 74: 205–212, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Morris GP, Phelan DL, Jendrisak MD, Mohanakumar T: Virtual crossmatch by identification of donor-specific anti-human leukocyte antigen antibodies by solid-phase immunoassay: A 30-month analysis in living donor kidney transplantation. Hum Immunol 71: 268–273, 2010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.