Abstract
Postoperative acute renal failure (ARF) is a serious complication which can result in a prolonged hospital stay and a high mortality and morbidity. Underlying renal disease, cardiac diseases, nephrotoxin exposure and renal hypoperfusion are the possible predisposing risk factors which can create a high probability for the development of ARF. The incidence of ARF is highest after major vascular, cardiac and high-risk thoraco-abdominal surgery. Among the various renal protection strategies, adequate peri-operative volume expansion and avoidance of hypovolemia is the most accepted and practiced strategy. Numerous bio-markers of renal injury are used to estimate the presence and extent of renal insult and various new are currently under trial. Traditional pharmacological interventions like dopamine, diuretics and calcium antagonists are not currently the first line of reno-protective agents. The new non-pharmacological and pharmacological methods may improve outcome in renal transplantation, contrast-induced nephropathy and in various other settings of ARF. The current review is an attempt to refresh the knowledge and put forth the various renal protection strategies during the peri-operative period.
Keywords: Acute renal failure, acute tubular necrosis, nephrotoxins, peri-operative renal protection strategies, renal protection
INTRODUCTION
In the recent past cardiac and pulmonary diseases have been given due priority by the anesthesiologists around the world whenever such patients present for any surgical procedure. Less research is being carried out presently in this arena but one cannot attribute it to more research in other areas. In the last three decades peri-operative acute renal failure (ARF) remains a serious complication, resulting in high morbidity and mortality rates.[1] Very few research studies have been carried out to establish the predictors of renal injury and their management. Acute tubular necrosis accounts for 20–25% of all cases of hospital-acquired renal failure.[2] Renal dysfunction after surgery may result in a mortality of up to 60%.[2] The chances of full recovery i.e. without leading to development of chronic renal failure in surgical settings are only 15%.[2] The most important elements in preserving renal function are peri-operative optimization of hemodynamics and intravascular volume and avoidance or cautious use of drugs and nephrotoxins.[2]
The need for revisiting these renal protection strategies was felt as the renal protection provided during the intra-operative period carries on to the postoperative period as well. The strategies are of immense significance to the surgeons also, as they have to manage such patients during the postoperative period. However, in a tertiary care center, renal protection is a multidisciplinary task by the involvement of various specialties during the peri-operative and postoperative period. For the present article a PUBMED and MEDLINE search was conducted using keywords: acute renal failure, acute tubular necrosis, renal protection, nephrotoxins, peri-operative renal protection strategies, and so on. Apart from indexed journals, peer-reviewed non-indexed journals were also analyzed. In-depth analysis was carried out by assessing the titles, abstracts, and/or the full-text papers retrieved from the electronic database, and manual searches for possible inclusion according to the predefined selection criteria.
Definition
ARF is defined as rapid decline in renal function (within 48 h) resulting in accumulation of nitrogenous waste products mainly blood urea nitrogen (BUN) and creatinine in blood. The quantitative estimation of serum creatinine is a universally accepted marker of renal failure and is indicated by a rise in serum creatinine ≥ 0.3 mg/dl or a rise of 1.5-fold value from the baseline.[3]
Clinical grading of ARF
An international interdisciplinary collaborative group, the acute dialysis qualitative initiative (ADQI)[4] has recently formulated a standard grading system for acute renal dysfunction. [Table 1]
Table 1.
The RIFLE classification of acute renal dysfunction
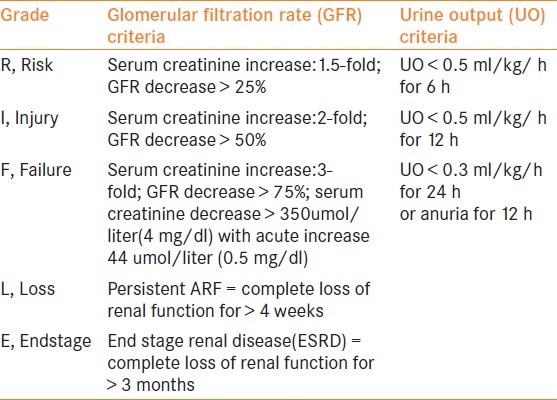
The acronym RIFLE defines three grades of increasing severity of acute renal dysfunction.[4]
R- Risk, I – Injury, F – Failure and two outcome variables L- Loss and E – End stage that are based on the changes in serum creatinine or urine output.
Etiology
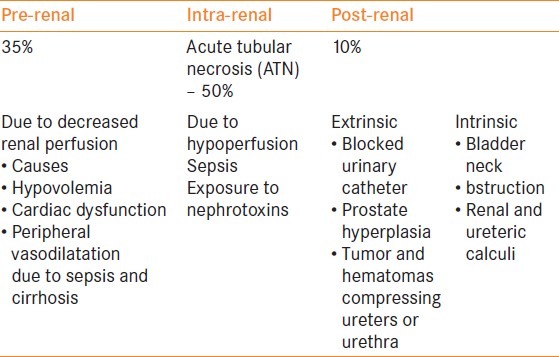
Renal failure is caused by a multitude of mechanisms and can be summarized as pre-renal, renal and post-renal failure. The causes can be classified as pre-renal, renal and post-renal [Table 2].
Table 2.
The etiological factors of acute renal failure [5]
Drugs with nephrotoxic potential[5]
Many of the drugs and chemicals can act as nephrotoxins in an already compromised renal status. The mechanism of nephrotoxicity varies for individual drugs and a detailed discussion is beyond the purview of the present article. However, many of the strategies preventing nephrotoxicity of these drugs have been explained later in the article. Great care has to be taken while administering these therapeutic agents in patients considered being at risk of possible development of renal failure. These include:
Antibiotics – aminoglyocosides, cephalosporins, amphotericin-B, penicillin sulphonamides
Calcium (hypercalcemia)
Chemotherapeutic/immunosuppressive agents–cisplatin, cyclosporine, tacrolimus, methotrexate, nitrosourea
Contrast agents
Non-steroidal anti-inflammatory
Pigments – hemoglobin myoglobin
Pathophysiology of acute tubular necrosis
Among the renal causes of acute renal failure, acute tubular necrosis (ATN) is the commonest cause and as such deserves a special mention here.[5] Clinically, the course of ischemic ATN is characterized by four phases[6] which can be briefly described as:
Initiation phase: This is the first phase of ATN and may last from a few hours to days and is characterized by decreased renal blood flow (RBF) leading to decreased GFR and finally decreased flow of filtrate in tubules due to obstruction by casts composed of shed epithelial cells and necrotic debris. The end results of these pathological changes cause back leak of glomerular filtrate through injured tubular epithelium. The prolonged duration of ischemia can be very detrimental to renal tissue and its consequences can be devastating [Figure 1].
Extension: It refers to a continuum of ischemic injury and inflammation of renal parenchyma and tubules.
Maintenance phase: This phase can last from one to two weeks and is characterized by intrarenal vasoconstriction and medullary ischemia mediated through tubulo-glomerular feedback and deregulated release of vasoactive substance from injured endothelial cells.
Recovery phase: It is characterized by tubular epithelial cells’ repair and regeneration as well as a gradual return of GFR towards pre-morbid level. It may take several days to weeks.
Figure 1.
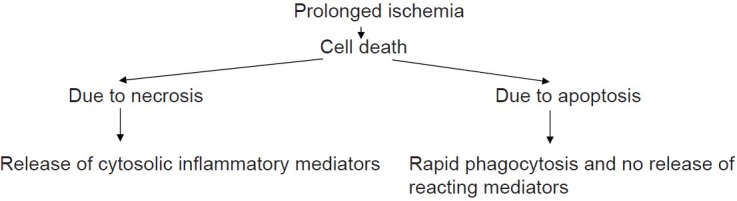
Prolonged ischemia
Predictors of renal failure in the peri-operative period
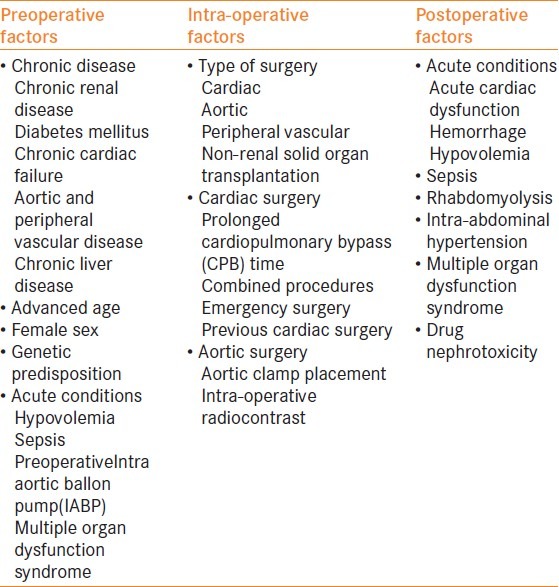
The predictors of renal injury in surgical patients have been observed by various research studies and can possibly be classified into preoperative, intra-operative and postoperative factors [Table 3].[2]
Table 3.
The various predisposing factors responsible for acute kidney injury during the preoperative, intra-operative and postoperative period
Besides, many patients are undergoing cardiac surgery nowadays. These are high-risk patients and are more prone to develop ARF during the peri-operative period. Several factors contribute to the development of ARF during cardiac and vascular surgery[2] and these include:
Systemic inflammatory response syndrome (SIRS) triggered by major surgery results in cell- mediated and cytotoxic injury.
During cardiopulmonary bypass (CPB) surgery, renal hypoperfusion outside the limits of auto regulatory reserve may cause development of ARF
ATN may be exacerbated by renal embolic injury due to thrombus, air, lipid and tissue; aortic atheroma may get disrupted by operative manipulation.
Renal excretion of haem derivatives produced by hemolysis in prolonged surgery may result in renal tubular injury.
Administration of large dose of contrast in endovascular surgery and in non-elective cardiac surgery results in toxic injury
Biomarkers of acute renal insult
Numerous biomarkers of renal injury are used to estimate the presence and extent of renal injury and failure and include:
-
Cystatin C[7]
A sensitive marker for GFR.
-
KIM – I (Kidney Injury Molecule I)[8,9]
A novel marker for human renal proximal tubule injury. Biomarker of acute kidney injury in renal transplant recipients.
-
Urine Interleukin (IL) – 18[10]
An early diagnostic marker for acute kidney injury and predicts mortality in the ICU.
-
NGAL (Neutrophil Gelatinase-associated Lipocalin)[11]
A marker for acute renal injury after cardiac surgery.
Strategies to prevent peri-operative development of acute renal failure
Numerous strategies and techniques have been used from time to time for prevention of acute renal insult during surgical procedures, especially during major surgeries where wide hemodynamic fluctuations and fluid shifts are common observations. The main objectives of these renal protection strategies can be summarized as:
To identify and optimize patients at risk.
To develop an appropriate anesthetic plan.
Peri-operative usage of sensitive and specific monitoring tools of renal functions.
To adopt efficacious interventions if renal function starts to deteriorate.
Further, these preventive strategies can be organized into two main types:
Primary prevention
Strategies to reduce the occurrence of renal injury in patients without evidence of acute renal dysfunction.
Secondary prevention
Avoidance of additional renal injury in the setting of established acute renal dysfunction
On a whole these strategies vary from application of non-pharmacological methods to administration of specific pharmacological agents and thus can be broadly classified into:
Non-pharmacological interventions
Pharmacological interventions
Non-pharmacological interventions
Various non-pharmacological methods of preventing renal insult include:[2]
Intravascular volume expansion – euvolemia
Maintenance of renal blood flow (RBF) and renal perfusion pressure
Avoidance of nephrotoxic agents
Strict glycemic control
Appropriate management of postoperative complications
Ischemic and pharmacological preconditioning.[5]
Intravascular volume expansion
Optimization of the hemodynamic status and correction of any volume deficit minimizes residual functional impairment of kidney. The newer terms are:
Volume-responsive acute kidney injury (AKI) (Pre-renal azotaemia)[12]
Volume-non-responsive AKI. Real hypovolemia is the most common cause of volume-responsive AKI.
-
Volume status monitoring
The intravascular volume status should be monitored with mean arterial pressure (MAP), urinary output, central venous pressure (CVP) and in certain rare high-risk circumstances pulmonary artery wedge pressure (PAWP) should be monitored as well. The goal of monitoring includes keeping these parameters within a specified targeted range [Table 4].[13,14]
-
Type of fluid therapy
It has been observed that intravenous hydration with saline was more effective than unrestricted oral fluid intake.[15] Controversies have always existed in literature about infusion of colloids and crystalloids. The dilemma still remains hugely unanswered.
Table 4.
The specified targeted range of various vital parameters during the peri-operative period

Colloids vs crystalloids?
The SAFE (Saline Versus Albumin Fluid Evaluation) study indicates that albumin is safe albeit not more effective than saline, for fluid resuscitation.[16] On the other side, hexaethyl starch (HES) has a negative effect on coagulation and the development of renal dysfunction is another major concern associated with its use. In a recent trial a ‘modern’ HES preparation with a low molecular weight and low molar substitution and a human albumin solution given in cardiac surgery patients with preoperative compromised kidney function showed that this type of HES solution had no negative influence on kidney integrity.[17] In a comprehensive Cochrane review there is some evidence that colloids (HES) may be associated with a higher incidence of AKI than Ringer's lactate in critically ill patients.[2]
Fluid choice in prevention of contrast induced nephropathy (CIN)
The sustained administration of isotonic saline before and after radiocontrast injection seems to be more protective than an equivalent volume of hypotonic saline and saline.[18] The incidence of CIN (defined as an increase of 25% of serum creatinine from baseline within 48 h) might decrease with intravenous (IV) sodium bicarbonate as was seen in recent trials as compared with saline.[19,20] It was noted that surgical patients receiving contrast benefited from the use of the lowest possible volume of non-ionic, iso-osmolar contrast in conjunction with isotonic IV fluids.[2]
Maintenance of renal blood flow and renal perfusion pressure
Renal blood flow depends on both cardiac output and systemic arterial pressure. Initial approach should be to reverse hypovolemia. Inotropic therapy should be started for management of low cardiac output. A vasopressor such as norepinephrine is an excellent first-line vasopressor agent. Vasopressin and Terlipressin may be useful agents in the treatment of postoperative catecholamine-resistant vasodilatory shock. Minimum MAP of 65–75 mm Hg is often targeted in clinical practice, however, a higher target may be necessary in patients with pre-existing hypertension.[2] It has been found that absolute hypotension (systemic blood pressure less than 90 mmHg is associated with development of AKI.[21] But in many patients the episodes of hypotension are absent. This form of AKI is called normotensive ARF[22,23] as auto-regulation of renal function is maintained at MAP 80–160 mm Hg. Hypotensive anesthesia is strictly contraindicated in CRF.
Avoidance of nephrotoxic drugs
Minimizing peri-operative exposure to nephrotoxic drugs is crucial in the prevention of ARF. Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) interfere with auto-regulation of renal blood flow and glomerular filtration rate.[24] Therefore, patients chronically treated with ACE inhibitors have an increased risk of postoperative renal dysfunction. Non-steroidal anti-inflammatory drugs (NSAIDs) causes acute inhibition of cyclooxygenase (Type I or II) and can reduce GFR as well as peripheral blood flow.[24] Antibiotics commonly associated with acute interstitial nephritis include Cephalosporins, Aminoglycosides and Vancomycin. Aminoglycosides like gentamicin can cause ATN and the use of once daily dosing reduces the incidence of tubular cell toxicity. Dosing should be based on creatinine clearance and peak and trough of drug levels.[25,26] Amphotericin B is also considered to be potentially nephrotoxic but its lipid formulations are less nephrotoxic. The use of aprotinin during coronary artery bypass grafting (CABG) surgery may be associated with an increased risk of ARF requiring dialysis.
Strict glycemic control
Strict glycemic control using intensive insulin therapy has improved survival and reduced the incidence of ARF requiring renal replacement therapy. Peri-operative hyperglycemia during cardiac and vascular surgery is associated with increased renal morbidity and overall mortality.[2]
Appropriate management of postoperative complications
Prompt diagnosis and management of acute cardiac dysfunction, hemorrhage, sepsis, rhabdomyolysis and intra-abdominal hypertension are essential to prevent development of ARF. Rhabdomyolysis is a devastating complication which can precipitate ARF and such patients should be treated with aggressive intravascular volume expansion, diuretic therapy and urinary alkanization. Intra-abdominal hypertension is associated with diminished renal perfusion and may precipitate ischemic ATN.[27] Timely recognition followed by decompresssive laparotomy may provide the optimal management.
Ischemic and pharmacological preconditioning[28]
The concept of preconditioning is based on the principle that short periods of injury (i.e. ischemia) followed by reperfusion temporarily increase the resistance to further ischemic damage. It has been shown to occur in several organ systems including the brain, spinal cord, liver and kidney. Volatile anesthetics are also capable of producing preconditioning. Anesthetic preconditioning also occurs in endothelial and smooth muscles cells, implying the possibility that anesthetic preconditioning could have beneficial effects on a number of different organs in protecting against ischemic injury, including the kidney. Its role as a therapeutic strategy to prevent the development of ARF requires laboratory and clinical investigation.
Cardiovascular surgeries have increased in recent times and so have the possibilities of acute renal injury in such patients. Numerous strategies can be of great help in reducing the incidence of possible acute renal injury in these patients. These include but are not limited to:
Cardiac surgery:
By limiting the duration of CPB
By maintaining adequate flow and perfusion pressure
Avoidance of excessive hemodilution
Avoidance of red cell transfusion
Extracorporal leucodepletion and hemofiltration
Off-pump surgery may theoretically offer renal protection[29]
Vascular surgery
endovascular aneurysm repair and open repair of abdominal aortic aneurysm, both are associated with worsening renal dysfunction in patients with pre-existing renal insufficiency.[30]
Pharmacological peri-operative renal protection strategies
Currently no drug therapy exists that has proven general protective properties against the development of ARF in the peri-operative settings. However, drugs used for renal protection [Table 5] are categorized as:
Table 5.
Various categories of reno-protective agents

Vasodilators
Vasodilators definitely have a very protective role in maintenance of renal tissue integrity by preserving the blood flow. These classes of agents can be classified further into various subgroups and their clinical characteristics include:
-
Dopamine agonist
-
-increases renal blood flow
-
-increases GFR
- -
Fenoldopam (selective dopamine I receptor)
-
-
-
Adenosine antagonists (theophylline)[39]
-
-these agents act by attenuation of the intra-renal vasoconstriction after the administration of radio-contrast media
-
-increase in GFR.
-
-
-
Calcium Channel Blockers[40]
-
-Induces preglomerular arteriolar dilatation leading to increased renal blood flow and GFR.
-
-
-
Sodium nitroprusside
-
-Its administration during the re-warming phase of CPB in patients undergoing CABG decreases the incidence of postoperative ARF.
-
-
Diuretics
Diuretics were once the most commonly used reno-protective agents and till date they are regarded as one the most common weapons to counter the insults of acute renal failure. They are classified according to their site and mechanism of action and include:
Natriuretic peptides – ANP, BNP[51]
Natriuretic peptides are used in renal protection because of their established protective role in preventing acute renal insult. The mechanism involved is - systemic and renal vasodilatation - inhibition of renal tubular sodium reabsorption - attenuating the activation of the rennin angiotensin aldosterone system - lowering the oxygen requirement in several nephrons.
Various natriuretic peptides are enumerated as under:
Anartide – synthetic analogue of ANP,[52] ularitide
rhANP – human recombinant ANP 50 ng/kg/min (decreases incidence of dialysis)
-
rh BNP (Nesritide) - 0.1ug/kg/min
-
-has a role in the prevention of AKI in heart failure and cardiac surgery[53]
-
-
-
Vessel dilator[54]
-
-recently identified cardiac peptide of the ANP family - ameliorates ischemic ATN with regeneration of the brush borders of proximal tubules - induces endogenous production of PGE2 - causes maintenance of glomerular hemodynamic blood flow redistribution to the medulla and improvement of microvascular permeability.
-
-
Antioxidants
The role of antioxidants is becoming popular nowadays as an active component in renal protection strategies during surgical intervention. These include N-acetyl cysteine (NAC), statins as well as corticosteroids. The mechanism of action of individual antioxidants is summarized as:
-
N acetyl cysteine[55]
-
-It is used in prevention of CIN and AKI post cardiac surgery.[56]
-
-CIN–contrast induced nephropathy–is defined as an increase in serum creatinine occurring within the first 24 h after contrast exposure and peaking up to five days afterwards. - the dose of NAC is 600 mg BD. - It decreases serum creatinine without affecting GFR by activating creatinine kinase and by increasing tubular secretion of creatinine.[57]
-
-
Statins: the effect of statins include - improvement of endothelial dysfunction - increased nitric oxide bioavailability - antioxidant properties - inhibitions of inflammatory responses.
It has been shown that patients who continued on statins during percutaneous coronary intervention (PCI) and CABG had lower rates of AKI and also lower incidence of CIN.[58]
Other agents
Other agents which have shown promise in providing renal protection during the peri-operative period include
Growth Factors - these promote repair of tubular renal cells after sublethal or nephrotoxic damage- have antiapoptotic effects - these growth factors include epidermal growth factors, insulin-like growth factor I, hepatocyte growth factor, bone morphogenetic protein 7.
-
Erythropoietin - it has tissue protective effects and prevents tissue damage during ischemia and inflammation.[24]
CONCLUSION
Peri-operative ARF is a major cause of morbidity and mortality. The incidence can be decreased by identifying the known risk factors like underlying medical illness, avoidance of renal hypoperfusion and nephrotoxin exposure. Traditional pharmacological interventions like dopamine, diuretics and calcium antagonists are not currently proposed drugs of choice. Mannitol and calcium may improve outcome in renal transplantation patients. Mannitol is effective in rhabdomyolysis. NAC has proven effective in CIN. Fenoldopam, vessel dilators and ANP have shown good results in prevention and treatment of ARF. The clinical efficacy of vessel dilators, growth factors, and preconditioning maneuvers are under trial and need to be assessed.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Mehta RL, Chertow GM. Acute renal failure definitions and classification: Time for change? JAmSoc Nephrol. 2003;14:2178–87. doi: 10.1097/01.asn.0000079042.13465.1a. [DOI] [PubMed] [Google Scholar]
- 2.Stephen T, Webb J, Stephen D, Allen Perioperative renal protection. Contin Educ Anaesth Crit Care Pain. 2008;8:176–80. [Google Scholar]
- 3.Tian J, Barrantes F, Amoateng-Adjepong Y, Manthous CA. Rapid Reversal of Acute Kidney Injury and Hospital Outcomes: A Retrospective Cohort Study. Am J Kidney Dis. 2009;53:974–81. doi: 10.1053/j.ajkd.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P Acute Dialysis Quality Initiative workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarnberg PO. Renal protection strategies in the perioperative period. Best Pract Res Clin Anaesthesiol. 2004;18:645–60. doi: 10.1016/j.bpa.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Lameire N, Van Biesen W, Vanholder R. Acute renal failure. Lancet. 2005;365:417. doi: 10.1016/S0140-6736(05)17831-3. [DOI] [PubMed] [Google Scholar]
- 7.Dworkin LD. Serum cystatin C as a marker of glomerular filtration rate. CurrOpinNephrolHypertens. 2001;10:551–3. doi: 10.1097/00041552-200109000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubuleinjury. Kidney Int. 2002;62:237–44. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 9.Perco P, Oberbauer R. Kidney injury molecule-1 as a biomarker of acute kidney injury in renal transplant recipients. NatClinPractNephrol. 2008;4:362–3. doi: 10.1038/ncpneph0828. [DOI] [PubMed] [Google Scholar]
- 10.Parikh CR, Abraham E, Ancukiewicz M, Edelstein CL. Urine IL-18 Is an Early Diagnostic Marker for Acute Kidney Injury and Predicts Mortality in the Intensive Care Unit. J Am SocNephrol. 2005;16:3046–52. doi: 10.1681/ASN.2005030236. [DOI] [PubMed] [Google Scholar]
- 11.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–8. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 12.Himmelfarb J, Joannidis M, Molitoris B, Schietz M, Okusa MD, Warnock D, et al. Evaluation and initial management of acute kidney injury. Clin J Am SocNephrol. 2008;3:962–7. doi: 10.2215/CJN.04971107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carmichael P, Carmichael AR. Acute renal failure in the surgical setting. ANZ J Surg. 2003;73:144–53. doi: 10.1046/j.1445-2197.2003.02640.x. [DOI] [PubMed] [Google Scholar]
- 14.Vincent JL, Gerlach H. Fluid resuscitation in severe sepsis and septic shock: An evidence-based review. Crit Care Med. 2004;32(11 Suppl):S451–4. doi: 10.1097/01.ccm.0000142984.44321.a4. [DOI] [PubMed] [Google Scholar]
- 15.Trivedi HS, Moore H, Nasr S, Aggarwal K, Agrawal A, Goel P, et al. A randomized prospective trial to assess the role of saline hydration on the development of contrast nephrotoxicity. Nephron ClinPract. 2003;93:C29–34. doi: 10.1159/000066641. [DOI] [PubMed] [Google Scholar]
- 16.Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R. SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247–56. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- 17.Boldt J, Brosch C, Ducke M, Papsdorf M, Lehmann A. Influence of volume therapy with a modern hydroxyethylstarch preparation on kidney function in cardiac surgery patients with compromised renal function: A comparison with human albumin. Crit Care Med. 2007;35:2740–6. doi: 10.1097/01.CCM.0000288101.02556.DE. [DOI] [PubMed] [Google Scholar]
- 18.Weisbord SD, Palevsky PM. Prevention of contrast-induced nephropathy with volume expansion. Clin J Am SocNephrol. 2008;3:273–80. doi: 10.2215/CJN.02580607. [DOI] [PubMed] [Google Scholar]
- 19.Briguori C, Airoldi F, D’Andrea D, Bonizzoni E, Morici N, Focaccio A, et al. Renal insufficiency following contrast media administration trial (REMEDIAL): A randomized comparison of 3 preventive strategies. Circulation. 2007;115:1211–7. doi: 10.1161/CIRCULATIONAHA.106.687152. [DOI] [PubMed] [Google Scholar]
- 20.Recio-Mayoral A, Chaparro M, Prado B, Cózar R, Méndez I, Banerjee D, et al. The reno-protective effect of hydration with sodium bicarbonate plus N-acetylcysteine in patients undergoing emergency percutaneous coronary intervention: The RENO Study. J Am CollCardiol. 2007;49:1283–8. doi: 10.1016/j.jacc.2006.11.034. [DOI] [PubMed] [Google Scholar]
- 21.Aronson S, Blumenthal R. Perioperative renal dysfunction and cardiovascular anesthesia: Concerns and controversies. J CardiothoracVascAnesth. 1998;12:567–86. doi: 10.1016/s1053-0770(98)90106-9. [DOI] [PubMed] [Google Scholar]
- 22.Bellomo R, Auriemma S, Fabbri A, D’Onofrio A, Katz N, McCullough PA, et al. The pathophysiology of cardiac surgery-associated acute kidney injury (CSA-AKI) Int J Artif Organs. 2008;31:166–78. doi: 10.1177/039139880803100210. [DOI] [PubMed] [Google Scholar]
- 23.Abuelo JG. Normotensive ischemic acute renal failure. N Engl J Med. 2007;357:797–805. doi: 10.1056/NEJMra064398. [DOI] [PubMed] [Google Scholar]
- 24.Lameire N, Biesen WV, Hoste E, Vanholder R. The prevention of acute kidney injury: An in-depth narrative review Part 1: Volume resuscitation and avoidance of drug- and nephrotoxin-induced AKI. NDT Plus. 2008;1:392–402. doi: 10.1093/ndtplus/sfn162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarubbi FA, Jr, Hull JH. Amikacin serum concentrations: Prediction of levels and dosage guidelines. Ann Intern Med. 1978;89:612–8. doi: 10.7326/0003-4819-89-5-612. [DOI] [PubMed] [Google Scholar]
- 26.Bajwa SJ, Kwatra I. Reno-endocrinal disorders: A basic understanding of the molecular genetics. Indian J Endocr Metab. 2012;16:158–63. doi: 10.4103/2230-8210.93731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De laet I, Malbrain ML, Jadoul JL, Rogiers P, Sugrue M. Renal implications of increased intra-abdominal pressure: Are the kidneys the canary for abdominal hypertension? ActaClinBelgSuppl. 2007;1:119–30. doi: 10.1179/acb.2007.62.s1.015. [DOI] [PubMed] [Google Scholar]
- 28.Bonventre JV. Kidney ischemic preconditioning. Curr Opin Nephrol Hypertens. 2002;11:43–8. doi: 10.1097/00041552-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Wijeysundera DN, Beattie WS, Djaiani G, Rao V, Borger MA, Karkouti K, et al. Off-pump coronary artery surgery for reducing mortality and morbidity: Meta-analysis of randomized and observational studies. J Am CollCardiol. 2005;46:872–82. doi: 10.1016/j.jacc.2005.05.064. [DOI] [PubMed] [Google Scholar]
- 30.Bown MJ, Norwood MG, Sayers RD. The management of abdominal aortic aneurysms in patients with concurrent renal impairment. Eur J VascEndovascSurg. 2005;30:1–11. doi: 10.1016/j.ejvs.2005.02.048. [DOI] [PubMed] [Google Scholar]
- 31.Hirschberg R, Kopple J, Lipsett P, Benjamin E, Minei J, Albertson T, et al. Multicenter clinical trial of recombinant human insulin-like growth factor I in patients with acute renal failure. Kidney Int. 1999;55:2423–32. doi: 10.1046/j.1523-1755.1999.00463.x. [DOI] [PubMed] [Google Scholar]
- 32.Bellomo R, Chapman M, Finfer S, Hickling K, Myburgh J. Low-dose dopamine in patients with early renal dysfunction: A placebo-controlled randomised trial. Australian and New Zealand Intensive Care Society (ANZICS) Clinical Trials Group. Lancet. 2000;356:2139–43. doi: 10.1016/s0140-6736(00)03495-4. [DOI] [PubMed] [Google Scholar]
- 33.Kellum JA. Use of dopamine in acute renal failure: A meta-analysis. Crit Care Med. 2001;29:1526–31. doi: 10.1097/00003246-200108000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Friedrich JO, Adhikari N, Herridge MS, Beyene J. Meta-analysis: Low dose dopamine increases urine output but does not prevent renal dysfunction or death. Ann Intern Med. 2005;142:510–24. doi: 10.7326/0003-4819-142-7-200504050-00010. [DOI] [PubMed] [Google Scholar]
- 35.Padmanabhan R. Renal dose dopamine—it's myth and the truth. J Assoc Physicians India. 2002;50:571–5. [PubMed] [Google Scholar]
- 36.Weldon BC, Monk TG. The patient at risk for acute renal failure. Recognition, prevention, and preoperative optimization. AnesthesiolClin North Am. 2000;18:705–17. doi: 10.1016/s0889-8537(05)70190-5. [DOI] [PubMed] [Google Scholar]
- 37.Halpenny M, Lakshmi S, O’Donnell A, O’Callaghan-Enright S, Shorten GD. Fenoldopam: Renal and splanchnic effects in patients undergoing coronary artery bypass grafting. Anaesthesia. 2001;56:953–60. doi: 10.1046/j.1365-2044.2001.02220.x. [DOI] [PubMed] [Google Scholar]
- 38.Cogliati AA, Vellutini R, Nardini A, Urovi S, Hamdan M, Landoni G, et al. Fenoldopam infusion for renal protection in high-risk cardiac surgery patients: A randomized clinical study. J CardiothoracVascAnesth. 2007;21:847–50. doi: 10.1053/j.jvca.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 39.Bagshaw SM, Ghali WA. Theophylline for prevention of contrastinduced nephropathy: A systematic review and meta-analysis. Arch Intern Med. 2005;165:1087–93. doi: 10.1001/archinte.165.10.1087. [DOI] [PubMed] [Google Scholar]
- 40.Rodicio JL, Campo C, Ruilope LM. Renal effects of calcium antagonists. Nephrol DialTransplant. 1995;10(Suppl9):17–22. [PubMed] [Google Scholar]
- 41.Bagshaw SM, Delaney A, Haase M, Ghali WA, Bellomo R. Loop diuretics in the management of acute renal failure: A systematic review and metaanalysis. Crit Care Resusc. 2007;9:60–8. [PubMed] [Google Scholar]
- 42.Bagshaw SM, Delaney A, Jones D, Ronco C, Bellomo R. Diuretics in themanagement of acute kidney injury: A multinational survey. ContribNephrol. 2007;156:236–49. doi: 10.1159/000102089. [DOI] [PubMed] [Google Scholar]
- 43.Lameire N, Biesen WV, Hoste E, Vanholder R. The prevention of acute kidney injury an in-depth narrative review: Part 2: Drugs in the prevention of acute kidney injury. NDT Plus. 2009;2:1–10. doi: 10.1093/ndtplus/sfn199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Townsend DR, Bagshaw SM. New insights on intravenous fluids,diuretics and acute kidney injury. Nephron ClinPract. 2008;109:c106–16. doi: 10.1159/000142930. [DOI] [PubMed] [Google Scholar]
- 45.Uchino S, Doig GS, Bellomo R, Morimatsu H, Morgera S, Schetz M, et al. Diuretics andmortality in acute renal failure. Crit Care Med. 2004;32:1669–77. doi: 10.1097/01.ccm.0000132892.51063.2f. [DOI] [PubMed] [Google Scholar]
- 46.Bajwa SJ, Kulshrestha A. Renal endocrine manifestations during polytrauma: A cause of concern for the anesthesiologist. Indian J Endocr Metab. 2012;16:252–7. doi: 10.4103/2230-8210.93744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sever MS, Vanholder R, Lameire N. Management of crushrelated injuries after disasters. N Engl J Med. 2006;354:1052–63. doi: 10.1056/NEJMra054329. [DOI] [PubMed] [Google Scholar]
- 48.Vanholder R, Sever MS, Erek E, Lameire N. Rhabdomyolysis. J Am SocNephrol. 2000;11:1553–61. doi: 10.1681/ASN.V1181553. [DOI] [PubMed] [Google Scholar]
- 49.Reddy VG. Prevention of postoperative acute renal failure. J Postgrad Med. 2002;48:64–70. [PubMed] [Google Scholar]
- 50.Schnuelle P, Van Der Woude JF. Perioperative fluid management in renal transplantation: A narrative review of the literature. TransplInt. 2006;19:947–59. doi: 10.1111/j.1432-2277.2006.00356.x. [DOI] [PubMed] [Google Scholar]
- 51.Vesely DL. Natriuretic peptides and acute renal failure. Am J Physiol Renal Physiol. 2003;285:F167–77. doi: 10.1152/ajprenal.00259.2002. [DOI] [PubMed] [Google Scholar]
- 52.Lewis J, Salem MM, Chertow GM, Weisberg LS, McGrew F, Marbury TC, et al. Atrial natriuretic factor in oliguric acute renal failure. Anaritide Acute Renal Failure Study Group. Am J Kidney Dis. 2000;36:767–74. doi: 10.1053/ajkd.2000.17659. [DOI] [PubMed] [Google Scholar]
- 53.Mentzer RM, Jr, Oz MC, Sladen RN, Graeve AH, Hebeler RF, Jr, Luber JM, Jr, et al. Effects of perioperative nesiritide in patients with left ventricular dysfunction undergoing cardiac surgery: The NAPA Trial. J Am CollCardiol. 2007;49:716–26. doi: 10.1016/j.jacc.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 54.Clark LC, Farghaly H, Saba SR, Vesely DL. Amelioration with vessel dilator of acute tubular necrosis and renal failure established for 2 days. American Journal of Physiology. Heart CircPhysiol. 2000;278:H1555–64. doi: 10.1152/ajpheart.2000.278.5.H1555. [DOI] [PubMed] [Google Scholar]
- 55.McCullough PA. Contrast-induced acute kidney injury. J Am CollCardiol. 2008;51:1419–28. doi: 10.1016/j.jacc.2007.12.035. [DOI] [PubMed] [Google Scholar]
- 56.Birck R, Krzossok S, Markowetz F, Schnülle P, van der Woude FJ, Braun C. Acetylcysteine for prevention of contrast nephropathy: Metaanalysis. Lancet. 2003;362:598–603. doi: 10.1016/S0140-6736(03)14189-X. [DOI] [PubMed] [Google Scholar]
- 57.Hoffmann U, Fischereder M, Krüger B, Drobnik W, Krämer BK. The value of Nacetylcysteine in the prevention of radiocontrast agent-induced nephropathy seems questionable. J Am SocNephrol. 2004;15:407–10. doi: 10.1097/01.asn.0000106780.14856.55. [DOI] [PubMed] [Google Scholar]
- 58.Khanal S, Attallah N, Smith DE, Kline-Rogers E, Share D, O’Donnell MJ, et al. Statin therapy reduces contrastinduced nephropathy: An analysis of contemporary percutaneous interventions. Am J Med. 2005;118:843–9. doi: 10.1016/j.amjmed.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 59.Sturiale A, Campo S, Crascì E, Aloisi C, Buemi M. Experimental models of acute renal failure and erythropoietin: What evidence of a direct effect? Ren Fail. 2007;29:379–86. doi: 10.1080/08860220701193290. [DOI] [PubMed] [Google Scholar]
- 60.Veys N, Van BW, Lameire N. Internal medicine, renal anaemia, and erythropoiesis-stimulating agents (ESAS) ActaClinBelg. 2007;62:396–407. doi: 10.1179/acb.2007.059. [DOI] [PubMed] [Google Scholar]


