Abstract
Metallothioneins (MTs) protect cells from oxidative damage by scavenging reactive oxygen species (ROS). Concurrent with protecting cells from ROS-mediated damage, MT transcription is induced by ROS. ROS activate transcription by affecting several signal transduction pathways, many of which have been implicated in regulating MT transcription. ROS-activated intracellular signaling is mediated by the stable lipid peroxide 4-hydroxynonenal (HNE). After determining the level of sensitivity of Hepa1-6 cells to HNE, MT-1 mRNA expression was shown to be induced in a concentration and time dependent manner after HNE exposure. Finally, using MT-based reporters, HNE was found to induce MT transcription via both antioxidant response and metal response elements. Thus, ROS may activate MT transcription by generating HNE which in turn affects signaling pathways that regulate MT transcription via the transcription factors AP-1 and MTF-1.
Keywords: 4-hydroxynonenal, metallothionein, transcription, oxidative stress, reactive oxygen species
Introduction
Metallothioneins (MTs) are an evolutionarily conserved family of cysteine-rich proteins that function in maintaining homeostatic levels of essential metals (copper, zinc) and detoxification of non-essential metals (cadmium, mercury) (1). The ability of MTs to bind metals is due to its high cysteine content, which also makes them efficient scavengers of reactive oxygen species (ROS) (2,3). The role of MT in ameliorating ROS toxicity is supported by the observations that increased MT expression can protect cells from ROS-mediated damage (4,5). Likewise, MT-null cells are hypersensitive to ROS generating toxicants (6).
Concurrent with protecting cells from ROS-mediated damage, MT transcription can be induced by ROS-generating compounds. Treatment with hydrogen peroxide, antimycin, ionizing radiation, paraquat, or redox cycling metals (e.g., copper) induces MT transcription (3,7–11). Transcriptional induction by ROS is mediated by both metal-response elements (MREs) and antioxidant response elements (AREs), which are located in the promoters of many MT genes, including the mouse metallothionein -1 gene (MT-1) (7,11).
While ROS can be cytotoxic, they can also serve as signaling molecules. Nitric oxide, superoxide, and hydrogen peroxide have been shown to modify signaling proteins and can activate transcription by affecting several signal transduction pathways including mitogen-activated protein kinase (MAPK) and NF-κB (12,13). Both of these signaling pathways are involved in MT transcriptional regulation (14,15). 4-Hydroxynonenal is one of the major end-products of lipid peroxidation that is produced when polyunsaturated fatty acids are oxidized. It has the ability to covalently bind proteins and form stable adducts that serve as signaling molecules (16,17). The ability of ROS to activate MAPK signaling is affected by HNE (12). Here we report on the ability of HNE to affect the steady state levels of MT-1 mRNA in a dose-and time-dependent manner, and activate MT transcription. This process appears to be transcriptionally regulated by AP-1 and MTF-1.
Materials and Methods
Cell Culture
COS-7 cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, and 100 μM nonessential amino acids (GIBCO® Invitrogen, Carlsbad, CA). Hepa 1–6 mouse hepatoma cells were grown in DMEM supplemented with 10% fetal bovine serum. Both cell lines were maintained in a humidified incubator at 37°C in 5% CO2.
Neutral Red Viability Assay
Hepa 1–6 cells were added to 6-well plates at an initial density of 1 × 105 cells/well. The next day, cells were treated with increasing concentrations of HNE (Cayman Chemical, Ann Arbor, MI) for 24 h. Control cells were treated with 0.16 % ethanol. After a 24 h incubation, cell viability was determined by Neutral Red assay, as previously described (11). Triplicate experiments were performed for each concentration and the results were expressed as percent viability, relative to ethanol-treated cells. A one-way ANOVA was performed followed by a Dunnett’s Test to compare each HNE treatment to ethanol-treated control cells.
Quantitative Real-Time RT-PCR
Hepa 1–6 cells were plated at a density of 1 × 105 cells/well in 6 well plates and exposed to several concentrations of HNE for 24 h or to 40 μM HNE for increasing periods of time. As a control, cells were exposed to ethanol (vehicle control) and process identically to the HNE-treated cells. After incubation, cells were washed twice with Hanks buffered saline and total RNA was isolated using RNeasy Mini kits, according to manufacturer’s instructions (Qiagen, Inc., Valencia, CA). cDNAs were generated from total RNA using the SuperScript First-Strand Synthesis System, according to manufacturer’s instructions (Invitrogen/Life Technologies, Carlsbad, CA). The resulting cDNAs were used in qRT-PCR reactions using Power SYBR Green RT-PCR kits, according to manufacturer’s instructions (Applied Biosystems, Foster City, CA). Quantitative PCR was performed in an ABI 7900 HT Fast Real-Time System and fold changes in mRNA levels were calculated using the ΔΔCt method with GAPDH as reference mRNA (18). Prior to statistical analysis the fold-change in MT-1 mRNA was natural log transformed. The effect different concentrations of HNE had on MT-1 expression; relative to untreated samples; was analyzed using one-way ANOVA followed by a Dunnett’s test. For time course experiments, changes in MT-1 expression were evaluated between treatments by a two-way ANOVA using untreated control samples at the 0 h time point as a reference. A Bonferroni post-hoc test was used to determine differences between each time point. These analyses were performed using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA).
Transient Transfection and Reporter Gene Assays
COS-7 cells were plated at a density of 8.0 × 104 cells/well in 24-well culture plates and allowed to grow for 18–24 h before transfection. Cells were transfected with chloramphenicol acetyltransferase (CAT)-containing reporter genes containing regions of the mouse MT-1 promoter (−42/+63-CAT, −153/+62-CAT, MREd5-CAT, and ARE4-CAT) as previously described (7,11). Following transfection, the cells were allowed to recover overnight. Cells were subsequently treated with 10 μM HNE for 24 h. The levels of reporter gene activity were then measured as previously described (11).Statistical analysis was performed using StatView software (JMP Software, Cary, NC). Results are presented as the mean ± standard error. The significance of mean differences for the reporter gene assays was determined by Student’s t-test.
Results and Discussion
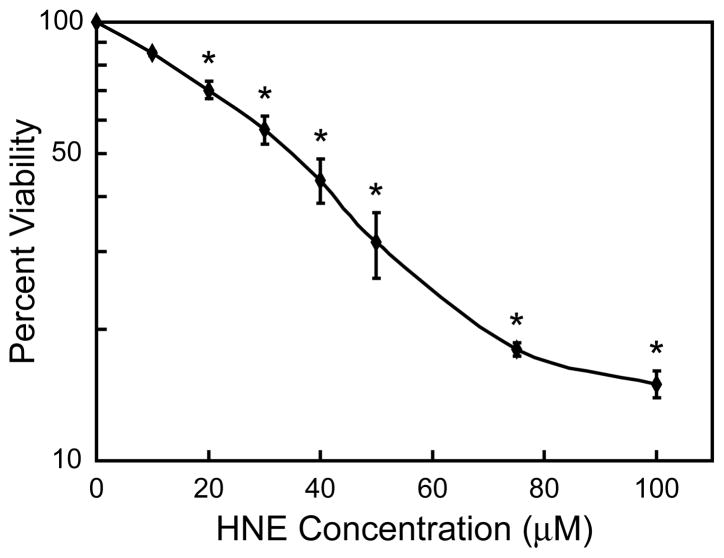
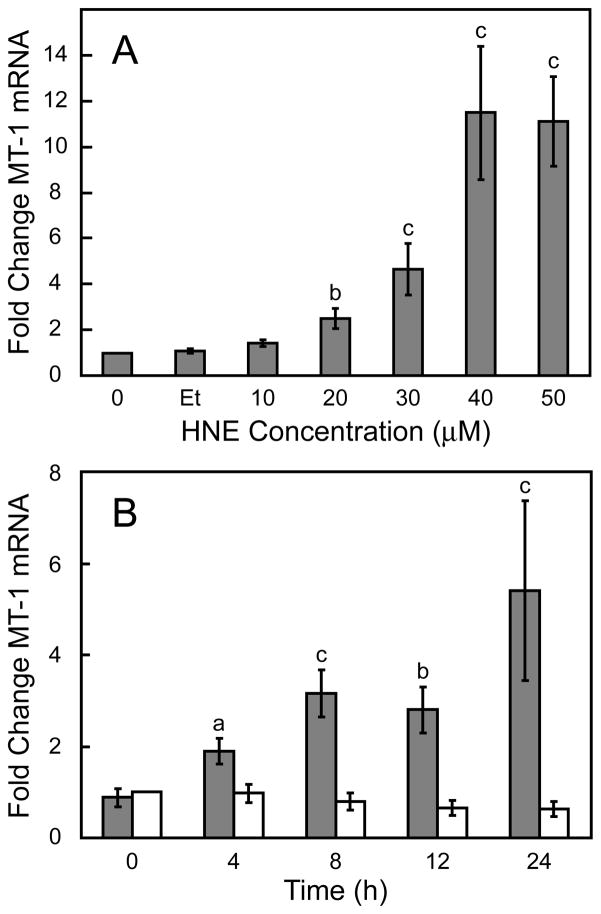
Cell viability was first performed to determine the level of sensitivity of exogenously added HNE on Hepa 1–6 cells. Hepa 1–6 cells showed a concentration dependent sensitivity to HNE. Maximum toxicity was observed at the highest concentration of 100 μM with an estimated EC50 of ~30 μM (Fig. 1). The steady state level of MT mRNA increased following exposure to increasing concentrations of HNE. MT mRNA levels increased in a dose-dependent manner after treatment with HNE for 24 h, with the maximal level of induction reached after a 40 μM exposure (Fig 2A). The kinetics for MT mRNA induction after exposure to 40 μM HNE was also determined. A steady increase in MT mRNA levels over the 24 h duration of the study was observed (Fig. 2B).
Figure 1. Effect of HNE on Hepa 1–6 cell viability.
Hepa 1–6 cells were exposed to increasing concentrations of HNE for 24 h and then cell survival was assessed using the Neutral Red viability assay. Percent viability was plotted at each concentration and represents the mean values of triplicate experiments ± standard error. * indicate significant difference from non-treated cells at p < 0.001.
Figure 2. Effect of HNE on steady state MT-1 mRNA levels.
panel A, concentration dependent induction of MT-1 mRNA in HNE-treated Hepa1–6 cells (Et, ethanol control). panel B, time dependent induction of MT-1 mRNA after exposure to 40 μM HNE. Steady state MT-1 mRNA levels were determined by qRT-PCR and presented as mean ratios ± standard error (gray bars), relative to non-treated cells (n = 4). Ethanol-treated cells (white bars) are shown as a control, as ethanol was used as a solvent for the HNE. a, b, and c indicate significant differences at p < 0.05, p < 0.01 and p < 0.001, respectively.
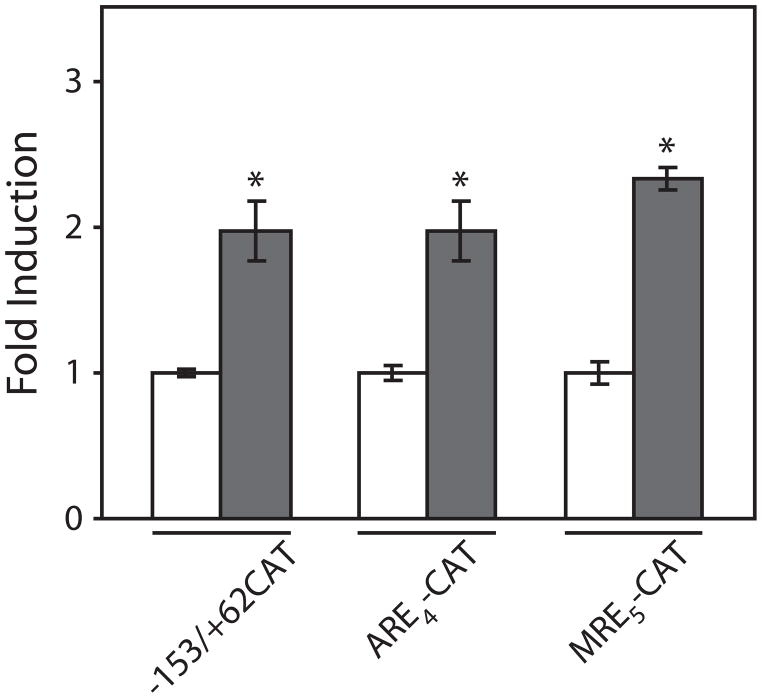
To identify specific transcription factors that may contribute to the regulation of MT gene expression, three different reporter genes were utilized that contain sequences derived from the mouse MT-1 promoter. These sequences included the fully functional 153 bp MT-1 promoter fragment, concatenated copies of MREs, or concatenated copies of AREs (7). After treatment with 10 μM HNE for 24 h, the levels of reporter gene expression significantly increased (Fig. 3). This increase was observed for both ARE- and MRE-regulated transcription, as well as intact the MT-1 promoter. These results suggest that HNE is capable of activating signal transduction pathways that ultimately affect MT transcription. Furthermore, these pathways converge on two independent transcription factors, the MRE binding transcription factor MTF-1 (19) and AP-1 (20,21).
Figure 3. Effect of HNE on metallothionein transcription.
COS-7 cells were transfected with metallothionein reporter genes: −153/+62-CAT, MREd5-CAT, and ARE4-CAT. Following transfection and recovery, cells were exposed to 10 μM HNE for 24 h (solid bars). The level of CAT was then measured by ELISA and normalized to the amount of β-galactosidase enzyme activity (11). Reporter gene expression in cells not exposed to HNE (open bars) in each treatment, n = 3 observations. * indicate significant difference at p < 0.01.
There are several mechanisms by which ROS can affect signal transduction pathways. It has been proposed that HNE acts as a second messenger to activate signaling transduction pathways (22). Correlated with HNE production is an increase in AP-1 activity and the expression of several genes including collagen type I, TGFβ1, γ-glutamylcysteine synthetase, and c-myc (23–27). In addition, HNE is capable of increasing c-Jun expression, and activating protein kinase C (PKC), p38, and c-Jun N-terminal kinase (JNK) (17,22,28,29).
The ability of HNE to affect the activity of MTF-1 has not been reported. Several studies have shown that ROS can activate the MTF-1/MRE-regulated transcription of MT (8,11). It has been proposed that MTF-1 is activated by post-translation modifications (i.e., phosphorylation) via one of several signal transduction pathways, including PKC and JNK (14,30). It is possible that HNE increases MT transcription by affecting these pathways which converge on MTF-1. An alternative model proposes that MTF-1 is activated by the binding of zinc in an unoccupied zinc finger (31). HNE-induced oxidative stress could cause a release of intracellular zinc by oxidizing metal-binding thiols or by glutathione mediated release of the metal from MT (8). Thus, it could facilitate the release of zinc from MT to activate MT transcription (12).
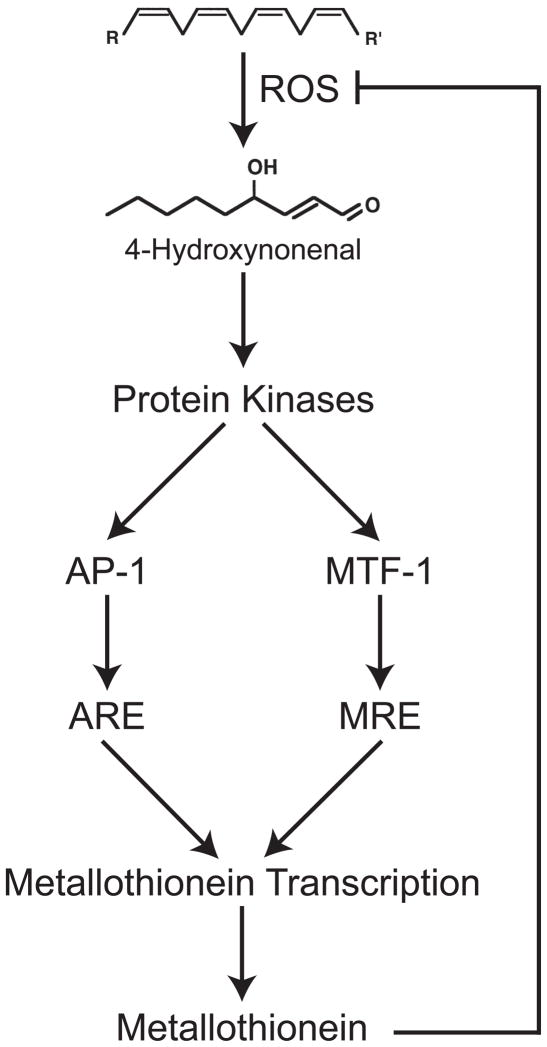
In summary, a mechanistic model is proposed in which exposure to ROS-generating chemicals causes an increase in the levels of HNE which subsequently activates MAPK signaling pathways. The downstream effect is an increase in PKC and JNK activities. The activation of these kinases leads to increased phosphorylation of AP-1 and MTF-1, which increases MT expression via both oxidative-stress and metal-responsive pathways (Fig. 4).
Figure 4. Model for the activation of MT transcription by ROS.
ROS reacts with lipids to produce HNE. HNE then activates protein kinases which ultimately affect MT expression. Once produced, MT can then scavenge ROS; decreasing HNE and kinase activation ultimately reducing MT expression.
Acknowledgments
This work was supported (in part) by the Intramural Research Program of the NIH (Z01ES102045). We thank Matthew McElwee (LMT, NIEHS) for assistance with the statistical analysis.
List of abbreviations
- HNE
4-Hydroxynonenal
- MT-1
mouse metallothionein-1
- MT
metallothionein
- ROS
reactive oxygen species
- qRT-PCR
quantitative real-time RT-PCR
References
- 1.Kagi JH, Schaffer A. Biochemistry of metallothionein. Biochemistry. 1988;27(23):8509–8515. doi: 10.1021/bi00423a001. [DOI] [PubMed] [Google Scholar]
- 2.Thornalley PJ, Vasak M. Possible role for metallothionein in protection against radiation-induced oxidative stress. Kinetics and mechanism of its reaction with superoxide and hydroxyl radicals. Biochim Biophys Acta. 1985;827(1):36–44. doi: 10.1016/0167-4838(85)90098-6. [DOI] [PubMed] [Google Scholar]
- 3.Kondoh M, Inoue Y, Atagi S, Futakawa N, Higashimoto M, Sato M. Specific induction of metallothionein synthesis by mitochondrial oxidative stress. Life Sci. 2001;69(18):2137–46. doi: 10.1016/s0024-3205(01)01294-2. [DOI] [PubMed] [Google Scholar]
- 4.Chimienti F, Jourdan E, Favier A, Seve M. Zinc resistance impairs sensitivity to oxidative stress in HeLa cells: protection through metallothioneins expression. Free Radic Biol Med. 2001;31(10):1179–90. doi: 10.1016/s0891-5849(01)00701-8. [DOI] [PubMed] [Google Scholar]
- 5.Reinecke F, Levanets O, Olivier Y, Louw R, Semete B, Grobler A, Hidalgo J, Smeitink J, Olckers A, Van der Westhuizen FH. Metallothionein isoform 2A expression is inducible and protects against ROS-mediated cell death in rotenone-treated HeLa cells. Biochem J. 2006;395(2):405–15. doi: 10.1042/BJ20051253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lazo JS, Kondo Y, Dellapiazza D, Michalska AE, Choo KH, Pitt BR. Enhanced sensitivity to oxidative stress in cultured embryonic cells from transgenic mice deficient in metallothionein I and II genes. J Biol Chem. 1995;270(10):5506–10. doi: 10.1074/jbc.270.10.5506. [DOI] [PubMed] [Google Scholar]
- 7.Dalton T, Palmiter RD, Andrews GK. Transcriptional induction of the mouse metallothionein-I gene in hydrogen peroxide-treated Hepa cells involves a composite major late transcription factor/antioxidant response element and metal response promoter elements. Nucleic Acids Res. 1994;22(23):5016–5023. doi: 10.1093/nar/22.23.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalton TP, Li Q, Bittel D, Liang L, Andrews GK. Oxidative stress activates metal-responsive transcription factor-1 binding activity. Occupancy in vivo of metal response elements in the metallothionein-I gene promoter. J Biol Chem. 1996;271(42):26233–26241. doi: 10.1074/jbc.271.42.26233. [DOI] [PubMed] [Google Scholar]
- 9.Robson TA, Lohrer H, Bailie JR, Hirst DG, Joiner MC, Arrand JE. Gene regulation by low-dose ionizing radiation in a normal human lung epithelial cell line. Biochem Soc Trans. 1997;25(1):335–42. doi: 10.1042/bst0250335. [DOI] [PubMed] [Google Scholar]
- 10.Tomita M, Okuyama T, Katsuyama H, Ishikawa T. Paraquat-induced gene expression in rat kidney. Arch Toxicol. 2006;80(10):687–93. doi: 10.1007/s00204-006-0092-2. [DOI] [PubMed] [Google Scholar]
- 11.Mattie MD, Freedman JH. Copper-inducible transcription: regulation by metal- and oxidative stress-responsive pathways. Am J Physiol Cell Physiol. 2004;286(2):C293–301. doi: 10.1152/ajpcell.00293.2003. [DOI] [PubMed] [Google Scholar]
- 12.Forman HJ, Fukuto JM, Miller T, Zhang H, Rinna A, Levy S. The chemistry of cell signaling by reactive oxygen and nitrogen species and 4-hydroxynonenal. Arch Biochem Biophys. 2008;477(2):183–95. doi: 10.1016/j.abb.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakraborti S, Chakraborti T. Oxidant-mediated activation of mitogen-activated protein kinases and nuclear transcription factors in the cardiovascular system: a brief overview. Cell Signal. 1998;10(10):675–83. doi: 10.1016/s0898-6568(98)00014-x. [DOI] [PubMed] [Google Scholar]
- 14.Saydam N, Adams T, Steiner F, Schaffner W, Freedman J. Regulation of metallothionein transcription by the metal-responsive transcription factor MTF-1: identification of signal transduction cascades that control metal-inducible transcription. Journal of Biological Chemistry. 2002;277(23):20438–20445. doi: 10.1074/jbc.M110631200. [DOI] [PubMed] [Google Scholar]
- 15.Sakurai A, Hara S, Okano N, Kondo Y, Inoue J, Imura N. Regulatory role of metallothionein in NF-kappaB activation. FEBS Letters. 1999;455(1–2):55–8. doi: 10.1016/s0014-5793(99)00839-x. [DOI] [PubMed] [Google Scholar]
- 16.Schwarzer E, Muller O, Arese P, Siems WG, Grune T. Increased levels of 4-hydroxynonenal in human monocytes fed with malarial pigment hemozoin. A possible clue for hemozoin toxicity. FEBS Lett. 1996;388(2–3):119–22. doi: 10.1016/0014-5793(96)00523-6. [DOI] [PubMed] [Google Scholar]
- 17.Parola M, Robino G, Marra F, Pinzani M, Bellomo G, Leonarduzzi G, Chiarugi P, Camandola S, Poli G, Waeg G, et al. HNE interacts directly with JNK isoforms in human hepatic stellate cells. J Clin Invest. 1998;102(11):1942–50. doi: 10.1172/JCI1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods (Duluth) 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Radtke F, Heuchel R, Georgiev O, Hergersberg M, Gariglio M, Dembic Z, Schaffner W. Cloned transcription factor MTF-1 activates the mouse metallothionein I promoter. Embo J. 1993;12(4):1355–62. doi: 10.1002/j.1460-2075.1993.tb05780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A. 1996;93(25):14960–5. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang H, Magilnick N, Lee C, Kalmaz D, Ou X, Chan JY, Lu SC. Nrf1 and Nrf2 regulate rat glutamate-cysteine ligase catalytic subunit transcription indirectly via NF-kappaB and AP-1. Mol Cell Biol. 2005;25(14):5933–46. doi: 10.1128/MCB.25.14.5933-5946.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uchida K, Shiraishi M, Naito Y, Torii Y, Nakamura Y, Osawa T. Activation of stress signaling pathways by the end product of lipid peroxidation. 4-hydroxy-2-nonenal is a potential inducer of intracellular peroxide production. J Biol Chem. 1999;274(4):2234–42. doi: 10.1074/jbc.274.4.2234. [DOI] [PubMed] [Google Scholar]
- 23.Parola M, Pinzani M, Casini A, Albano E, Poli G, Gentilini A, Gentilini P, Dianzani MU. Stimulation of lipid peroxidation or 4-hydroxynonenal treatment increases procollagen alpha 1 (I) gene expression in human liver fat-storing cells. Biochem Biophys Res Commun. 1993;194(3):1044–50. doi: 10.1006/bbrc.1993.1927. [DOI] [PubMed] [Google Scholar]
- 24.Leonarduzzi G, Scavazza A, Biasi F, Chiarpotto E, Camandola S, Vogel S, Dargel R, Poli G. The lipid peroxidation end product 4-hydroxy-2,3-nonenal up-regulates transforming growth factor beta1 expression in the macrophage lineage: a link between oxidative injury and fibrosclerosis. Faseb J. 1997;11(11):851–7. doi: 10.1096/fasebj.11.11.9285483. [DOI] [PubMed] [Google Scholar]
- 25.Liu RM, Gao L, Choi J, Forman HJ. gamma-glutamylcysteine synthetase: mRNA stabilization and independent subunit transcription by 4-hydroxy-2-nonenal. Am J Physiol. 1998;275(5 Pt 1):L861–9. doi: 10.1152/ajplung.1998.275.5.L861. [DOI] [PubMed] [Google Scholar]
- 26.Fazio VM, Barrera G, Martinotti S, Farace MG, Giglioni B, Frati L, Manzari V, Dianzani MU. 4-Hydroxynonenal, a product of cellular lipid peroxidation, which modulates c-myc and globin gene expression in K562 erythroleukemic cells. Cancer Res. 1992;52(18):4866–71. [PubMed] [Google Scholar]
- 27.Leonarduzzi G, Arkan MC, Basaga H, Chiarpotto E, Sevanian A, Poli G. Lipid oxidation products in cell signaling. Free Radic Biol Med. 2000;28(9):1370–8. doi: 10.1016/s0891-5849(00)00216-1. [DOI] [PubMed] [Google Scholar]
- 28.Chiarpotto E, Domencotti C, Paola D, Vitali A, Nitti M, Pronzato MA, Biasi F, Cottaloasso D, Marinari UM, Dragonetti A, Cesaro P, Isidoro C, Poli G. Regulation of rat hepatocyte protein kinase C beta isozymes by the lipid peroxidation product 4-hyrdroxy-2, 3-nonenal. A signaling pathway to modulate vesicular transport of glycoproteins. Hepatology. 1999;29:1565–1572. doi: 10.1002/hep.510290510. [DOI] [PubMed] [Google Scholar]
- 29.Camandola S, Poli G, Mattson MP. The lipid peroxidation product 4-hydroxy-2,3-nonenal increases AP-1-binding activity through caspase activation in neurons. J Neurochem. 2000;74(1):159–68. doi: 10.1046/j.1471-4159.2000.0740159.x. [DOI] [PubMed] [Google Scholar]
- 30.LaRochelle O, Gagne V, Charron J, Soh JW, Seguin C. Phosphorylation is involved in the activation of metal-regulatory transcription factor 1 in response to metal ions. J Biol Chem. 2001;276(45):41879–88. doi: 10.1074/jbc.M108313200. [DOI] [PubMed] [Google Scholar]
- 31.Laity JH, Andrews GK. Understanding the mechanisms of zinc-sensing by metal-response element binding transcription factor-1 (MTF-1) Arch Biochem Biophys. 2007;463(2):201–10. doi: 10.1016/j.abb.2007.03.019. [DOI] [PubMed] [Google Scholar]