Abstract
Aberrant signaling through the class I phosphatidylinositol 3-kinase (PI3K)-Akt axis is frequent in human cancer. Here we show that Beclin 1, an essential autophagy and tumor suppressor protein, is a target of the protein kinase Akt. Expression of a Beclin 1 mutant resistant to Akt-mediated phosphorylation increased autophagy, reduced anchorage-independent growth, and inhibited Akt-driven tumorigenesis. Akt-mediated phosphorylation of Beclin 1 enhanced its interactions with 14-3-3 and vimentin intermediate filament proteins, and vimentin depletion increased autophagy and inhibited Akt-driven transformation. Thus, Akt-mediated phosphorylation of Beclin 1 functions in autophagy inhibition, oncogenesis, and the formation of an autophagy-inhibitory Beclin 1/14-3-3/vimentin intermediate filament complex. These findings have broad implications for understanding the role of Akt signaling and intermediate filament proteins in autophagy and cancer.
Mutations leading to activation of the serine/threonine kinase Akt are frequent in human cancer (1). Akt has many downstream targets involved in tumorigenesis, including mTOR (mammalian target of rapamycin) (2). Akt also inhibits autophagy (3), a lysosomal degradation pathway that removes unwanted or damaged cellular constituents and functions in tumor suppression (4). Akt suppression of autophagy can be mediated by activation of mTOR, which inhibits the autophagy-initiating ULK1 kinase complex (4).
We investigated whether Akt inhibits autophagy by directly regulating the core autophagy machinery independently of mTOR. Expression of constitutively active myristoylated (5) and tagged Akt1 (Flag-tagged myr-Akt) in HeLa cells inhibited autophagy during growth in normal medium, in response to serum and amino acid starvation (a physiological inducer of autophagy), in response to treatment with an ATP-competitive inhibitor of mTOR, Torin1 (6), and in response to both starvation and Torin1 treatment (Fig. 1A and 1B). In all conditions, cells expressing myr-Akt1 had decreased numbers of puncta upon transfection with a fusion protein of green fluorescent protein with LC3 (GFP-LC3), a fluorescent marker of autophagosomes; increased amounts of p62 (a substrate that is degraded by autophagy); and increased amounts of the cytosolic non-lipidated form of LC3, LC3-I, and of total LC3 (7). Amounts of phospho-4E-BP1, a phosphorylation target of mTOR, were decreased in Torin1-treated cells, including those expressing myr-Akt1. Thus, myr-Akt1 suppresses basal autophagy, starvation-induced autophagy, and Torin1-induced autophagy, indicating that active Akt can inhibit autophagy through mTOR-independent mechanisms.
Fig. 1.
Akt suppression of autophagy, interaction with Beclin 1, and phosphorylation of Beclin 1. (A) Biochemical assessment of autophagy (p62 and LC3) and mTOR activity (p-4E-BP1) in HeLa cells expressing constitutively active Akt (myr-Akt1) or control vector, grown in normal medium or starved in Earles’ Balance Salt Solution (EBSS) for 2 hours, and treated with 250 nM Torin 1 or control solution. (B) GFP-LC3 dots (autophagosomes) in HeLa/GFP-LC3 cells treated as in (A). Bars represent mean ± SEM of triplicate samples with >50 cells analyzed per sample. Similar results observed in 3 independent experiments. (C) Immunoprecipitation of endogenous Akt with endogenous Beclin 1 in HeLa cells with or without starvation for 2 hours. (D) In vitro phosphorylation of Flag-Beclin 1 S295 by GST-Akt1 with or without 1 μM indicated Akt inhibitors. (E) Phosphorylation of endogenous Beclin 1 S295 and Beclin 1 S234 in HeLa cells transfected with control vector or HA-myr-Akt1 with or without Torin1 for 4 hours. (E) Phosphorylation of endogenous Beclin 1 S295 in paired melanoma (WM793 and 451Lu), glioblastoma (U87-MG, U87-MG + PTEN), and breast cancer (MCF-10DCIS and MDMB-231) cells with high and low activities of Akt, respectively. *P<0.05; **P<0.01; Tukey test. WCL, whole cell lysates.
We examined whether autophagy execution proteins could be targets of Akt. We focused on Beclin 1 because of its role in autophagy and tumor suppression (4). Endogenous Akt co-immunoprecipitated with endogenous Beclin 1 in HeLa cells, and this interaction was weakened by starvation (Fig. 1C). In contrast, the interaction of myr-Akt1 with a Flag epitope-tagged construct of Beclin 1 was not affected by starvation (Fig. S1). Kinase prediction algorithms (8, 9) showed Beclin 1 to contain a motif (R-X-X-R-X-X-S295) that resembles the consensus Akt phosphoryation motif (R-X-R-X-XS/T) (10) and another sequence (R-X-X-S234) that corresponds to a 14-3-3 protein binding motif (which may be generated by Akt phosphorylation) (Fig. S2A). Phosphospecific antibodies against these two candidate phosphorylation sites in Beclin 1 (S234 and S295) recognized wild-type Flag-Beclin 1 expressed in HeLa cells and immunoreactivity was decreased with the corresponding Flag-Beclin 1 ala-nine substitution mutant (Fig. S2B). GST-Akt1 phosphorylated Beclin 1 S295 but not Beclin 1 S234 in vitro (Fig. S2C), and this was partially blocked by treatment with two Akt inhibitors, MK-2206 and Akt inhibitor X (Fig. 1D). Expression of active Akt1 (myr-Akt1) increased and expression of a catalytically inactive, non-phosphorylatable Akt1 mutant (K179M/T308A/S473A; DN-Akt1) decreased, respectively, phosphorylation of Flag-Beclin 1 S295 and Flag-Beclin 1 S234 in HeLa cells (Fig. S2D). Expression of myr-Akt1 also led to phosphorylation of endogenous Beclin 1 S295 and endogenous Beclin 1 S234 which was not reversed by mTOR inactivation with Torin1 (Fig. 1E). Endogenous Beclin 1 S295 phosphorylation increased when starved HeLa cells were fed with normal medium (Fig. S2E). Together, these studies demonstrate that Beclin 1 is phosphorylated by Akt on residue 295 (and possibly 234) in an mTOR-independent manner.
We compared the phosphorylation of Beclin 1 S295 in three paired sets of tumor cell lines with and without Akt activation (Fig. 1F). Melanoma cells with mutant PTEN (WM793) had more phosphorylation of Beclin 1 S295 than did those with wild-type PTEN (451Lu) (11). U87-MG glioblastoma cells with high Akt activity due to inactivating mutations in PTEN showed more phosphorylation of Beclin 1 S295 than did U87-MG cells in which wild-type PTEN was reintroduced (12). In breast carcinoma cells, S295 phosphorylation was detected in MCF10A-DCIS cells with an activating H1047R mutation in PIK3CA but not in MDA-MB231 cells lacking constitutive Akt activation (13). Thus, in three different tumor types, activation of Akt is associated with phosphorylation of Beclin 1 S295, indicating that phosphorylation of Beclin 1 S295 may be common in human tumors with activated Akt.
We transfected MCF7 human breast carcinoma cells [which express low amounts of endogenous Beclin 1 (14)] with GFP-LC3 and wild-type Beclin 1 or Beclin 1 S295A or AA mutants (Fig. S3A). Cells transfected with S295A and AA mutants had increased basal (but not starvation-induced) autophagy (Fig. S3B and C). Inhibition of Akt by MK-2206 (Fig. S3D) increased basal autophagy in MCF7 cells to a lesser extent in cells transfected with Beclin 1 AA than in cells transfected with wild-type Beclin 1 (Fig. S3E). Conversely, expression of active Akt decreased basal autophagy in all MCF7 cells, but cells expressing Beclin 1 AA showed more autophagy than cells expressing wild-type Beclin 1 or vector alone (Fig. S3F and S3G). Thus, Akt appears to inhibit basal autophagy both through Beclin 1 phosphorylation-dependent and independent mechanisms.
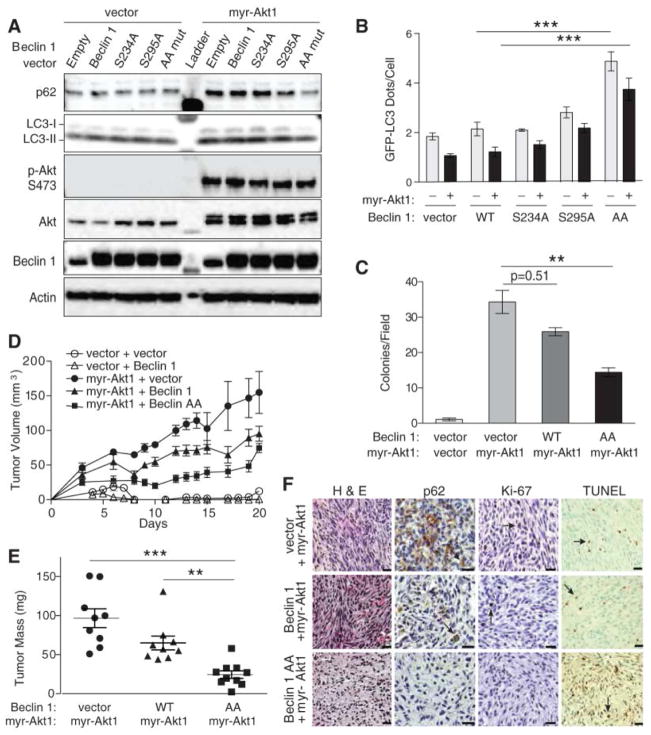
To examine the role of Akt-mediated Beclin 1 phosphorylation in Akt-driven tumorigenesis, we transduced Rat2 fibroblasts with myr-Akt1 (which transforms rat fibroblasts (15)) and either wild-type Beclin 1 or Beclin 1 phosphorylation site mutants. Myr-Akt1 suppressed autophagy in Rat2 fibroblasts (Fig. 2A and B), reduced co-immunoprecipitation of Class III PI3K Vps34 with Beclin 1 (Fig. S4A), and decreased Beclin 1-associated lipid kinase activity (Fig. S4B). The autophagy suppressive effects of active Akt were largely prevented by expression of the Beclin 1 AA mutant and mildly decreased by the Beclin 1 S295A mutant (Fig. 2A and B). Expression of myr-Akt1 had minimal effects on the interaction of Beclin 1 AA and Vps34 or on the amounts of Beclin 1 AA-associated Vps34 activity (Fig. S4A and S4B). Thus, myr-Akt1 suppresses Beclin 1-associated Vps34 activity and autophagy in a manner that is partially reversed by a Beclin 1 mutant resistant to Akt-mediated phosphorylation.
Fig. 2.
Effect of a non-phosphorylatable mutant of Beclin 1 (Beclin 1 S234A/S295A (AA)) on autophagy, anchorage-independent growth, and Akt-mediated tumorigenesis. (A) Biochemical assessment of autophagy in Rat2 fibroblasts expressing indicated Flag-Beclin 1 and Akt constructs. (B) GFP-LC3 dots in Rat2 cells transduced with indicated lentiviral vectors and transfected with GFP-LC3. Bars represent mean ± SEM of triplicate samples with >50 cells analyzed per sample. Similar results observed in 3 independent experiments. (C) Soft agar colonies formed by Rat2 fibroblasts transduced with indicated vectors. Bars represent mean ± SEM of triplicate samples of ≥12 random images analyzed by ImageJ. Similar results observed in 3 independent experiments. (D) Xenograft tumor volumes formed by Rat2 fibroblasts transduced with indicated vectors. (P<0.001 for myr-Akt1 + Beclin 1 AA group vs. myr-Akt1 + vector or myr-Akt1 + Beclin 1 groups; linear-mixed effect model). (E) Tumor weights at day 21. (F) Representative images of tumors formed by Rat2 fibroblasts. Arrows denote representative p62-, Ki-67-, and TUNEL-positive cells. Scale bars, 20 μm. For (D) and (E), results represent mean ± SEM for all tumors in each genotype (9–10 per group). *P<0.05, **P<0.01, ***P<0.001; Tukey-test.
In an anchorage-independence growth assay, shRNA depletion of Beclin 1 (Fig. S5) or myr-Akt1 expression caused Rat2 fibroblasts to form numerous colonies in soft agar (Fig. 2C). Both the number and size of colonies formed by myr-Akt1-expressing cells were significantly reduced by co-expression of Beclin 1 AA (Fig. 2C, Fig. S6A and S6B). Rat2 cells expressing myr-Akt1 also had higher amounts of endogenous Beclin 1 S295 phosphorylation than control cells (Fig. 3B). Thus, active Akt1 promotes Beclin 1 S295 phosphorylation, and expression of a non-phosphorylatable mutant of Beclin 1 suppresses Akt-mediated transformation in vitro. Inactivation of Beclin 1 by Akt-mediated phosphorylation may therefore contribute to Akt’s transforming properties.
Fig. 3.
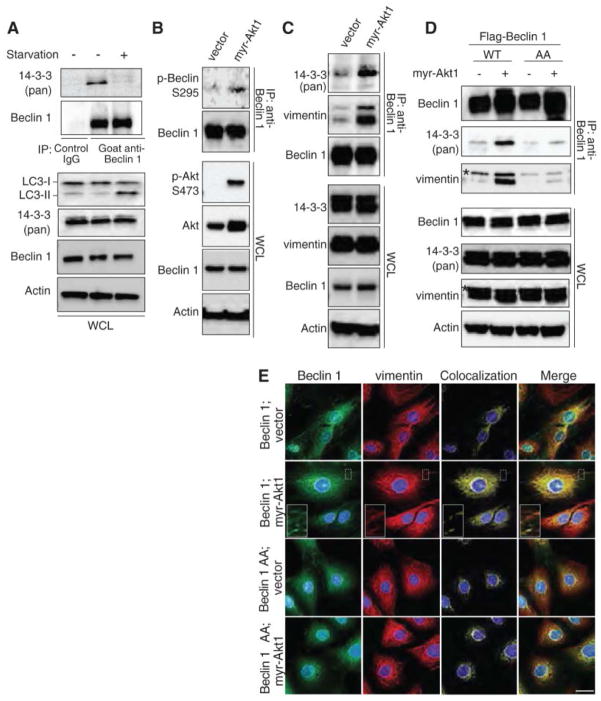
Interactions between Beclin 1 and 14-3-3 proteins or Beclin 1 and vimentin promoted by active Akt. (A) Immunoprecipitation of 14-3-3 proteins with endogenous Beclin 1 in HeLa cells with or without starvation for 2 hours. (B to C) Endogenous Beclin 1 S295 phosphorylation (B) and immunoprecipitation of endogenous 14-3-3 and vimentin with endogenous Beclin 1 (C) in Rat2 fibroblasts tranduced with control vector or myr-Akt1. (D) Immunoprecipitation of endogenous 14-3-3 and vimentin with Flag-Beclin 1 in Rat2 cells transduced with indicated Flag-Beclin 1 and Akt constructs. *, non-specific band. (E) Localization of Flag-Beclin 1 with endogenous vimentin in Rat2 cells transduced with the indicated vectors. WCL, whole cell lysates.
Beclin 1 AA also suppressed myr-Akt1-driven tumorigenesis in vivo. All myr-Akt1-expressing Rat2 cells formed tumors in immunodeficient NOD SCID® mice, but tumor growth rate and tumor mass upon necropsy were less for cells expressing Beclin 1 AA than for cells expressing wild-type Beclin 1 or myr-Akt1 alone (Fig. 2D and 2E, Fig. S6C). Tumors expressing myr-Akt1 alone had numerous cells displaying p62 immunoreactivity (indicating autophagy suppression) whereas very few p62 immunoreactive cells were detected in tumors from cells expressing both myr-Akt1 and Beclin 1 AA (Fig. 2F). Tumors expressing myr-Akt1 alone or active Akt and Beclin 1 resembled fibrosarcomas (Fig. 2F and table S1); 94% (17/18) showed tissue invasion and 50% (9/18) exhibited nuclear pleomorphism and decreased nucleus:cytoplasmic ratios. Tumors expressing myr-Akt1 and Beclin 1 AA formed less cellular, more disorganized tumors with limited or no tissue invasion; 60% (6/10) displayed degenerative features with numerous pyknotic nuclei. Tumors expressing active Akt alone or with Beclin 1 had higher mean mitotic counts (n=8.39; n=8.37, respectively) than tumors expressing myr-Akt1 and Beclin 1 AA (n=3.67) (P<0.0001). They also had increased Ki-67 labeling (Fig. S6D), and decreased TUNEL staining (Fig. S6E). Thus, the expression of a mutant of Beclin 1 that cannot be phosphorylated by Akt increases tumor cell autophagy, decreases tumor growth rate and size, decreases tumor cellular proliferation, and increases tumor cell death. These results suggest a role for Akt-mediated Beclin 1 phosphorylation in the tumorigenic effects of Akt.
To investigate how Akt phosphorylation of Beclin 1 inhibits autophagy, we determined whether Akt-mediated phosphorylation of Beclin 1 generates a 14-3-3 binding motif (10). Endogenous 14-3-3 proteins and Beclin 1 co-immunoprecipitated in HeLa cells and this interaction decreased during starvation (Fig. 3A). Mutation of predicted Beclin 1 14-3-3 binding sites, S234A or S295A, weakened the Beclin 1/14-3-3 interaction and the double mutation (AA) nearly completely abolished Beclin 1/14-3-3 binding (Fig. S7A). Similar amounts of Atg14, a component of the autophagy-inducing Beclin 1/Class III PI3K complex (16), immunoprecipitated with WT and non-phosphorylatable mutants of Beclin 1, indicating these mutations do not cause major alterations in protein stability or folding. Expression of myr-Akt1 blocked starvation-induced disruption of 14-3-3/Beclin 1 binding and conversely, expression of DN-Akt1 inhibited 14-3-3/Beclin 1 binding during growth in normal medium (Fig. S7B). Thus, Beclin 1 interacts with 14-3-3 proteins through S234 and S295, and this interaction is negatively regulated by starvation and Akt inhibition.
14-3-3 proteins can regulate their binding partners’ functions through interactions with intermediate filaments (17, 18). The intermediate filaments, keratin 18 (K18) and vimentin, immunoprecipitated with Flag-Beclin 1 in HeLa cells and this was reduced by expression of DN-Akt1 (Fig. S7C). Immunoprecipitation of Flag-Beclin 1 was increased by mutants of vimentin and K18 with increased binding to 14-3-3 proteins (K18 R89C (19)) but decreased with mutants with decreased binding to 14-3-3 proteins (K18S33A (19) and vimentin S39A) (Fig. S7D-E). The Flag-Beclin 1 AA mutant did not immunoprecipitate with wild-type vimentin-GFP (Fig. S7F). siRNA targeted against 14-3-3ε abolished the interaction between Flag-Beclin 1 and vimentin-GFP (Fig. S7G). Thus, Beclin 1 interacts with 14-3-3 proteins and intermediate filament proteins through a mechanism involving the S234 and S295 Akt phosphorylation/14-3-3 binding sites of Beclin 1 and the 14-3-3 binding sites of intermediate filament proteins.
In Rat2 cells, expression of myr-Akt1 increased the interactions of endogenous 14-3-3 proteins and vimentin (the major intermediate filament protein expressed in fibroblasts (20)) with endogenous Beclin 1 in parallel with its increased phosphorylation (Fig. 3B and C). Expression of active Akt had little effect on the binding of Beclin 1 AA with 14-3-3 proteins and vimentin (Fig. 3D). Thus, active Akt may promote the interaction of Beclin 1 with vimentin through phosphorylation of Beclin 1 and generation of 14-3-3 binding sites.
Wild-type Beclin 1 had a diffuse cytoplasmic localization in the absence of active Akt expression, and localization of Beclin 1 with vimentin was observed primarily in a perinuclear pattern (Fig. 3E). Expression of myr-Akt1 redistributed wild-type Beclin 1 into a reticular pattern and increased Beclin 1/vimentin colocalization, but did not have these effects on Beclin 1 AA. Conversely, starvation decreased Beclin 1/vimentin colocalization in a reticular pattern (Fig. S7H). Thus, active Akt enhances the colocalization of Beclin 1 with vimentin in Rat2 cells in a manner that requires the Beclin 1 Akt phosphorylation and 14-3-3 binding sites S234 and S295.
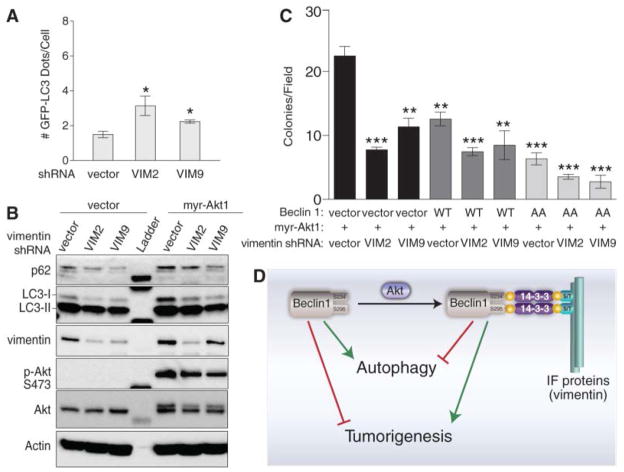
In Rat2 cells, two different shRNAs that target vimentin (Fig. S8A) increased autophagy (Fig. 4A). Vimentin appears to inhibit autophagy downstream of Beclin 1 phosphorylation because depletion of vimentin also increased autophagy in Rat2 fibroblasts expressing myr-Akt1 (Fig. S8B and Fig. 4B). This increased autophagy was associated with inhibition of Akt-mediated transformation; vimentin shRNAs significantly inhibited the number and size of Rat2 colonies formed in soft agar (Fig. 4C and Fig. S8C). Thus, the regulation of Beclin 1/vimentin interactions by active Akt may be a mechanism for both inhibition of autophagy by intermediate filaments and for Akt-mediated transformation. As in the case of the interaction of AMBRA1 with the dynein moter complex (21), these data indicate that interactions between core autophagy proteins and cytoskeletal elements may regulate autophagy.
Fig. 4.
Effects of vimentin on autophagy and Akt-mediated transformation. (A) GFP-LC3 dots in Rat2 cells transduced with indicated vimentin shRNA and transfected with GFP-LC3. Bars represent mean ± SEM of triplicate samples with >50 cells analyzed per sample. Similar results observed in 3 independent experiments. (B) Biochemical assessment of autophagy in Rat2 cells transduced with indicated vimentin shRNA and myr-Akt1 or control vector. (C) Soft agar colonies formed by Rat2 fibroblasts transduced with indicated vectors. Bars represent mean ± SEM of triplicate samples of ≥12 random images analyzed by ImageJ. (D) Speculative model for relations between Akt phosphorylation of Beclin 1; formation of a complex with Beclin 1, 14-3-3 proteins, and intermediate filament (IF) proteins; autophagy, and tumorigenesis. *P<0.05, **P<0.01, ***P<0.001; t test. In (A) and (C), all comparisons are with the first bar in graph.
Our findings demonstrate a link between oncogenic signaling, the core autophagy machinery, and cytoskeletal proteins in the intermediate filament family. Akt signaling, intermediate filaments and 14-3-3 proteins may be mechanistically linked to autophagy inhibition and tumorigenesis through regulation of the Beclin 1 complex (Fig. 4D). These findings also demonstrate a specific mechanism by which autophagy may be suppressed in human cancer. Cross-talk between oncogenic kinases and autophagy proteins might represent a fundamental mechanism underlying the regulation of mammalian cell growth control and cancer.
Supplementary Material
Acknowledgments
We thank R. De Berardinis, W. Hahn, D. Lev, P. Mischel, N. Mizushima, B. Omary, G. Pearson, W. Sellers, R. Weinberg, and M. Yaffe for providing critical reagents; and A. Diehl and H. Harrington for help with manuscript preparation. This work was supported by NIH/NCI grants ROI CA84254-S1 and RO1 CA109618 to B.L., ROI CA129451 to M.W., a Dermatology Foundation Physician Scientist Career Development Award to R.W., and a German Research Foundation Grant RE2673/1-1 to J.R.
Footnotes
References and Notes
- 1.Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24:7455. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- 2.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 3.Degtyarev M, et al. Akt inhibition promotes autophagy and sensitizes PTEN-null tumors to lysosomotropic agents. J Cell Biol. 2008;183:101. doi: 10.1083/jcb.200801099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boehm JS, et al. Integrative genomic approaches identify IKBKE as a breast cancer oncogene. Cell. 2007;129:1065. doi: 10.1016/j.cell.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 6.Thoreen CC, et al. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem. 2009;284:8023. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Obenauer JC, Cantley LC, Yaffe MB. Scansite 2.0: Proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res. 2003;31:3635. doi: 10.1093/nar/gkg584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xue Y, et al. GPS 20, a tool to predict kinase-specific phosphorylation sites in hierarchy. Mol Cell Proteomics. 2008;7:1598. doi: 10.1074/mcp.M700574-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paraiso KH, et al. PTEN loss confers BRAF inhibitor resistance to melanoma cells through the suppression of BIM expression. Cancer Res. 2011;71:2750. doi: 10.1158/0008-5472.CAN-10-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang MY, et al. Mammalian target of rapamycin inhibition promotes response to epidermal growth factor receptor kinase inhibitors in PTEN-deficient and PTEN-intact glioblastoma cells. Cancer Res. 2006;66:7864. doi: 10.1158/0008-5472.CAN-04-4392. [DOI] [PubMed] [Google Scholar]
- 13.Kalaany NY, Sabatini DM. Tumours with PI3K activation are resistant to dietary restriction. Nature. 2009;458:725. doi: 10.1038/nature07782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang XH, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 15.Carpten JD, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448:439. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- 16.He C, Levine B. The Beclin 1 interactome. Curr Opin Cell Biol. 2010;22:140. doi: 10.1016/j.ceb.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Margolis SS, et al. Role for the PP2A/B56delta phosphatase in regulating 14-3-3 release from Cdc25 to control mitosis. Cell. 2006;127:759. doi: 10.1016/j.cell.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim S, Wong P, Coulombe PA. A keratin cytoskeletal protein regulates protein synthesis and epithelial cell growth. Nature. 2006;441:362. doi: 10.1038/nature04659. [DOI] [PubMed] [Google Scholar]
- 19.Ku NO, Liao J, Omary MB. Phosphorylation of human keratin 18 serine 33 regulates binding to 14-3-3 proteins. EMBO J. 1998;17:1892. doi: 10.1093/emboj/17.7.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franke WW, Schmid E, Winter S, Osborn M, Weber K. Widespread occurrence of intermediate-sized filaments of the vimentin-type in cultured cells from diverse vertebrates. Exp Cell Res. 1979;123:25. doi: 10.1016/0014-4827(79)90418-X. [DOI] [PubMed] [Google Scholar]
- 21.Di Bartolomeo S, et al. The dynamic interaction of AMBRA1 with the dynein motor complex regulates mammalian autophagy. J Cell Biol. 2010;191:155. doi: 10.1083/jcb.201002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.