Abstract
Obesity is epidemic among adolescents in the United States. We sought to analyze the anthropometric measures of adiposity and fasting indices of insulin resistance, including insulin-like growth factor–binding protein-1 (IGFBP-1), and to provide a clinical estimate of intraperitoneal (IP) fat in obese adolescents (BMI ≥95th percentile), between ages 13 and 17 years. Subjects had baseline testing to determine eligibility for a subsequent randomized, placebo-controlled trial of metformin XR therapy. Anthropometry and dual-energy X-ray absorptiometry (DXA) were used to quantify total body fat while abdominal computed tomography (CT) was used to measure IP (CT-IP) and subcutaneous (CT-SQ) fat. Using anthropometry and fasting laboratory data, we constructed regression models for both CT-IP and CT-SQ. A total of 92 subjects, 33 males, were evaluated. Of the 92 subjects, 19 were black. Fasting insulin concentrations were highly associated with other measures of insulin resistance. Median percent body fat across all subjects, as measured by DXA, was 41%. Using CT measures, 67% of abdominal cross-sectional area was fat, 14% of which was IP fat. In multiple regression analysis, waist circumference (WC) and BMI, jointly and independently, were strongly associated with both CT-IP and CT-SQ fat. BMI and WC explained 62% of variance of CT-SQ fat, but only 26% of variance of CT-IP fat. Adding triglyceride:high-density lipoprotein (TG:HDL) ratio and IGFBP-1 (among nonblacks) to the regression model increased the explained variance for estimating CT-IP fat to 45%. When evaluating the metabolic morbidity of an obese adolescent, a model using fasting IGFBP-1, TG:HDL, BMI, and WC may be worthwhile as an estimate of IP fat.
INTRODUCTION
Obesity is epidemic among adolescents in the United States. As in adults, risks associated with childhood and adolescent obesity include elevated blood pressure and dyslipidemia, predis-posing these individuals to cardiovascular disease. In addition, a significant number of obese youth have abnormally severe insulin resistance with an attendant increased risk of developing type 2 diabetes mellitus (1).
The magnitude of insulin resistance and risk for other metabolic complications among equally obese adolescents differs depending on their physique and body fat distribution, which can be measured by a cross-sectional computed tomography (CT) scan of the abdomen. In fact, there is increased recognition that visceral or intraperitoneal (IP) fat is associated with disorders of glucose and lipid metabolism (2–6), while sub-cutaneous (SQ) fat may even be somewhat protective of these disorders (7).
As part of the baseline evaluation for entry into a multi-center, randomized, placebo-controlled, double-blind trial of metformin XR in obese adolescents, we sought to quantify the associations between different measures of adiposity (including IP fat as determined by CT) and insulin resistance. We used simple anthropometric and fasting biochemical measures to statistically estimate CT-IP.
METHODS AND PROCEDURES
Setting
The study was conducted at the five clinical sites (see Supplementary Appendix 1 online) of the Glaser Pediatric Research Network, with a center for data management and statistical analysis at Children's Hospital Boston. The study was approved by the institutional review boards of all five centers.
Subjects
Adolescents, aged 13.0–17.99 years, were eligible if they were obese (BMI ≥95th percentile for age and gender (8) but weighed less than 136 kg (the weight limit for the dual-energy X-ray absorptiometry (DXA) machine). No specific fasting insulin concentration was required for study entry. Subjects were excluded if they had known diabetes; had ever used a medication to treat diabetes or insulin resistance; had ever used a medication to aid in weight loss; were taking any medications known to increase metformin levels (e.g., cimetidine); received recent glucocorticoid therapy; had any syndrome or medical disorder predisposing to obesity; had surgical therapy for obesity; had attended a formal weight-loss program within the previous 6 months; admitted to significant alcohol use in the past 6 months; had elevated creatinine (>1.2 mg/dl) or liver enzymes (aspartate aminotransferase or alanine aminotransferase of >80 U/l) or untreated disorders of thyroid function; had a mobility impairment; or had, if a female, ever been pregnant. Parental consent and subject assent were obtained. All eligible subjects were recruited.
Procedures
Height was measured twice using a wall-mounted stadiometer and weight was measured twice using an electronic scale. A third reading was taken if the difference between the first two readings was >0.5 cm for height or 0.3 kg for weight. The means were used for data analysis. BMI (kg/m2) was calculated from the mean weight and height measurements and converted to a sex- and age-specific z-score (8).
Waist circumference (WC) was measured by two methods and each measurement was done twice. For the UMB method, subjects were measured around the smallest area below the rib cage and above the umbilicus (9). The NHANES method involved measuring subjects at the high point of the iliac crest at minimal expiration (10).
Tanner staging was assessed for breasts for females, and genitalia for males, and pubic hair for both sexes, by direct inspection. The degree of acanthosis nigricans was assessed using Burke's method (11).
Race was determined by self-identification. Subjects were asked to choose one or more of the following categories: American Indian or Alaska Native; Asian; Native Hawaiian or other Pacific Islander; black or African American; white; or other. Ethnicity was also determined by self-identification by asking whether the subjects considered themselves to be Hispanic or Latino.
Two-slice CT scans were obtained on each subject to evaluate both the distribution of abdominal fat and the degree of fatty infiltration of the liver. Abdominal fat content and distribution were measured using a modification of the methods of Borkan et al., obtaining a slice aligned with the L4–L5 intervertebral disk found by using a low-dose abdominal scout radiograph to standardize the position of the scan to the nearest millimeter (12). The cross-sectional area (in cm2) was determined using measurement software available on the CT review console. Percent body fat was measured by whole-body DXA.
Following 3 days of a normal carbohydrate diet (at least 150 g/day) and a 10-h fast, glucose, insulin, insulin-like growth factor–binding protein-1 (IGFBP-1), and lipid panel were obtained. Subjects then underwent a 3-hour oral glucose tolerance test (OGTT; 75 g of glucose) with insulin and glucose at time 0 (just before), and at 15, 30, 60, 90, 120, and 180 min after glucose load, C-peptide at time 0 (just before), and at 15, 30, 60, 120, and 180 min after glucose load. All assays were performed at Esoterix Clinical Trials Services (Calabasas Hills, CA). Insulin and IGFBP-1 were measured by two-site immunochemiluminometric assays with sensitivities of 0.6 μU/ml and 1 ng/ml, respectively. The Esoterix reference ranges for IGFBP-1 are prepubertal (fasting) 30–1,000 ng/ml and pubertal (fasting) 20–200 ng/ml.
Calculated insulin parameters
A number of indices based on OGTT have been proposed as estimators of insulin resistance. The simplest of these is fasting insulin. The other indices included the homeostasis model assessment of insulin resistance (HOMAIR) calculated the (fasting glucose (mmol/l) × fasting insulin (μU/dl))/22.5 (ref. 13). Using the full OGTT, the composite insulin sensitivity index (CISI) (14,15) was calculated as
where FI is fasting insulin, FBG is fasting glucose, and MI and MG are the mean insulin and glucose between 0 and 120 min inclusively.
β-Cell activity was estimated using the CIRgp (corrected insulin release at the glucose peak) (16) calculated as
where Ggp is the peak glucose (maximum glucose value of all seven measurements between 0 and 180 min) and Igp is the insulin concentration at time of glucose peak.
Statistics
Data were reported as median and interquartile range (25th to 75th percentile) because many variables had skewed distribution. Bivariate associations were assessed by Fisher exact test (two dichotomies), Pearson and Spearman correlation (two continuous measures), or Student's t-test (one dichotomy, one continuous measure). Comparisons of anthropometric measures across sex and race were made by two-way ANOVA with a test for interaction. In cases of skewed distribution, the Student's t-test was confirmed by a Wilcoxon rank-sum test and ANOVA by the Kruskal–Wallis test. We used mixed-model analysis of variance to compare to WC measurements within subject (WC-UMB and WC-NHANES) and to estimate the precision of each technique (standard deviation for replicate measurements).
We used simple and multiple linear regression analyses to construct formulas for estimating CT-IP fat and CT-SQ fat from demographic and anthropometric variables and serum levels of lipids and IGFBP-1. Where we had a hypothesis that race might modify the effect of an independent variable, we tested interaction terms in the regression model. The dependent variables were log-transformed to reduce skew. SAS software (Cary, NC) was used for all computations.
RESULTS
Subjects
In the course of screening for the subsequent randomized intervention trial, we obtained baseline data from 92 obese adolescents. Of them, 33 (36%) subjects were males. Of the subjects 55 (60%) described themselves solely as white and 19 (21%) solely as black; 24 subjects (26%) identified themselves as Hispanic or Latino. Given our interest in associations between adiposity and insulin measures with gender and race, we present the baseline data (Tables 1 and 2) divided by gender and race, comparing the subjects those who identified themselves solely as “black”) with all others (“nonblack”). Thus, the nonblack population is a mixed population. Age was similar across gender and race (P = 0.56).
Table 1.
Anthropometric parameters in 92 obese adolescents
| All | Black males | Nonblack males | Black females | Nonblack females | |
|---|---|---|---|---|---|
| N | 92 | 5 | 28 | 14 | 45 |
| Median (25th–75th percentile) | |||||
| Age (years) | 14.6 (13.7–15.8) | 14.0 (13.5–14.2) | 14.7 (13.8–15.8) | 15.0 (13.9–16.1) | 14.4 (13.6–15.5) |
| Weight (kg) | 100.1 (88.6–113.6) | 100.6 (89.4–123.2) | 101.5 (87.6–113.0) | 108.2 (95.1–118.6) | 95.8 (88.5–113.4) |
| BMI (kg/m2) | 35.87 (32.37–39.66) | 36.23 (33.69–36.55) | 33.00 (31.22–37.11) | 41.24 (34.90–44.33) | 36.77 (32.86–39.66) |
| BMI z-score | 2.34 (2.14–2.54) | 2.49 (2.40–2.54) | 2.27 (2.15–2.56) | 2.44 (2.24–2.56) | 2.34 (2.12–2.52) |
| Waist (UMB) (cm) | 104.0 (96.9–112.8) | 103.1 (101.4–113.4) | 104.7 (98.0–112.9) | 107.3 (91.7–118.8) | 103.6 (96.5–111.8) |
| Waist (NHANES) (cm) | 111.4 (104.1–118.5) | 115.4 (104.4–119.2) | 110.1 (97.0–117.9) | 113.1 (102.1–128.5) | 110.5 (106.5–117.5) |
| DXA fat:total mass, % | 41.4 (35.9–44.0) | 35.0 (34.6–41.3) | 35.8 (33.2–42.1) | 42.7 (39.9–46.5) | 42.1 (40.2–44.9) |
| CT-IP fat area, cm2 | 72 (46–94) | 64 (61–77) | 83 (48–116) | 60 (36–88) | 72 (46–93) |
| CT-SQ fat area, cm2 | 498 (419–600) | 472 (465–498) | 478 (400–565) | 572 (430–673) | 504 (425–607) |
| Tanner score n (%) | |||||
| Pubic hair | |||||
| 1–2 | 5 (5) | 0 (0) | 5 (18) | 0 (0) | 0 (0) |
| 3 | 17 (18) | 2 (40) | 11 (39) | 1 (7) | 3 (7) |
| 4 | 30 (33) | 3 (60) | 6 (21) | 4 (29) | 17 (38) |
| 5 | 40 (43) | 0 (0) | 6 (21) | 9 (64) | 25 (56) |
| Genital/breasta | |||||
| 1–2 | 3 (3) | 0 (0) | 3 (11) | 0 (0) | 0 (0) |
| 3 | 21 (23) | 3 (60) | 10 (37) | 1 (7) | 7 (16) |
| 4 | 30 (33) | 2 (40) | 9 (33) | 1 (7) | 18 (40) |
| 5 | 37 (41) | 0 (0) | 5 (19) | 12 (86) | 20 (44) |
CT-IP, intraperitoneal fat measured by computed tomography; CT-SQ, subcutaneous fat measured by computed tomography; DXA, dual-energy X-ray absorptiometry; NHANES, National Health and Nutrition Examination Survey; UMB, umbilicus.
Genital score for males, breast score for females. Not recorded for one nonblack male.
Table 2.
Biochemical parameters in 92 obese adolescents
| All | Black males | Nonblack males | Black females | Nonblack females | |
|---|---|---|---|---|---|
| N | 92 | 5 | 28 | 14 | 45 |
| Median (25th to 75th percentile) | |||||
| IGFBP-1 (ng/ml) | 1.0 (0.5–3.0) | 0.5 (0.5–2.0) | 1.5 (0.5–3.0) | 1.0 (0.5–4.0) | 1.0 (0.5–2.0) |
| Gluc OGTT 0 min (mg/dl) | 91 (85–97) | 88 (86–98) | 94 (86–98) | 92 (86–99) | 89 (84–95) |
| Insu OGTT 0 min (μU/ml) | 18.0 (11.0–27.0) | 21.0 (20.0–23.0) | 18.0 (10.0–30.0) | 14.0 (9.3–24.0) | 19.0 (11.0–26.5) |
| HOMAIR index | 3.91 (2.42–5.94) | 4.83 (4.20–5.73) | 4.26 (1.89–6.86) | 2.97 (2.27–5.56) | 4.02 (2.44–5.94) |
| CISI (mg/dl × μU/ml)–1 | 2.66 (1.72–3.98) | 2.14 (1.88–3.09) | 2.60 (1.75–3.98) | 3.30 (1.93–5.38) | 2.69 (1.64–3.99) |
| CIRgp (μU/l × (mg/dl)–2) | 1.03 (0.58–1.75) | 1.30 (1.09–2.15) | 0.87 (0.50–1.42) | 1.44 (0.60–2.31) | 1.01 (0.62–1.77) |
| HDL, mg/dl | 40 (33–44) | 50 (46–51) | 34 (32–40) | 42 (39–51) | 40 (32–42) |
| Triglycerides, mg/dl | 106 (67–146) | 73 (65–94) | 137 (76–193) | 73 (51–105) | 109 (69–128) |
| Triglyceride:HDL ratio | 2.5 (1.7–4.5) | 1.5 (1.4–2.0) | 4.5 (1.9–5.3) | 1.6 (1.2–2.6) | 2.6 (1.9–3.7) |
CIRgp, corrected insulin release at the glucose peak; CISI, composite insulin sensitivity index; HDL, high-density lipoprotein; HOMAIR, homeostasis model assessment for insulin resistance; IGFBP-1, insulin-like growth factor–binding protein-1; OGTT, oral glucose tolerance test.
Obesity measures
Black subjects tended to have higher BMI z-scores than did nonblack subjects (P = 0.12). Although black females had greater BMI (P = 0.02) than the other subgroups, their weights (P = 0.13) and WCs (P = 0.47) were similar. The mean of the WC-NHANES was greater than the WC-UMB (111.4 cm vs. 104.0 cm). The precision of each WC (standard deviation for replicate measurements) was similar (UMB s.d. 0.96 cm, 95% confidence interval 0.81–1.10; NHANES s.d. 1.05 cm, 95% confidence interval 0.81–1.22 cm). The correlation between the two measures of WC was only moderately high (r = 0.83). Moreover, although the correlations of WC with measures of insulin resistance and β-cell activity were generally low, the correlations with the NHANES method of measuring WC were consistently better (Table 3).
Table 3.
Spearman correlations between obesity measures and insulin resistance measures in obese adolescents
| n | Fasting insulin | CIRgp | CISI | IGFBP-1 | |
|---|---|---|---|---|---|
| BMI z-score | 92 | 0.29 (P = 0.006) | 0.24 (P = 0.022) | –0.31 (P = 0.003) | –0.39 (P < 0.001) |
| Waist (UMB) | 90 | 0.18 (P = 0.09) | 0.09 (P > 0.40) | –0.17 (P > 0.10) | –0.27 (P = 0.01) |
| Waist (NHANES) | 63 | 0.29 (P = 0.020) | 0.17 (P > 0.10) | –0.27 (P = 0.03) | –0.47 (P < 0.001) |
| DXA lean mass | 91 | 0.25 (P = 0.02) | 0.21 (P = 0.047) | –0.24 (P = 0.02) | –0.39 (P < 0.001) |
| DXA fat mass | 91 | 0.10 (P > 0.30) | 0.13 (P > 0.20) | –0.06 (P > 0.50) | –0.22 (P = 0.03) |
| CT-IP fat area | 87 | 0.35 (P = 0.001) | 0.01 (P > 0.90) | –0.34 (P = 0.002) | –0.44 (P < 0.001) |
| CT-SQ fat area | 87 | 0.14 (P > 0.10) | 0.11 (P > 0.30) | –0.13 (P > 0.20) | –0.23 (P = 0.03) |
| IGFBP-1 | 92 | –0.47 (P < 0.001) | –0.34 (P = 0.001) | 0.51 (P < 0.001) | – |
CIRgp, corrected insulin release at the glucose peak; CISI, composite insulin sensitivity index; CT-IP, intraperitoneal fat measured by computed tomography; CT-SQ, subcutaneous fat measured by computed tomography; DXA, dual-energy X-ray absorptiometry; IGFBP-1, insulin-like growth factor-binding protein-1; NHANES, National Health and Nutrition Examination Survey; UMB, umbilicus.
Median percent body fat across all subjects, as measured by DXA, was 41%. Using fat distribution derived for areal CT measures, 67.3% (interquartile range 62.0–70.8%) of abdominal cross-sectional area was fat, 14% of which was IP fat. Total body fat was higher among females using either measure (P < 0.0001 for DXA, P = 0.004 for CT).
Correlation among obesity measures
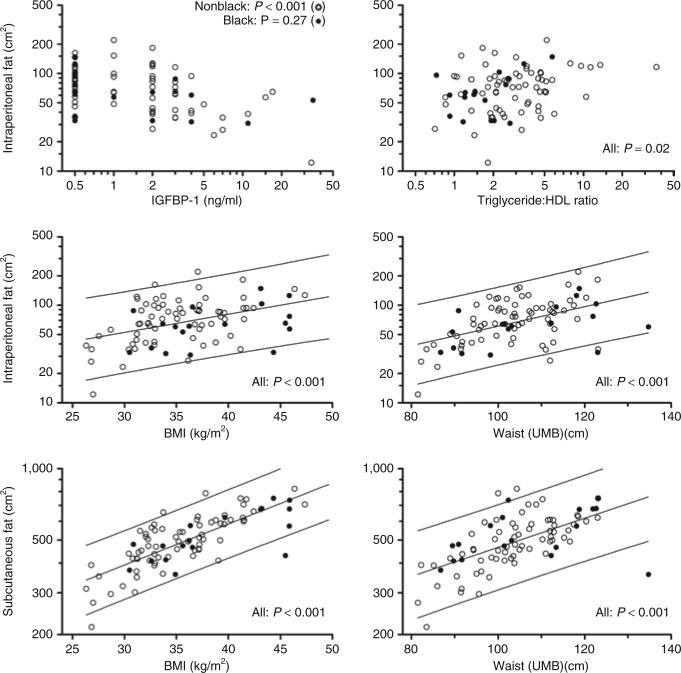
As illustrated in Figure 1, WC-UMB was highly associated (P < 0.001) with CT-determined IP fat (CT-IP; Spearman r = 0.61), CT-determined subcutaneous fat (CT-SQ; r = 0.74), and CT-total fat (r = 0.79). Likewise, BMI was highly associated with CT-SQ fat (r = 0.67) and CT-total fat (r = 0.66). The association between BMI and CT-IP fat was weaker (r = 0.30, P = 0.006). BMI z-score, DXA fat mass, and body weight showed essentially the same associations with the CT fat measures.
Figure 1.
Association of abdominal fat compartments with anthropometric and serum measures (unadjusted). For fully adjusted regression analysis, see table 4. Association of intraperitoneal fat with serum IGFBP-1 (upper left) was significant for nonblack subjects (open circles), nonsignificant for blacks (filled circles) as indicated by race × IGFBP-1 interaction in multiple regression (P = 0.01); no other associations differed by race. Lines indicate 95% confidence interval for estimating IP or SQ fat area from simple regression on BMI or waist (UMB) in a new individual from a population of obese adolescents comparable to this sample. IGFBP-1, insulin-like growth factor–binding protein-1; IP, intraperitoneal; UMB, umbilicus; SQ, subcutaneous.
Modeling CT fat measures
In multiple regression analysis, WC-UMB and BMI, jointly and independently, were strongly associated with both CT-IP fat and CT-SQ fat (Table 4). WC-NHANES methodology was just as strongly associated as WC-UMB with CT-SQ fat (data not shown). BMI and WC-UMB explained 62% of variance of CT-SQ fat, but only 26% for CT-IP fat.
Table 4.
Association of abdominal fat compartments with anthropometric and serum measures
| Intraperitoneal fat area |
Subcutaneous fat area |
||||||
|---|---|---|---|---|---|---|---|
| Association | Variable | Coefficient | R 2 | P | Coefficient | R 2 | P |
| Simplea | Waist (UMB) (cm) | 2.3 (1.4, 3.2) | 0.24 | <0.001 | 1.4 (1.0, 1.8) | 0.37 | <0.001 |
| BMI (kg/m2) | 4.4 (2.3, 6.5) | 0.17 | <0.001 | 4.1 (3.3, 4.8) | 0.59 | <0.001 | |
| Age (years) | 5.4 (–2.9, 14.4) | 0.02 | 0.20 | 1.6 (–2.4, 5.9) | 0.01 | 0.43 | |
| Male | 7.3 (–14.7, 35.0) | 0.00 | 0.54 | –9.9 (–19.4, 0.8) | 0.04 | 0.07 | |
| Black | –13.2 (–48.6, 13.8) | 0.01 | 0.37 | 6.6 (–6.9, 18.4) | 0.01 | 0.32 | |
| TG:HDL ratio | 3.0 (0.4, 5.6) | 0.06 | 0.02 | – c | |||
| IGFBP-1 (ng/ml) | – | ||||||
| Black | –1.5 (4.3, 1.2) | 0.01 | 0.27 | ||||
| Nonblack | –4.8 (–6.9, –2.6) | 0.18 | <0.001 | ||||
| Adjustedb | Waist (UMB) (cm) | 1.4 (0.3, 2.4) | 0.09 | 0.01 | 0.5 (0.1, 0.9) | 0.07 | 0.02 |
| BMI (kg/m2) | 2.6 (0.0, 5.2) | 0.05 | 0.05 | 3.5 (2.4, 4.5) | 0.35 | <0.001 | |
| Age (years) | 3.7 (–3.1, 11.0) | 0.01 | 0.29 | 0.2 (–2.5, 2.9) | 0.00 | 0.90 | |
| Male | 17.5 (–4.6, 44.8) | 0.03 | 0.13 | –1.2 (–8.8, 7.0) | 0.00 | 0.77 | |
| Black | –20.3 (–50.5, 3.9) | 0.07 | 0.11 | –7.1 (–17.2, 2.1) | 0.03 | 0.13 | |
| TG:HDL ratio | 2.1 (–0.0, 4.4) | 0.05 | 0.05 | – c | |||
| IGFBP-1 (ng/ml) | – | ||||||
| Black | –0.1 (–2.5, 2.4) | 0.01 | 0.96 | ||||
| Nonblack | –4.1 (–6.1, –2.1) | 0.17 | 0.0002 | ||||
HDL, high-density lipoprotein; IGFBP-1, insulin-like growth factor–binding protein-1; TG, triglyceride; UMB, umbilicus.
From simple regression of log-transformed fat area on indicated variable. Coefficient is percentage increase or decrease in fat area per unit change in indicated variable (or attributable to presence of dichotomous variable), with 95% confidence interval. R2 is fraction of variance explained by variable. P tests for zero coefficient.
Coefficient adjusted by multiple regression for all listed variables. R2 is fraction of variance explained in addition to that explained by all other variables. P tests for zero coefficient.
Not included in analysis.
Estimation of CT fat measures
The tiglyceride:high-density lipoprotein (TG:HDL) ratio showed a significant association with both IP fat (P = 0.02) and SQ fat (P = 0.05), whereas IGFBP-1 was associated with CT-IP fat only among nonblacks. Adding TG:HDL ratio and the interaction term IGFBP-1 × black to the regression model increased the explained variance to 45%. Neither age nor race contributed significantly to the regression model for either CT-SQ or CT-IP fat after adjustment for the other variables. Gender was marginally associated with SQ fat (P = 0.07).
Several other variables indicative of insulin resistance showed simple associations with CT-IP fat and were tested for inclusion in the multiple regression model. Fasting insulin was mildly associated with CT-IP fat (r = 0.35, Table 3) though not as strongly as IGFBP-1 (r = −0.47). When added to the model, fasting insulin did not show statistical significance (P = 0.33) or substantially attenuate the effects of other regression variables, despite its moderate correlation with IGFBP-1 (r = −0.50). HOMAIR, CISI, CIRgp, impaired fasting glucose (>100 mg/dl), impaired glucose tolerance (2-h OGTT glucose >140 mg/dl), and the area under the OGTT insulin curve likewise failed to show statistical significance when added to the model or materially affect the significance of the other regressors. Excluding the 25 subjects with impaired fasting glucose or impaired glucose tolerance produced only minor changes in the regression models reported in Table 4. The effects of WC and TG:HDL ratio were attenuated and became nonsignificant, but the remaining regression coefficients and significance levels were essentially unchanged. In particular, the interaction of IGFBP-1 with black race remained significant (P = 0.01), with a coefficient of −4% in IP fat per ng/ml increment in IGFBP-1 in nonblacks (P = 0.001) and a virtually null coefficient in blacks (P = 0.58).
Glucose and insulin indices
Fasting glucoses were normal (<100 mg/dl) in 81 of 91 (89%) subjects, and no subject had a baseline fasting glucose above 125 mg/dl. Two hours after the standard glucose load, 76 of 92 subjects had glucose concentrations of <140 mg/dl and no subject had 2-h glucoses exceeding 199 mg/dl. Fasting insulin concentrations were highly associated (P < 0.001) with other measures of insulin resistance including HOMA (r = 0.99), CISI (r = −0.93), CIRgp (r = 0.47), TG:HDL (r = 0.44), and IGFBP-1 (r = −0.46). Black females had similar degrees of insulin resistance when measured by fasting insulin (P = 0.31), HOMA (P = 0.37), or CISI (P = 0.30). Likewise, black females also had similar β-cell activity as measured by CIRgp (P = 0.40).
Correlation between insulin indices and obesity
BMI z-score, WC-NHANES, and DXA lean mass were moderately associated with measures of both insulin resistance (CISI, insulin, and IGFBP-1) and insulin secretion (CIRgp) (Table 3). CT-IP fat was associated with measures of insulin resistance, but not insulin secretion. Neither CT-SQ fat nor DXA fat mass showed association with any index of insulin resistance or secretion.
DISCUSSION
Body mass is made up of three compartments, which are associated with longevity (muscle, bone, and SQ fat), and one compartment associated with mortality (IP fat). Quantification of body composition by anthropometric indices, such as weight, BMI, and WC, are only variably indicative of the amount of IP fat; accounting for a wide variance in metabolic outcome based on these measures alone.
Although an excess of SQ fat can contribute to comorbidi-ties, numerous studies have demonstrated the primary pathogenicity of IP fat in mediating the cardio vascular and metabolic comorbidities associated with obesity (17,18). Unfortunately, direct measurement of IP fat by CT or magnetic resonance imaging is expensive and generally impractical. One of the goals of our analysis, therefore, was to determine how well we could estimate IP fat from a combination of anthropometric and simple fasting laboratory measures. Using a combination of BMI, WC, fasting IGFBP-1, and the TG:HDL ratio, we were able to account for about 45% of the variance in IP fat (Table 4, equations in Supplementary Appendix 2 online).
IGFBP-1 levels are affected by insulin levels, as portal insulin strongly downregulates IGFBP-1 (ref. 19). Previously, Travers et al. found IGFBP-1 levels to have a strong univariate inverse correlation with body fatness as measured by underwater weighing (20). However, the univariate relationship between IGFBP-1 and body fatness in their study did not persist when subjected to a multiple model that included insulin sensitivity. In our analysis, we examined the utility of fasting IGFBP-1 as a measurement of IP fat. Simple regression demonstrates the inverse correlation between IGFBP-1 and IP fat in nonblack adolescents. More importantly, this relationship is also seen when multiple regression analysis is completed. Therefore, a lower IGFBP-1 value can be used to estimate IP area particularly in nonblack adolescents. The same inverse correlation between IGFBP-1 and IP fat is seen in black adolescents as well; however, we did have a smaller number of black subjects compared to nonblack subjects. Although a racial difference in IGFBP-1 values is seen, the authors could not find any previously published studies that document this difference in adolescents.
The effects of gender and puberty on IGFBP-1 levels have been variable depending on the population (20–23). In our study, a gender difference in IGFBP-1 level is only seen in nonblack males and all females. Interestingly, black and non-black females had the same IGFBP-1 levels whereas the black males seemed to have a lower IGFBP-1 value than nonblack males. However, our sample size for black males is quite small. Regardless of gender, the measurement of IGFBP-1 was a good estimator of IP fat for obese nonblack adolescents. In addition, our subjects were all adolescents aged 13–17 years who were at minimum midway through pubertal development. Therefore, pubertal effects were not seen.
In addition, previous work by Motaghedi et al. found that IGFBP-1 levels could be used as a serum marker for insulin resistance (24). Furthermore, fasting peripheral insulin levels can be normal in children with mild insulin resistance, while IGFBP-1 levels are decreased reflecting the early stage of portal hyperinsulinemia (25). The explanation is thought to be that periodic postprandial hyperinsulinemia may first occur and later abnormal fasting insulin levels are seen (26). Interestingly in our analysis, fasting insulin was a significant estimator of IP fat by itself (P = 0.001), but not after controlling for all the variables in Table 4. However, IGFBP-1 is associated with IP fat both alone and after controlling for all the same variables. Thus, our findings further support the utility of fasting IGFBP-1 levels as an additional tool for assessing insulin resistance and as a part of a model to estimate IP fat without costly and time consuming CT or magnetic resonance imaging scans of the abdomen.
TG:HDL ratio
In adults, the TG:HDL ratio provides a reasonable estimation of insulin sensitivity (27). Under the influence of insulin, HDL particles increase in size and buoyancy with increasing cholesterol acquisition and esterification, at which point the cholesterol stored can be exchanged with TG-rich lipoproteins via cholesterol ester transfer protein activity, thus lowering HDL-cholesterol levels (28). As insulin sensitivity is highly correlated with IP fat, it is not surprising that the TG:HDL ratio lent power to estimation of CT-IP in nonblack subjects. However, we found a lower TG:HDL ratio in blacks, a phenomenon documented in many studies. This is almost certainly due to the finding of apolipoprotein CIII polymorphisms in blacks, accounting for their lower triglyceride levels (29,30).
Insulin sensitivity
We measured insulin sensitivity as a combined group; thus, there is the limitation that the HOMA will be affected by impaired fasting glucose and CISI will be altered by both impaired fasting and impaired glucose tolerance. However, our analysis has taken these alterations into account. We found HOMA values very similar to those found in the 1999–2002 NHANES data among adolescents with BMI >95th percentile (31). In this study, Lee et al. examined HOMAIR levels for each year of adolescence (12–19 years); normal and overweight females had an earlier peak in HOMAIR compared to normal and overweight males, reflecting the effects of puberty on insulin resistance. However, our, age range was smaller, most subjects were at least midway through puberty and all of our subjects were obese. Therefore, we captured the highest HOMAIR values and did not see a peak in HOMAIR based on age or pubertal development. Interestingly, we found that black females had the lowest HOMAIR value; therefore this insulin sensitivity reflects the racial and gender difference of a black female.
Racial differences
Racial differences are thought to play a role in the expression and severity of insulin resistance (32,33). The Bogalusa Heart Study demonstrated racial dichotomies in insulin dynamics during an OGTT. Obese black children demonstrated both insulin resistance and insulin hypersecretion (34). Later, Jiang et al. proposed that the elevated insulin levels, particularly in black females, may be attributed to a decrease in hepatic clearance rather than in secretion (35). More recently, after adjusting for clearance, Hannon et al. found that there remained a race difference in first-phase insulin concentration between black and nonblack adolescents and perhaps there is upregulation of β-cell function in blacks. In our study, the median fasting insulin was lower among black black females compared to the other groups (Table 2). The black females were less insulin resistant by fasting insulin, HOMA, and CISI. Black females also had greater insulin release as measured by CIR. Overall, insulin hypersecretion as measured by CISI was also noted in blacks, although measures of insulin resistance, as measured by fasting insulin, HOMA, and CISI, were not as consistent. Of interest is that blacks measured lower CT-IP fat, despite a higher BMI and increased WC.
Conclusion
We sought to analyze the anthropometric measures of adiposity and fasting indices of insulin resistance, including IGFBP-1 and to provide a clinical estimate of IP fat in obese adolescents (BMI ≥95th percentile), between ages 13 and 17 years. We recruited and enrolled all eligible participants and as a result, there were more females than males represented in the study and fewer blacks than nonblacks. Our study supports the racial differences established between black and nonblack individual; however, our number of black adolescents, particularly males, is limited. We did not include an analysis of the same factors in nonobese adolescents as our subjects were included to be part of the baseline evaluation for entry into a multicenter, randomized, placebo-controlled, double-blind trial of metformin XR in obese adolescents. Most of our subjects were at least Tanner 3 or greater at the time of enrollment and therefore we cannot make pre- and postpubertal comparisons. Although our population consisted of obese adolescents, surrogate measures of insulin resistance from OGTT parameters are still valid (36). However, despite these limitations, we provide a useful recommendation for the clinician. In addition to the usual glucose, insulin, and lipid profile obtained to assess for insulin resistance when evaluating an obese, nonblack adolescent, we recommend measuring a fasting IGFBP-1 as a component of our statistical model (see Supplementary Appendix 2 online) to estimate IP fat without a costly and time consuming CT or magnetic resonance imaging scans of the abdomen. Furthermore, lifestyle intervention programs using diet and exercise might include fasting IGFBP-1 levels as a marker of progress in the nonblack population.
Supplementary Material
ACKNOWLEDGMENTS
The Glaser Pediatric Research Network (GPRN) consists of five clinical research centers and a Data Coordinating Center (listed below) devoted to clinical research involving disorders important in pediatrics. The GPRN is funded by the Elizabeth Glaser Pediatric Research foundation (EGPRF), a program of the Elizabeth Glaser Pediatric aIDS foundation. The study was funded by the EGPRf and the NIH-supported Clinical Research Centers (Stanford University: grant number MO1-RR00070; Baylor College of Medicine: grant number MO1-RR00188; University of California, San francisco: grant number UL-RR024131-01; University of California, Los angeles: grant number MO1-RR00865; Harvard Medical School: grant number MO1-RR02172). Bristol-Myers Squibb generously provided active drug and placebo for the subsequent intervention phase of the study.
Footnotes
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http://www.nature.com/oby
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1.Deckelbaum RJ, Williams CL. Childhood obesity: the health issue. Obes Res. 2001;9(Suppl 4):239S–243S. doi: 10.1038/oby.2001.125. [DOI] [PubMed] [Google Scholar]
- 2.Montague CT, O'Rahilly S. The perils of portliness: causes and consequences of visceral adiposity. Diabetes. 2000;49:883–888. doi: 10.2337/diabetes.49.6.883. [DOI] [PubMed] [Google Scholar]
- 3.Asayama K, Dobashi K, Hayashibe H, et al. Threshold values of visceral fat measures and their anthropometric alternatives for metabolic derangement in Japanese obese boys. Int J Obes Relat Metab Disord. 2002;26:208–213. doi: 10.1038/sj.ijo.0801865. [DOI] [PubMed] [Google Scholar]
- 4.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 5.Matsuzawa Y, Nakamura T, Shimomura I, Kotani K. Visceral fat accumulation and cardiovascular disease. Obes Res. 1995;3(Suppl 5):645S–647S. doi: 10.1002/j.1550-8528.1995.tb00481.x. [DOI] [PubMed] [Google Scholar]
- 6.De Simone M, Verrotti A, Iughetti L, et al. Increased visceral adipose tissue is associated with increased circulating insulin and decreased sex hormone binding globulin levels in massively obese adolescent girls. J Endocrinol Invest. 2001;24:438–444. doi: 10.1007/BF03351044. [DOI] [PubMed] [Google Scholar]
- 7.Buemann B, Sørensen TI, Pedersen O, et al. Lower-body fat mass as an independent marker of insulin sensitivity--the role of adiponectin. Int J Obes (Lond) 2005;29:624–631. doi: 10.1038/sj.ijo.0802929. [DOI] [PubMed] [Google Scholar]
- 8.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;27 [PubMed] [Google Scholar]
- 9.Wang J, Thornton JC, Bari S, et al. Comparisons of waist circumferences measured at four sites. Am J Clin Nutr. 2003;77:379–384. doi: 10.1093/ajcn/77.2.379. [DOI] [PubMed] [Google Scholar]
- 10.Anthropometry Procedures Manual [February 2008];2000 < www.cdc.gov/nchs/data/nhanes/nhanes_03_04/BM.pdf>.
- 11.Burke JP, Hale DE, Hazuda HP, Stern MP. A quantitative scale of acanthosis nigricans. Diabetes Care. 1999;22:1655–1659. doi: 10.2337/diacare.22.10.1655. [DOI] [PubMed] [Google Scholar]
- 12.Borkan GA, Gerzof SG, Robbins AH, et al. Assessment of abdominal fat content by computed tomography. Am J Clin Nutr. 1982;36:172–177. doi: 10.1093/ajcn/36.1.172. [DOI] [PubMed] [Google Scholar]
- 13.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 14.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 15.Lustig RH, Mietus-Snyder ML, Bacchetti P, et al. Insulin dynamics predict body mass index and z-score response to insulin suppression or sensitization pharmacotherapy in obese children. J Pediatr. 2006;148:23–29. doi: 10.1016/j.jpeds.2005.08.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sluiter WJ, Erkelens DW, Reitsma WD, Doorenbos H. Glucose tolerance and insulin release, a mathematical approach I. Assay of the β-cell response after oral glucose loading. Diabetes. 1976;25:241–244. doi: 10.2337/diab.25.4.241. [DOI] [PubMed] [Google Scholar]
- 17.Després JP, Lemieux I, Bergeron J, et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28:1039–1049. doi: 10.1161/ATVBAHA.107.159228. [DOI] [PubMed] [Google Scholar]
- 18.Hamdy O, Porramatikul S, Al-Ozairi E. Metabolic obesity: the paradox between visceral and subcutaneous fat. Curr Diabetes Rev. 2006;2:367–373. doi: 10.2174/1573399810602040367. [DOI] [PubMed] [Google Scholar]
- 19.Yki-Jarvinen H, Makimattila S, Utriainen T, Rutanen EM. Portal insulin concentrations rather than insulin sensitivity regulate serum sex hormone-binding globulin and insulin-like growth factor binding protein 1 in vivo. J Clin Endocrinol Metab. 1995;80:3227–3232. doi: 10.1210/jcem.80.11.7593430. [DOI] [PubMed] [Google Scholar]
- 20.Travers SH, Labarta JI, Gargosky SE, et al. Insulin-like growth factor binding protein-I levels are strongly associated with insulin sensitivity and obesity in early pubertal children. J Clin Endocrinol Metab. 1998;83:1935–1939. doi: 10.1210/jcem.83.6.4857. [DOI] [PubMed] [Google Scholar]
- 21.Buyalos RP, Pekonen F, Halme JK, Judd HL, Rutanen EM. The relationship between circulating androgens, obesity, and hyperinsulinemia on serum insulin-like growth factor binding protein-1 in the polycystic ovarian syndrome. Am J Obstet Gynecol. 1995;172:932–939. doi: 10.1016/0002-9378(95)90024-1. [DOI] [PubMed] [Google Scholar]
- 22.Yeoh SI, Baxter RC. Metabolic regulation of the growth hormone independent insulin-like growth factor binding protein in human plasma. Acta Endocrinol (Copenh) 1988;119:465–473. doi: 10.1530/acta.0.1190465. [DOI] [PubMed] [Google Scholar]
- 23.Holly JM, Smith CP, Dunger DB, et al. Levels of the small insulin-like growth factor-binding protein are strongly related to those of insulin in prepubertal and pubertal children but only weakly so after puberty. J Endocrinol. 1989;121:383–387. doi: 10.1677/joe.0.1210383. [DOI] [PubMed] [Google Scholar]
- 24.Motaghedi R, Gujral S, Sinha S, et al. Insulin-like growth factor binding protein-1 to screen for insulin resistance in children. Diabetes Technol Ther. 2007;9:43–51. doi: 10.1089/dia.2006.0056. [DOI] [PubMed] [Google Scholar]
- 25.Saitoh H, Kamoda T, Nakahara S, Hirano T, Nakamura N. Serum concentrations of insulin, insulin-like growth factor(IGF)-I, IGF binding protein (IGFBP)-1 and -3 and growth hormone binding protein in obese children: fasting IGFBP-1 is suppressed in normoinsulinaemic obese children. Clin Endocrinol (Oxf) 1998;48:487–492. doi: 10.1046/j.1365-2265.1998.00476.x. [DOI] [PubMed] [Google Scholar]
- 26.Le Stunff C, Bougneres P. Early changes in postprandial insulin secretion, not in insulin sensitivity, characterize juvenile obesity. Diabetes. 1994;43:696–702. doi: 10.2337/diab.43.5.696. [DOI] [PubMed] [Google Scholar]
- 27.McLaughlin T, Reaven G, Abbasi F, et al. Is there a simple way to identify insulin-resistant individuals at increased risk of cardiovascular disease? Am J Cardiol. 2005;96:399–404. doi: 10.1016/j.amjcard.2005.03.085. [DOI] [PubMed] [Google Scholar]
- 28.Mietus-Snyder M. Lipid metabolism in children and adolescents: impact on vascular biology. J Clin Lipidol. 2008;2:127–137. doi: 10.1016/j.jacl.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Florez H, Mendez A, Casanova-Romero P, et al. Increased apolipoprotein C-III levels associated with insulin resistance contribute to dyslipidemia in normoglycemic and diabetic subjects from a triethnic population. Atherosclerosis. 2006;188:134–141. doi: 10.1016/j.atherosclerosis.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 30.Shin MJ, Kanaya AM, Krauss RM. Polymorphisms in the peroxisome proliferator activated receptor-α gene are associated with levels of apolipoprotein CIII and triglyceride in African-Americans but not Caucasians. Atherosclerosis. 2008;198:313–319. doi: 10.1016/j.atherosclerosis.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JM, Okumura MJ, Davis MM, Herman WH, Gurney JG. Prevalence and determinants of insulin resistance among U.S. adolescents: a population-based study. Diabetes Care. 2006;29:2427–2432. doi: 10.2337/dc06-0709. [DOI] [PubMed] [Google Scholar]
- 32.Hannon TS, Bacha F, Lin Y, Arslanian SA. Hyperinsulinemia in African American (AA) adolescents compared with their American white (AW) peers despite similar insulin sensitivity: a reflection of up-regulated β-cell function? Diabetes Care. 2008;31:1445–1447. doi: 10.2337/dc08-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arslanian S, Danadian K. Insulin secretion, insulin sensitivity and diabetes in black children. Trends Endocrinol Metab. 1998;9:194–199. doi: 10.1016/s1043-2760(98)00049-6. [DOI] [PubMed] [Google Scholar]
- 34.Preeyasombat C, Bacchetti P, Lazar AA, Lustig RH. Racial and etiopathologic dichotomies in insulin hypersecretion and resistance in obese children. J Pediatr. 2005;146:474–481. doi: 10.1016/j.jpeds.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 35.Jiang X, Srinivasan SR, Radhakrishnamurthy B, Dalferes ER, Berenson GS. Racial (black-white) differences in insulin secretion and clearance in adolescents: the Bogalusa heart study. Pediatrics. 1996;97:357–360. [PubMed] [Google Scholar]
- 36.Yeckel CW, Weiss R, Dziura J, et al. Validation of insulin sensitivity indices from oral glucose tolerance test parameters in obese children and adolescents. J Clin Endocrinol Metab. 2004;89:1096–1101. doi: 10.1210/jc.2003-031503. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.