Abstract
Here, we explore the established and potential roles for intradermal adipose tissue in communication with hair follicle biology. The hair follicle delves deep into the rich dermal macroenvironment as it grows to maturity where it is surrounded by large lipid-filled adipocytes. Intradermal adipocytes regenerate with faster kinetics than other adipose tissue depots and in parallel with the hair cycle, suggesting an interplay exists between hair follicle cells and adipocytes. While adipocytes have well-established roles in metabolism and energy storage, until recently, they were overlooked as niche cells that provide important growth signals to neighboring skin cells. We discuss recent data supporting adipocytes as niche cells for the skin and skin pathologies that may be related to alterations in skin adipose tissue defects.
Keywords: adipocyte, hair follicle, skin, preadipoctyes, subcutis, hypodermis
Scope
White adipocytes compose a major component of the skin, yet their role in skin biology has largely been ignored. This Viewpoint article aims to discuss and speculate on potential roles of intradermal adipocytes in cutaneous biology with an emphasis on communication during hair follicle growth and regeneration. We discuss the organization of adipose tissue associated with the skin, and describe how the size of this specialized adipocyte layer is regulated in parallel with the hair follicle cycle. We emphasize the potential role of adipocytes in hair growth and explore how alterations in the intradermal adipose tissue may support clinical manifestations of alopecia and hypertrichosis.
Cutaneous Adipocytes : Intradermal and subcutaneous depots
White adipose tissue (WAT) is composed of unilocular cells that function to store energy through their ability to accumulate and release fatty acids. While the mature adipocytes comprise the majority of WAT mass, WAT also contains several other cell types including immature adipocyte lineage precursors, blood cells, macrophages, and endothelial cells1,2. In addition to energy storage, WAT also has endocrine functions that are involved in food intake, glucose homeostasis, lipid metabolism, inflammation and angiogenesis. Adipose tissue also provides non-energetic functions such as thermal insulation and mechanical cushioning of the body.
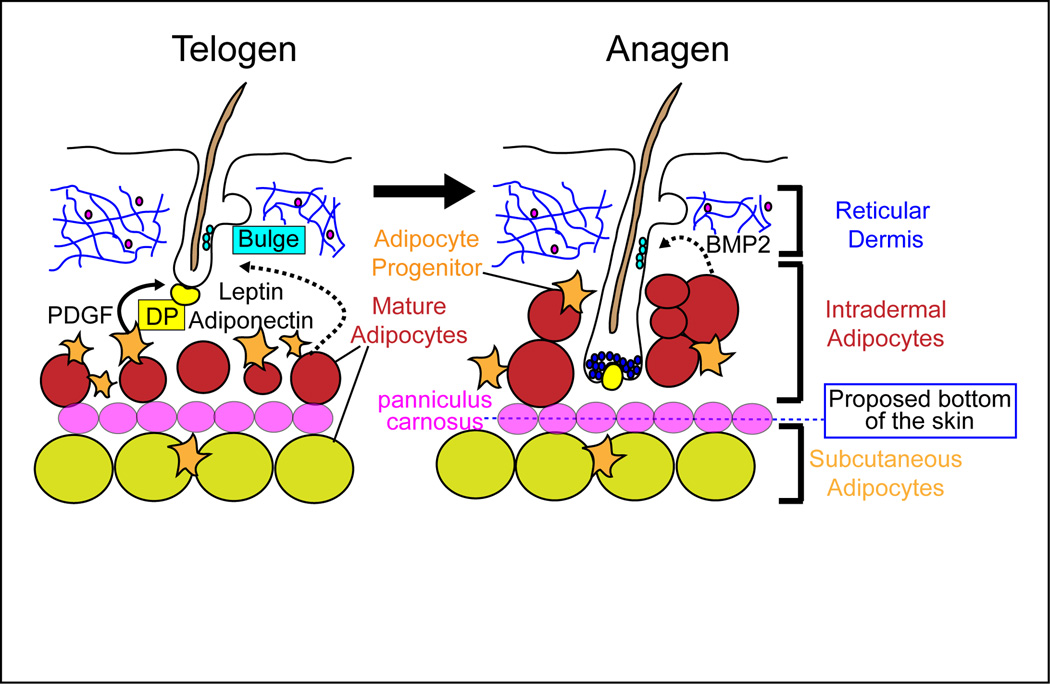
WAT develops at several specific locations called depots that display distinct cellular and molecular characteristics1,2. Two major adipocyte depots are the subcutaneous depot below the skin and the visceral depot within the abdominal cavity. Previously, the skin-associated WAT was described as being located below the dermis or skin in the hypodermis and subcutis, respectively. However, a WAT depot exists below the reticular dermis in the skin and is clearly separated from the subcutaneous WAT depot in rodents by a striated muscle, the panniculus carnosus (Figure 1). The majority of the human body lacks the panniculus carnosus, and yet, two histologically and anatomically distinct layers of adipose tissue exist under the reticular dermis3–6. The most superficial adipocyte layer in human skin is morphologically and metabolically distinct from the deeper adipocyte layer. We refer to the upper layer of adipose as the “intradermal” WAT depot since this layer surrounds hair follicles in both humans and rodents. This terminology ensures that the entire mature anagen hair follicle is included within the definition of the dermis and that the intradermal WAT depot is distinguished from the underlying “subcutaneous” adipose tissue.
Figure 1. Model for potential roles of intradermal adipocytes in the regulation of hair follicle cycling.
A distinct intradermal adipocyte layer exists in the skin, which underlies the fibroblast and extracellular matrix rich reticular dermis and lies above the panniculus carnosus in rodents and the subcutaneous adipocytes. During the transition of the hair follicle from rest (telogen) to growth (anagen), adipocyte progenitor cells are activated to proliferate and form new mature adipocytes that surround the new hair follicle. These immature adipocytes express platelet derived growth factor (PDGF) which can signal to activate anagen. Mature adipocytes also express leptin, adiponectin, and BMP2, which may facilitate hair growth. Dotted lines indicate potential interactions that have not yet been shown conclusively.
The murine intradermal adipocyte depot forms postnatally, resulting in a direct interaction of the growing hair follicles with adipocytes, a relationship reminiscent to the mammary gland epithelium, which grows and branches within a WAT depot7. The organization of the intradermal WAT varies among different mammals from a continuous layer of adipocytes to a discontinuous layer of small adipocyte clusters specifically around compound hair follicles8–10. During follicular morphogenesis, intradermal adipocytes form and grow via lipogenesis resulting in adipocyte hypertrophy and the formation of the intradermal adipocytes that surround each hair follicle. The mechanisms that induce intradermal adipocyte formation are not known.
The hair follicles of the skin continuously cycle through stages of growth and activation (anagen), regression (catagen), and quiescence (telogen). The hair follicle contains a unique population of stem cells that are located in a specific niche known as the bulge. During skin homeostasis, the stem cells of the bulge are quiescent, and become activated to initiate a new anagen phase11. The dermal environment that surrounds each hair follicle is rich in multiple cell types including intradermal white adipocytes, dermal fibroblasts, smooth muscle and endothelial cells of the vasculature, neurons, smooth muscle cells of the arrector pili muscle, and resident immune cells. Recent data suggest that multiple cell types in the dermal macroenvironment are important in the maintenance of the bulge cell population and hair follicle growth9,12,13. Here, we focus on the interplay between adipocytes within the skin, which have become an interesting topic of study, as recent research has shown that adipocytes have intriguing regulatory properties during hair follicle homeostasis.
In general, adipose tissue is long-lived, as adipocytes are estimated to persist in humans for 10 years14. Early histological studies of the skin demonstrated that the layer of intradermal adipocytes expands as the hair follicle enters its growth stage and then thins during telogen8,10,15–17. Since mitotic nuclei were not identified by the histological methods of the time, it was hypothesized that hypertrophy was the primary mode of thickening during this hair growth transition. Indeed, hypertrophy of individual adipocytes does occur in the skin7. However, the growth of the adipocyte layer during during anagen induction also occurs through proliferation and differentiation of resident adipocyte precursor cells7,18. Together, these data support the ability of intradermal adipocytes to form a thick depot through both adipogenesis and hypertrophy.
Hair growth in mice with abnormal adipocyte or sebocyte formation
Mouse models with defects in intradermal adipocytes have been reported. For instance, transgenic mice overexpressing human apolipoprotein C-I in the skin19, fatty acid transport protein (FATP)-4-deficient mice20, and Dgat1−/− or Dgat2−/− mice21,22 have decreased intradermal adipose tissue due to defects in lipid accumulation in mature adipocytes (Table 1). Interestingly, these mice also display abnormalities in skin structure and function such as hair loss and epidermal hyperplasia. However, these mutations also result in abnormal sebaceous gland function due to the role of these proteins in sebaceous gland differentiation.
Table 1.
Mouse models with adipocyte and skin defects
| Mouse Line | Adipose Defects | Skin Defects | Reference |
|---|---|---|---|
| Early B-cell factor-1 (Ebf1−/−) | Reduced intradermal adipocytes, lack of adipocyte progenitors in postnatal skin, increased number of bone marrow and liver adipocytes | Hair follicles fail to re-enter anagen | Hesslein, et. al. 2009 |
| AZIP | Lack of subcutaneous and visceral WAT, decreased BAT, elevated numbers of adipocyte progenitors in putative visceral fat pad and skin | None reported | Moitra, et. al. 1998, Rodeeffer, et. al. 2008, Festa, et. al. 2011 |
| Waved-5 (EGFR−/−) | Reduced intradermal adipocytes | Delayed entry into anagen | Maklad, et. al. 2009, Sugawara, et. al. 2010 |
| Agpat6−/− | Reduced intradermal adipose tissue in adult mice | None reported | Vergnes, et. al. 2006 |
| Fatp4 −/− | Low body weight, reduced intradermal adipose tissue | Epidermal barrier defects, compact dermis, reduced number of sebaceous glands | Hermann, et. al. 2003 |
| Dgat1 −/−, Dgat2−/− | Lack of ability to store triglycerides (Dgat2−/−) | Hair loss at 7 weeks, atrophic sebaceous glands at 3 months | Chen, et. al. 2002, Stone, et. al. 2004 |
| APOC1 | Lack of subcutaneous fat, reduced visceral adipose tissue | Scaly skin, loss of hair, atrophic sebaceous glands | Jong, et. a. 1998 |
Several parallels exist between adipogenesis and sebocyte differentiation. Specification of the sebaceous gland first becomes evident postnatally with the identification of cells in the upper hair follicle that express the adipogenic transcription factor PPARγ23. Further maturation of sebocytes results in large differentiated cells that are filled with lipids. Given that both adipocytes and sebocytes mature through PPARγ and lipid accumulation, variations in multiple genes that modify adipocytes may also affect sebaceous gland function. Since defective sebaceous glands can change hair follicle stem cell activity and lead to skin abnormalities23,24, the skin defects in the apolipoprotein C-I transgenic, FATP4−/−, Dgat1–/, or Dgat2−/− mice were not clearly attributable to deficiencies in adipocytes in the skin but could be due to defects in sebocytes.
In contrast to the mouse models described above, several mutant mice with defects in adipocytes, but not sebocytes, have allowed the elucidation of the roles of adipocytes in the skin11 (Table 1). A genetic mouse model lacking Early B cell factor 1 (Ebf1−/−), which is expressed in the skin in intradermal adipocytes, sebaceous glands, and dermal papillae of anagen hair follicles, exhibits reduced intradermal adipocytes25 due in part to a lack of adipocyte precursor cells postnatally. Hair follicles in these mice also fail to re-enter anagen, and instead remain in late catagen or telogen, which indicates the failure of bulge SC to become active following initial hair follicle growth.
The importance of immature adipocytes in the promotion of the telogen to anagen transition during the hair follicle cycle is clearly evident when these findings are compared to the AZIP mouse model. AZIP mice lack mature fat cells throughout the body, although these mice do have resident, and even elevated, numbers of intradermal adipocyte progenitor cells. The absence of mature adipocytes is due to the expression of a dominant-negative form of C/EBP in the late stages of adipogenesis, which blocks mature adipocyte formation7,26. The hair follicles of these mice enter anagen at the same rate as wild-type mice.
In addition to the requirement of adipocytes in the skin for anagen induction, adipocyte lineage cells are sufficient to induce the hair follicle cycle. Transplantation of adipocyte progenitor cells intradermally into the backskin of shaved mice at the extended 3–4 week telogen stage of the hair follicle cycle that occurs around 7 weeks of age resulted in adipocyte graft formation and corresponding precocious hair growth. Anagen was induced in these mice injected with the enriched adipocyte progenitor cells from WT or AZIP, but not with cells of the entire stromal vascular fraction (SVF), supporting that the hair-inducing activity was specific for immature adipocyte lineage cells7.
The mechanism behind this interaction is not completely understood, but PDGF signaling may play a role7. PDGFA mRNA is significantly elevated in adipocyte precursor cells, and mice lacking PDGFA show a delay in hair follicle stem cell activation that mirrors the phenotype of Ebf1−/− mice27,28. The lack of mouse models that allow genetic manipulation in immature adipocytes to date precludes the specific genetic deletion of PDGF in intradermal adipocyte precursor cells. Future experiments can clarify the role of secreted factors produced by adipocytes during the hair cycle using engraftment of adipocyte lineage cells isolated from mice lacking specific factors.
In addition to adipocyte derived induction mechanisms for hair growth, intrinsic mechanisms within hair follicles can activate the telogen to anagen transition29. For instance, activation of calcineurin/NFAT signaling or hair plucking can induce hair cycling independent from adipogenesis29–32. Whether hair activation can induce adipogenesis has not been explored. In addition, vibrissae hair follicles reside within an encapsulated blood sinus, that lacks differentiated adipocytes, yet can cycle very fast and efficiently. Whether immature adipocytes or another cell type within the sinus promote the telogen to anagen transition is not clear.
Factors secreted from mature adipocytes may influence hair growth
Mature adipocytes also express signaling molecules that can modulate hair cycling. Intradermal adipocytes express BMP2 maximally in late anagen and early telogen, causing follicles to be refractory to activation cues30. Given that BMP signaling blocks anagen induction33–36, these data suggest that adipocyte derived BMPs may promote and maintain follicular stem cell quiescence. Thus, when BMP signals are reduced in the macroenvironment, the hair follicles are open to activation signals, enter into a competent telogen phase and the follicles can re-enter anagen. The contribution of adipocyte derived BMP proteins is unknown since the dermal papillae also express BMP mRNAs37.
Epidermal growth factor (EGF) signaling may be another possible connection between the hair follicle and intradermal adipocytes. Mutations in the EGF receptor that result in the expression of a dominant-negative EGFR38 cause a slightly delayed regression of the follicle during catagen, as well as a wavy hair phenotype. Interestingly, during early postnatal stages, the intradermal adipocyte layer is reduced during the initial stages of anagen39. Since mice that conditionally lack EGFR in the skin epithelium also display defects in intradermal adipocytes40, the hair follicle may regulate intradermal adipocyte growth in signaling pathways downstream of EGFR or through the hair follicle cycle itself. Future work will be needed to tease out the interplay between the hair follicles and intradermal adipogenesis.
The expression of classic adipokines and long chain free fatty acids (FFAs) may also impact hair follicle biology. The leptin receptor is expressed on dermal papillae cells41. In addition, adiponectin has been shown to influence keratinocyte growth and differentiation42. In addition to functioning as energy sources, long-chain fatty acids have been recognized as crucial elements of signaling pathways, particularly those involved in the endocrine, nervous, and immune systems4,43,44. FFAs can act as secondary messengers and ligands to act both intra- and intercellularly to elicit or amplify metabolic responses45–47. FFAs, particularly oleic and palmitic acids, have been shown to regulate gene expression at the transcriptional level in adipocytes48–50. FFA have additionally been shown to reduce dermal fibroblast proliferation51. Adipose tissue is the source of most FFA found systemically, and alterations in lipid metabolism can affect the amount of FFA found throughout the body52. Excess FFA, such as in cases of obesity, can result in altered metabolic function of numerous body organs, including, but not limited to, the liver, heart, and skeletal muscle. It is possible that FFA and other adipokines in the skin also have similar functions, and may directly or indirectly contribute to gene or protein expression in the hair follicle or appendages. The possibility that these molecules are “feeding” or signaling to the hair follicle cells has not yet been explored. AZIP mice may be useful to tease out the impact of mature adipocytes on the homeostasis of the skin.
Potential cutaneous manifestations of lipid-associated diseases in humans
Extrinsic regulation of the hair follicle cells from the macroenviroment has the potential to alter the clinical perspective of disorders affecting human hair follicle biology. Several disorders are known to increase or involve an absence of fat mass including obesity and lipodystrophy, respectively, and intradermal adipocytes are clearly becoming recognized for more than just their roles in energy storage53. Lipodystrophy can be associated with alopecia54,55 and hirsutism56. In addition, production of certain adipokines, such as lepin, adiponectin, and visfatin is increased in patients with psoriasis57. Furthermore, patients with Goltz syndrome or focal dermal hypoplasia display alterations in skin adipose tissue associated with sparse, brittle hair with patchy alopecia17,58. Obesity can also be associated with hirsutism and hypertrichosis, or excessive hair in areas that are not usually androgen responsive59. Interestingly, mice lacking androgen receptor (AR) become obese suggesting a link between androgen signaling and adipocytes60. Furthermore, expression of AR has also been shown in vitro to downregulate the expression of PPARγ mRNA61, as well as to reduce the proliferation of adipocyte progenitors62. Since dysfunction of adipocytes can alter insulin signaling and other metabolic signaling pathways, establishing the role of intradermal adipocytes in many of these disorders may alter treatment options for cutaneous manifestations of these diseases.
Acknowledgements
We thank Ralf Paus and Matthew Rodeheffer for critical reading of this manuscript. V.H. is a Pew Scholar in Biomedical Research and is funded by the NIAMS (AR060295). B.S. prepared the figures and wrote the manuscript. V.H. edited the figures and wrote the manuscript.
Footnotes
Conflicts of Interest
The authors do not have any conflicts of interest to declare.
References
- 1.Lundgren M. Glucocorticoids Down-Regulate Glucose Uptake Capacity and Insulin-Signaling Proteins in Omental But Not Subcutaneous Human Adipocytes. Journal of Clinical Endocrinology & Metabolism. 2004;89:2989–2997. doi: 10.1210/jc.2003-031157. [DOI] [PubMed] [Google Scholar]
- 2.Ostman J, Arner P, Engfeldt P, Kager L. Regional differences in the control of lipolysis in human adipose tissue. Metabolism. 1979;28:1198–1205. doi: 10.1016/0026-0495(79)90131-8. [DOI] [PubMed] [Google Scholar]
- 3.Smith SR, et al. Contributions of total body fat abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism. 2001;50:425–435. doi: 10.1053/meta.2001.21693. [DOI] [PubMed] [Google Scholar]
- 4.Miyazaki Y, et al. Abdominal fat distribution and peripheral and hepatic insulin resistance in type 2 diabetes mellitus. Am. J Physiol. Endocrinol. Metab. 2002;283:E1135–E1143. doi: 10.1152/ajpendo.0327.2001. [DOI] [PubMed] [Google Scholar]
- 5.Walker GE, et al. Deep subcutaneous adipose tissue: a distinct abdominal adipose depot. Obesity (Silver Spring) 2007;15:1933–1943. doi: 10.1038/oby.2007.231. [DOI] [PubMed] [Google Scholar]
- 6.Sbarbati A, et al. Subcutaneous adipose tissue classification. Eur J Histochem. 2010;54:e48. doi: 10.4081/ejh.2010.e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Festa E, et al. Adipocyte Lineage Cells Contribute to the Skin Stem Cell Niche to Drive Hair Cycling. Cell. 2011;146:761–771. doi: 10.1016/j.cell.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chase HB, Montagna W, Malone JD. Changes in the skin in relation to the hair growth cycle. Anat Rec. 1953;116:75–81. doi: 10.1002/ar.1091160107. [DOI] [PubMed] [Google Scholar]
- 9.Plikus MV, et al. Self-Organizing and Stochastic Behaviors During the Regeneration of Hair Stem Cells. Science. 2011;332:586–589. doi: 10.1126/science.1201647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen LS, Coggle JE, Wells J, Charles MW. The influence of the hair cycle on the thickness of mouse skin. Anat Rec. 1984;210:569–573. doi: 10.1002/ar.1092100404. [DOI] [PubMed] [Google Scholar]
- 11.Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Botchkarev VA, et al. Neurotrophins in skin biology and pathology. J Invest Dermatol. 2006;126:1719–1727. doi: 10.1038/sj.jid.5700270. [DOI] [PubMed] [Google Scholar]
- 13.Fujiwara H, et al. The Basement Membrane of Hair Follicle Stem Cells Is a Muscle Cell Niche. Cell. 2011;144:577–589. doi: 10.1016/j.cell.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spalding KL, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 15.Hausman GJ. Adipocyte development in subcutaneous tissues of the young rat. Acta Anat (Basel) 1982;112:185–196. doi: 10.1159/000145510. [DOI] [PubMed] [Google Scholar]
- 16.Hausman GJ, Campion DR, Richardson RL, Martin RJ. Adipocyte development in the rat hypodermis. Am. J Anat. 1981;161:85–100. doi: 10.1002/aja.1001610107. [DOI] [PubMed] [Google Scholar]
- 17.Hausman GJ, Martin RJ. The development of adipocytes located around hair follicles in the fetal pig. J. Anim. Sci. 1982;54:1286–1296. doi: 10.2527/jas1982.5461286x. [DOI] [PubMed] [Google Scholar]
- 18.Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240–249. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 19.Jong MC, et al. Hyperlipidemia and cutaneous abnormalities in transgenic mice overexpressing human apolipoprotein C1. J Clin Invest. 1998;101:145–152. doi: 10.1172/JCI791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrmann T, et al. Mice with targeted disruption of the fatty acid transport protein (Fatp 4, Slc27a4) gene show features of lethal restrictive dermopathy. J Cell Biol. 2003;161:1105–1115. doi: 10.1083/jcb.200207080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen HC, Smith SJ, Tow B, Elias PM, Farese RV. Leptin modulates the effects of acyl CoA:diacylglycerol acyltransferase deficiency on murine fur and sebaceous glands. J Clin Invest. 2002;109:175–181. doi: 10.1172/JCI13880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stone SJ, et al. Lipopenia and skin barrier abnormalities in DGAT2-deficient mice. J Biol Chem. 2004;279:11767–11776. doi: 10.1074/jbc.M311000200. [DOI] [PubMed] [Google Scholar]
- 23.Horsley V, et al. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell. 2006;126:597–609. doi: 10.1016/j.cell.2006.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petersson M, et al. TCF/Lef1 activity controls establishment of diverse stem and progenitor cell compartments in mouse epidermis. EMBO J. 2011;30:3004–3018. doi: 10.1038/emboj.2011.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hesslein DGT, et al. Ebf1-dependent control of the osteoblast and adipocyte lineages. Bone. 2009;44:537–546. doi: 10.1016/j.bone.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moitra J, et al. Life without white fat: a transgenic mouse. Genes Dev. 1998;12:3168–3181. doi: 10.1101/gad.12.20.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karlsson L, Bondjers C, Betsholtz C. Roles for PDGF-A and sonic hedgehog in development of mesenchymal components of the hair follicle. Development. 1999;126:2611–2621. doi: 10.1242/dev.126.12.2611. [DOI] [PubMed] [Google Scholar]
- 28.Tomita Y, Akiyama M, Shimizu H. PDGF isoforms induce and maintain anagen phase of murine hair follicles. J Dermatol Sci. 2006;43:105–115. doi: 10.1016/j.jdermsci.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 29.Hsu Y-C, Pasolli HA, Fuchs E. Dynamics between Stem Cells, Niche, and Progeny in the Hair Follicle. Cell. 2011;144:92–105. doi: 10.1016/j.cell.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plikus MV, et al. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451:340–344. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horsley V, Aliprantis AO, Polak L, Glimcher LH, Fuchs E. NFATc1 balances quiescence and proliferation of skin stem cells. Cell. 2008;132:299–310. doi: 10.1016/j.cell.2007.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldstein J, Horsley V. Home sweet home: skin stem cell niches. Cell. Mol. Life Sci. 2012 doi: 10.1007/s00018-012-0943-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobielak K, Stokes N, la Cruz de J, Polak L, Fuchs E. Loss of a quiescent niche but not follicle stem cells in the absence of bone morphogenetic protein signaling. Proc Natl Acad Sci USA. 2007;104:10063–10068. doi: 10.1073/pnas.0703004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, et al. Bone morphogenetic protein signaling inhibits hair follicle anagen induction by restricting epithelial stem/progenitor cell activation and expansion. STEM CELLS. 2006;24:2826–2839. doi: 10.1634/stemcells.2005-0544. [DOI] [PubMed] [Google Scholar]
- 35.Plikus MV, Widelitz RB, Maxson R, Chuong C-M. Analyses of regenerative wave patterns in adult hair follicle populations reveal macroenvironmental regulation of stem cell activity. Int J Dev Biol. 2009;53:857–868. doi: 10.1387/ijdb.072564mp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Millar SE. Smad7: licensed to kill beta-catenin. Developmental Cell. 2006;11:274–276. doi: 10.1016/j.devcel.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 37.Rendl M, Polak L, Fuchs E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev. 2008;22:543–557. doi: 10.1101/gad.1614408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee D, et al. Wa5 is a novel ENU-induced antimorphic allele of the epidermal growth factor receptor. Mamm. Genome. 2004;15:525–536. doi: 10.1007/s00335-004-2384-2. [DOI] [PubMed] [Google Scholar]
- 39.Sugawara K, Schneider MR, Dahlhoff M, Kloepper JE, Paus R. Cutaneous consequences of inhibiting EGF receptor signaling in vivo: normal hair follicle development, but retarded hair cycle induction and inhibition of adipocyte growth in Egfr(Wa5) mice. J Dermatol Sci. 2010;57:155–161. doi: 10.1016/j.jdermsci.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 40.Maklad A, et al. The EGFR is required for proper innervation to the skin. J Investig Dermatol. 2009;129:690–698. doi: 10.1038/jid.2008.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rendl M, Lewis L, Fuchs E. Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biol. 2005;3:e331. doi: 10.1371/journal.pbio.0030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawai K, et al. Effects of adiponectin on growth and differentiation of human keratinocytes--implication of impaired wound healing in diabetes. Biochem. Biophys. Res. Commun. 2008;374:269–273. doi: 10.1016/j.bbrc.2008.07.045. [DOI] [PubMed] [Google Scholar]
- 43.Steneberg P, Rubins N, Bartoov-Shifman R, Walker MD, Edlund H. The FFA receptor GPR40 links hyperinsulinemia, hepatic steatosis, and impaired glucose homeostasis in mouse. Cell Metabolism. 2005;1:245–258. doi: 10.1016/j.cmet.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 44.Brunkhorst BA, Kraus E, Coppi M, Budnick M, Niederman R. Propionate induces polymorphonuclear leukocyte activation and inhibits formylmethionylleucyl-phenylalanine-stimulated activation. Infect. Immun. 1992;60:2957–2968. doi: 10.1128/iai.60.7.2957-2968.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nunez E. Biological complexity is under the “strange attraction” of non-esterified fatty acids. Prostaglandins Leukot Essent Fatty Acids. 1997;57:107–110. doi: 10.1016/s0952-3278(97)90500-7. [DOI] [PubMed] [Google Scholar]
- 46.Sumida C, Graber R, Nunez E. Role of fatty acids in signal transduction: modulators and messengers. Prostaglandins Leukot Essent Fatty Acids. 1993;48:117–122. doi: 10.1016/0952-3278(93)90019-s. [DOI] [PubMed] [Google Scholar]
- 47.Ichimura A, et al. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature. 2012:1–8. doi: 10.1038/nature10798. [DOI] [PubMed] [Google Scholar]
- 48.Dennis EA, Rhee SG, Billah MM, Hannun YA. Role of phospholipase in generating lipid second messengers in signal transduction. FASEB J. 1991;5:2068–2077. doi: 10.1096/fasebj.5.7.1901288. [DOI] [PubMed] [Google Scholar]
- 49.Distel RJ, Robinson GS, Spiegelman BM. Fatty acid regulation of gene expression. Transcriptional and post-transcriptional mechanisms. J Biol Chem. 1992;267:5937–5941. [PubMed] [Google Scholar]
- 50.Clarke SD, Jump DB. Polyunsaturated fatty acid regulation of hepatic gene transcription. J. Nutr. 1996;126:1105S–1109S. doi: 10.1093/jn/126.suppl_4.1105S. [DOI] [PubMed] [Google Scholar]
- 51.Ezure T, Amano S. Negative regulation of dermal fibroblasts by enlarged adipocytes through release of free fatty acids. J Investig Dermatol. 2011;131:2004–2009. doi: 10.1038/jid.2011.145. [DOI] [PubMed] [Google Scholar]
- 52.Bergman RN, Ader M. Free fatty acids and pathogenesis of type 2 diabetes mellitus. Trends Endocrinol Metab. 2000;11:351–356. doi: 10.1016/s1043-2760(00)00323-4. [DOI] [PubMed] [Google Scholar]
- 53.Klein J, et al. What are subcutaneous adipocytes reallygood for…? Experimental Dermatology. 2007;16:45–70. doi: 10.1111/j.1600-0625.2006.00519_1.x. [DOI] [PubMed] [Google Scholar]
- 54.Fukumoto D, Kubo Y, Saito M, Arase S. Centrifugal lipodystrophy of the scalp presenting with an arch-form alopecia: a 10-year follow-up observation. J. Dermatol. 2009;36:499–503. doi: 10.1111/j.1346-8138.2009.00685.x. [DOI] [PubMed] [Google Scholar]
- 55.Agarwal AK, Garg A. Genetic Disorders of Adipose Tissue Development, Differentiation, and Death. Annu. Rev. Genom. Human Genet. 2006;7:175–199. doi: 10.1146/annurev.genom.7.080505.115715. [DOI] [PubMed] [Google Scholar]
- 56.Savage DB, et al. A clinical approach to severe insulin resistance. Endocr Dev. 2007;11:122–132. doi: 10.1159/000111067. [DOI] [PubMed] [Google Scholar]
- 57.Gerdes S, et al. Leptin, adiponectin, visfatin and retinol-binding protein-4 -mediators of comorbidities in patients with psoriasis? Experimental Dermatology. 2012;21:43–47. doi: 10.1111/j.1600-0625.2011.01402.x. [DOI] [PubMed] [Google Scholar]
- 58.Goltz RW. Focal dermal hypoplasia syndrome. An update. Arch Dermatol. 1992;128:1108–1111. [PubMed] [Google Scholar]
- 59.Samara-Boustani D, et al. High prevalence of hirsutism and menstrual disorders in obese adolescent girls and adolescent girls with type 1 diabetes mellitus despite different hormonal profiles. Eur. J Endocrinol. 2012;166:307–316. doi: 10.1530/EJE-11-0670. [DOI] [PubMed] [Google Scholar]
- 60.Sato T, et al. Late onset of obesity in male androgen receptor-deficient (AR KO) mice. Biochem. Biophys. Res. Commun. 2003;300:167–171. doi: 10.1016/s0006-291x(02)02774-2. [DOI] [PubMed] [Google Scholar]
- 61.Kajita K, et al. Dehydroepiandrosterone down-regulates the expression of peroxisome proliferator-activated receptor gamma in adipocytes. Endocrinology. 2003;144:253–259. doi: 10.1210/en.2002-220039. [DOI] [PubMed] [Google Scholar]
- 62.Fujioka K, et al. Dehydroepiandrosterone Reduces Preadipocyte Proliferation via Androgen Receptor. AJP: Endocrinology and Metabolism. 2012 doi: 10.1152/ajpendo.00112.2011. [DOI] [PubMed] [Google Scholar]