Abstract
MicroRNAs (miRNAs) are evolutionarily conserved small non-coding RNAs that repress target genes at post-transcriptional level. Langerhans cells (LCs) are skin-residential dendritic cells (DCs) with a life cycle distinct from other types of DCs. miRNA deficiency interrupts the homeostasis and function of epidermal LCs, suggesting the critical roles of miRNAs in LC development and function. However, the roles of individual miRNAs in regulating LC development and function remain completely unknown. miRNA miR-150 is highly expressed in mature lymphocytes, and regulates T and B cell development and function. Here, we reported that miR-150 is also expressed in epidermal LCs and its expression is significantly down-regulated during in vitro LC maturation. Using a miR-150 knockout mouse model, we found that lack of miR-150 reduces the capacity of LCs to cross-present a soluble antigen to antigen-specific CD8+ T cells, but does not disturb the development, maturation, migration and phagocytic capacity of LCs. Thus, our data indicate that miR-150 is required for LC cross-presentation.
Keywords: microRNA, Langerhans cells, cross-presentation
Background
MicroRNAs (miRNAs) are evolutionarily conserved small non-coding RNAs and repress their target genes posttranscriptionally. Emerging evidence suggests that miRNAs-mediated interference is an important regulatory pathway for various biological processes, including skin development and skin diseases (1–3). In the past few years, the cell lineage-specific changes of miRNA expressions have been identified during the differentiation of hematopoietic stem cells (HSCs), suggesting that miRNAs could be key regulators of immune cell development and function (4, 5). We and others recently reported that deletion of Dicer, the enzyme required for processing mature miRNAs, in specific immune cell lineages results in defective development and function in T, B, NK and NKT cells (4–6). Langerhans cells (LCs) are skin-residential dendritic cells (DCs) with a life cycle distinct from other types of DCs. LCs have the ability to traffic peripherally acquired antigens to skin-draining lymph nodes (LNs), where they present processed antigens to T cells and to initiate adaptive immune responses or immune tolerance (7–9). The mechanisms involved in LC homeostasis and immunological functions are still not clear. A recent study showed that DC-specific Dicer deletion interrupted the homeostasis and function of LCs, but not other types of DCs (10), suggesting the specific roles of miRNAs on LC development and function. However, the roles of individual miRNAs in regulating LC development and function are still completely unknown. miRNA miR-150 is highly expressed in resting mature lymphocytes, but not in their progenitors (11, 12). Using gain-of-function and loss-of-function mouse models, we and others reported that miR-150 regulate the development of B cells, NK, and NKT cells (13–15). It’s unclear whether miR-150 is also expressed in LCs and involved in LC development and function.
Questions addressed
To test if LCs express miR-150 and whether lack of miR-150 affects LC development and function.
Experimental design
Results
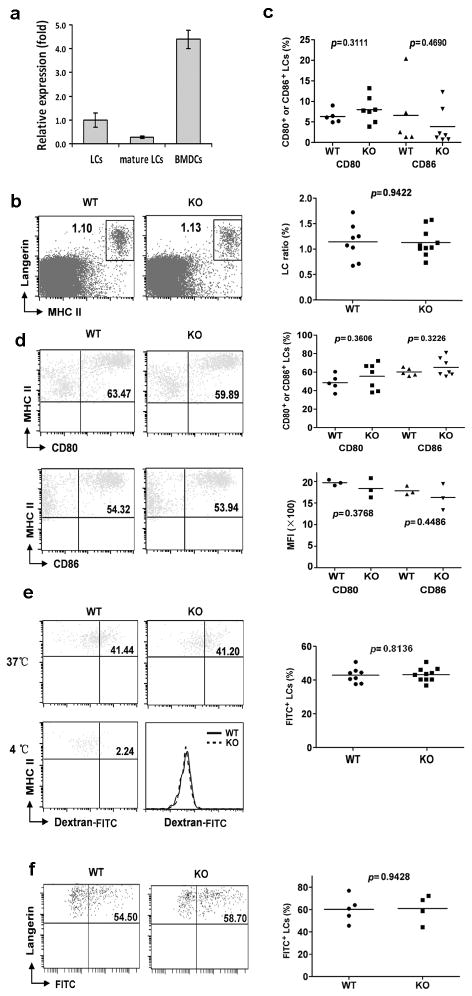
We found that miR-150 is expressed in freshly-isolated epidermal LCs, even though the expression level is lower than that of bone marrow-derive DCs (Fig. 1a). Most interestingly, expression level of miR-150 was significantly down-regulated (3-fold reduction) during in vitro LC maturation. To test the potential role of miR-150 in epidermal LC development and homeostasis, epidermal cell suspensions prepared from miR-150KO and wild-type (WT) mice were stained with anti-Langerin and anti-MHCII antibodies. As shown in (Fig. 1b), the percentage of epidermal LCs was comparable between miR-150KO and WT mice, indicating miR-150 is not critically required for normal LC development. Epidermal LCs are immature skin-residential DCs. When matured, LCs highly express co-stimulatory molecules including MHCII, CD80, and CD86, which is necessary for the stimulation of T cells (7–9). Given the down-regulation of miR-150 expression during the maturation of LCs, we next tested if lack of miR-150 can enhance LC spontaneous maturation. The frequencies of CD86- and CD80-expression LCs in freshly-prepared LCs were comparable between miR150KO and WT mice (Fig. 1c), suggesting that lack of miR-150 did not promote the spontaneous maturation of LCs The expression of these maturation makers were further evaluated after LCs were in vitro cultured for 48 hours to induce their maturation. The frequencies of CD86 and CD80 as well as their relative expression levels in LCs appeared similar comparing miR-150KO with WT mice (Fig. 1d). Thus, lack of miR-150 did not affect LC maturation during in vitro maturation. In addition, no significant differences of LC frequencies or numbers after 48h culture were identified comparing miR-150KO with WT control mice (Fig. S1), further suggseting that loss of miR-150 did not affect the survival or homeostasis of LCs.
Figure 1. miRNA miR-150 is not required for LC development, maturation, antigen-uptake, and migration.
(a) TaqMan real-time PCR analysis of miR-150 expression in epidermal LCs and bone marrow-derive DCs (BMDCs). BMDCs and freshly isolated epidermal LCs enriched with anti-MHCII-PE and anti-PE beads using the AutoMACS were sorted by a FACSAria™II Cell Sorter (CD11c for BMDCs, CD45.2 and MHCII for LCs). For mature LCs, epidermal cells were cultured for 60h, and then autoMACS-enriched LCs were sorted to get mature LCs (CD45.2+MHCIIhigh). Total RNAs of sorted LCs and BMDCs were extracted and miR-150 expression was detected using TaqMan® MicroRNA Assays. snoRNA135 was used as an endogenous control. (b) miR-150 is not required for ontogeny of epidermis LCs. Epidermal cell suspensions prepared from ears and trunk skin of miR-150KO and WT mice were stained with anti-Langerin and MHCII antibodies and analyzed by flow cytometry. Data are representative of 3 independent experiments, 2–4 mice/experiment. (c) miR-150 is not required to maintain the pool of immature LCs in the epidermis. The frequencies of CD80- and CD86-positive cells in gated LCs shown in Fig. 1b were comparable between miR-150KO and WT mice. (d) miR-150 is not an essential factor to control in vitro epidermal LC maturation. Epidermal cell suspensions were cultured in complete culture medium for 48h, then stained with anti-CD45.2, MHCII, CD80, CD86 antibodies, and analyzed by flow cytometry. (e) LCs from miR-150KO mice uptake FITC-Dextran normally. Epidermal cell suspension of miR-150KO and WT mice were incubated with 0.25 mg/ml FITC-Dextran for 45 min at 37°Cor 4°C (served as a control), then stained with anti-MHCII and anti-CD45.2 antibodies. The percentage of FITC+ cells in gated CD45.2+MHCII+ LCs was determined. (f) Lack of miR-150 did not affect the migration of LCs in vivo. miR-150KO and WT mice were painted with 200ul 5mg/ml FITC in acetone/dibutylphthalate (1:1), and 24 hrs later the draining LNs were collected. LN cells were then stained with anti-Langerin and anti-EpCAM. The percentage of migrated FITC+ LCs was analyzed on gated Langerin+ and EpCAM+ LCs.
Like other types of DCs, LCs acquire and process antigens in skin, and subsequently migrate into skin draining LNs to present antigens to T cells, leading to the induction of immunity or tolerance (7–9). As shown in (Fig. 1e), no significant differences of LC phagocytic capacities were detected comparing miR-150KO with WT mice. We further assessed the in vivo antigen capture and migration capability of LCs in miR-150KO mice after FITC painting. There are at least 3 distinct skin DC subsets based on the differential expressions of EpCAM and Langerin, and epidermal LCs are Langerin+EpCAM+ DCs (16). The frequencies of FITC+Langerin+EpCAM+ migrated LCs in LNs were comparable between WT and miR-150KO mice (Fig. 1f). Thus, miR-150 is not critically required for in vivo LC antigen capture and migration to drain LNs. We also analyzed the Langerin+EpCAM− DC population in LNs for the FITC expression. However, few Langerin+EpCAM− cells were FITC positive, and there was no significant difference between KO and WT mice.
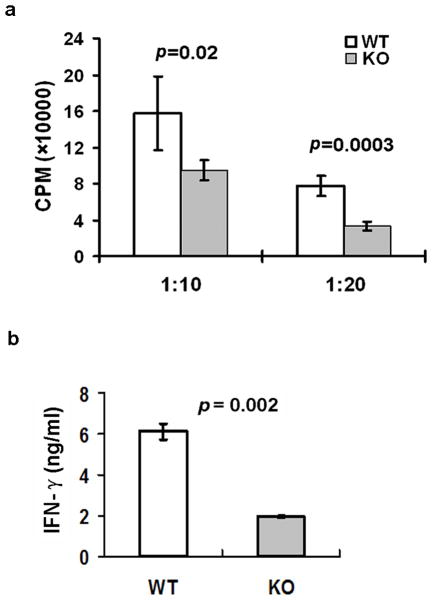
Recent studies indicate that LCs can also cross-present soluble exogenous antigens and cellular antigens from the epidermis to CD8+ T cells (17, 18). Loss of LCs confers a larger deficit in the responding CD8+ T cells compared to the loss of the CD8+ DCs (19). Although miRNA-deficient LCs from Dicer.KO mice could properly stimulate CD8+ T cells (10), it could not rule out the possibilities that specific miRNAs are involved in LC-mediated cross-presentation, as overall deleted miRNAs in Dicer.KO mice could include miRNAs that both positively and negatively regulate LC cross-presentation. To investigate if miR-150 is involved in LC cross-presentation process, epidermal LCs pulsed with ovalbumin were co-cultured with antigen-specific CD8+ or CD4+ T cells from OT-I/OT-II mice for 3 days. As shown in Fig. 2, LCs from miR-150KO mice showed significantly decreased capacity of stimulating antigen-specific CD8+ T cell proliferation (Fig. 2a) and IFN-γ production (Fig. 2b) compared to LCs from WT mice. Interestingly, their capacity of stimulating antigen-specific CD4+ T cells was not significantly changed (Fig S2). Thus, miR-150 is required for normal LC cross-presentation to CD8+ T cells.
Figure 2. miRNA miR-150 is required for LC cross-presentation.
(a) Epidermal cell suspensions from miR-150KO and WT mice pulsed with 0.5 mg/ml ovalbumin protein overnight. After extensive washing, cells were further cultured for 36h. LCs from cultured epidermal cells were first enriched by AutoMACS and then sorted by a FACSAria™II Cell Sorter (the purity of LCs was close to 99%). Sorted LCs were co-cultured with 2.5 × 104 antigen-specific CD8+ T cells from OT-I mice for 3 days. Incorporation of radioactivity during the last 16h of culture was measured. Data are representative of 3 independent experiments, 3–4 mice/experiment. (b) The supernatant from LC/CD8+ T cell co-culture was collected after 48h culture, and the IFNγ production in the co-culture supernatant was analyzed by ELISA. Data are representative of two experiments.
Conclusions
MiRNA miR-150 is expressed in epidermal LCs and its expression is significantly down-regulated during in vitro LC maturation. Lack of miR-150 interrupts the capacity of LCs to cross-present a soluble antigen to antigen-specific CD8+ T cells, but does not disturb the development, maturation, migration and phagocytic capacity of LCs. To the best of our knowledge, this is the first identified miRNA that positively regulates LC cross-presentation. The exact antigen-processing mechanisms that lead to cross-presentation in DCs/LCs are still incompletely understood. Further identification of miR-150 downstream targets involved in LC cross-presentation may illuminate new underlying molecular mechanisms, which will help to develop new intervention strategies for LCs-related therapies.
Supplementary Material
Acknowledgments
We thank Matthew Weiland and Min Liu for their efforts in maintaining our mouse colony and Matthew Weiland for his assistance to sort LCs and T cells. Q.S.M and L.Z. designed experiments and wrote the manuscript, and all authors have read and revised the manuscript critically; R.Q., Y.P.X, Y.L.S and Q.S.M performed the experiments; R.Q., Y.P.X, Y.L.S, L.Z. and Q.S.M analyzed data. This work was supported by grant from National Institutes of Health Grant 1R21AR059976 and Henry Ford Health System Start-up Grant for the Immunology Program (T71016 and T71017).
Footnotes
Conflict of interests
The authors have declared no conflicting interests.
References
- 1.Holst LM, Kaczkowski B, Glud M, Futoma-Kazmierczak E, Hansen LF, Gniadecki R. Exp Dermatol. 2011;20:278–280. doi: 10.1111/j.1600-0625.2010.01163.x. [DOI] [PubMed] [Google Scholar]
- 2.Wei T, Orfanidis K, Xu N, et al. Exp Dermatol. 2010;19:854–856. doi: 10.1111/j.1600-0625.2010.01118.x. [DOI] [PubMed] [Google Scholar]
- 3.Yi R, Poy MN, Stoffel M, Fuchs E. Nature. 2008;452:225–229. doi: 10.1038/nature06642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou L, Park JJ, Zheng Q, Dong Z, Mi Q. Cell Mol Immunol. 2011;8:380–387. doi: 10.1038/cmi.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou L, Seo KH, Wong HK, Mi QS. IntImmunopharmacol. 2009;9:524–527. doi: 10.1016/j.intimp.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Zhou L, Seo KH, He HZ, et al. ProcNatlAcadSciUSA. 2009;106:10266–10271. [Google Scholar]
- 7.Romani N, Clausen BE, Stoitzner P. Immunol Rev. 2010;234:120–141. doi: 10.1111/j.0105-2896.2009.00886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merad M, Ginhoux F, Collin M. Nat RevImmunol. 2008;8:935–947. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan DH. Trends Immunol. 2010;31:446–451. doi: 10.1016/j.it.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuipers H, Schnorfeil FM, Fehling HJ, Bartels H, Brocker T. J Immunol. 2010;185:400–409. doi: 10.4049/jimmunol.0903912. [DOI] [PubMed] [Google Scholar]
- 11.Monticelli S, Ansel KM, Xiao C, et al. Genome Biol. 2005;6:R71. doi: 10.1186/gb-2005-6-8-r71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou B, Wang S, Mayr C, Bartel DP, Lodish HF. Proc Natl Acad Sci U S A. 2007;104:7080–7085. doi: 10.1073/pnas.0702409104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao C, Calado DP, Galler G, et al. Cell. 2007;131:146–159. doi: 10.1016/j.cell.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 14.Bezman NA, Chakraborty T, Bender T, Lanier LL. J Exp Med. 2011;208:2717–2731. doi: 10.1084/jem.20111386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng Q, Zhou L, Mi QS. J Immunol. 2012;188:2118–2126. doi: 10.4049/jimmunol.1103342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagao K, Ginhoux F, Leitner WW, et al. Proc Natl Acad Sci U S A. 2009;106:3312–3317. doi: 10.1073/pnas.0807126106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stoitzner P, Tripp CH, Eberhart A, et al. Proc Natl Acad Sci U S A. 2006;103:7783–7788. doi: 10.1073/pnas.0509307103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kissenpfennig A, it-Yahia S, Clair-Moninot V, et al. MolCell Biol. 2005;25:88–99. doi: 10.1128/MCB.25.1.88-99.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oh JZ, Kurche JS, Burchill MA, Kedl RM. Blood. 2011;118:3028–3038. doi: 10.1182/blood-2011-04-348839. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.