Abstract
ETS transcription factors ETV2, FLI1 and ERG1 specify pluripotent stem cells into endothelial cells (ECs). However, these ECs are unstable and drift towards non-vascular cell fates. We show that human mid-gestation c-Kit− lineage-committed amniotic cells (ACs) can be readily reprogrammed into induced vascular endothelial cells (iVECs). Transient ETV2 expression in ACs generated proliferative but immature iVECs, while co-expression with FLI1/ERG1 endowed iVECs with a vascular repertoire and morphology matching mature stable ECs. Brief TGFβ-inhibition functionalized VEGFR2 signaling, augmenting specification of ACs to iVECs. Genome-wide transcriptional analyses showed that iVECs are similar to adult ECs in which vascular-specific genes are turned on and non-vascular genes are silenced. Functionally, iVECs form long-lasting patent vasculature in Matrigel plugs and regenerating livers. Thus, short-term ETV2 expression and TGFβ-inhibition along with constitutive ERG1/FLI1 co-expression reprogram mature ACs into durable and functional iVECs with clinical-scale expansion potential. Public banking of HLA-typed iVECs would establish a vascular inventory for treatment of genetically diverse disorders.
Introduction
The generation of human endothelial cells (ECs) from non-vascular cell sources has great therapeutic potential for treatment of injured organs. However, the cultivation of stable ECs to clinically relevant scales has not been achieved. Adult-derived ECs have limited expansion potential. Likewise, ECs derived from human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSC) proliferate poorly and drift into non-vascular lineages (James et al., 2010). Endothelial progenitor cells (EPCs) (Lyden et al., 2001; Rafii et al., 2002; Rafii and Lyden, 2003; Jin et al., 2006) and endothelial colony forming cells (ECFCs) show significant expansion potential (Yoder et al., 2007) when grown in plasma (Reinisch et al., 2009). However, whether EPCs and ECFCs could maintain their vascular identity after serial passaging is unknown. The short-comings of existing strategies to generate mature and stable ECs are likely attributable to an insufficient appreciation of the transcription factors and microenvironmental cues that establish durable tissue-specific vascular cells.
Members of the E-twenty six (ETS)-family of transcription factors (TFs), including ETV2 (Lee et al., 2008), FLI1 (Liu et al., 2008), and ERG (McLaughlin et al., 2001) regulate vascular development and angiogenesis (De Val and Black, 2009). These TFs drive the expression of genes associated with EC development and function. Adult ECs constitutively express several ETS factors, such as FLI1 and ERG (isoforms 1 and 2), while ETV2 is transiently expressed during embryonic development and is absent in adult ECs (Hollenhorst et al., 2007). Although many of these TFs play key roles in vascular specification (Liu and Patient, 2008; Pham et al., 2007), it is not known whether defined sets of these TFs can switch on EC genes in non-vascular cells. Here, we show that differentiation of hESCs into embryonic ECs is driven by the expression of ETV2, ERG1 and FLI1. However, ECs generated by this approach are unstable and often loose their vascular identity.
In search of readily accessible and proliferative human cells for generation of ECs, we identified human amniotic fluid-derived cells (ACs) as an ideal source of genetically diverse, non-vascular cells that are amenable to transcriptional reprogramming into induced vascular ECs (iVECs). Freshly isolated ACs are obtained from individuals with broad genetic and ethnic backgrounds, and are routinely cultured from the amniotic fluid of mid-gestation human fetus for diagnostic purposes. They display high proliferative potential, can be HLA-typed, cryopreserved, and stored for clinical use.
Three subclasses of lineage-committed ACs have been identified: epithelioid (E-type), amniotic fluid (AF-type) and fibroblastic (F-type) (Bossolasco et al., 2006). E-type ACs presumably originate from fetal skin, while F-type cells are derived from connective tissue. A rare subset of ACs comprise c-Kit+ (CD117+) multipotent amniotic fluid stem cells (AFS) (De Coppi et al., 2007). Incubation of naïve ACs or pre-selected c-Kit+ AFSs in angiogenic culture conditions have led to the outgrowth of EC-like cells (Benavides et al., 2012; De Coppi et al., 2007; Konig et al., 2012; Zhang et al., 2009). However, it is unknown whether these EC-like cells have acquired a complete repertoire of EC genes, nor has it been verified that their original AC signature is erased.
Here, we report that within 4 days of enforced expression of ETV2, FLI1, ERG1 and TGFβ inhibition in mature lineage-committed c-Kit− ACs, EC-specific genes are induced. Modular two-week ETV2 expression and three-week TGFβ suppression, along with constitutive FLI1/ERG1 co-expression, not only turned on and locked in the expression of EC genes in ACs, but also suppressed expression of non-vascular genes. Attenuation of TGFβ signaling functionalized VEGFR2 signaling pathway, supporting expansion of abundant iVECs without loss of EC identity. Genome-wide transcriptome analyses showed that iVECs express a complete angiogenic signature similar to adult ECs. IVECs established functional, patent, and long-lasting vessels in immunocompromised mice. These data set forth two important findings: 1) Mid-gestation lineage-committed ACs are endowed with a unique plastic epigenetic profile that enables reprogramming of these cells into a large number of vascular cells; 2) Constitutive expression of FLI1/ERG1 in combination with transient expression of ETV2 and TGFβ pathway inhibition provide for an efficient means to reprogram non-vascular cells into a proliferative population of stable and long-lasting iVECs that maintain their vascular identity upon serial passaging.
Results
ETV2, FLI1 and ERG1 differentiate hESCs into ECs that are unstable and have limited proliferative potential
To identify the TFs that are essential for the generation of ECs, we used an established model of hESC differentiation into embryonic ECs (James et al., 2010) (Sup Fig. S1a). Using microarray profiling, we found that ETV2, FLI1 and ERG are key ETS-family TFs that are expressed during differentiation of hESCs into ECs (Sup Fig. S1b). Since, as compared to ERG2, ERG1 isoform was more abundant and functionally active in ECs, we used ERG1 in protocols for the derivation of ECs from hESCs and ACs.
Human ESCs were incubated with BMP2 and VEGF-A for 10 days to generate VEGFR2+CD31− VE-cadherin− cells, which are vascular precursors of early embryonic ECs. Subsequently, these cells were transduced with lentiviral vectors expressing cDNA for ETV2, FLI1, and ERG1 (‘ETS-TFs’) or control virus (Sup Fig. S1a). After culturing cells with VEGF-A, FGF-2, and TGFβ inhibitor, we observed a modest increase in VEGFR2+CD31+VE-cadherin+ ECs among ETS-TF transduced cells compared to controls (Sup Fig. S1c). However, ECs generated from both ETS-TF transduced and untransduced VEGFR2+CD31+VE-cadherin+ precursors failed to proliferate beyond 3 weeks and transdifferentiated into non-EC cell types, such as smooth muscle cells. Therefore, despite enforced expression of ETS-TFs, hESC-derived ECs are unable to sustain their proliferative potential and EC identity.
ETS-TFs reprogram ACs into proliferative and stable iVECs
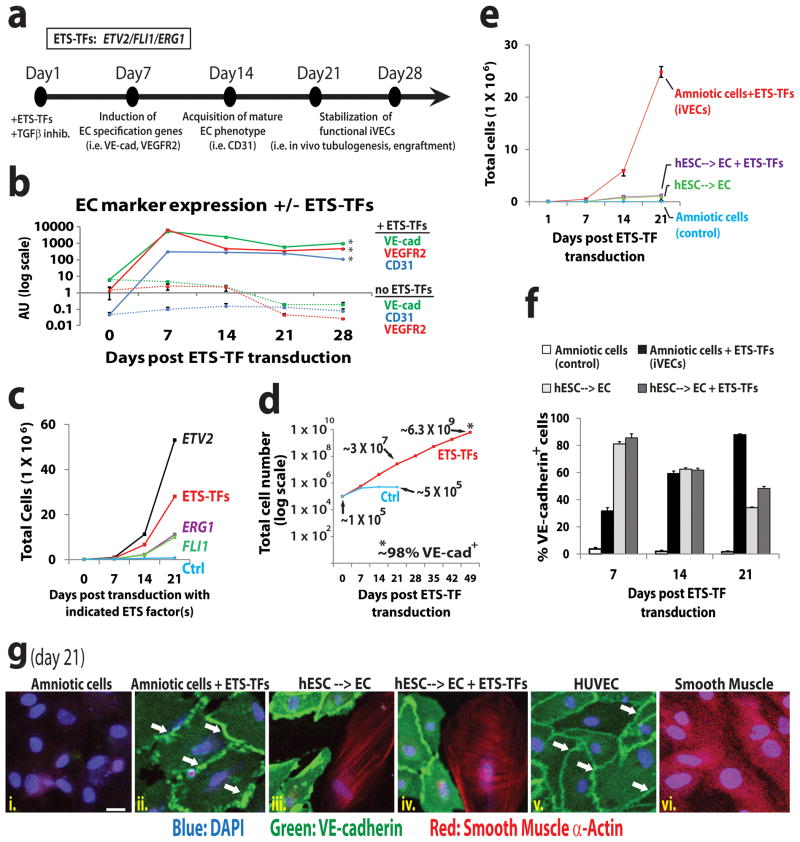
In screening for proliferative non-vascular cells amenable to reprogramming into ECs, we found that EC-specific genes in adult fibroblasts and mesenchymal cells were only minimally induced by ETS-TFs (data not shown). However, mid-gestation ACs proved to be highly reprogrammable. Cultured ACs were transduced singularly and in combination with lentiviral ETS-TFs (ETV2, FLI1, ERG1) (Fig. 1a). As TGFβ inhibition is essential in the derivation of ECs from hESCs (James et al., 2010), ACs were also cultured in the presence of TGFβ receptor inhibitor or neutralizing mAbs to TGFβ ligands. Transduction of ACs with lentiviruses encoding ETV2, FLI1, or ERG1 led to measurable levels of corresponding mRNA and protein expression for several months (Sup Figs. S2a–b). While individual ETS-TFs turned on distinct EC genes (data not shown), ACs transduced with all three ETS-TFs displayed strong induction of multiple EC markers within 7 days and lasting for beyond one month thereafter (Fig. 1b), suggesting that a combination of these factors is necessary to activate the full-compliment of genes associated with EC identity and maturity. Transduction of ACs with ETS-TFs, especially ETV2, led to a rapid increase in cell numbers compared to untransduced cells (Fig. 1c). Starting with 105 ACs transduced with ETV2, FLI1 and ERG1, nearly 30 million VE-cadherin+ iVECs grew out by week 3, and increased to over 6 billion cells by week 7 (Fig. 1d).
Figure 1. ACs transduced with ETS-TFs and TGFβ inhibition display a proliferative and stable vascular phenotype.
a) Schematic of iVEC reprogramming platform. ETV2, FLI1 and ERG1 (ETS-TFs) transduced ACs were cultured in the presence of TGFβ inhibitor (SB431542 – 5μM) and assayed for expression of EC markers. b) EC marker expression was measured in ACs transduced with lentiviral vectors encoding ETV2, ERG1, and FLI1 plus TGFβ inhibition. [“+” = cells transduced with ETV2, ERG1, and FLI1 lentivirus; “−” = cells transduced with equivalent doses of empty-vector lentivirus. All subsequent control (‘ctrl’) samples in this report were transduced with empty-vector virus unless otherwise noted]. (Triplicate samples: *p<.01 compared to control ACs for day 7 – day 28). c) Cellular expansion of ACs transduced with ETV2, FLI1 and/or ERG1 plus TGFβ inhibition for 3 weeks (n=3, p<0.05 for all conditions). d) Cellular expansion of ACs transduced with ETS-TFs plus TGFβ inhibition for 7 weeks (n=4 independent experiments). Fluorescence-activated cell sorting (FACS) performed at week 7 reveals nearly 100% of these cells express VE-cadherin (VE-cad). e) Cellular expansion of ACs and hESC-derived ECs transduced with ETS-TFs plus TGFβ inhibition for 3 weeks (n=3, p<0.05). f) FACS reveals % of VE-cadherin+ cells following transduction by ETS-TFs. g) Immunofluorescence micrographs stained with antibodies to VE-cadherin and Smooth Muscle α-Actin are shown for ETS-TF transduced ACs (iVECs) and ETS-TF transduced hESC-derived VE-cadherin+CD31+VEGFR2+ ECs plus TGFβ inhibition for 3 weeks. HUVECs and smooth muscle cells serve as controls. VE-cadherin (green stain), Smooth Muscle α-Actin (red stain), DAPI (blue stain), white arrows indicate junctional staining of VE-cadherin. Scale bar – 25 μm. See also Sup Figures S1 and S2.
Following transduction with ETS-TFs and TGFβ inhibition, iVECs generated from ACs expanded to numbers nearly 30-fold higher than ETS-TF transduced or untransduced hESCs--> ECs (Fig. 1e). Furthermore, a noticeable decrease in VE-cadherin+ cells was seen over successive passages of ETS-TF transduced or untransduced hESC-derived ECs compared to AC-derived iVECs transduced with ETS-TFs (Fig. 1f). This ‘drift’ away from an EC phenotype within the hESC-derived EC population was verified by staining with Smooth Muscle α-Actin (SMA) antibody (Fig. 1g – boxes iii., iv., and vi.). ACs transduced with ETS factors are negative for this non-EC marker (Fig. 1g – box ii.); rather, they exhibit the typical VE-cadherin expression pattern found on other adult EC types, such as human umbilical vein ECs (HUVECs: Fig. 1g – box v.) and human adult liver sinusoidal ECs (LSECs: Sup Fig. S2c – box iii.). Thus, ACs are more amenable to transcriptional reprogramming into stable ECs than are hESCs or adult fibroblasts.
ACs are devoid of EC precursor cells
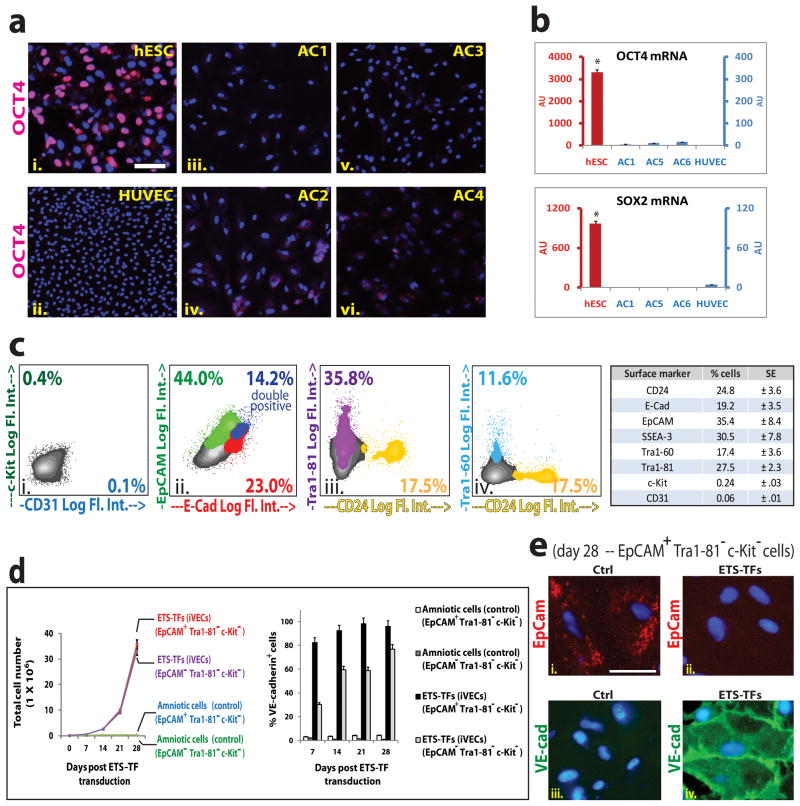
Subsets of c-Kit+ ACs, which compose ≤1% of the AC population (De Coppi et al., 2007), could potentially differentiate into ECs. To exclude the possibility that ECs are exclusively borne out from an undifferentiated sub-population of ACs, we assessed the presence of pluripotency markers. The overwhelming majority of ACs was negative for OCT4 protein (Fig. 2a), confirming results from other groups (Jezierski et al., 2010). Near undetectable mRNA expression levels for OCT4 (Fig. 2b – top panel), SOX2 (Fig. 2b – bottom panel) and NANOG (data not shown) further suggest only a very small subset of ACs cells could be pluripotent or multipotent.
Figure 2. Lineage-committed mature epithelioid and mesenchymal/fibroblastic (Tra1-81− c-Kit−) ACs are reprogrammable into iVECs.
a) hESCs(i.), HUVECs (ii.), and ACs (AC1 to AC4: iii.–vi.) were stained for OCT4 protein (pink). DAPI (blue). Scale bar – 100 μm. b) OCT4 (upper graph) and SOX2 (lower graph) mRNA expression was measured in hESCs, HUVECs, and ACs (AC1, AC5, AC6). Red bars: hESC data (y-axis scale, left side of graph). Blue bars: AC and HUVEC data (y-axis scale, right side of graph). (OCT4 and SOX2: *p<.002 compared to ACs and to HUVECs). c) FACS of ACs indicate presence of lineage-committed and uncommitted cells. One representative cell-line is depicted (i.–iv.). Specific markers tested are indicated on ‘x’ and ‘y’ axes as Log fluorescent intensity, (Log. Fl. Int.) showing the percentage of cells positive for that marker. Chart (right panel) displays mean values for percentage of cells expressing designated markers across fifteen independent cultured AC samples (SE: Standard Error). d) (left graph) Cellular expansion of ETS-TF transduced EpCAM+Tra1-81− c-Kit− and EpCAM − Tra1-81− c-Kit− ACs plus TGFβ inhibition for 4 weeks. (Right graph) FACS reveals % of VE-cadherin+ cells following transduction with ETS-TFs (n=3, P<0.05). e) Immunofluorescence micrographs are shown for ETS-TF transduced EpCAM+Tra1-81−c-Kit− ACs in the presence of TGFβ inhibition for 4 weeks. EpCAM (red stain), VE-cad (green stain), DAPI (blue stain). Scale bar – 25 μm. See also Sup Figure S3.
Notwithstanding, to determine whether ECs could grow out from a pre-existing EC precursor cell, we cultured ACs without transduction of ETS-TFs in optimal EC growth media (EM) and TGFβ inhibitor in a normoxic conditions. We did not detect growth of any stable and fully-committed ECs, verifying that cultured ACs are devoid of pre-existing vascular precursor cells that would generate authentic ECs (Sup Fig. S3a–b).
c-Kit− lineage-committed mature ACs are reprogrammed into iVECs
ACs compose of both undifferentiated cells expressing c-Kit, Tra1-60, Tra1-81, and SSEA-3, SSEA-4, as well as lineage-committed cells, phenotypically marked as E-Cadherin+ (E-Cad, CD324), EpCAM+ (CD326) and CD24+ cells (Fig 2c). To determine whether ETS-TFs could directly reprogram the proliferative and well-defined populations of lineage-committed ACs into iVECs, we depleted c-Kit+ cells from the AC pool. Next, mature epithelioid cells (EpCAM+Tra1-81−c-Kit−) and mesenchymal/fibroblastic cells (EpCAM− Tra1-81−c-Kit−) were purified separately, transduced with ETS-TFs and propagated in the presence of TGFβ inhibition. We observed a significant increase in VE-cadherin+ cells among the ETS-TF transduced EpCAM+Tra1-81−c-Kit− and EpCAM− Tra1-81− c-Kit− subpopulations (Fig. 2d). Immunostaining for EpCAM and VE-cadherin confirmed the transition from an epithelioid-type AC to an iVEC (Fig. 2e). Therefore, lineage-committed ACs are amenable to TF-mediated reprogramming into iVECs.
ETS-TFs induce expression of a vascular signature in ACs
To assess the extent to which iVECs acquire a vascular signature, we temporally measured VE-cadherin and VEGFR2 surface expression on emerging nascent iVECs (Fig. 3a). Four days after transducing TGF-β inhibited ACs with ETS-TFs, VE-cadherin+ cells and to a lesser extent VEGFR2+ cells were generated (Fig. 3a – box ii.). The percentage of cells expressing these markers increased significantly over several weeks (Fig. 3a – boxes iv. and vi.), such that after 4 weeks of reprogramming, the entire population (~99%) of transduced ACs were VE-cadherin+ (Fig. 3b – box ii.) of which nearly two-thirds also showed VEGFR2 expression. In addition, the size and shape of switched cells were similar to HUVECs (Fig. 3b – boxes iv. - ix.). We also performed Comparative Genomic Hybridization (CGH) analysis to assess the genomic integrity of day 28 iVECs. This analysis revealed no genomic abnormalities, demonstrating that proliferating iVECs are genetically stable (Sup Fig. S4a).
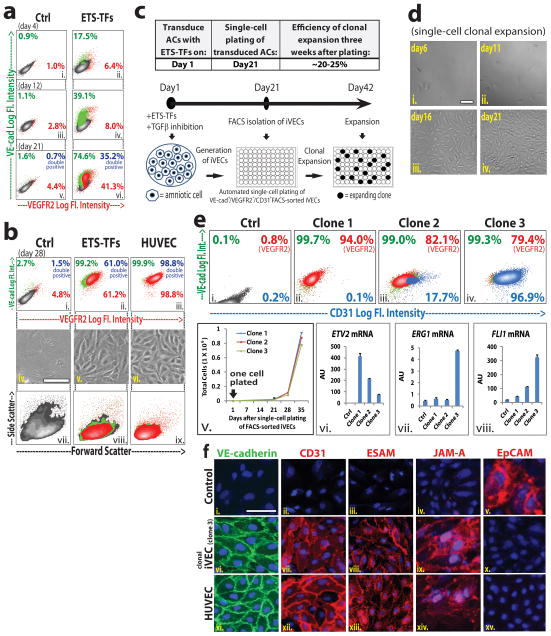
Figure 3. Optimal stoichiometric ratios of ETV2, ERG1, and FLI1 are essential for generation of clonal mature iVECs.
FACS reveals VE-cad and VEGFR2 expression on emerging iVECs at 4 days (a: i.– ii.), 12 days (a: iii.– iv.), 21 days (a: v.– vi.), and 28 days (b: i.–ii.) following transduction with ETS-TFs plus TGFβ inhibition. (control ECs: HUVECs - b: iii.). The morphology (b: iv. – vi.) and size (b: vii. – ix.) of emerging iVECs approximated those of control ECs. Scale bar – 50 μm. c) Schematic of single-cell clonal expansion protocol: ACs were transduced with ETS-TFs and cultured in the presence of TGFβ inhibition. At day 21, monoclonal antibodies (mAb) were used to isolate VE-cad+VEGFR2+CD31+ cells. These iVECs then underwent automated single-cell plating into a 96-well format for clonal expansion for several weeks. On average, 20 to 25% of individual plated cells formed colonies. d) Single-cell clonal expansion over three weeks following VE-cad+VEGFR2+CD31+ isolation (day 21 through day 42). Scale bar – 100 μm. e) FACS of specific iVEC Clone-1, Clone-2, and Clone-3 (boxes ii.– iv.) reveals VE-cad, VEGFR2 and CD31 surface expression at day 42 of clonal expansion protocol (i.e. 3 weeks post VE-cad+VEGFR2+CD31+ isolation). Cellular expansion of iVEC clones from time of single-cell plating (‘day 21’ of clonal expansion protocol) over subsequent 5 weeks (box v.). Expression levels for ETV2, FLI1, and ERG1 of iVEC clones at day 42 of clonal expansion protocol (boxes vi. – viii.). f) Immunofluorescence micrographs are shown for control (ctrl) ACs, clonal iVECs (Clone-3 – day 42) and HUVECs. VE-cad (green stain: i.,vi.,xi.), CD31 (red stain: ii.,vii.,xii.), ESAM (red stain: iii.,viii.,xiii.), JAM-A (red stain: iv.,ix.,xiv.), EpCAM (red stain: v.,x.,xv.), DAPI (blue stain). Scale bar – 50 μm. See also Sup Figure S4 and S5.
Optimal stoichiometry of ETS-TF expression establishes mature and stable iVECs
To create a homogenous population of mature iVECs, we performed clonal analyses. iVECs generated from ACs by transduction with ETS-TFs and TGFβ inhibition were cultured for 21 days. We then isolated single cells expressing VE-cadherin, VEGFR2 and CD31 (VE-cad+VEGFR2+CD31+ cells) and plated them at a density of one cell per well in 96-well plates for clonal expansion (Fig. 3c). Within 21 days, 20–25% of single-cell clones showed expansion potential (Fig. 3d), with individual clones yielding progeny with different phenotypes (Fig. 3e – boxes i. – iv.). While cells from clones 1 to 3 were all VE-cadherin+, CD31 expression varied greatly. Notably, nearly all Clone-3 cells expressed CD31, whereas Clone-1 yielded no CD31+ cells and Clone-2 produced both CD31+ (18%) and CD31− cells. Regardless of CD31 expression, all three clones were able to expand beyond 4 weeks (Fig. 3e – box v.).
These differences in EC marker induction among different clones could be due to levels of ETS-TF expression (Fig. 3e – boxes vi. – viii.). Both ERG1 and FLI1 were expressed in Clone-3, suggesting these factors turn on CD31 expression (Fig. 3e). By contrast, ETV2 correlated negatively with CD31 expression. Thus, Clone-3 has the appropriate combination of ETS-TF expression to induce VE-cadherin, VEGFR2 and CD31. Immunostaining of this clone confirmed the presence of VE-cadherin, CD31, ESAM and JAM-A at cell junctions (Fig. 3f and Sup Fig. S5a). The disappearance of EpCAM in iVECs (Fig. 3f) indicates that the original non-vascular signature of these cells is being stripped away. Thus, differential expression levels of ETS-TFs are important for generating iVECs capable of maintaining optimal EC identity.
Clonally derived iVECs display a transcriptome profile similar to mature ECs
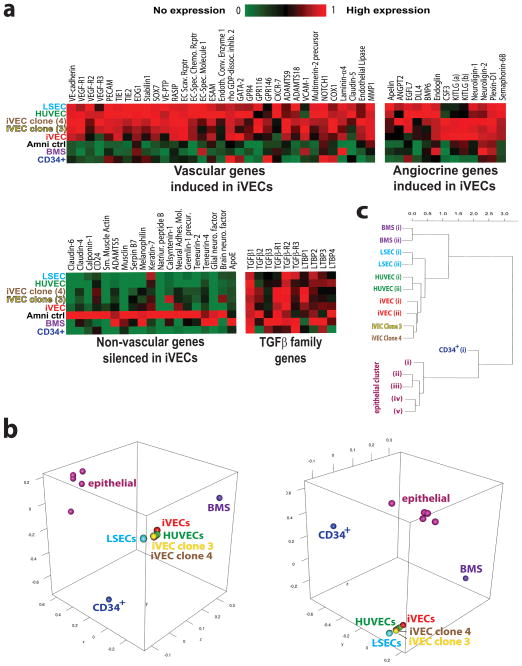
Transcriptome sequencing (RNA-seq) was performed on iVECs derived from ACs. These genome-wide analyses of iVECs were then compared to the transcriptomes of HUVECs, LSECs, and non-EC types, including CD34+ hematopoietic cells (‘CD34+’), bone marrow stromal cells (‘BMS’), and naïve control ACs (‘Amni ctrl’). A significant number of vascular genes were upregulated in non-clonal (‘iVEC’) and clonal (‘iVEC clone-3’ and ‘iVEC clone-4’) derived iVECs compared to naïve ACs (Fig. 4a). Furthermore, the expression levels of these induced EC genes approached those seen in in vitro cultured HUVECs and LSECs, supporting the notion that iVECs have attained a complete EC identity. Angiocrine factors that regulate EC-driven organ regeneration (Butler et al., 2010b; Ding et al., 2010; Ding et al., 2011; Kobayashi et al., 2010) and tumor growth (Butler et al., 2010a), including BMPs, Notch-ligands, IGFs, CSFs, Kit-ligand, and semaphorins, were all switched on in the iVECs as well. Importantly, non-EC genes normally expressed in ACs, such as smooth muscle actin, musclin and calponin-1 were silenced in iVECs.
Figure 4. iVEC global gene transcriptome reveals vascular genes are turned on while non-vascular genes are silenced, matching expression profile of mature ECs.
a) ETS-TF transduced ACs (‘iVEC’) as well as two iVEC clones (‘iVEC Clone-3’ and ‘iVEC Clone-4’) cultured for two months with TGFβ inhibition underwent RNA-seq analysis. (iVEC Clone-4 has a similar ETS-TF expression profile to that of iVEC Clone-3 – data not shown). The expression profile of these iVEC samples were compared to human umbilical cord-blood derived CD34+ cells (‘CD34+’), human bone marrow stromal cells (‘BMS’), naïve human ACs (‘Amni ctrl’), ‘HUVEC’ and ‘LSEC’. Heat-maps of relative transcription levels are shown. b) Three dimensional MDS plots (3D MDS) was generated based on all pairwise distances between the global transcriptome-wide RNA-seq profiles of the samples shown here, including adult lung airway epithelial cells (Hackett et al., 2012). Distances were defined as one minus the Pearson correlation between two profiles. Multidimensional scaling (MDS) was used to identify the set of points in 3D space such that the distances between the points are approximately equal to the true distances between samples. This analysis shows a tight colocalization of clonal and nonconal iVECs with HUVECs and LSECs, thus indicating that their genome-wide expression profiles are highly similar. Contrarily, non-vascular cell types, such as BMS, epithelial cells, and CD34+ hematopoietic cells manifest no similarities to iVECs. This analysis was not biased towards any gene set or processes: all >30,000 RefSeq transcripts whose expression was quantified by RNA-seq were used to calculate distances between samples. c) Hierarchical clustering of iVEC and non-iVEC samples. The same samples and pairwise distances between global transcriptome-wide RNA-seq profiles were used as those used for 3D-MDS, by performing average linkage clustering. Like the 3D-MDS analysis, hierarchical clustering shows that clonal and non-clonal iVECs, but not non-vascular cells cluster closely with HUVECs and LSECs. See also 3D-MDS Movie.
We then compared iVEC and adult EC transcriptomes obtained from RNA-sequencing by performing 3D multi-dimensional scaling (3D-MDS) (Fig. 4b and 3D-MDS movie) and hierarchical clustering analyses (Fig. 4c). A tight association of clonal and non-clonal derived iVECs was seen with HUVECs and LSECs, while BMS, lung-derived epithelial cells (Hackett et al., 2012), and CD34+ hematopoietic cells showed no similarities to iVECs. Notably, iVECs did not express hematopoietic markers, ruling out the possibility that FLI1 and ERG1 induced hematopoietic identity. Therefore, genome-wide analyses demonstrate that enforced expression of ETV2, FLI1 and ERG1 with TGFβ inhibition reprogram ACs into mature iVECs (while silencing non-vascular genes) that resemble authentic mature ECs in global gene expression.
Short-term TGFβ inhibition permanently functionalizes VEGFR2 signaling in iVECs
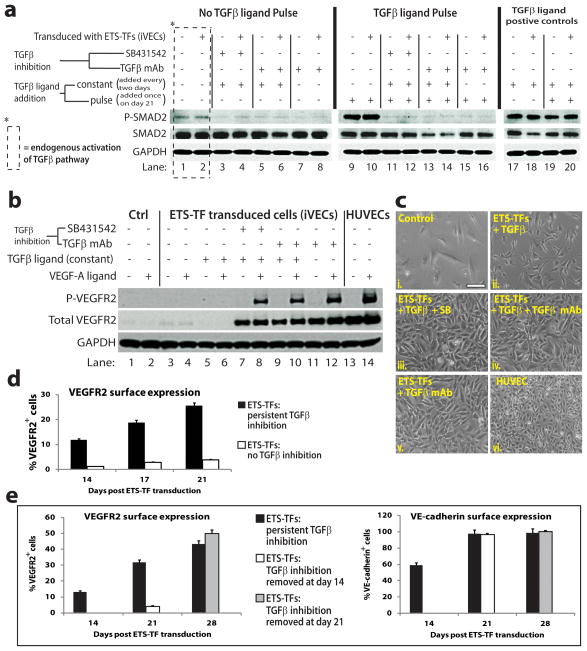
ETS-TF transduced ACs produce both TGFβ and its receptors (Fig. 4a), raising the possibility that an autocrine/juxtacrine loop might favor endothelial-mesenchymal transition (Endo-MT) and prevent iVEC generation (Zeisberg et al., 2007). Thus, ETS-TF transduced ACs were cultured with and without neutralizing mAb to TGFβ ligands, or a TGFβ inhibitor for 21 days. Even in the absence of exogenous TGFβ ligand, basal levels of phosphorylated SMAD2 (P-SMAD2) was active in both control and ETS-TF transduced ACs (Fig. 5a - lanes 1,2). This correlated with absence of total and phosphorylated VEGFR2 protein in these TGFβ activated control and ETS-TF transduced ACs (Fig. 5b - lanes 1–4). However, addition of mAb to TGFβ ligands abrogates P-SMAD2 expression in iVECs (Fig. 5a - lanes 7,8) upregulating VEGFR2 protein (Fig. 5b - lanes 11,12). Importantly, iVECs treated with mAb to TGFβ ligands were responsive to VEGF-A stimulation, as shown by the phosphorylation of VEGFR2 (Fig. 5b - lane 12). Despite supplementation with TGFβ ligand (Fig. 5a - lanes 3–6) or pulsed with TGFβ ligand stimulation (Fig. 5a - lanes 11–16), the presence of TGFβ inhibitors prevented SMAD-2 phosphorylation allowing for upregulation of VEGFR2 protein (Fig 5b - lane 7–10) and VEGF-A dependent phosphorylation (Fig. 5b - lanes 8,10). Notably, TGFβ-activated iVECs failed to attain EC morphology (Fig. 5c - box ii. versus box vi.), while inhibition of TGFβ signaling (Fig. 5c - box iii., iv., v.) endows iVECs with typical cobblestone morphology of EC monolayers (Fig. 5c - box vi.). Thus, suppression of TGFβ signaling functionalizes VEGFR2 preserving the vascular identity of iVECs (Fig. 5d).
Figure 5. TGFβ inhibition upregulates and confers functionality to VEGFR2 in iVECs.
a) Western blot analysis of day 21 iVECs that were treated with or without TGFβ ligand neutralizing mAB (TGFβ ligand mAb, directed against β1, β2, and β3) or TGFβ inhibition. This experiment was performed ± TGFβ ligands (TGFβ1 and TGFβ3) to delineate the extent of TGFβ receptor activity. ETS-TF transduced ACs were incubated ± TGFβ ligands (10ng/ml) every two days (‘constant’) with or without TGFβ ligand mAb (10 μg/ml) or TGFβ inhibitor. On day 21, ETS-TF transduced ACs were serum-starved for 4 hours, and then treated ± one dose of TGFβ ligands (10ng/ml) for 45 minutes (‘pulse’) with or without TGFβ ligand mAb or TGFβ inhibitor. Following day 21 treatment, all cells were assayed for phosphorylated SMAD2 (P-SMAD2), total SMAD2, and GAPDH. b) Western blot analysis of day 21 iVECs that were treated ± ‘constant’ TGFβ ligands, with or without TGFβ ligand mAb or TGFβ inhibitor. On day 21, ETS-TF transduced ACs were serum-starved for 4 hours, and then treated ± VEGF-A (50ng/ml) for 5 minutes. Following day 21 treatment, cells were assayed for phosphorylated VEGFR2 (P-VEGFR2), total VEGFR2, and GAPDH. c) Cell morphology of control ACs (i.), ETS-TF transduced ACs (ii.–v.) and HUVECs (vi.) was noted for indicated treatments with TGFβ ligands, TGFβ ligand mAb, and/or TGFβ small molecule inhibitor. Scale bar – 100 μm. d) FACS reveals expression of VEGFR2 in day 21 ETS-TF transduced ACs in the presence (black bars) or absence (white bars) of persistent TGFβ inhibition. e) FACS reveals surface expression of VEGFR2 (left graph) and VE-cad (right graph) in day 28 ETS-TF transduced ACs. Black bars: cells subjected to persistent TGFβ inhibition – EC marker expression was assessed on day 14, day 21, and day 28. White bars: cells subjected to TGFβ inhibition for 14 days – EC marker expression was assessed on day 21. Gray bars: cells subjected to TGFβ inhibition for 21 days – EC marker expression was assessed on day 28.
To determine whether continuous suppression of TGFβ signaling was necessary to sustain iVEC stability, we sequentially removed TGFβ inhibition following ETS-TF transduction (Fig. 5e – left panel). Upon removal of TGFβ inhibition at day 14 after transduction with ETS-TFs, we noticed a drop in VEGFR2+ cells during the ensuing 7 days of cell growth (Fig. 5e – left panel, white bar). However, when TGFβ inhibition was removed at day 21 after transduction with ETS-TFs, the percentage of VEGFR2+ cells was retained (Fig. 5e – left panel, gray bar). Notably, manipulation of the TGFβ pathway had no effect on VE-cadherin expression in iVECs (Fig. 5e – right graph). Thus, abrogation of TGFβ signaling for only 3 weeks is sufficient to permanently functionalize VEGFR2 signaling, maintaining long-term vascular identity and stability of iVECs.
AC-derived iVECs are engraftable and form functional perfused vessels
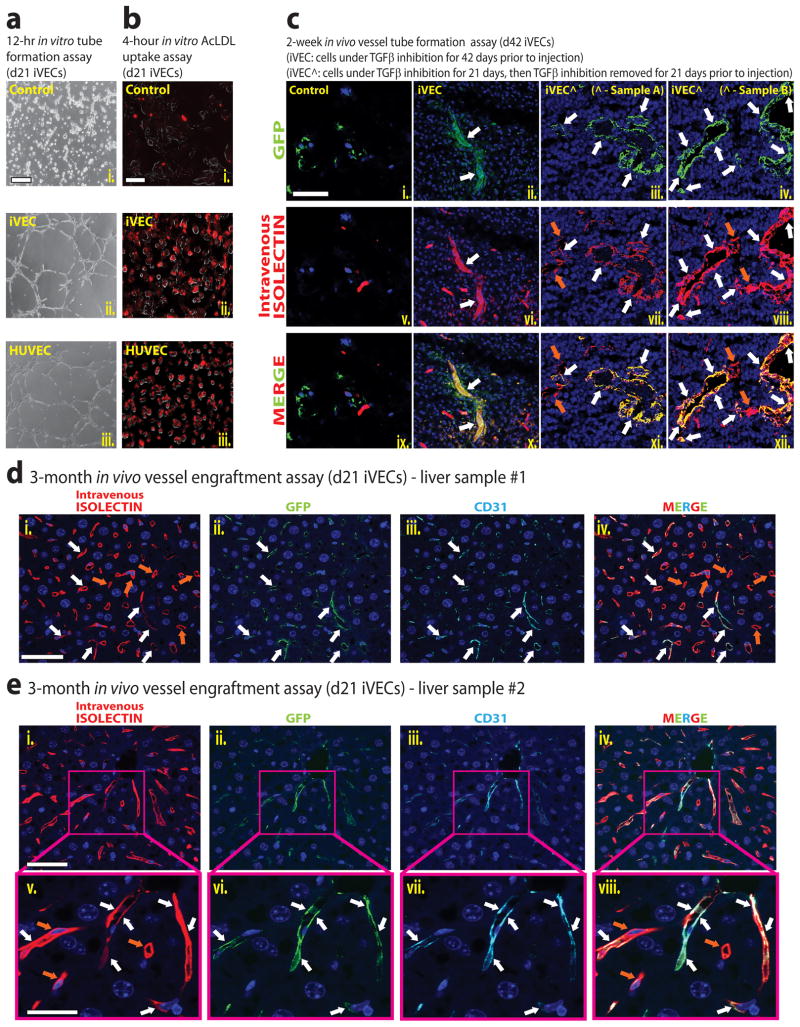
We employed in vitro and in vivo models to assess whether iVECs have acquired full angiogenic potential. A tube formation assay using Matrigel as a substrate showed that day 21 iVECs, but not naïve ACs, were capable of forming tubes in vitro (Fig. 6a). Additionally, uptake of acetylated-LDL (Ac-LDL) in iVECs was similar to HUVECs (Fig. 6b).
Figure 6. iVECs establish functionally perfused vessels in vitro and in vivo.
a) In vitro tube formation assay of control ACs, day 21 iVECs, and HUVECs cultured on Matrigel in EM + TGFβ inhibition for 12 hours. Phase-contrast microscopy images of different groups are shown. Scale bar – 100μm. b) Control ACs, day 21 iVECs, and HUVECs were treated with labeled Ac-LDL (DiI label) for 4 hours. Scale bar – 100μm. c) In vivo tube formation assay of GFP-labeled control ACs (i., v., ix.), GFP-labeled day 42 iVECs (ii., vi., x.), and GFP-labeled day 42 iVECs in which TGFβ inhibition was removed at day 21 (Sample A - iii., vii., xi.; Sample B - iv., viii., xii.). Cells were loaded separately into Matrigel plugs and implanted subcutaneously into NSG mice. Two weeks after implantation, mice were intravenously perfused with Alexa568-Isolectin-B4. After 10 minutes plugs were removed and sectioned. Immunofluorescence show control ACs and iVECs in green (GFP), intravital labeling of perfused vasculature in red (Isolectin), and nuclear counterstain (DAPI) in blue. Scale bar – 50μm. White arrows indicate the colocalization of GFP- and isolectin-marked (anastomosed) vessels. Orange arrows indicate only isolectin-marked host mouse vessels. d) and e) In vivo engraftment of day 21 iVECs (GFP-labeled) into liver sinusoidal vessels of two mice. After performing 70% partial hepatectomy on NSG mice, 5×105 GFP-labeled day 21 iVECs were transplanted via intrasplenic route, which will drain into portal circulation and incorporate into liver vasculature. Thirty minutes later, the spleen was removed to prevent migration back to spleen. Three months following transplantation, the mice were perfused with Alexa568-Isolectin-B4 and the liver was removed. Immunofluorescence show control iVECs in green (GFP), intravital labeling of perfused vasculature in red (Isolectin), human CD31 staining in cyan, and nuclear counterstain (DAPI) in blue. Liver sample #1 (d) was imaged at low magnification (i. – iv.: scale bar - 50 μm). Liver sample #2 (e) was imaged at low (i. – iv.: scale bar - 50 μm) and high magnification (v. – viii.: scale bar - 25 μm). White arrows indicate the colocalization of GFP-, CD31- and isolectin-marked (anastomosed) vessels. Orange arrows indicate only isolectin-marked host mouse vessels.
We then tested for the ability of iVECs to establish functional vasculature in two in vivo models. In one model, GFP-labeled day 42 iVECs under either constant or short-term (21 days) TGFβ inhibition in culture were loaded into Matrigel plugs supplemented with VEGF-A and FGF-2, and injected into immunocompromised NOD-SCID/IL2Rγ−/− (NSG) mice for a duration of 2 weeks (Fig. 6c). Following intravital labeling of the vasculature by perfusion with Alexa568-Isolectin-B4 (10 minutes prior to sacrificing the mice) to identify patent vessels, Matrigel plugs were removed for analysis. Although naïve ACs failed to form any capillaries (Fig. 6c – box ix.), iVECs under constant TGFβ inhibition formed numerous functional vessels of varying caliber that anastomosed to the host vasculature. This was corroborated by colocalization of GFP signal with isolectin marker, as seen in the ‘merge’ image (Fig. 6c – box x.). Similarly, iVECs subjected to short-term TGFβ inhibition showed an abundance of perfused vessels, underscoring their functionality in vivo (Fig. 6c – box xi., box xii.).
Intrasplenic transplantation of ECs results in the engraftment of these cells into the liver sinusoidal vessels of NSG mice that have undergone 70% partial hepatectomy (Ding et al., 2010). We used this in vivo model to interrogate the potential of iVECs to incorporate long-term into the NSG mouse liver vasculature. Three months following intrasplenic transplantation of 5×105 GFP-labeled day 21 iVECs, the mice were perfused with Alexa568-Isolectin-B4 to identify functional vessels. The number of perfused vessels engrafted with human GFP+ iVECs was determined by staining with mAbs specific to human CD31 (hCD31). Notably, we detected the presence of human GFP+Isolectin+hCD31+ ECs within 5–10% of the regenerated liver sinusoidal vessels (Figs. 6d and 6e). Thus, iVECs can engraft and form durable patent vasculature in regenerating liver and establish functional perfused vessels in Matrigel plugs.
Function and vascular identity of iVECs are preserved following suppression of ETV2
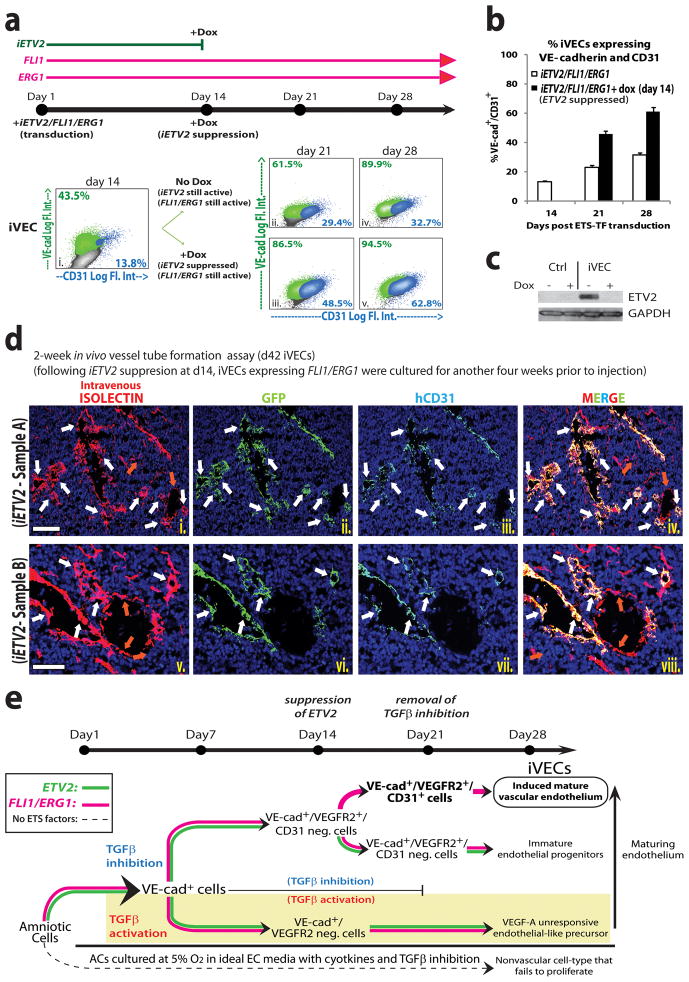
ETV2 is transiently expressed during vascular development but is absent from adult ECs (Kataoka et al., 2011). This is similar to our observation that iVEC clones with low ETV2 levels and high ERG1 and FLI1 levels could represent populations of iVECs that have achieved a mature vascular fate. Therefore, we examined whether short-term expression of ETV2, in conjunction with sustained expression of FLI1 and Erg1, might initiate reprogramming and then lock in optimal iVEC identity. Using a doxycycline-dependent inducible (“Tet-off”) expression system to control ETV2 production (inducible ETV2: “iETV2”), iETV2/FLI1/ERG1 were transduced in ACs and cultured with TGFβ inhibition for 14 days (Fig. 7a). Next, cells were treated with doxycycline to suppress iETV2 expression, without interfering with the expression of FLI1 and ERG1. ACs transduced with iETV2/FLI1/ERG1 expressed VE-cadherin and CD31 in both dox-treated (iETV2 suppressed) and untreated cells beyond 4 weeks. Notably, 7 and 14 days after iETV2 was silenced, there were a higher percentage of VE-cadherin+CD31+ iVECs compared to untreated iVECs (Fig. 7b). Western blot analysis confirmed that ETV2 protein expression had indeed been silenced (Fig. 7c) in dox-treated cells, indicating that ETV2 was no longer required for maintaining VE-cadherin expression in iVECs.
Figure 7. Transient ETV2 and constitutive FLI1 and ERG1 expression, concomitant with TGFβ inhibition, generate long-lasting iVECs without loss of vascular identity.
a) FACS reveals expression of VE-cad and CD31 in ACs transduced with iETV2/FLI1/ERG1 for 14 (i.), 21 (ii.– iii.), and 28 (iv.– v.) days plus TGFβ inhibition. (‘iETV2’: inducible ETV2 lentivirus). A subset of these cells (iii. and v.) was treated with doxycycline (Dox) at day 14 to suppress ETV2 protein. b) Percentage of VE-cad+CD31+ cells is shown. White bars: no Dox treatment. Black bars: Dox treatment at day 14. c) iETV2 suppression by Dox was confirmed by Western blot analysis. d) In vivo tube formation assay of GFP-labeled day 42 iVECs in which iETV2 expression was suppressed at day 14. iVECs were subjected to Matrigel plug analysis in NSG mice as previously described in figure 6c. Immunofluorescence micrographs show iVECs in green (GFP), intravital labeling of perfused vasculature in red (Isolectin), human CD31 staining in cyan, and nuclear counterstain (DAPI) in blue. Sample A was imaged at low magnification (i. – iv.: scale bar - 100 μm). Sample B was imaged at high magnification (v. – viii.: scale bar - 50 μm). White arrows indicate the colocalization of GFP-, CD31- and isolectin-marked (anastomosed) vessels. Orange arrows indicate only isolectin-marked host mouse vessels. e) Modular ETS-TF mediated reprogramming of ACs into abundant mature iVECs. Even when cultured in ‘optimal EC conditions’, untransduced ACs do not reprogram into iVECs, nor do they proliferate (bottom panel). In the absence of TGFβ inhibition, ETS-TF transduced ACs are VE-cad+, but fail to express other essential EC-markers, including functional VEGFR2, resulting in the generation of ‘VEGF-A unresponsive’ EC-like precursors (shaded panel). Transient inhibition of TGFβ signaling for approximately 3 weeks upregulates and functionalizes VEGFR2, allowing for VEGF-A dependent signaling events to proceed (upper panel). Co-expression of FLI1/ERG1 with ETV2 along with TGFβ inhibition maintains VE-cadherin and VEGFR2 expression; however constitutive ETV2 inhibits CD31+ expression, resulting in the production of immature EC progenitors (upper panel - low). Upon suppression of ETV2 at day 14, CD31 is induced, facilitating the generation of mature iVECs (upper panel - high). Thus, modular TGFβ inhibition and ETV2 expression along with constitutive FLI1/ERG1 co-expression provide for an efficient approach to reprogram lineage-committed ACs into long-lasting functional iVECs. See also Sup Figure S6 and S7.
ERG1 synergizes with FLI1 to enhance and sustain CD31 expression in maturing iVECs (Fig. 3e and Fig. 7b). Thus, its importance in reprogramming was assayed by transducing TGFβ inhibited ACs with iETV2/FLI1 in the absence of ERG1 (Sup Fig. S6a). These cells were then split into two fractions at day 14, one of which was treated with doxycycline to suppress iETV2 expression without perturbing FLI1 expression. There was an increase in VE-cadherin+CD31+ iVECs generated from ACs transduced with iETV2/FLI1/ERG1 compared to ACs lacking ERG1 transduction (Fig. 7b vs. Sup Fig. S6b). In day 28 iETV2/FLI1/ERG1 transduced ACs, in which iETV2 expression has been suppressed for two weeks, there was colocalization of VE-cadherin and CD31 (Sup Fig. S6c), as well as induction of numerous EC markers (Sup Fig. S7a).
To assess the functionality of iVECs generated via transient iETV2 expression in vivo, GFP-labeled iETV2/FLI1/ERG1 transduced ACs were cultured for 14 days, at which time iETV2 expression was suppressed. Subsequently, these cells were grown for another 28 days (d42 iVECs) and then loaded into Matrigel plugs for injection into NSG mice. Two weeks later, following intravital labeling of the vasculature by perfusion with Alexa568-Isolectin-B4, Matrigel plugs were removed for analysis (Fig. 7d and Sup Fig. S7b). There was colocalization of GFP, human CD31 (hCD31), and isolectin-marked cells, verifying that iVECs no longer expressing iETV2 were capable of anastomosing to the host vasculature. Therefore, expression of ETV2 for a duration of only two weeks along with constitutive co-expression of FLI1 and ERG1 are sufficient to confer iVECs permanent EC identity.
Discussion
ETS-TFs reprogram ACs into durable and expandable iVECs
Derivation of abundant and engraftable human ECs could benefit patients with vascular disorders. However, purification and expansion of adult ECs is technically cumbersome. Moreover, current strategies to derive ECs from pluripotent stem cells even after transduction with ETS-TFs results in the generation of unstable ECs that have limited expansion potential (Fig. 1e–1g). Here, we have identified an alternative source of human lineage-committed ACs that can be reprogrammed into proliferative functional ECs, which we refer to as iVECs. We show that enforced expression of FLI1/ERG1 with transient expression of ETV2 and TGFβ inhibition reprograms ACs into iVECs, which possess the morphology and long-lasting angiogenic repertoire of mature ECs (Fig. 7e).
Notably, transduction of ACs with ETS-TFs not only resulted in complete induction of a vascular signature, it also turned off non-vascular programs in ACs. iVECs are highly proliferative and stable, capable of undergoing 6×104-fold expansion in 50 days, while maintaining expression of adhesion molecules, ECMs and angiocrine factors, all of which establish their vascular function. With an increasing number of amniocentesis being performed world-wide, we expect that genetically matched ACs will be available to generate sufficient numbers of durable and engraftable iVECs for the treatment of vascular disorders and promoting organ regeneration.
Lineage-committed ACs are amenable to reprogramming into iVECs
While it is plausible that certain cultured AC samples may compose of small population of pluripotent cells, in the numerous specimens tested here we did not detect any significant expression of the pluripotency genes, such as OCT4, NANOG, and SOX2. c-Kit+ cells compose of a rare AC subpopulation (≤ 1%) that are multipotent (De Coppi et al., 2007). However, the majority of ACs is c-Kit−, comprising of lineage-committed cell types (Fig. 2c). Thus, it is unlikely that iVECs spawned from pluripotent or c-Kit+ multipotent cells because of their scarcity, and because our attempts to generate iVECs without ETS-TF transduction were unsuccessful. Regardless, our reprogramming strategy is effective in generating iVECs from mature c-Kit− ACs, which constitute the vast majority of ACs. Within 4 days of ETS-TF transduction, nearly 20% of transitioning c-Kit− ACs expressed VE-cadherin. Both lineage-committed EpCAM+Tra1-81−c-Kit− epithelioid and EpCAM−Tra1-81−c-Kit− mesenchymal/fibroblastic ACs could be transcriptionally reprogrammed into iVECs, supporting the notion that iVECs are primarily derived from mature ACs.
What could be the underlying mechanism by which such large numbers of mature ACs are readily reprogrammed into iVECs? It is plausible that ETV2, FLI1, and ERG1 require interaction with other TFs to switch on vascular specific genes, while silencing non-vascular genes. For example, the mechanism by which ETS-TFs switch on EC-specific genes in embryonic tissues is mediated through interaction with cofactors, such as FoxC2 (De Val et al., 2008). Binding of Etv2 and FoxC2 to the specific enhancer FOX:ETS regions increases the expression of EC specification genes. Notably, FOXC2 is expressed constitutively in ACs and its expression is maintained during ETS-TF mediated reprogramming into iVECs. Such observations will allow for hierarchical reconstruction of molecular pathways that regulate organ-specificity of the vasculature.
ETS-TFs switch on vascular-specific genes and silence the expression of non-vascular genes in ACs
Hierarchical clustering and 3D-MDS analyses demonstrate that on a genome-wide scale the transcriptome of iVECs is highly similar to prototypical fetal (HUVEC) and adult (LSEC) mature ECs, and differs drastically from non-vascular cells. So far, very few studies have assessed the efficiency of direct reprogramming approaches as comprehensively as is demonstrated here at the genome-wide level. Thus, our analyses set a new standard in direct reprogramming protocols, in which reprogrammed cells exclusively acquire the global transcriptome of a specific target cell-type, while simultaneously silencing its original transcriptional signature.
ETS-TF mediated suppression of non-vascular transcription programs in iVECs is clinically relevant, since persistent expression of non-vascular genes may result in malfunction of in vivo engrafted iVECs predisposing to vascular deformity and thrombus formation. Indeed, engraftment of iVECs into Matrigel plugs or regenerating mouse liver sinusoidal vessels for three months led to the generation of patent vessels without thrombosis. Thus, engraftable iVECs have silenced pro-coagulation and inflammatory mediators to allow for the generation of durable vessels.
ETS-TF-mediated generation of functional iVECs requires TGFβ inhibition
Transduction of ETS-TF into ACs without TGFβ inhibition failed to generate functional proliferative iVECs. Notably, iVECs not only express TGFβ1,β2,β3 and LTBPs, but also TGFβRII and TGFβRI resulting in constitutive SMAD2 phosphorylation. Active TGFβ signaling favors Endo-MT (Zeisberg et al., 2007) and might hinder the formation of iVECs. TGFβ also modulates acetylation of FLI1 protein, leading to a decrease in FLI1 stability and its capacity to bind DNA (Asano and Trojanowska, 2009). TGFβ signaling abrogates VEGFR2 expression in ECs, impairing proliferative and vasculogenic functions. Thus, the generation of iVECs initially requires TGFβ inhibition to functionalize VEGFR2 and possibly FLI1 as well as other unknown EC-specific signaling and transcriptional pathways (Fig. 5).
Transient expression of ETV2 and inhibition of TGFβ pathways are sufficient to sustain vascular identity
Most adult ECs do not express ETV2, but do constitutively express FLI1 and ERG1 (De Val and Black, 2009), suggesting that transient expression of ETV2 is sufficient to specify and lock in the vascular fate. Similarly, ETV2 expression in ACs for 2 weeks was sufficient to switch on multiple downstream EC-specific ETS-family TFs exclusive of FLI1 and ERG1. The irreversible epigenetic mechanism by which expression of ETV2 for 14 days results in permanent specification of EC-specific genes in ACs is intriguing. It is plausible that upon silencing of ETV2, constitutive expression of FLI1, ERG1 and other TFs maintain long-term expression of EC-specific genes. The mechanism for the ease of reprogramability of ACs may rely on unrecognized epigenetic factors that modulate cell fate determination. Additionally, the notion that iVEC identity can be sustained even after initiating inputs such as ETV2 expression or TGFβ inhibition are removed will improve the safety of using iVECs in the clinical setting.
After ETV2 suppression, FLI1 and ERG1 sustain vascular maturity and quiescence of iVECs
The genes turned on by ETV2 were factors that are essential for specification of ACs into functional ECs, including VEGFR2, VE-cadherin, TIE1, TIE2, EDG-1, VEGFR1 and Notch signaling pathway genes. However, ETV2 alone was insufficient to switch on all EC genes. FLI1 and ERG1, meanwhile, turned on EC genes associated with maturity, such as CD31, ECMs, and angiocrine factors. Hence, ETV2 specifies ACs into immature endothelial progenitor cells, while FLI1 synergizes with ERG1 to induce genes that render these progenitors into mature functional ECs (Fig. 7e).
Upon silencing of ETV2 in iVECs, FLI1 and ERG1 preserve EC identity. Indeed, deletion of FLI1 gene in adult ECs results in down-regulation of vascular-specific genes that modulate EC identity (Asano et al., 2010). Thus, as we demonstrate here, constitutive expression of FLI1 plays a seminal role in stabilizing IVECs. Similarly, ERG may also maintain the quiescence of ECs by repressing the activity of NF-kB (Dryden et al., 2012; Yuan et al., 2009) and safeguarding vascular permeability by enforcing expression of Claudin5 (Yuan et al., 2012) and VE-cadherin (Birdsey et al., 2008).
FLI1 and ERG have been implicated in the specification of hematopoietic cells (De Val and Black, 2009; Lee et al., 2008). However, combinatorial transduction of ETV2 concomitant with FLI1/ERG1 (along with TGFβ inhibition) did not induce hematopoietic-specific genes (i.e. CD45, Thrombopoietin receptor) in iVECs. Notably, SCL/TAL1, VAV3, and LMO2 genes that are co-expressed in both ECs and hematopoietic cells were induced by ETV2/FLI1/ERG1. Furthermore, derivation of true hematopoietic cells may require introduction of other TFs (i.e. RUNX1, PU.1), which are not expressed in ACs. Thus, transient expression of ETV2 may alter FLI1/ERG1 predilection for hematopoietic specification directing nascent iVECs transition towards a vascular fate.
We have found that in mature ECs, ERG is expressed in at least two isoforms (ERG1 and ERG2) with ERG1 being expressed up to 5 times more than ERG2 transcript. Moreover, as initial screening demonstrated that ERG1 accelerates the expression of CD31 compared to ERG2, we focused on ERG1 to drive ACs into mature iVECs. Whether co-expression of ERG2 in addition to ERG1 plays a physiological role in enhancing vascular identity to iVECs is unknown.
Optimal ratios of ETS-TFs augment production of mature iVECs
Singular transduction with FLI1 and/or ERG1 not only failed to confer EC-identity upon ACs, but also was ineffective in supporting proliferation of these cells. Based on the clonal analyses (Figs. 3c–3e), we found that proper stoichiometric ratios of ETV2 relative to FLI1 and ERG1 were key to the generation of mature and proliferative iVECs.
By day 21, a time point in which iVECs have achieved their maximal maturity, the clonal efficiency of mature iVECs, such as Clone-3 is ~20%. Genome-wide 3D-MDS analyses demonstrated that the transcriptomes of Clone-3 and other similar clones (i.e. Clone-4) match those of HUVECs and LSECs. Although the majority of vascular specific genes were induced specifically in these clones, certain vascular expressed genes, such as eNOS and GATA3, were not completely induced. Microenvironmental cues may play an essential role in induction of these genes. Nonetheless, the remarkable proliferative capacity (ETV2-dependent) and vascular maturity (ERG1/FLI1-dependent) of specific iVEC clones suggest that the optimal combinatorial ratios of these ETS-TF within ACs is the major determinant of generating plentiful stable iVECs.
Constitutive expression of FLI1 and ERG1 is not associated with malignant transformation
Aberrant expression of FLI1 can induce leukemia (Cui et al., 2009) and Ewing Sarcoma (Zhang et al., 1993). ERG overexpression has also been associated with leukemias (Martens, 2012). However, in vivo transplantation of iVECs expressing FLI1 and ERG1 in Matrigel plugs for 14 days or in regenerating liver for 3 months did not lead to emergence of angiomas or angiosarcomas. Long-term expansion of iVECs beyond 80 days showed no evidence of malignant transformation. Employing CGH, we also show that expansion of iVECs beyond 28 days did not lead to any chromosomal abnormalities. Therefore, iVECs represent a stable and expandable cell population that provide for a safe source of vascular cells.
Potential use of iVECs for therapeutic vascularization and organ regeneration
The genetic repertoire of iVECs approximates that of generic naïve ECs. As such, iVECs may have the vascular plasticity to undergo further tissue-specific specialization once reintroduced into the microenvironment of a given organ. In support of this premise, we show that iVECs acquired the morphology of sinusoidal ECs upon transplantation into regenerating liver. Ultimately, identification of the TFs that endows ECs with tissue-specific signature will enable generation of iVECs that will adapt to the physiological and metabolic needs of that particular organ.
In this regard, we have identified a readily available source of human lineage-committed proliferative cells with allogeneic compatibility that are amenable to reprogramming into abundant functional iVECs. The generation of iVECs will not only increase our understanding of the hierarchy of transcriptional pathways involved in the induction and maintenance of organ-specific ECs, but also open the door for vascularization of ischemic tissues. With thousands of amniocenteses being performed worldwide each year, this ensures that sufficient genetically matched ACs will be available for reprogramming into iVECs that may benefit a broad cross-section of the ethnically diverse population. Given that ACs and iVECs could be HLA-typed, cryopreserved, and publicly banked, these cells could establish an inventory for generating abundant vascular cells for promoting angiocrine-dependent organ regeneration as well as lay the foundation for treating genetically diverse populations of patients with vascular disorders.
Experimental Procedures
Reprograming Protocol
Cell culture
Human embryonic stem cells (hESCs) were maintained on Matrigel™ using hESC medium (KOSR) conditioned by mouse embryonic fibroblasts (MEF, Chemicon), and grown at 37º, at 5% CO2. Amniotic cells (ACs) were cultured in Amniotic Media (AM) and grown at 37º, at 5% CO2.
In reprogramming assays, ACs were cultured in Endothelial Growth Media (EM: Medium 199, 15% FBS, 20μg/ml endothelial cell supplement, 1X Pen/Strep, and 20 units/ml Heparin), and where indicated, supplemented with 5μM TGFβ inhibitor or 10μg/ml TGFβ ligand neutralizing mAB (R&D) to inhibit TGFβ signaling. Liver sinusoidal ECs (LSECs, ScienCell) were cultured in EM with 20% FBS. Human umbilical vein ECs (HUVECs) were obtained as previously described (Rafii et al., 1995; Rafii et al., 1994) and cultured in EM with 20% FBS. For ACs undergoing reprogramming, LSECs, and HUVECs, plates were coated with 1μg/ml Fibronectin (Sigma) and cells were grown at 37º, and at 5% CO2 and 5% O2. See extended experimental procedures for details of strains and media.
Cytokine Treatments
For TGFβ experiments, 10ng/ml TGFβ ligands β1 (R&D) and β3 (R&D) were added for indicated times. For VEGFR2 phosphorylation experiments, 50ng/ml VEGF-A was added for 5minutes. For Acetylated-LDL uptake assays, cells were treated with10μg/ml DiI-labeled Ac-LDL (Biomedical Technical Inc).
Lentiviral Vectors and transduction strategy
Multiple cDNAs, including ETV2, ERG1, and FLI1 were cloned into the pCCL-PGK lentivirus vector. A triple Flag-tag was subcloned into the ETV2 construct at the amino terminus. For conditional expression/suppression experiments, Flag-tag ETV2 was re-subcloned into an inducible pTA-dependent construct, in which suppression of Flag-tag ETV2 was achieved via treatment with 2μg/ml doxycycline (Clonetech). A scrambled shRNA construct was cloned into the pLKO vector for use as a control. See extended experimental procedures for details of construct strategy and lentiviral production.
In vitro Matrigel™ tubulogenesis and in vivo angiogenesis assays
To test the capacity of iVECs to undergo tubulogenesis in vivo, ACs infected with ETS-TFs were transduced with GFP-lentivirus, and were mixed with Matrigel (BD), 100ng/ml of VEGF-A, and 50ng/ml of FGF-2, and subcutaneously implanted at the flanks of NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NOD-SCID/IL2Rγ−/−, NSG) mice (Jackson Laboratories). To test the capacity of iVECs to functionally incorporate into regenerating liver sinusoidal vasculature, GFP-labeled iVECs were intrasplenically injected into NSG mice 2 days after they were subjected to 70% partial hepatectomy, as previously described (Ding et al., 2010). 30 minutes after intrasplenic injection, the spleen was removed. Two weeks (Matrigel plugs) or three months (intrasplenic) after iVEC injection, the mice were injected intravenously with Isolectin-B4 conjugated with Alexa568 (Invitrogen) (2mg/kg), and sacrificed 10 minutes later. The Matrigel plug or liver were then fixed in 4% paraformaldehyde, followed by 48 hour saturation in 30% sucrose. 20 μm cryosections were made and counterstained with Hoechst 33342. Incorporation of GFP-labeled iVECs was identified, and the functional engraftment into the host vasculature was revealed by co-staining with Isolectin-B4 fluorescence. Anti-human CD31 (BD) staining was also performed to distinguish iVECs from host mouse ECs. Fluorescent signal was analyzed by confocal microscopy (710 META Zeiss). The area of GFP-positive field was quantified by ImageJ, and the number of isolectin-positive functional vessels was determined by counting cells present in three fields of view taken at random. For in vitro assays, 200μl Matrigel was coated onto 12-well TC plates, and 200,000 iVECs were seeded on Matrigel, and tubulogenesis was determined as previously described (Kobayashi et al., 2010).
Immunofluorescence/Western Blot Analysis
Samples were stained as previously described (James et al., 2010). Briefly, samples were permeabilized in PBS-Tween and blocked in 5% donkey serum. Samples were incubated for 1hr with conjugated-antibodies in blocking solution, washed, and counterstained for nucleic acids by DAPI or ToPro3 (Invitrogen) for imaging. Western Blot analysis was performed as previously described (Kobayashi et al., 2010). See extended experimental procedures for details of antibodies used in these assays.
Supplementary Material
Highlights.
Short-term ETV2 and constitutive FLI1/ERG1 co-expression reprograms lineage-committed amniotic cells (ACs) into induced vascular endothelial cells (iVECs)
Transient TGFβ inhibition functionalizes VEGFR2, preserving long-term vascular identity of iVECs
Genome-wide transcriptional profiles of iVECs are similar to adult ECs
AC-derived iVECs are proliferative and form long-lasting functional blood vessels
Acknowledgments
S.R. are supported by Howard Hughes Medical Institute; Ansary Stem Cell Institute; Empire State Stem Cell Board and New York State Department of Health grants (NYSTEM, C024180, C026438, C026878); NHLBI R01s HL097797 and DK095039; Qatar National Priorities Research Foundation NPRP08-663-3-140 and Qatar foundation BioMedical Research Program (BMRP). The authors acknowledge Agnes Stachnik and Pooja Rana for their technical contribution to this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asano Y, Stawski L, Hant F, Highland K, Silver R, Szalai G, Watson DK, Trojanowska M. Endothelial FLI1 deficiency impairs vascular homeostasis: a role in scleroderma vasculopathy. Am J Pathol. 2010;176:1983–1998. doi: 10.2353/ajpath.2010.090593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano Y, Trojanowska M. Phosphorylation of FLI1 at threonine 312 by protein kinase C delta promotes its interaction with p300/CREB-binding protein-associated factor and subsequent acetylation in response to transforming growth factor beta. Mol Cell Biol. 2009;29:1882–1894. doi: 10.1128/MCB.01320-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavides OM, Petsche JJ, Moise KJ, Jr, Johnson A, Jacot JG. Evaluation of Endothelial Cells Differentiated from Amniotic Fluid-Derived Stem Cells. Tissue Eng Part A. 2012 doi: 10.1089/ten.tea.2011.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsey GM, Dryden NH, Amsellem V, Gebhardt F, Sahnan K, Haskard DO, Dejana E, Mason JC, Randi AM. Transcription factor Erg regulates angiogenesis and endothelial apoptosis through VE-cadherin. Blood. 2008;111:3498–3506. doi: 10.1182/blood-2007-08-105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossolasco P, Montemurro T, Cova L, Zangrossi S, Calzarossa C, Buiatiotis S, Soligo D, Bosari S, Silani V, Deliliers GL, et al. Molecular and phenotypic characterization of human amniotic fluid cells and their differentiation potential. Cell Res. 2006;16:329–336. doi: 10.1038/sj.cr.7310043. [DOI] [PubMed] [Google Scholar]
- Butler JM, Kobayashi H, Rafii S. Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat Rev Cancer. 2010a;10:138–146. doi: 10.1038/nrc2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JM, Nolan DJ, Vertes EL, Varnum-Finney B, Kobayashi H, Hooper AT, Seandel M, Shido K, White IA, Kobayashi M, et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010b;6:251–264. doi: 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui JW, Vecchiarelli-Federico LM, Li YJ, Wang GJ, Ben-David Y. Continuous Fli-1 expression plays an essential role in the proliferation and survival of F-MuLV-induced erythroleukemia and human erythroleukemia. Leukemia. 2009;23:1311–1319. doi: 10.1038/leu.2009.20. [DOI] [PubMed] [Google Scholar]
- Da Sacco S, Sedrakyan S, Boldrin F, Giuliani S, Parnigotto P, Habibian R, Warburton D, De Filippo RE, Perin L. Human amniotic fluid as a potential new source of organ specific precursor cells for future regenerative medicine applications. J Urol. 2010;183:1193–1200. doi: 10.1016/j.juro.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Coppi P, Bartsch G, Jr, Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ, et al. Isolation of amniotic stem cell lines with potential for therapy. Nature biotechnology. 2007;25:100–106. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- De Val S, Black BL. Transcriptional control of endothelial cell development. Dev Cell. 2009;16:180–195. doi: 10.1016/j.devcel.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Val S, Chi NC, Meadows SM, Minovitsky S, Anderson JP, Harris IS, Ehlers ML, Agarwal P, Visel A, Xu SM, et al. Combinatorial regulation of endothelial gene expression by ets and forkhead transcription factors. Cell. 2008;135:1053–1064. doi: 10.1016/j.cell.2008.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding BS, Nolan DJ, Butler JM, James D, Babazadeh AO, Rosenwaks Z, Mittal V, Kobayashi H, Shido K, Lyden D, et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468:310–315. doi: 10.1038/nature09493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding BS, Nolan DJ, Guo P, Babazadeh AO, Cao Z, Rosenwaks Z, Crystal RG, Simons M, Sato TN, Worgall S, et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell. 2011;147:539–553. doi: 10.1016/j.cell.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryden NH, Sperone A, Martin-Almedina S, Hannah RL, Birdsey GM, Khan ST, Layhadi JA, Mason JC, Haskard DO, Gottgens B, et al. The transcription factor Erg controls endothelial cell quiescence by repressing activity of nuclear factor (NF)-kappaB p65. J Biol Chem. 2012;287:12331–12342. doi: 10.1074/jbc.M112.346791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett NR, Butler MW, Shaykhiev R, Salit J, Omberg L, Rodriguez-Flores JL, Mezey JG, Strulovici-Barel Y, Wang G, Didon L, et al. RNA-Seq quantification of the human small airway epithelium transcriptome. BMC Genomics. 2012;13:82. doi: 10.1186/1471-2164-13-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenhorst PC, Shah AA, Hopkins C, Graves BJ. Genome-wide analyses reveal properties of redundant and specific promoter occupancy within the ETS gene family. Genes Dev. 2007;21:1882–1894. doi: 10.1101/gad.1561707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James D, Nam HS, Seandel M, Nolan D, Janovitz T, Tomishima M, Studer L, Lee G, Lyden D, Benezra R, et al. Expansion and maintenance of human embryonic stem cell-derived endothelial cells by TGFbeta inhibition is Id1 dependent. Nat Biotechnol. 2010;28:161–166. doi: 10.1038/nbt.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezierski A, Gruslin A, Tremblay R, Ly D, Smith C, Turksen K, Sikorska M, Bani-Yaghoub M. Probing stemness and neural commitment in human amniotic fluid cells. Stem cell reviews. 2010;6:199–214. doi: 10.1007/s12015-010-9116-7. [DOI] [PubMed] [Google Scholar]
- Jin DK, Shido K, Kopp HG, Petit I, Shmelkov SV, Young LM, Hooper AT, Amano H, Avecilla ST, Heissig B, et al. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med. 2006;12:557–567. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka H, Hayashi M, Nakagawa R, Tanaka Y, Izumi N, Nishikawa S, Jakt ML, Tarui H, Nishikawa S. Etv2/ER71 induces vascular mesoderm from Flk1+PDGFRalpha+ primitive mesoderm. Blood. 2011;118:6975–6986. doi: 10.1182/blood-2011-05-352658. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Butler JM, O’Donnell R, Kobayashi M, Ding BS, Bonner B, Chiu VK, Nolan DJ, Shido K, Benjamin L, et al. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nature cell biology. 2010;12:1046–1056. doi: 10.1038/ncb2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig J, Huppertz B, Desoye G, Parolini O, Frohlich JD, Weiss G, Dohr G, Sedlmayr P, Lang I. Amnion-derived mesenchymal stromal cells show angiogenic properties but resist differentiation into mature endothelial cells. Stem cells and development. 2012;21:1309–1320. doi: 10.1089/scd.2011.0223. [DOI] [PubMed] [Google Scholar]
- Lee D, Park C, Lee H, Lugus JJ, Kim SH, Arentson E, Chung YS, Gomez G, Kyba M, Lin S, et al. ER71 acts downstream of BMP, Notch, and Wnt signaling in blood and vessel progenitor specification. Cell stem cell. 2008;2:497–507. doi: 10.1016/j.stem.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Patient R. Genome-wide analysis of the zebrafish ETS family identifies three genes required for hemangioblast differentiation or angiogenesis. Circ Res. 2008;103:1147–1154. doi: 10.1161/CIRCRESAHA.108.179713. [DOI] [PubMed] [Google Scholar]
- Liu F, Walmsley M, Rodaway A, Patient R. FLI1 acts at the top of the transcriptional network driving blood and endothelial development. Curr Biol. 2008;18:1234–1240. doi: 10.1016/j.cub.2008.07.048. [DOI] [PubMed] [Google Scholar]
- Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- Martens JH. Acute myeloid leukemia: a central role for the ETS factor ERG. Int J Biochem Cell Biol. 2012;43:1413–1416. doi: 10.1016/j.biocel.2011.05.014. [DOI] [PubMed] [Google Scholar]
- McLaughlin F, Ludbrook VJ, Cox J, von Carlowitz I, Brown S, Randi AM. Combined genomic and antisense analysis reveals that the transcription factor Erg is implicated in endothelial cell differentiation. Blood. 2001;98:3332–3339. doi: 10.1182/blood.v98.12.3332. [DOI] [PubMed] [Google Scholar]
- Pham VN, Lawson ND, Mugford JW, Dye L, Castranova D, Lo B, Weinstein BM. Combinatorial function of ETS transcription factors in the developing vasculature. Dev Biol. 2007;303:772–783. doi: 10.1016/j.ydbio.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–712. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- Rafii S, Lyden D, Benezra R, Hattori K, Heissig B. Vascular and haematopoietic stem cells: novel targets for anti-angiogenesis therapy? Nat Rev Cancer. 2002;2:826–835. doi: 10.1038/nrc925. [DOI] [PubMed] [Google Scholar]
- Rafii S, Shapiro F, Pettengell R, Ferris B, Nachman RL, Moore MA, Asch AS. Human bone marrow microvascular endothelial cells support long-term proliferation and differentiation of myeloid and megakaryocytic progenitors. Blood. 1995;86:3353–3363. [PubMed] [Google Scholar]
- Rafii S, Shapiro F, Rimarachin J, Nachman RL, Ferris B, Weksler B, Moore MA, Asch AS. Isolation and characterization of human bone marrow microvascular endothelial cells: hematopoietic progenitor cell adhesion. Blood. 1994;84:10–19. [PubMed] [Google Scholar]
- Reinisch A, Hofmann NA, Obenauf AC, Kashofer K, Rohde E, Schallmoser K, Flicker K, Lanzer G, Linkesch W, Speicher MR, et al. Humanized large-scale expanded endothelial colony-forming cells function in vitro and in vivo. Blood. 2009;113:6716–6725. doi: 10.1182/blood-2008-09-181362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Le Bras A, Sacharidou A, Itagaki K, Zhan Y, Kondo M, Carman CV, Davis GE, Aird WC, Oettgen P. ETS-related gene (ERG) controls endothelial cell permeability via transcriptional regulation of the claudin 5 (CLDN5) gene. J Biol Chem. 2012;287:6582–6591. doi: 10.1074/jbc.M111.300236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Nikolova-Krstevski V, Zhan Y, Kondo M, Bhasin M, Varghese L, Yano K, Carman CV, Aird WC, Oettgen P. Antiinflammatory effects of the ETS factor ERG in endothelial cells are mediated through transcriptional repression of the interleukin-8 gene. Circ Res. 2009;104:1049–1057. doi: 10.1161/CIRCRESAHA.108.190751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- Zhang L, Lemarchandel V, Romeo PH, Ben-David Y, Greer P, Bernstein A. The Fli-1 proto-oncogene, involved in erythroleukemia and Ewing’s sarcoma, encodes a transcriptional activator with DNA-binding specificities distinct from other Ets family members. Oncogene. 1993;8:1621–1630. [PubMed] [Google Scholar]
- Zhang P, Baxter J, Vinod K, Tulenko TN, Di Muzio PJ. Endothelial differentiation of amniotic fluid-derived stem cells: synergism of biochemical and shear force stimuli. Stem cells and development. 2009;18:1299–1308. doi: 10.1089/scd.2008.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.