Abstract
The cortical microtubule array provides spatial information to the cellulose-synthesizing machinery within the plasma membrane of elongating cells. Until now data indicated that information is transferred from organized cortical microtubules to the cellulose-synthesizing complex, which results in the deposition of ordered cellulosic walls. How cortical microtubules become aligned is unclear. The literature indicates that biophysical forces, transmitted by the organized cellulose component of the cell wall, provide a spatial cue to orient cortical microtubules. This hypothesis was tested on tobacco (Nicotiana tabacum L.) protoplasts and suspension-cultured cells treated with the cellulose synthesis inhibitor isoxaben. Isoxaben (0.25–2.5 μm) inhibited the synthesis of cellulose microfibrils (detected by staining with 1 μg mL−1 fluorescent dye and polarized birefringence), the cells failed to elongate, and the cortical microtubules failed to become organized. The affects of isoxaben were reversible, and after its removal microtubules reorganized and cells elongated. Isoxaben did not depolymerize microtubules in vivo or inhibit the polymerization of tubulin in vitro. These data are consistent with the hypothesis that cellulose microfibrils, and hence cell elongation, are involved in providing spatial cues for cortical microtubule organization. These results compel us to extend the microtubule/microfibril paradigm to include the bidirectional flow of information.
Cell elongation is necessary for normal plant morphogenesis. During this developmental event, highly organized microfibrils of cellulose confine turgor-driven cellular expansion to a single major axis of growth (Green and Poethig, 1982; Delmer and Armor, 1995). Therefore, correctly ordered cellulose microfibrils are essential for proper cell differentiation (Green and Selker, 1991).
In the primary wall of an elongating cell, cellulose microfibrils are deposited in an ordered configuration at right angles to the major axis of elongation (Gertel and Green, 1977). The ordering of nascent cellulose microfibrils is controlled by cortical microtubules (Williamson, 1991; Cyr and Palevitz, 1995). When cortical microtubules are disrupted with anti-microtubule agents, ordered cellulose deposition does not occur and the cell fails to elongate properly (Morejohn, 1991). Precisely how cortical microtubules affect cellulose alignment is uncertain, but the available data indicate that cortical microtubules act indirectly by limiting the avenues available for the movement of cellulose synthase complexes as they glide within the fluid milieu of the plasma membrane (Giddings and Staehelin, 1991). Although it is clear that microtubules provide the spatial information necessary to ensure proper cellulose microfibril alignment, it is less clear how microtubules acquire their own alignment cues.
Changes in the arrangements of cortical microtubules follow (or accompany) alterations in the growth status of cells (Cyr and Palevitz, 1995). Rearrangements have also been noted following hormonal and light treatments and application of exogenous forces (mechanical, centrifugal, and electrical; Williamson, 1991; Shibaoka, 1994; Cyr and Palevitz, 1995; Hush and Overall, 1996; Wymer et al., 1996b). Although it is known that the cortical array changes as a result of these treatments, it is unclear how these treatments provide the spatial informational cues that act to guide the cortical microtubules to their proper locations. It has been suggested that cortical microtubules are sensitive to mechanical strain and therefore use the vector of cell expansion as a spatial cue (Green et al., 1970). If this hypothesis is correct, then treatments that affect the mechanical properties of the growing wall should alter the arrangement of cortical microtubules. We tested this hypothesis using a compound that inhibits cellulose synthesis.
Isoxaben is a herbicide that inhibits the incorporation of Glc into the cellulose-rich, acid-insoluble fraction of isolated walls and is an extremely powerful and specific inhibitor of cell wall biosynthesis (Heim et al., 1990b; Corio-Costet et al., 1991b). Cell wall-fractionation studies have revealed that the herbicidal action of isoxaben can be explained entirely by its effect on cellulose biosynthesis (Heim et al., 1991). Its probable mode of action is to directly inhibit cellulose synthesis, because resistant cell lines show an unaltered uptake or detoxification of the herbicide (Heim et al., 1991) and only two genetic loci in Arabidopsis thaliana have been shown to confer resistance (Heim et al., 1989, 1990a). Exhaustive studies have revealed that other cellular processes are unaffected by isoxaben (e.g. seed germination, mitosis, respiration, photosynthesis, and lipid and RNA synthesis, Lefebvre et al., 1987; Corio-Costet et al., 1991a). Treated cells fail to elongate with high fidelity and consequently grow isodiametrically (Lefebvre et al., 1987). This herbicide acts at much lower concentrations (< 40×) than dichlobenil, another cellulose synthesis inhibitor (Heim et al., 1990b). Therefore, the properties of isoxaben make it an ideal agent for perturbing the mechanical properties of the primary cell wall.
The regenerating protoplast was chosen as our experimental model. A freshly isolated tobacco (Nicotiana tabacum L.) protoplast lacks a wall and is spherical. These spherical cells contain cortical microtubules in a relatively random configuration (Hasezawa et al., 1988). Shortly after the removal of protoplasting enzymes, the wall begins to regenerate and the cortical microtubule array begins to reorder. Within 3 d, a new cell wall is regenerated, the cortical microtubules acquire full order, and the cells begin elongation (Hasezawa et al., 1988; Kuss-Wymer and Cyr, 1992; Wymer et al., 1996a). This system allows us to closely examine the relationship among cell wall synthesis, microtubule organization, and cell growth.
The data presented here support the hypothesis that the mechanical properties of the wall influence the organization of the cortical microtubule array. These results are discussed in terms of a broadened view of the microtubule/microfibril paradigm (Giddings and Staehelin, 1991), in which the cortical microtubules supply positional information to the cellulose microfibrils, and the cellulose microfibrils, in turn, provide biophysical information back to the underlying cortical microtubules. In short, microtubules and microfibrils make up a self-rectifying, feedback organizational system.
MATERIALS AND METHODS
Plant Material and Culturing
Tobacco (Nicotiana tabacum L. cv Bright Yellow-2) suspension cultures were grown in 100 mL of a culture medium containing 4.3 g L−1 Murashige-Skoog salts (GIBCO-BRL), 100 mg L−1 myo-inositol, 1 mg L−1 thiamine-HCl, 0.2 mg L−1 2,4-D, and 30 g L−1 Suc, pH 5.0, in 500-mL Erlenmeyer flasks on a rotary shaker at 26°C in the dark. All chemicals listed here were from Sigma unless otherwise noted.
Protoplast Preparation and Culturing
Protoplasts were isolated enzymatically using 1% (w/v) Cellulase-YC and 0.1% (w/v) Pectyolase Y-23, pH 5.0 (Seishan Pharmaceutical Co., Ltd., Tokyo, Japan) with 0.35 m mannitol added as an osmoticum. Three- to 5-d-old cells were incubated in the enzyme solution for approximately 2 h at room temperature with gentle agitation on a rotary shaker. Protoplasts were filtered from cellular debris passively through lightly packed cotton in a 10-mL syringe base and then washed twice with PMM buffer (50 mm Pipes, pH 6.9, 1 mm MgSO4, 1 mm EGTA, and 0.35 m mannitol). All of the above steps were done aseptically. Protoplasts were typically resuspended in a medium that favored elongative growth (FMS medium: 4.3 g L−1 Murashige-Skoog salts, 10 g L−1 Suc, 0.30 m mannitol, 100 mg L−1 myo-inositol, and 1.0 mg L−1 each of nicotinic acid and pyridoxine-HCl; modified from Hasezawa and Syono, 1983). The protoplasts were cultured at 26°C in the dark.
Isoxaben Treatment
Isoxaben (95%, N-3-[1-ethyl-1-methyl-propyl]-5-isoxazolyl-2, 6-dimethoxybenzamide) was graciously supplied by DowElanco (Indianapolis, IN) and was made up as a concentrated ethanol stock solution and used at concentrations ranging from 0.01 to 5.0 μm. The final concentration of ethanol was less than 0.05%. Control cultures without isoxaben contained the same amount of ethanol as the isoxaben-treated cultures; no effect of ethanol was noted. Isoxaben-treated protoplasts were cultured as described above.
Monitoring Cell Wall Regeneration
Cell wall regeneration was monitored immediately after the protoplasts were released and cultured with or without isoxaben. Cellefluor (1 μg mL−1, Polysciences, Inc., Washington, PA) was used to visualize cellulose microfibrils and, hence, the initiation and progress of cell wall formation. In older cells the orientation of microfibrils is difficult to resolve because of excessive out-of-focus fluorescent flare, but can be analyzed by observing the birefringent properties of the wall with a microscope (Axioskop, Zeiss) equipped for polarizing microscopy using a 100-W mercury arc lamp for illumination. Images were captured with an integrating charge-coupled device camera and digitally processed (Argus 20, Hammamatsu Corp., Bridgewater, NJ).
Cellular Morphometry
Diameters of 30 individual control and isoxaben-treated cells were measured at selected time intervals from digitized images (Image-Pro Plus, Media Cybernetics, Silver Spring, MD). Volume calculations for a sphere were made for isoxaben-treated protoplasts and mean volumes were determined.
Immunolocalization of Cortical Microtubules
Microtubules in lysed and nonlysed cells were examined via immunolocalization techniques. Cells were settled onto poly-l-Lys-coated slides (applied as a 1 mg mL−1 solution, Mr 300,000) for 3 min. Excess medium was removed by wicking, and detergent lysis buffer (50 mm Pipes, pH 6.9, 1 mm MgSO4, 10 mm 3-[(cholamidopropyl) dimethyl- ammonio]- 1-propanesulfonic acid , and 5 μg mL−1 each of the following protease inhibitors: antipain, aprotinin, chymostatin, leupeptin, and pepstatin C) was applied for 5 min. After lysing, the buffer was wicked away and the cells were fixed for 30 min with 4% (w/v) formaldehyde (made fresh from paraformaldehyde), 0.1% (v/v) glutaraldehyde, 50 mm Pipes, pH 6.9, 5 mm EGTA, 2 mm MgSO4, 1% (v/v) glycerol, and 0.30 m mannitol. Fixative was wicked off and the slides were rinsed in a beaker of PBS for 3 min, followed by dehydration with methanol at −20°C for 3 min, and then blocked with 3% (w/v) BSA in PBS for 5 min. A polyclonal antibody raised against soybean tubulin in a rabbit was applied for at least 60 min. After the slides were rinsed for 15 min in PBS, they were incubated in a goat anti-rabbit fluorescein isothiocyanate-conjugated antibody for at least 1 h and then rinsed for 15 min in PBS.
The same regime was followed for the nonlysed cells as for the lysed cells except, prior to the methanol step, the cells were incubated for 3 min in an enzyme solution that included 0.5% (w/v) Cellulase-YC, 0.05% (w/v) Pectyolase Y-23, 0.5% (v/v) Triton X-100, PMM buffer, and 1 μg mL−1 protease inhibitors (listed above). This step aids in increased antibody accessibility (primary and secondary antibodies were applied for at least 4 h). All slides were mounted with 4 m glycerol and 100 mm Tris, pH 9.0, containing 1 mg mL−1 phenylenediamine (to reduce fluorescent fading) and 1 mg mL−1 Hoescht 33258 (Calbiochem) to visualize nuclei. The slides were viewed with an Axioskop microscope and a laser-scanning confocal microscope (model LSM 410, Zeiss) using the 488-nm line of the argon-ion laser for excitation, a 488 dichroic mirror (Zeiss), and 515- to 540-nm emission filters (Zeiss). Images were either captured from one focal plane or the depth-of-focus option was used to simultaneously view more than one plane.
Isolation of Tubulin and in Vitro Assembly of Microtubules
Carrot tubulin was isolated and purified using the methods described by Moore et al. (1997). The purified tubulin was preincubated on ice with an equal stoichiometry of isopropyl N-(3-chlorophenyl)-carbamate or isoxaben prior to assembly and visualized using dark-field microscopy.
RESULTS
Isoxaben Inhibits Normal Cell Wall Deposition and Elongation in Cultured Protoplasts
Freshly isolated tobacco BY-2 protoplasts were devoid of demonstrable cellulose microfibrils, as detected by Cellefluor (Fig. 1a). However, detectable microfibrils appeared within 15 min of culturing, after enzyme removal (Fig. 1b). The deposition of microfibrils was cumulative and became very pronounced by 45 min (Fig. 1c). The cellulose deposited by 15 min rendered the protoplasts osmotically stable, as evidenced by their inability to burst when transferred into hypotonic medium (data not shown).
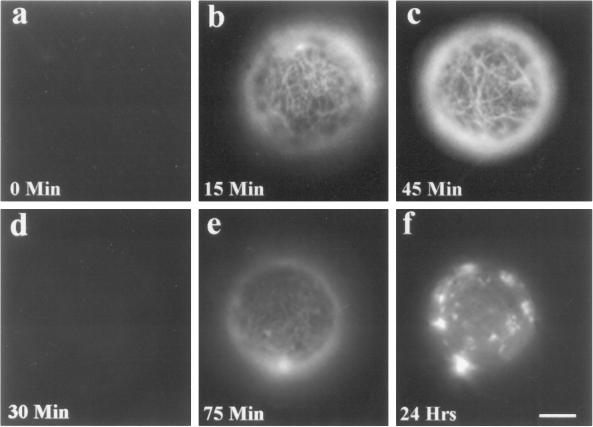
Figure 1.
Isoxaben inhibits the deposition of cellulose microfibrils. Freshly isolated tobacco BY-2 protoplasts do not exhibit cellulose microfibrils when treated with Cellefluor (a). Microfibrils become visible within 15 min of culturing (b) and are pronounced by 45 min (c). When similarly treated protoplasts are exposed to 2.5 μm isoxaben, microfibrils are not visible by 30 min (d), and by 75 min only faint staining is observed (e). By 24 h the prominent fluorescing material is observed as bright aggregates (f). Bar = 4 μm.
Isoxaben dramatically affects cellulose microfibril detection via Cellefluor staining. Protoplasts treated with 2.5 μm isoxaben were virtually devoid of detectable fluorescent microfibrils by 30 min (Fig. 1d). By 75 min, faint fibrils were observable (Fig. 1e). After 24 h, the prominent, fluorescing material appeared only as bright aggregates (Fig. 1f). Additionally, isoxaben-treated protoplasts were sensitive to hypotonic lysis (data not shown). The effects of 0.25 to 2.5 μm isoxaben were similar.
Ordered cellulose microfibrils provide plant cells with walls that have anisotropic properties (Green and Poethig, 1982). Such ordering alters the optical properties of the wall, causing them to become birefringent. The primary cell wall deposited by a tobacco BY-2 protoplast that had been cultured for 7 d had a birefringent pattern when examined with polarized light microscopy (Fig. 2a), which is characteristic of a highly organized arrangement of cellulose microfibrils (Richmond, 1983). Characteristically, birefringence was lost when the cell was turned at a 45° angle (Fig. 2b). Birefringence was not discernible at either angle in the cell walls of protoplasts that were similarly cultured for 7 d in the presence of 2.5 μm isoxaben (Fig. 2, c and d). Therefore, the ordered cellulose microfibrils of tobacco BY-2 cells permitted cell elongation (Fig. 2e), but, presumably, the lack of ordered cellulose microfibrils in isoxaben-treated protoplasts caused the cell to remain spherical (Fig. 2f).
Figure 2.
Isoxaben-treated protoplasts exhibit neither birefringent cell walls nor cellular elongation. Ordered cellulose microfibrils have optical properties that render them birefringent by polarized light microscopy. Cultured tobacco BY-2 protoplasts have highly organized cellulose microfibrils, as evidenced by their birefringent properties (a). When birefringent cells are turned at a 45° angle, they lose their birefringent character (b). Birefingence is not observed at either angle when protoplasts are cultured for 7 d in the presence of 2.5 μm isoxaben (c and d, smaller apparent size is due to the focal plane being at the top of the spherical protoplast). Ordered cellulose microfibrils permit cell elongation (e), and the lack of ordered microfibrils in isoxaben-treated protoplasts results in the inability of cells to elongate (f). The cells shown in e and f were both cultured for 7 d. Bars = 20 μm (a and b), 6 μm (c and d), and 40 μm (e and f).
Isoxaben Does Not Inhibit Growth or Irreversibly Damage Protoplasts
Does isoxaben inhibit the growth of tobacco BY-2 protoplasts, and could such an inhibition explain why protoplasts fail to deposit a normal cell wall and elongate? To answer this question, the growth of isoxaben-treated protoplasts was examined.
Freshly isolated tobacco BY-2 protoplasts are typically 25 μm in diameter. When these cells are cultured, they elongate rapidly (Kuss-Wymer and Cyr, 1992). Identically cultured 2.5 μm isoxaben-treated protoplasts failed to elongate but, rather, grew spherically. The mean volume of isoxaben-treated protoplasts was calculated for 14 d and was found to steadily increase until approximately 11 d, when their growth slowed (Fig. 3). Also noteworthy was the extremely active streaming of cytoplasmic strands observed in these robust cells (even after 1 month of culturing in the presence of isoxaben; data not shown). Extremely high concentrations (50–100 μm) of isoxaben did not affect growth, and no evidence of lethality was observed after treating protoplasts with this agent; this was not the case if such high concentrations were added to walled suspension- cultured cells (data not shown).
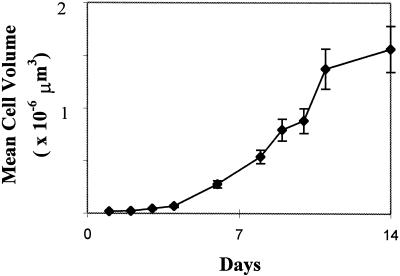
Figure 3.
Isoxaben-treated cells increase in volume. Tobacco BY-2 protoplasts cultured with 2.5 μm isoxaben have mean volumes that increase steadily during culture. Growth slows but does not cease after d 11. Error bars = sd; n = 25.
Does isoxaben irreversibly damage protoplasts? One criterion used to ascertain whether drugs have a general toxic mode of action is to determine whether the effects are reversible. Therefore, protoplasts were cultured for 48 h in the presence of 2.5 μm isoxaben and then cultured in its absence. Within 7 d the cells elongated with a morphology similar to untreated, control cells (compare Figs. 4a and 2e). If cells were treated for significantly longer times (> 14 d) or at high isoxaben concentrations (> 5 μm) and then cultured in its absence, they also elongated but typically with bulging morphologies (data not shown). Additionally, freshly isolated protoplasts treated for 2 h with 2.5 μm isoxaben lacked detectable cellulose microfibrils (Fig. 1), but after the protoplasts were washed with isoxaben-free FMS medium, the cells stained positively for cellulose microfibrils within 60 min (Fig. 4b). This reveals a relatively quick reversal of the effects of isoxaben on normal cell wall regeneration.
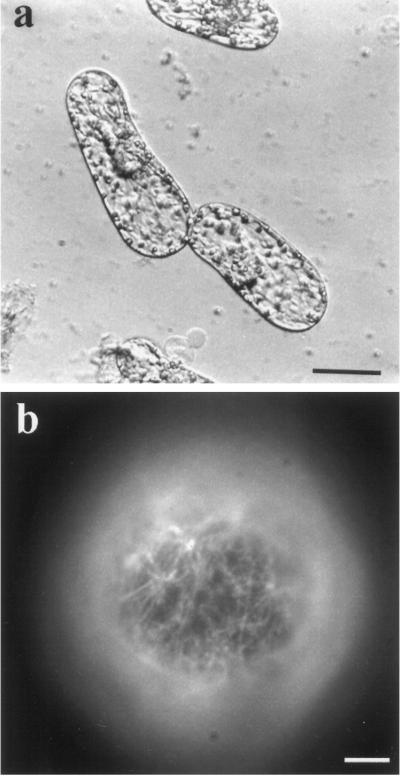
Figure 4.
Isoxaben does not irreversibly damage protoplasts. Tobacco BY-2 protoplasts were cultured for 48 h in the presence of 2.5 μm isoxaben, rinsed three times thoroughly with culture medium, and then cultured for 7 d without isoxaben. Isoxaben's effects were reversed by removal of the cellulose inhibitor, and cell elongation proceeded normally (a). When the protoplasts were treated for a shorter time (2 h) with 2.5 μm isoxaben and were then washed and cultured in isoxaben-free culture medium, the cells stained positively for cellulose microfibrils via Cellefluor (1 μg mL−1) within 60 min, revealing a quick reversal time for isoxaben's effects. Bars = 20 μm (a) and 2.5 μm (b).
Isoxaben Inhibits the Acquisition of Cortical Microtubule Order in Regenerating Protoplasts
The microtubule/microfibril paradigm postulates that spatial information is conveyed from the cortical microtubules to the newly synthesized cellulose microfibrils (Giddings and Staehelin, 1991). However, it does not explain how cortical microtubules acquire their spatial information. Biophysical forces may be involved in the alignment of these cortical elements (Green and Selker, 1991; Williamson, 1991; Cyr, 1994a; Zandomeni and Schopfer, 1994; Hush and Overall, 1996). If this hypothesis is correct, then a perturbation of cellulose deposition (which controls the direction of cellular strain) should inhibit the acquisition of the order of cortical microtubules. Isoxaben was chosen to examine this hypothesis because it provides a perturbation without being irreversibly harmful to protoplasts.
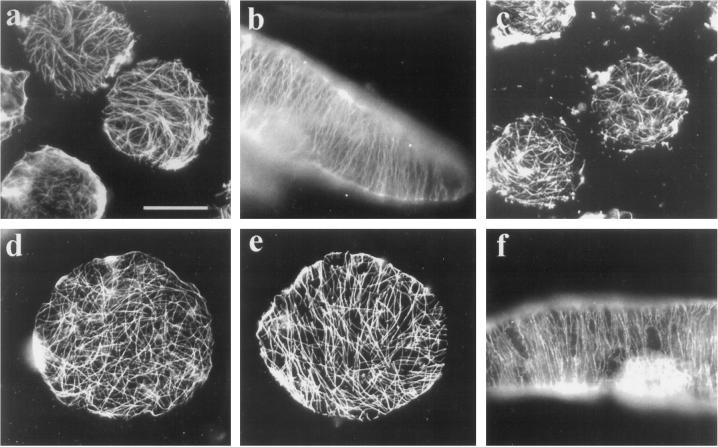
Freshly isolated tobacco BY-2 protoplasts possessed randomly organized cortical microtubules predominantly (Fig. 5a). Upon culturing, the cortical microtubules became organized, and concomitantly the cells elongated (Fig. 5b). The majority (96%) of freshly isolated protoplasts, treated with 2.5 μm isoxaben for 2 h, also possessed randomly arranged cortical microtubules (Fig. 5c), which indicates that this herbicide does not depolymerize microtubules in vivo. These cortical microtubules remained predominantly random in protoplasts cultured for 7 d in the presence of this herbicide (Fig. 5d). Whereas cortical microtubules in isoxaben-treated protoplasts did not become ordered, we did see some cells (< 25%, n = >300) that had partially ordered microtubules (Fig. 5e). Inhibition of normal cell elongation and microtubule ordering by isoxaben requires that the herbicide be present continuously. Protoplasts treated for 4 h with 2.5 μm isoxaben and then cultured in its absence for 7 d elongated normally and had highly organized cortical microtubules (Fig. 5f). These data support the hypothesis that cellulose synthesis is required for cortical microtubules to acquire full order. The related question of what role cellulose synthesis plays in the maintenance of order was next addressed.
Figure 5.
Isoxaben inhibits the acquisition of cortical microtubule order in regenerating protoplasts. Freshly isolated tobacco BY-2 protoplasts have randomly organized cortical microtubules that are demonstrable with a plant anti-tubulin antibody (a). Culturing of these protoplasts results in elongate cells with highly organized cortical microtubules by 7 d (b). Similarly isolated protoplasts exposed for 2 h to 2.5 μm isoxaben also have randomly organized cortical microtubules (c). After 7 d of culturing in the presence of isoxaben, the majority of cells still possess randomly organized microtubules and the overall shape of the cell remains round (d). In < 25% of such treated cells, some regional ordering of microtubules was observed, but even these cells retained their round shape (e). Protoplasts treated with 2.5 μm isoxaben for 4 h and cultured in the absence of isoxaben also elongated and possessed highly organized cortical microtubules by 7 d (f). Bar = 20 μm.
Isoxaben Inhibits the Maintenance of Cortical Microtubule Order in Growing, Walled Cells
The geometry of walled, suspension-cultured tobacco BY-2 cells, and the organization of their cortical microtubules, changes as a function of the cell cycle and growth state. Recently divided cells are typically small, relatively isodiametric, and possess cortical microtubules that are relatively unordered (Hasezawa et al., 1991). As the cells begin elongating, the cortical microtubule arrays become highly ordered, with order being maintained throughout cell expansion (Hasezawa et al., 1988). Elongative growth requires that newly synthesized cellulose be deposited in the proper orientation to ensure continued anisotropic expansion. We hypothesized that these newly intercalated cellulose microfibrils were required to maintain the cortical microtubule array in its highly ordered configuration.
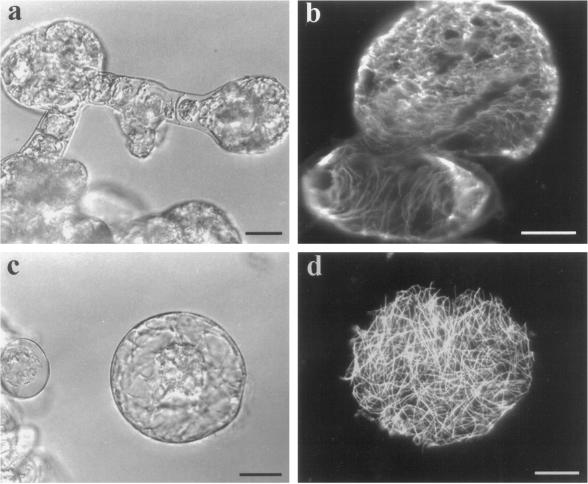
To test this hypothesis, walled tobacco BY-2 suspension-cultured cells were cultured with 1.0 μm isoxaben. Within 3 d, numerous cells within a file began to bulge, and by d 5 these cells became spherical (Fig. 6a). The spherical portion of these cells possessed cortical microtubules that appeared to be losing their tight, ordered configurations, whereas the nonspherical portion retained a more ordered appearance (Fig. 6b). Cellefluor staining revealed a thin, dim fluorescence at the bulging portions of these cells and a brighter signal at the nonbulging portions (data not shown). A high proportion of these bulging cells were eventually released from their weakened cell walls (no longer producing cellulose) into the culture medium (Fig. 6c). These released cells resembled freshly isolated protoplasts, and they too had randomly organized cortical microtubules (Fig. 6d). These data support the hypothesis that continued cellulose synthesis is required for the maintenance of order within the cortical microtubule array.
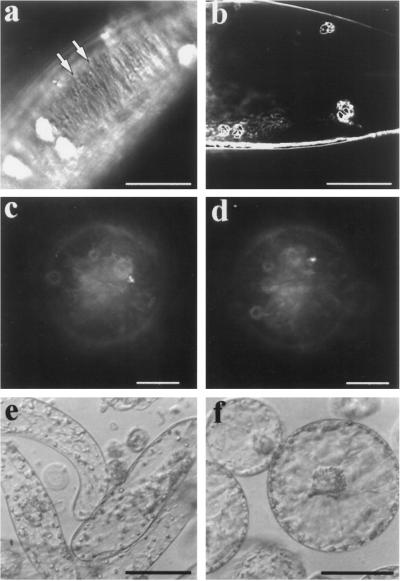
Figure 6.
Isoxaben inhibits the maintenance of cortical microtubule order in growing, walled, suspension-cultured cells. Tobacco BY-2 suspension-cultured cells were cultured with 1.0 μm isoxaben, and within 5 d numerous cells within a file of cells began to bulge and became spherical (a). The spherical portion of the cells had cortical microtubules that were more unorganized than their nonspherical portion, as demonstrated with a plant anti-tubulin antibody (b, confocal image of five stacked images, 1 μm each). Many of these spherical cells were eventually released from their weakened cell walls and observed as protoplast-like entities (c). These released cells also possessed randomly organized cortical microtubules (d). Bars = 20 μm.
Isoxaben Does Not Directly Affect the Polymerization of Plant Tubulin Assembled in Vitro
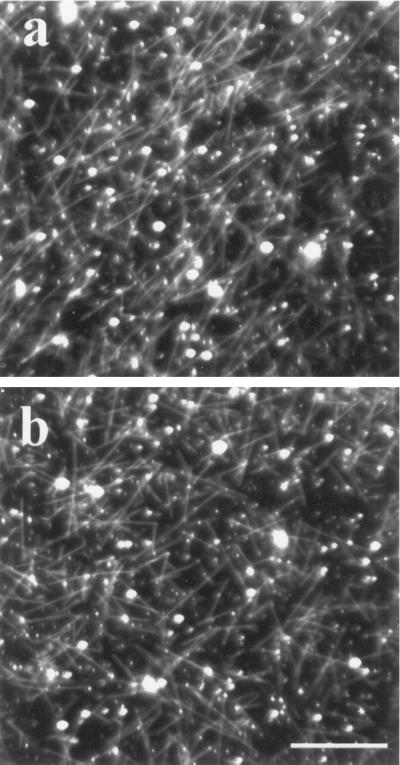
Some herbicides, notably the phenylcarbamates, can disorder microtubules in vivo (Clayton and Lloyd, 1984) and cause their depolymerization in vitro (Mizuno and Suzaki, 1990). Therefore, isoxaben was tested to determine whether it has two specific and independent modes of action: (a) to inhibit cellulose synthesis and (b) to disorder microtubules in a pathway that initially involves microtubule depolymerization. Although we found no evidence that isoxaben depolymerizes microtubules in vivo, we wanted to corroborate this finding in vitro. To do this, purified tubulin was obtained from suspension-cultured carrot cells (the yield is higher from carrot cells than from tobacco) and was assembled into microtubules in the absence (Fig. 7a) and in the presence (Fig. 7b) of isoxaben. Isoxaben, at equal stoichiometry (40 μm), did not affect the assembly of tubulin into microtubules. However, tubulin did not assemble into microtubules when an equal stoichiometry of isopropyl N-(3-chlorophenyl)-carbamate (a phenylcarbamate herbicide) was similarly tested (data not shown), indicating that isoxaben and the phenylcarbamates have different modes of action.
Figure 7.
Isoxaben does not affect tubulin assembly in vitro. Purified carrot tubulin (40 μm) assembles readily into microtubules, which are observable by dark-field microscopy (a). When isoxaben is added at an equal stoichiometry (40 μm) to a similar tubulin preparation, assembly of tubulin is unaffected (b). Bar = 5 μm.
DISCUSSION
Isoxaben inhibits cellulose synthesis in a number of plant species (Lefebvre et al., 1987; Heim et al., 1989, 1990a, 1990b, 1991, 1993; Corio-Costet et al., 1991a, 1991b; Schneegurt et al., 1994). We confirmed this mode of action in isoxaben-treated tobacco BY-2 protoplasts with four lines of evidence: (a) deposition of microfibrils, demonstrable by Cellefluor, is inhibited; (b) cells fail to grow anisotropically; (c) cell walls never acquire a birefringent character; and (d) protoplasts show prolonged sensitivity to hypoosmotic lysis. Although cellulose synthesis is inhibited, other cell wall carbohydrates are probably synthesized, because some amorphic Cellefluor-stained material is observed after prolonged culture, and eventually the protoplasts become resistant to hypoosmotic lysis. The presence of some amorphic cell wall material is not surprising, because pectin synthesis continues in the presence of this herbicide (K. Vaughn, personal communication); moreover, cellulose-binding dyes (such as Cellefluor) stain a variety of carbohydrate polymers (Maeda and Ishida, 1967; Hughes and McCully, 1975). In spite of the presence of some wall material, the isoxaben-treated protoplasts are incompetent to undergo normal morphogenesis and fail to show any sign of anisotropic growth even after 2 months of culture (data not shown). We conclude that normal, cellulosic-containing walls are not synthesized in isoxaben-treated tobacco protoplasts.
The isodiametric character of isoxaben-treated protoplasts indicates that these cells are incapable of constraining turgor force in an anisotropic manner (i.e. there is no preferential reinforcement axis). Although these protoplasts do not elongate, their growth is evidenced by their steady increase in volume, which indicates that membrane pumps, ion channels, and any needed cell wall-loosening factors are not adversely affected by this herbicide. Additionally, cytoplasmic streaming is as active in isoxaben-treated cells as is normally observed in untreated, control cells. The available data indicate that the sole consequence of isoxaben treatment is to inhibit cellulose synthesis (Heim et al., 1991) and, consequently, the cells grow round.
It is curious that isoxaben, even at extremely high concentrations (50–100 μm), is not toxic to protoplasts but is toxic to many of the walled suspension-cultured cells within a file (except those that showed the “bulging” phenomenon). The lethal effect on walled cells and the nonlethal effect on protoplasts are reminiscent of the action of pectic enzymes implicated in the hypersensitive host-defense interaction, whereby walled cells are killed by these pathogenically produced enzymes, but isolated protoplasts are left undamaged (Basham and Bateman, 1975; Stephens and Wood, 1975). The hypersensitive response involves the release of endogenous wall components, which then act to kill the affected cells (Bucheli et al., 1990). We postulate that isoxaben, by interfering with cellulose synthesis in walled cells, leads to the accumulation or release of noncellulosic wall constituents that induce a hypersensitive reaction. Protoplasts that lack walls are not susceptible to this secondary lethal action of isoxaben.
The cortical microtubule array in isoxaben-treated protoplasts does not acquire order, and this disorganization is reminiscent of the action of phenylcarbamates and griseo-fulvin. Although the ultimate effect of isoxaben is in some instances similar to these two other types of inhibitors (Giddings and Staehelin, 1991; Hoffman and Vaughn, 1994), its mechanism of action is different. Griseofulvin and phenylcarbonate likely affect the ordering of cortical microtubule arrays by directly interacting with the microtubules themselves. Both inhibitors block the assembly of purified tubulin in vitro (Morejohn, 1991; Zhang, 1995), and short-term treatment causes the depolymerization of microtubules (although after prolonged treatment they can return, albeit in a disordered state). However, isoxaben does not have any noticeable short-term effect on cortical microtubules, and in vitro tubulin assembly is unaffected by this compound. We conclude that isoxaben does not act directly to alter the intrinsic character of the microtubule polymer; rather, the data indicate that isoxaben's effect on the ordering of microtubules is consequential to its inhibitory action on cellulose synthesis.
The microtubule/microfibril paradigm is based on evidence that cortical microtubules affect the spatial organization of newly deposited cellulose, but it does not include the possibility that cellulose affects the organization of microtubules. Therefore, these current data necessitate a re-evaluation of the paradigm. Specifically, the model needs to embrace the bidirectional flow of information between the cytoskeleton and the cell wall. What is the nature of this information? One possibility is that the flow of information from the wall to the underlying cortical microtubules is biophysical in nature.
What role could cellulose play in the transduction of biophysical forces? Cellulose is the strongest component of the primary cell wall and, hence, is normally the dominant load-bearing component. This crystalline polymer's importance in morphogenic modeling is evident by the failure of isoxaben-treated protoplasts to express a preferential growth axis (i.e. they lack a major strain axis; strain is defined here as deformation resulting from turgor. An elongating cell has a major strain axis parallel to the elongative axis and a minor strain axis at right angles [Green and Selker, 1991]). The failure of cortical microtubules to fully acquire order in these cells is consistent with the hypothesis that the microtubule alignment process requires a morphogenically competent cell wall that can properly contain turgor-based growth forces. Additionally, isoxaben affects the organization of microtubules in cells with walls but only after there is a marked change in their growth character and the cells lose their morphogenic competency (i.e. they fail to express a major strain axis).
When cells with walls are treated with isoxaben for short periods, they maintain relatively ordered cortical microtubules. This order remains only until the cells exhibit isodiametric growth. Holdaway et al. (1995) found that cortical microtubule alignment in cells with walls was un-affected by relatively short treatment times with another cellulose inhibitor, dichlobenil. However, in addition to reporting only short treatment times, a significant amount of the initial cell wall was in place. This would predictably permit normal biophysical force transduction and thereby allow for normal biophysical cues to be transmitted to the microtubules. Like isoxaben, dichlobenil inhibits only newly deposited cellulose microfibrils, although it may act at a later biochemical step (Durso and Vaughn, 1997). We found that 10 μm dichlobenil-treated protoplasts behaved similarly to those treated with isoxaben (i.e. longer treatment times result in round cells lacking ordered microtubules, data not shown). We interpret these data as support of a biophysical strain model for microtubule alignment. Microtubules can acquire order via the acquisition of spatial information, as long as the cell possesses the ability to express a dominant strain axis. As was depicted in Figure 6, this alignment process apparently can function regionally in the cell; that region of the cell that is spherical has disorganized microtubules, whereas that region retaining an elongate character has ordered microtubules.
In summary, we propose the following restatement of the microtubule/microfibril paradigm: Cortical microtubules can direct the orientation of cellulose microfibrils, which, because of their great tensile strength, typically limit growth to one major axis. As a result of this unidirectional wall compliance, biophysical forces are generated parallel to the major strain axis and are relayed back to the plasma membrane and to the attached cortical microtubules. The underlying cortical microtubules are thereby exposed to the forces of cell growth and this leads to the alignment of cortical microtubules. Thus, the cell wall, plasma membrane, and underlying cortical microtubules provide a self-reinforcing system to ensure that plant cell expansion occurs continuously in the proper direction. The challenge for future research will be to understand how this cellular system, which is regulated by biophysical feedback, is integrated within the context of developing tissues and organs to ensure normal plant growth and development.
ACKNOWLEDGMENTS
We would like to thank Dr. Kevin Vaughn, who recommended the use of isoxaben for these experiments, for many helpful conversations, and for sharing prepublication data. We would also like to thank Dr. Simon Gilroy for critically reading the manuscript.
Footnotes
This work was supported by a grant from the U.S. Department of Agriculture-National Research Initiative Competitive Grants Program and the Department of Energy Bioscience Program.
LITERATURE CITED
- Basham HG, Bateman DF. Killing of plant cells by pectic enzymes: the lack of direct injurious interaction between pectic enzymes or their soluble reaction products and plant cells. Phytopathology. 1975;65:141–153. [Google Scholar]
- Bucheli P, Doares SH, Albersheim P, Darvill A. Host-pathogen interactions. XXXVI. Partial purification and characterization of heat-labile molecules secreted by the rice blast pathogen that solubilize plant cell wall fragments that kill plant cells. Physiol Mol Plant Pathol. 1990;36:159–173. [Google Scholar]
- Clayton L, Lloyd CW. The relationship between the division plane and spindle geometry in Allium cells treated with CIPC and griseofulvin: an anti-tubulin study. Eur J Cell Biol. 1984;34:248–253. [PubMed] [Google Scholar]
- Corio-Costet M-F, Agnese MD, Scalla R. Effects of isoxaben on sensitive and tolerant plant cell cultures. I. Metabolic fate of isoxaben. Pestic Biochem Physiol. 1991a;40:246–254. [Google Scholar]
- Corio-Costet M-F, Lherminier J, Scalla R. Effect of isoxaben on sensitive and tolerant plant cell cultures. II. Cellular alterations and inhibition of the synthesis of acid-insoluble cell wall material. Pestic Biochem Physiol. 1991b;40:255–265. [Google Scholar]
- Cyr RJ. Microtubules in plant morphogenesis: role of the cortical array. Annu Rev Cell Biol. 1994a;10:153–180. doi: 10.1146/annurev.cb.10.110194.001101. [DOI] [PubMed] [Google Scholar]
- Cyr RJ, Palevitz BA. Organization of cortical microtubules in plant cells. Curr Opin Cell Biol. 1995;7:65–71. doi: 10.1016/0955-0674(95)80046-8. [DOI] [PubMed] [Google Scholar]
- Delmer DP, Amor Y. Cellulose biosynthesis. Plant Cell. 1995;7:987–1000. doi: 10.1105/tpc.7.7.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durso NA, Vaughn KC. The herbicidal manipulation of callose levels in cell plates radically affects cell plate structure (abstract no. 351) Plant Physiol. 1997;114:S-87. [Google Scholar]
- Gertel ET, Green PB. Cell growth pattern and wall microfibrillar arrangement. Plant Physiol. 1977;60:247–254. doi: 10.1104/pp.60.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giddings TH, Jr, Staehelin LA. Microtubule-mediated control of microfibril deposition: a re-examination of the hypothesis. In: Lloyd CW, editor. The Cytoskeletal Basis of Plant Growth and Form. San Diego, CA: Academic Press; 1991. pp. 85–100. [Google Scholar]
- Green PB, Ercikson RO, Richmond PA. On the physical basis of cell morphogenesis. Ann NY Acad Sci. 1970;175:712–731. [Google Scholar]
- Green PB, Selker JML. Mutual alignments of cell walls, cellulose and cytoskeletons: their role in meristems. In: Lloyd CW, editor. The Cytoskeletal Basis of Plant Growth and Form. San Diego, CA: Academic Press; 1991. pp. 303–322. [Google Scholar]
- Green PL, Poethig SP (1982) Biophysics of the extension and initiation of plant organs. In S. Subtelny, PB Green, eds, Developmental Order: Its Origin and Regulation. Alan R Liss, New York, pp 485–509
- Hasezawa S, Hogetsu T, Syono K. Rearrangement of cortical microtubules in elongating cells derived from tobacco protoplasts—a time-course observation by immunofluorescence microscopy. J Plant Physiol. 1988;133:46–51. [Google Scholar]
- Hasezawa S, Marc J, Palevitz BA. Microtubule reorganization during the cell cycle in synchronized BY-2 tobacco suspensions. Cell Motil Cytoskeleton. 1991;18:94–106. [Google Scholar]
- Hasezawa S, Syono K. Hormonal control of elongation of tobacco cells derived from protoplasts. Plant Cell Physiol. 1983;24:127–132. [Google Scholar]
- Heim DR, Bjelk LA, James J, Scheegurt MA, Larrinua IM. Mechanism of isoxaben tolerance in Agrostis palustris var. Penncross. J Exp Bot. 1993;44:1185–1189. [Google Scholar]
- Heim DR, Roberts JL, Pike PD, Larrinua IM. Mutation of a locus of Arabidopsis thaliana confers resistance to the herbicide isoxaben. Plant Physiol. 1989;90:146–150. doi: 10.1104/pp.90.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim DR, Roberts JL, Pike PD, Larrinua IM. A second locus, ixr B1 in Arabidopsis thaliana, that confers resistance to the herbicide isoxaben. Plant Physiol. 1990a;92:858–861. doi: 10.1104/pp.92.3.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim DR, Skomp FR, Tschabold ED, Larrinua IM. Plant Physiol. 1990b;93:695–700. doi: 10.1104/pp.93.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim DR, Skomp JR, Waldron C, Larrinua IM. Differential response to isoxaben of cellulose biosynthesis by wild-type and resistant strains of Arabidopsis thaliana. Pestic Biochem Physiol. 1991;39:93–99. [Google Scholar]
- Hoffman JC, Vaughn KC. Griseofulvin destabilizes plant microtubules. Cytobios. 1994;77:253–258. [Google Scholar]
- Holdaway NJ, White RG, Overall RL. Is the recovery of microtubule orientation in pea roots dependent on the cell wall? Cell Biol Int. 1995;19:913–919. [Google Scholar]
- Hughes J, McCully ME. The use of an optical brightener in the study of plant structure. Stain Technol. 1975;50:319–329. doi: 10.3109/10520297509117082. [DOI] [PubMed] [Google Scholar]
- Hush JM, Overall RL. Cortical microtubule reorientation in higher plants: dynamics and regulation. J Microsc. 1996;181:129–139. [Google Scholar]
- Kuss-Wymer CL, Cyr RJ. Tobacco protoplasts differentiate into elongate cells without net microtubule depolymerization. Protoplasma. 1992;168:64–72. [Google Scholar]
- Lefebvre A, Maizonnier D, Gaudry JC, Clair D, Scalla R. Some effects of the herbicide EL-107 on cellular growth and metabolism. Weed Res. 1987;27:125–134. [Google Scholar]
- Maeda H, Ishida N. Specificity of binding of hexopyransoyl polysaccharides with fluorescent brightener. J Biochem. 1967;62:276–278. doi: 10.1093/oxfordjournals.jbchem.a128660. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Suzaki T. Effects of anti-microtubule drugs on in vitro polymerization of tubulin from mung bean. Bot Mag Tokyo. 1990;103:435–448. [Google Scholar]
- Moore RC, Zhang M, Cassimeris L, Cyr RJ. In vitro assembled plant microtubules exhibit a high state of dynamic instability. Cell Motil Cytoskeleton. 1997;38:278–286. doi: 10.1002/(SICI)1097-0169(1997)38:3<278::AID-CM6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Morejohn LC. The molecular pharmacology of plant tubulin and microtubules. In: Lloyd CW, editor. The Cytoskeletal Basis of Plant Growth and Form. San Diego, CA: Academic Press; 1991. pp. 29–44. [Google Scholar]
- Richmond P. Patterns of cellulose microfibril deposition and rearrangement in Nitella: in vivo analysis by a birefringement index. J Appl Polymer Sci. 1983;37:107–122. [Google Scholar]
- Schneegurt MA, Heim DR, Larrinua IM. Investigation into the mechanism of isoxaben tolerance in dicot weeds. Weed Sci. 1994;42:163–167. [Google Scholar]
- Shibaoka H. Plant hormone-induced changes in the orientation of cortical microtubules: alterations in the cross-linking between microtubules and the plasma membrane. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:527–544. [Google Scholar]
- Stephens GJ, Wood RKS. Killing of protoplasts by soft-rot bacteria. Physiol Plant Pathol. 1975;5:165–181. [Google Scholar]
- Williamson RE. Orientation of cortical microtubules in interphase plant cells. Int Rev Cytol. 1991;129:135–206. [Google Scholar]
- Wymer CL, Fisher DD, Moore RC, Cyr RJ. Elucidating the mechanism of cortical microtubule reorientation in plant cells. Cell Motil Cytoskeleton. 1996a;35:162–173. doi: 10.1002/(SICI)1097-0169(1996)35:2<162::AID-CM8>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Wymer CL, Wymer SA, Cosgrove DJ, Cyr RJ. Plant cell growth responds to external forces and the response requires intact microtubules. Plant Physiol. 1996b;110:425–430. doi: 10.1104/pp.110.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandomeni K, Schopfer P. Mechanosensory microtubule reorientation in the epidermis of maize coleoptiles subjected to bending stress. Protoplasma. 1994;162:96–101. doi: 10.1007/BF01403471. [DOI] [PubMed] [Google Scholar]
- Zhang M (1995) Effect of griseofulvin on microtubule dynamics in plant cells. MS thesis. The Pennsylvania State University, University Park