Abstract
This study explored the possibility that specific, theoretically consistent profiles of reactivity could be identified in a sample of cocaine-exposed infants and whether these profiles were associated with a range of infant and/or maternal characteristics. Cluster analysis was used to identify distinct groups of infants based on physiological, behavioral and maternal reported measures of reactivity. Five replicable clusters were identified which corresponded to 1) Dysregulated/High Maternal Report Reactors, 2) Low Behavioral Reactors, 3) High Reactors, 4) Optimal Reactors and 5) Dysregulated/Low Maternal Report Reactors. These clusters were associated with differences in prenatal cocaine exposure status, birthweight, maternal depressive symptoms, and maternal negative affect during mother-infant interactions. These results support the presence of distinct reactivity profiles among high risk infants recruited on the basis of prenatal cocaine exposure and demographically similar control group infants not exposed to cocaine.
Keywords: Regulatory Profiles, Prenatal Cocaine Exposure, Regulation, Reactivity
Children prenatally exposed to cocaine are at risk for a wide range of poor developmental outcomes (Bateman & Chiriboga, 2000; Behnke et al., 2006; Lester, LaGasse, & Brunner, 1997; Mayes, 2002). Recent studies have suggested that one particular area of concern is the effects of prenatal exposure to cocaine on regulatory processes in infants and young children. During later infancy, one of the primary developmental tasks for infants is to cope with sensory challenges from the external environment (DeGangi, DiPietro, Greenspan, & Porges, 1991). Reactivity is conceptualized as consisting of both physiological and behavioral processes. Thus, an emerging developmental task for infants is learning to modulate positive and negative emotional experiences, both behaviorally and physiologically (e.g., Kopp, 1989; Ungerer, Dolby, Waters, Barnett, Kelk, & Lewin, 1990). Increasingly, studies have indicated that prenatal exposure to cocaine increases the risk for regulatory problems from birth into childhood. Cocaine has been shown to inhibit the reuptake of monoamines at the presynaptic junction, leading to higher concentrations of norepinephrine, serotonin, and dopamine in the synaptic cleft and higher levels of activation in the catecholaminergic systems (Gawin & Ellinwood, 1988; Nassogne, Evrard, & Courtoy, 1998). The regions of the brain that are rich in monoamines are the centers involved in reactivity to stress (Robbins, 1997; Tucker & Williamson, 1984).
During the neonatal period, cocaine-exposed neonates display signs of altered behavioral and physiological regulation including state lability, altered sleep patterns, deficits in orienting and attention, increased irritability, decreased heart rates, and greater overall heart rate variability (Chasnoff, Griffith, MacGregor, Dirkes, & Burns, 1989; Coles, Platzman, Smith, James & Falek, 1992; Karmel & Gardner, 1996; Regalado, Schechtman, Del Angel, & Bean, 1995; Regalado, Schechtman, Del Angel, & Bean, 1996; Regalado, Schechtman, Khoo, & Bean, 2001; Silvestri, Long, Weese-Mayer, & Barkov, 1991). Beyond the neonatal period, prenatal exposure to cocaine has been associated with decreased inhibitory control (Bendersky, Gambini, Lastella, Bennett & Lewis, 2003; Bendersky & Lewis, 1998; Mayes, Bornstein, Chawarska, & Granger, 1996), higher negative affect in a variety of paradigms (Azuma & Chasnoff, 1993; Eiden, Lewis, Croff & Young, 2002), increased disruptive behavior (Delaney-Black et al., 2004), reduced cortisol response (Jacobson, Bihun, & Chiodo, 1999), poorer physiological regulation during a baseline period and during environmental challenge throughout the first year of life (Schuetze & Eiden, 2006; Schuetze, Eiden & Coles, 2007; Schuetze, Eiden & Danielewicz, 2009). Taken together, these studies indicate that cocaine-exposure is associated with altered reactivity throughout infancy.
To date, however, studies on regulatory processes in cocaine-exposed infants have utilized a variable-centered approach in which group differences in individual variables (typically either behavioral or physiological) are examined. Such an approach ignores the fact that behavioral and physiological regulatory components are not independent traits but rather function as part of an integrated regulatory system within an individual (Bergman & Magnusson, 1997; Hart, Atkins, & Fegley, 2003). In contrast, a person-centered approach views an individual as an integrated whole in which individual aspects of a process are most meaningful when considered in the context of the overall functioning of an individual (Bergman & Magnusson, 1997). In such an approach, subgroups of individuals are identified on the basis of shared characteristics. Individuals within a subgroup are assumed to be more similar to each other than to individuals in other subgroups. Furthermore, it is assumed that membership in specific subgroups can be predicted a priori by other factors (Bergman & Magnusson, 1997). An increasing number of studies have examined regulatory profiles in nonexposed children using a person-centered approach. The majority of these studies have focused on early childhood and have primarily identified profiles on the basis of behavioral (e.g., Aksan, et al., 1999; Caspi & Silva, 1995; Hill, Degnan, Calkins & Keane, 2006; Janson & Mathieson, 2008) or physiological processes (Wilson, Lengua, Tininenko, Taylor & Trancik, 2009). Physiological variables have been found to identify three profiles 1) an under-controlled profile which consisted of children with both low electrodermal and heart rate reactivity, 2) an over-controlled profile which consisted of children with high electrodermal reactivity and moderate heart rate reactivity and 3) a well-regulated group which consisted of children with low electrodermal reactivity and moderate heart rate reactivity (Wilson et al., 2009). Behaviorally, studies have found evidence for four distinct profiles of externalizing behaviors over time: 1) consistently high levels from 2 to 5 years of age, 2) initially high levels at 2 years of age with lower levels at ages 4 and 5 years, 3) a normative profile which consisted of moderate levels at age 2 and lower levels at 4 and 5 years of age, and 4) a low profile which consisted of children with low levels of externalizing behavior at each age (e.g., Hill et al., 2006). Similarly, a recent study found that four distinct profiles of disruptive behavior in preschool-aged children could be identified using a combination of behavioral and physiological variables (Degnan, Calkins, Keane & Hill-Soderlund, 2008). These profiles consisted of children with 1) high levels, which were associated with high reactivity combined with low regulation from 2 to 5 years of age, 2) moderate levels, which are associated with initially high levels of disruptive behavior followed by lower levels at later ages, 3) normative, which are associated with both higher reactivity and regulation across early childhood, and 4) low levels of disruptive behavior, which consisted of children who were less reactive and more regulated across early childhood.
It is unclear, however, whether there are distinct reactivity profiles among substance-exposed children and whether these profiles exist for process variables (behavioral and physiological reactivity) as opposed to outcome variables (externalizing or disruptive behavior problems). Consequently, the first goal of this study was to explore the possibility that reactivity profiles could be identified in a sample of cocaine-exposed and demographically similar control group infants. Since a major developmental task during infancy is to cope with sensory challenges from the external environment (DeGangi et al., 1991), we selected measures that a) index both physiological and behavioral reactivity, and b) have been associated with cocaine and other substance exposure during pregnancy. The three variables selected were 1) latency to negative affect during a frustration paradigm (behavioral reactivity), 2) physiological responses during a frustration paradigm (physiological reactivity), and 3) a maternal report of their infant’s predominant style of behavioral reactivity across a range of contexts (temperamental reactivity). From a theoretical perspective, these three variables, taken together, capture the essence of reactivity during infancy.
Behavioral Reactivity
A number of studies have found evidence for increased behavioral reactivity among cocaine-exposed infants and children. For example, cocaine-exposed infants exhibit greater irritability and crying during habituation procedures at 3 months of age (Mayes, Grillon, Granger & Schottenfeld, 1998), more negative expressions during the re-engagement phase of the still-face paradigm at 4 months of age (Bendersky & Lewis, 1998) and are more reactive to increases in stress during an arm-restraint procedure at 7 months of age (Eiden, McAuliffe, Kachadourian, Coles, Colder & Schuetze, 2008). In addition, cocaine-exposed boys were quicker to react with frustration (higher reactivity) during a problem solving task at 4 years of age (Dennis, Bendersky, Ramsay, & Lewis, 2006).
Physiological Reactivity
Over the past several decades, the physiological correlates of individual differences in reactivity have been described in the general developmental literature (Calkins, 1997; Gunnar, 1986; Stifter & Fox, 1990). Much of this work has focused on the association between physiological indices and negative emotionality (Buss, Davidson, Kalin & Goldsmith, 2004; Calkins & Fox, 1992; Gunnar, 1989). In particular, respiratory sinus arrhythmia (RSA), which is a measure of heart rate variability due to the influence of breathing rate, has been associated with behaviors reflecting negative emotional reactivity and temperament in infants (Calkins, 1997; DiPietro, Larson, & Porges, 1987; Fox, 1989; Stifter et al., 1999). This variability in heart rate is influenced by the parasympathetic branch of the autonomic nervous system via one branch of the vagus nerve. According to Porges’ polyvagal theory (Porges et al., 1996), the vagal system responds to both internal and external demands.
One commonly used measure of RSA quantifies changes in RSA during environmental demands (RSA regulation; Bornstein & Suess, 2000; Calkins, 1997). When an infant faces environmental demands, the myelinated vagal system optimally responds by applying a “brake” to regulate cardiac output (Porges et al., 1996) such that RSA is suppressed during stressful situations. This suppression of RSA allows heart rate to increase and the infant to meet environmental demands. Thus, RSA suppression may serve a key role in increasing an infant’s orientation to exogenous stimulation, allowing the infant to coordinate internal physiological needs with environmental demands (Porges et al., 1996). Thus, greater suppression during environmental challenge is believed to be indicative of a more adaptive physiological system which facilitates the ability of infants to modulate their behavioral response to environmental challenge. RSA suppression, quantified as a negative change in RSA from baseline to environmental challenge, is associated with more optimal state regulation in infancy (DeGangi et al., 1991), decreased behavior problems in preschool-aged children (Porges et al., 1996), and more adaptive behavior during attention and affect eliciting tasks in both preschool and school-aged children (Calkins, 1997; Suess, Porges, & Plude, 1994), and during social approach (Stifter & Corey, 2001). Thus, the measurement of change in RSA from baseline to challenging situations is an important concurrent and predictive index of behavioral regulation in infants.
Temperament
Temperament is often defined as constitutionally based individual differences in reactivity and regulation (Rothbart & Derryberry, 1981). Infant temperament is often assessed using maternal report of infant behavior. Maternal reports of temperament, however, have been criticized as not reflecting the actual temperament of the child. In fact, the correspondence between maternal reports and observed temperament are often poor (correlations ranging from .10–.40; Mangelsdorf, McHale, Diener, Goldstein & Lehn, 2000; Seifer, Sameroff, Barrett & Krafchuk, 1994). One possible explanation for this low concurrent validity is that the frame of reference for assessing child behavior is so different in direct observations and maternal reports. Maternal reports are typically based on a wide range of child behaviors across numerous contexts over a much longer period of time (Rothbart & Bates, 1998) while laboratory assessments are typically based on child behavior at one point in time in a context that is unfamiliar to the child. Thus, Rothbart and Bates (1998) have argued that the maternal perspective on their child’s behavior is particularly useful because it is reliant on a wide range of infant behaviors across numerous contexts and over time. Studies that have used both maternal reports and direct observation of child behavior have found that, although the two measures of child behavior have little convergence, they both predict behavior during early childhood (Mangelsdorf et al., 2000). Thus, maternal reports may provide a unique perspective on infant reactivity that is not captured in direct observations of reactivity by observers who are unfamiliar with the infant. Together, maternal reports and laboratory observation may provide important and unique information regarding infant behavior.
The Current Study
The purpose of this study was to explore the possibility that specific, theoretically consistent regulatory profiles could be identified in a sample of cocaine-exposed and demographically similar control group infants not exposed to cocaine, but exposed to other substances. The first goal was to identify patterns of reactivity in response to frustration using observational, physiological, and maternal report methods. In particular, based on findings from studies using a variable-centered approach, we hypothesized that a theoretically expected profile of dysregulation would be associated with higher risk for prenatal cocaine and other substance exposure.
Since one of the advantages of using a person-centered approach to explore development is that membership in specific subgroups can be predicted a priori by other factors (Bergman & Magnusson, 1997), the logical next step after identifying distinct reactivity profiles is to determine what developmental influences may differ between these subgroups. Individual differences in reactivity are thought to reflect the impact of a wide range of developmental influences. Maternal cocaine use is a marker variable for a number of other risk variables that may have a negative impact on reactivity. These risk variables include other substance use, poor intrauterine growth and maternal negative affect. The majority of women who use cocaine also use heavier amounts of alcohol and cigarettes compared to non-cocaine using women. Alcohol and cigarettes are known to have significant teratological influences on regulatory processes, including reactivity (Fried & Makin, 1987; Fried, Watkinson, & Dillon, 1987; Schuetze, Lopez, Granger & Eiden, 2008; Streissguth, 1984). Thus, the impact of maternal cocaine use can only be studied in the context of polydrug cocaine exposure and by measuring the use of other substances in addition to cocaine. Similarly, poor intrauterine growth is a factor that is consistently associated with both prenatal substance exposure and the regulatory system (Handler, Kistin, Davis & Ferre, 1991; Heffelfinger, Craft, White & Shyken, 2002). Cocaine using mothers have also been reported to have higher negative affect (Eiden, Stevens, Schuetze & Dombkowski, 2006). Parents play a critical role in helping children manage their arousal (Feldman, Greenbaum & Yirmiya, 1999; Schore, 1994) by reading their children’s emotional signals and responding with appropriate levels of soothing or stimulation. Parents who have high negative affect are more likely to have infants who have difficulty regulating arousal in affect arousing situations. Finally, cocaine-exposed infants experience higher levels of environmental risk as indicated by a number of factors such as lower maternal education, single parenting and lower socioeconomic status (Bendersky, Bennett, & Lewis, 2006; Lewis et al., 2004; Platzman, Coles, Lynch, Bard & Brown, 2001). Several studies have noted the importance of examining the potential effects of these differences in the caregiving environment of cocaine exposed infants on developmental outcomes (e.g., Bendersky et al., 2006). Thus, the second goal was to examine whether distinct patterns of behavioral and physiological reactivity were associated with other infant characteristics including fetal growth, maternal substance use or with caregiver characteristics including demographics, and maternal negative affect.
Method
Participants
Participants consisted of mother-infant dyads recruited postpartum from two local area hospitals into a longitudinal study of maternal cocaine use and child development. All mothers were screened after delivery for initial eligibility and matching criteria. Once a family was recruited into the cocaine group, the closest matching non-cocaine group family was recruited. The two groups were matched on maternal education, age, race/ethnicity and on infant gender. However, a significantly higher proportion of mothers in the non-cocaine group declined participation or withdrew before formal enrollment, resulting in a smaller number of families in the control group.
Interested and eligible mothers were given detailed information about the study and asked to sign consent forms. About 2 weeks after delivery, mothers were contacted and scheduled for their first laboratory visit, which took place at the time that their infant was approximately 4–8 weeks old. Additional visits were scheduled when the infant was 7, 13, and 24 months old. All visits consisted of a combination of maternal interviews, observations of mother-infant interactions, and infant assessments.
Of the 220 infants recruited into the study, only the 114 with complete physiological and observational data at 13 months of age were included in these analyses. One additional family was excluded from analyses because it was a multivariate outlier (cocaine, alcohol, cigarette use and gestational age were more than three standard deviations from the sample mean). Thus, the final sample was 113 (56 cocaine-exposed, 57 nonexposed) dyads. There were no significant differences between families with complete versus missing data at 13 months on demographic or substance use variables.
Mothers ranged in age from 18 to 42 years (M = 29.69, SD = 5.95). 74% (n = 84) of mothers were African-American, 18% (n = 20) were Caucasian, 6% (n = 7) were Hispanic-American and the remaining were other. 81% (n = 92) of mothers were receiving Temporary Assistance for Needy Families and 90% (n = 102) were single. Women were classified as either cocaine-users or “abstainers”. 46% (n = 52) of the infants were male. 12.5% of the cocaine-exposed (CE; ranged from 33 to 41 weeks; n = 7) and 3.5% of the nonexposed (NE infants; ranged from 36 to 42 weeks; n = 2) were preterm (<37 weeks gestational age). CE infants were not significantly more likely to have been preterm than NE infants, (X2(1) = 3.12, p > .05). All testing was conducted after age corrected for prematurity (based on the infant’s due date rather than date of birth). Infants ranged from 1531 to 5072 grams at birth (M =3166.33, SD = 556.51) and their average birth weight was 3137.26 after adjusting for gestational age. 10.7% were low birth weight (<2500 grams) and none of the infants were very low birth weight (<1500 grams). Results of an analysis of covariance (ANCOVA) revealed that the groups did differ with respect to birth weight when the results were adjusted for gestational age (F(1, 109) = 7.42, p <.05), such that the NE infants had higher birth weights (adjusted M = 3286.79) compared to the CE infants (adjusted M = 3042.11). Additional exclusionary criteria consisted of maternal age less than 18 years, use of illicit substances other than cocaine or marijuana during pregnancy, diagnosis of fetal alcohol spectrum disorder, and significant medical problems in the infant (e.g., genetic disorders, major perinatal complications, baby in critical care for over 48 hours). See Table 1 for descriptive information of the sample by prenatal exposure status.
Table 1.
Descriptive Information of the Sample by Prenatal Exposure Status
| Nonexposed (n = 57) | Cocaine Exposed (n = 56) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| M | SD | M | SD | t | p | |
| Maternal Age (in years) | 28.61 | 5.37 | 30.91 | 6.11 | −2.12 | .036 |
| Maternal Years of Education | 12.35 | 1.73 | 11.41 | 1.72 | 2.90 | .005 |
| Parity (# of live births) | 3.33 | 1.70 | 4.30 | 2.37 | −2.50 | .014 |
| Prenatal Number of Cigarettes Per Week | 5.97 | 14.84 | 41.49 | 45.64 | −5.50 | <.001 |
| Prenatal Number of Drinks Per Week | .07 | .14 | 4.02 | 9.73 | −3.01 | .004 |
| Prenatal Number of Joints Per Week | 1.48 | 7.32 | 1.41 | 3.16 | .067 | .95 |
| Postnatal Average Number of Cigarettes Per Day | 1.74 | 4.08 | 7.11 | 7.78 | −4.58 | <.001 |
| Postnatal Average Number of Drinks Per Day | 2.63 | 3.47 | 3.98 | 6.89 | −1.31 | .19 |
| Postnatal Average Number of Joints Per Day | .60 | 1.60 | 1.27 | 2.90 | −1.52 | .13 |
| Postnatal Number of Days Used Cocaine | 0.00 | .00 | 6.78 | 22.74 | −2.23 | .03 |
| Gestational Age (in weeks) | 39.37 | 1.22 | 38.54 | 2.05 | 2.62 | .01 |
| Birth Weight (grams) | 3347.42 | 503.20 | 2979.27 | 551.15 | 3.69 | <.001 |
| Head Circumference (centimeters) | 33.65 | 1.30 | 32.96 | 1.55 | 2.57 | .01 |
The study received approval from the institutional review boards of the hospitals as well as the primary institutions with which the authors are affiliated. A Federal Certificate of Confidentiality was obtained and informed written consent was obtained from all recruited participants. Participants received $35.00 and $80.00 in the form of monetary incentives, gift cards, and toys at the 1-month and 13-month visits, respectively.
Identification of Substance Use
Cocaine status was determined by a combination of maternal report, chart review, and urine and maternal hair analysis. Urine toxicologies were routinely conducted at the first prenatal visit on maternal urine and/or at delivery (for those mothers who tested positive prenatally, obtained prenatal care elsewhere, or did not receive any prenatal care) on infant and maternal urine by participating hospitals. Mothers were included in the cocaine group if self-reports were positive, regardless of urine toxicology or hair-sample results. Similarly, mothers who reported that they did not use cocaine but had positive urine toxicology or hair samples were included in the cocaine group.
Urine toxicologies consisted of standard urine screening for drug level or metabolites of cocaine, opiates, benzodiazepines, and tetrahydrocannabinol. Urine was rated positive if the quantity of drug or metabolite was >300 g/ ml. Hair samples were collected from the mothers at the first laboratory visit and sent to the Psychemedics Corporation for radioimmunoanalyses (RIAH). Hair samples were screened for cocaine followed by a gas chromatography/mass spectrometry (GC/MS) confirmation for positive cocaine screens. Drugs and their metabolites are absorbed into the hair and can be extracted and measured. As hair grows at an average rate of 1/2 inch per month, it can record a pattern of drug consumption related to the amount and frequency of use (see Baumgartner, Hill, & Blahd, 1989). Thus, a 2-inch length of hair could contain a record of approximately 4 months of use, and given adequate hair length (i.e., about 4–5 inches), use per trimester may be recorded. Drugs become detectable in hair about 3 to 4 days after use, a time when cocaine is rendered undetectable by urinalysis. RIAH is the most well-established hair-analysis technique and has been replicated by independent laboratories across the world (see Magura, Freeman, Siddiqi, & Lipton, 1992). GC/MS confirmations of RIAH have not revealed any false positives because of testing errors (see Magura et al., 1992). Special washing techniques and data pertaining to kinetics of washing were used to distinguish external contamination from intentional use.
Eleven mothers were identified based on self-report alone, none of the mothers were identified on the basis of positive urine toxicology alone and 8 mothers were identified based on hair analysis alone. Thirty-seven mothers had multiple indicators of cocaine use. Approximately 32% (n = 36) of mothers in the study (55% of the mothers in the cocaine group; n = 31) had positive urine toxicologies at delivery, and 25% (n = 28) of mothers (79% of the mothers in the cocaine group; n = 44) had hair samples that tested positive for cocaine during pregnancy. The remainder of mothers in the cocaine group admitted having used cocaine in the brief self-report screening instrument administered after delivery. Mothers in the comparison group reported not having used any illicit substances other than marijuana.
The Timeline Follow-Back Interview (TLFB; Sobell, Sobell, Klajner, Pavan, & Basian, 1986) was used to assess maternal substance use before, during, and after pregnancy at the 1-month visit and in the time since the last laboratory visit. Participants were provided a calendar and asked to identify events of personal interest (i.e., holidays, birthdays, vacations, etc.) as anchor points to aid recall. This method has been established as a reliable and valid method of obtaining longitudinal data on substance-use patterns, has good test-retest reliability, and is highly correlated with other intensive self-report measures (Brandon, Copeland, & Saper, 1995; Brown et al., 1998). The TLFB yielded data about the average number of days of cocaine use per week, the average number of joints smoked per week, average number of cigarettes smoked per week, and average number of standard drinks per week, for each trimester of pregnancy and for the postnatal period.
Procedure
Assessments from 1 and 13 months of age were used in current analyses. Data from maternal interviews (to obtain accurate information about prenatal substance use) and hair analysis conducted at the 1 month visit were used in these analyses. The 13-month visit consisted of a maternal interview, video-taped observation of free play, and video-taped observations and physiological measures of infant reactivity and regulation during tasks designed to elicit positive and negative affect. The typical order of assessment during the 13-month visit was: 1) assessment of infant reactivity/regulation (behavioral and physiological, 2) free play, 3) assessment of physical growth, 4) maternal interview. In cases where the infant was asleep or fussy, the maternal interview was conducted before beginning the procedures with the infant. All visits and behavioral coding were conducted by research assistants who were blind to the group status of the caregiver-infant dyads.
Assessment of growth and risk status
Three measures of growth were taken by obstetrical nurses in the delivery room: birth weight (gm), birth length (cm), and head circumference (cm). Medical chart review at the time of recruitment was used to complete the Obstetrical Complications Scale (OCS; Littman & Parmelee, 1978), a scale designed to assess perinatal risk factors. Higher numbers indicate a more optimal score.
Laboratory Frustration Paradigm
Infant reactivity was assessed using an anger/frustration paradigm (Goldsmith & Rothbart, 1999; Stifter & Braungart, 1995). The anger/frustration paradigm consisted of a gentle arm restraint episode which is a widely-used, well-validated measure of anger/frustration used to assess infant regulation and reactivity (Goldsmith & Rothbart, 1999; Stifter & Braungart, 1995). First, disposable electrodes were triangulated on the infant’s chest. A respiration bellows was placed around the lower sternum to measure inspiration and expiration. The physiological data were recorded during a 3-minute baseline period, a 2-minute puppet show, and the two arm restraint trials (2 minutes) by examiners blind to infant group status. Infants were tested while seated in a high-chair. Recording of the physiological data began once the infant was observed to be in a stable, quiet, alert state. A resting state was induced by having the infant watch a three minute segment of a neutral videotape “Baby Einstein”; (see Calkins, 1997 for similar procedures for inducing rest). Although this condition was not a true baseline because infant attention was engaged, it served to keep the infant seated quietly without eliciting affect, thereby minimizing movement artifact. All physiological data were recorded continuously on-line directly into a data acquisition computer.
Following the baseline period, infants viewed a puppet show designed to elicit positive affect which was followed by the frustration task. In this episode, the child was allowed to play with an attractive toy for 30 seconds, until the child was engaged with the toy (first negative affect trial). The caregiver was asked to stand behind the child, place her hands on the child’s forearms, move them to the child’s sides, and hold them there for 30 seconds, while maintaining a neutral expression. An event marker was pressed when the caregiver restrained the child to mark the beginning of the data acquisition period for RSA reactivity. After the first trial, the caregiver was again asked to play with the child for 30 seconds. An event marker was again pressed when the play period began to mark the end of the data acquisition period for RSA reactivity. The play period was then followed by a second trial (negative affect trial 2) the beginning and end of which was again indicated by an event marker. The session was stopped at the caregiver’s request or if the child reached a maximum distress code, defined as the child reaching the highest intensity of negative affect of a full cry.
Behavioral Reactivity
A series of behavioral measures were used to assess. Each of the two 30-second trials of the arm restraint procedure was divided up into six 5-second epochs. Latency to negative affect was used obtained as the behavioral measure of reactivity. First, latencies to anger and sadness were obtained using the guidelines of the LabTAB manual, developed by Goldsmith and Rothbart (1991) for each of the two trials. These latency scores were then used to create a composite variable for latency to negative affect by taking the lowest of the scores from the two trials for each infant. This score ranged from 0 to 30, with higher scores indicating a longer latency and lower reactivity. Two coders blind to all information about the families coded latency to negative affect. Inter-rater reliability was calculated for 14% of the tapes. The inter-rater reliability for latency to anger and sadness ranged from 97% to 99% across the two trials (κ = .94 and .95).
Physiological Reactivity
IBI Analysis software (James Long Company, 1999) was used to process the heart rate data and to calculate RSA. Heart rate samples, which were collected every 10 ms, were used to calculate mean HR per one-second period. A level detector was triggered at the peak of each R-wave. The interval between sequential R-waves was calculated to the nearest millisecond. Data files of R-wave intervals were later manually edited to remove incorrect detection of the R-wave or movement artifacts. The software computes RSA using respiration and interbeat interval (IBI) data as suggested by Grossman (1983). The difference between maximum IBI during expiration and the minimum IBI during inspiration was calculated. The difference, which is measured in seconds, is considered to be a measure of RSA, and is measured twice for each respiration cycle (once for each inspiration and once for each expiration). The time for inspirations and expirations is assigned as the midpoint for each. The time for each arrhythmia sample is assigned as the midpoint between an inspiration time and an expiration time. The software synchronizes with respiration and is, thus, relatively insensitive to arrhythmia due to tonic shifts in heart rate, thermoregulation, and baroreceptor. Unlike some methods of calculating RSA including Porges’ moving polynomial algorithm (Porges, 1986), this method corrects for RSA using tidal volume which has been found to influence RSA independent of vagal tone (Grossman, Karemaker & Wieling, 1991). However, despite the differences in calculating RSA, studies suggest that the various estimates of RSA are highly correlated with each other (Beauchaine, 2001). Average RSA was calculated for the 3-minute baseline period and for the arm restraint paradigm. To assess physiological regulation during the arm restraint task, a change score for RSA was calculated from baseline to the arm restraint task by subtracting baseline RSA from RSA during the arm restraint task. Thus, RSA reactivity scores that are greater than zero indicate an increase in RSA relative to baseline and RSA reactivity scores that are less than zero indicate a decrease (RSA suppression/withdrawal) relative to baseline. Because there was no significant difference in RSA between the two negative affect (NA) trials, we created mean RSA for the two trials using only the physiological data acquired while the infant was physically restrained by the caregiver. This composite variable was used in all subsequent analyses.
Maternal Reports of Infant Reactivity
Infant temperamental reactivity was assessed with the distress to limitations subscale of the revised Infant Behavior Questionnaire (IBQ-R; Garstein & Rothbart, 2003) at 13 months of age. The IBQ yields information about discrete categories of behavior that has been shown to have good internal consistency and discriminate validity. The distress to limitations subscale demonstrated adequate reliability (a = .77) in the current sample.
Assessment of Maternal Depression/Anxiety (MDA)
The Brief Symptom Inventory (BSI; Derogatis, 1993), with well established psychometrics, was used to assess MDA at 13-months. The BSI is a brief form of the Symptom Checklist 90-Revised. It consists of 53 items rated on a 5-point scale. The items are grouped into nine scales: Anxiety, Hostility, Somatization, Obsessive-Compulsive, Interpersonal Sensitivity, Depression, Phobic Anxiety, Paranoid Ideation and Psychoticism. Because numerous studies have found that mothers who used cocaine during pregnancy reported higher levels of depression and anxiety (e.g., Singer et al., 1995; Woods, Eyler, Behnke & Conlon, 1993), we used the Anxiety and Depression subscales in these analyses.
Assessment of Maternal and Infant Negative Affect
Maternal and infant negative affect was assessed during a free play task. These interactions were coded using a collection of global 5-point rating scales developed by Clark, Musick, Scott, and Klehr (1980), with higher scores indicating more negative affect. These scales have been found to be applicable for children ranging in age from 2 months to 5 years (Clark, 1999; Clark et al., 1980). Composite scales of maternal negative affect and of infant negative affect were derived from these items. The scale for maternal negative affect consisted of items such as angry, hostile tone of voice; expressed negative affect; angry, hostile mood; and displeasure or disapproval or criticism. The scale for infant negative affect consisted of items such as expressed negative affect, irritable/angry mood, avoiding/averting/resistance, consolability/soothability. Both the maternal and infant negative affect scales had high internal consistency with Cronbach’s alpha of .94 and .81 respectively.
Mothers were asked to interact with their infants as they normally would at home for 5 minutes in a room filled with toys. Two coders rated infant and maternal behavior. Both coders were trained on the Clark scales by the second author and were unaware of group membership. Inter-rater reliability was conducted on a random selection of 14% (n = 24) of the tapes and ranged from r = .89 for infant items to r = .92 for maternal items.
Statistical Analyses
To determine the regulatory profiles, a hierarchical cluster analysis using Ward’s procedure (squared Euclidian distances between subjects) was performed on RSA reactivity (M = −.002., SD = .021) maternal report of infant reactivity (M = 4.24, SD = .91), and behavioral infant reactivity (M = 7.06, SD = 9.31). All calculations were performed with SPSS for Windows, version 18.
More specifically, as in Busseri, Sadava, Molnar, and DeCourville (2009), a six-step approach was applied to identify the optimal cluster solutions within each sample. First, to equate scores across measures, RSA reactivity, maternal report of infant reactivity, and behavioral infant reactivity were standardized (i.e., z scores) within each sample. Second, a hierarchical (agglomerative) cluster analysis was performed using Ward’s method and squared Euclidean distance as the dissimilarity measure. A number of solutions were estimated, ranging from two up to six clusters. Third, the cluster centers (i.e., the mean values of RSA reactivity, maternal report of infant reactivity, and behavioral infant reactivity) from these solutions were used as start values for a series of k-means cluster analyses, again comprising between two and six clusters. With this approach, assignments of participants to clusters based on the hierarchical procedure are optimized using the k-means procedure by maximizing both the separation among clusters and homogeneity within clusters. Fourth, to assess the replicability of these k-means cluster solutions within-samples, the previous three steps were repeated using randomly-selected sub-samples comprising random halves of the respondents. Fifth, the third step was repeated within each sub-sample using the final cluster centers from the full sample as the start values. Sixth, to determine the overall within-sample replicability of the cluster analytic results in each sub-sample, the assignments of respondents to clusters from step four for each sub-sample was cross-tabulated with results from step five, and agreement was estimated by the kappa coefficient. The amount of variance in each of the indicators explained by the cluster solutions also was examined. Consistent with previous research applying a person-centered approach (e.g., Asendorpf, 2003; Asendorpf, Borkeneau, Ostendorf, & Van Aken, 2001; Costa et al., 2002), kappas of .60 or greater were considered adequate. A well-fitting cluster solution also was expected to explain a substantial proportion of variance in physiological regulation, maternal report of child reactivity, and child behavioral reactivity (Bergman, Magnusson, & El-Khouri, 2003).
Finally, we tested whether the clusters differed with respect to relevant demographics, prenatal and postnatal substance use, maternal negative affect, infant birth outcomes, and mother-infant interactions. Chi-square tests were conducted to assess differences among the clusters when the dependent variables were categorical (i.e., child gender, prenatal cocaine exposure, race, and whether the children were placed in foster care or not). Multinomial logistic regression was used when a dependent variable was categorical and when we needed to assess whether differences among the clusters remained after accounting for the effects of covariates in the model (i.e., determining whether prenatal cocaine exposure was associated with cluster membership after accounting for relevant covariates, such as infant growth and other prenatal substance use). To control for high Type I error rate, multivariate analysis of variance (MANOVA) with Bonferroni pair-wise comparisons was conducted to assess differences among the clusters when multiple theoretically associated constructs measured on a continuous scale were the dependent measures (i.e. prenatal and postnatal alcohol, cigarette, and marijuana use, infant birth outcomes, and maternal negative affect). Unlike univariate tests that inflate overall type I error and ignore theoretically and empirically significant associations among dependent variables, MANOVA incorporates correlations among variables into the test statistic, reduces probability of type I error, and is more powerful in detecting potential group differences (see Stevens, 1986). Finally, analysis of variance (ANOVA) was utilized to assess differences among the clusters when a dependent variable was assessed on a continuous scale and analysis of covariance was used when a dependent variable was measured on a continuous scale and we wanted to assess differences among the clusters after accounting for the effects of relevant covariates.
Results
Cluster Analyses
Determining clusters
Results from the within-sample replicability analyses are presented in Table 2, as are the amounts of explained variance in the indicators (i.e., RSA reactivity, maternal report of infant reactivity, and behavioral infant reactivity). From these results, three solutions were found that met the combined criteria of 60% of total explained variance or greater and a kappa of .60 or greater (Asendorpf et al., 2001; Busseri et al., 2009): the 4-, 5-, and 6-cluster solutions. The five-cluster solution was chosen as the best fitting solution based on the within-sample replicability assessments, because the five-cluster solution was consistent within each of the subsamples and made the most sense theoretically. Results from discriminant function analyses further supported a five-cluster solution because 99% of the infants were correctly classified using the five-cluster solution which was substantially higher than what would be expected by chance alone, which was calculated to be 24% of the infants (Tabachnick & Fidell, 2001).
Table 2.
Amount of Explained Variance and Cluster Replicability Results
| Solution | Within-Sample | |
|---|---|---|
|
| ||
| Mean Kappa | Explained Variance | |
| 2 clusters | 1.00 | .29 |
| 3 clusters | .68 | .49 |
| 4 clusters | .77 | .65 |
| 5 clusters | .77 | .71 |
|
| ||
| 6 clusters | .70 | .74 |
Cluster profiles
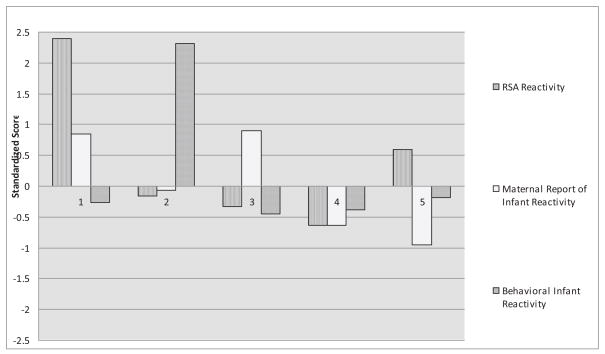
Descriptive information regarding the regulatory profiles, including means and standard deviations for each of the five clusters, as well as the means and standard deviations for the total sample are presented in Table 3. Figure 1 displays the configurations of standardized scores for each of the five clusters (i.e., z scores, which are defined as the differences between the total sample mean and the cluster mean, divided by the sample standard deviation).
Table 3.
Descriptives for the Five-Cluster Solution
| Label | RSA Reactivity | Maternal Report of Infant Reactivity | Behavioral Infant Reactivity | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| M | SD | M | SD | M | SD | ||
| Cluster 1 n=9 | Dysregulated/High Maternal Report Reactors | .049 | .02 | 5.01 | .66 | 4.56 | 3.84 |
| 2.39 | .84 | .85 | .72 | −.26 | .41 | ||
| Cluster 2 n=15 | Low Behavioral Reactors | −.006 | .02 | 4.18 | .89 | 28.53 | 3.18 |
| −.16 | .72 | −.07 | .98 | 2.31 | .34 | ||
| Cluster 3 n=37 | High Reactors | −.009 | .01 | 5.06 | .47 | 2.86 | 2.58 |
| −.33 | .66 | .90 | .51 | −.45 | .28 | ||
| Cluster 4 n=31 | Optimal Reactors | −.016 | .01 | 3.67 | .45 | 3.52 | 3.60 |
| −.63 | .37 | −.63 | .49 | −.38 | .39 | ||
| Cluster 5 n=21 | Dysregulated/Low Maternal Report Reactors | .011 | .01 | 3.38 | .64 | 5.43 | 6.15 |
| .60 | .42 | −.95 | .70 | −.18 | .66 | ||
|
| |||||||
| Total Sample n=113 | −.002 | .02 | 4.24 | .91 | 7.06 | 9.31 | |
Notes. Standardized and unstandardized means (standard deviations) are reported; standardized scores are italicized. High scores on RSA reactivity reflect an increase in RSA from baseline, an indicator of poor parasympathetic regulation. High scores on behavioral infant reactivity measure reflect higher latency to negative affect during arm restraint and indicate low behavioral reactivity. High scores on maternal report of infant reactivity indicate high temperamental reactivity.
Figure 1.
Five-factor solution. Note: High scores on RSA reactivity reflect an increase in RSA from baseline, an indicator of poor parasympathetic regulation. High scores on behavioral infant reactivity measure reflect higher latency to negative affect during arm restraint and indicate low behavioral reactivity. High scores on maternal report of infant reactivity indicates high temperamental reactivity. Scores between ± 0.5 indicate reactivity/regulation in the moderate range. Scores above ± 1.0 indicate high or low reactivity/regulation. Note. Cluster 1 refers to the Dysregulated/High Maternal Report Reactors, Cluster 2 refers to the Low Behavioral Reactors, Cluster 3 refers to the High Reactors, Cluster 4 refers to the Optimal Reactors, and Cluster 5 refers to the Dysregulated/Low Maternal Report Reactors.
Relative to the other clusters, children who were included in Cluster 1 (Dysregulated/High Maternal Report Reactors; 8% of the sample) had high (standardized) RSA reactivity scores, indicating a nonoptimal failure to release the vagal brake during environmental challenge; their mothers reported high levels of reactivity; and they displayed moderate latencies to negative reactivity. Relative to the other clusters, the children who comprised Cluster 2 (Low Behavioral Reactors; 13% of the sample) had moderate levels of physiological reactivity; their mothers reported moderate levels of reactivity; and they showed high latencies to negative reactivity, indicating low behavioral reactivity. Relative to the other clusters, the children in Cluster 3 (High Reactors; 33% of the sample) were typified by moderate levels of physiological reactivity; their mothers reported high levels of reactivity; and they exhibited marginally small latencies to negative reactivity, indicating high behavioral reactivity. Relative to the other clusters, the children included in Cluster 4 (Optimal Reactors; 27% of the sample) had large decreases in RSA, indicating that they reacted optimally by releasing the vagal brake during the frustration challenge; their mothers reported low levels of reactivity; and they exhibited moderate levels of latencies to negative affectivity. Finally, relative to the other clusters, the children who comprised Cluster 5 (Dysregulated/ Low Maternal Report Reactors; 19% of the sample) had increases in their RSA scores, indicating a failure to release the vagal brake; their mothers reported low levels of reactivity; and they displayed moderate latencies to negative reactivity.
Cluster comparisons
Demographics
After determining the best-fitting cluster solution, clusters were compared on child gender, race, and whether the children were placed in foster care or not, using chi-square tests. Results indicated that the clusters did not differ in terms of child gender (χ2(4) = 2.65, p = .63), race (χ2 (4) = 15.00, p = .52), or foster care (χ2 (4) = 6.92, p = .14). Using MANOVA, clusters were then compared on maternal age, education, and parity. Results were not statistically significant (Wilks’ λ = .93, F(12,280.74) = .63, p > .05, η2 = .02).
Prenatal Substance Exposure
Results from chi-square analyses revealed that the clusters did differ with respect to prenatal cocaine exposure (χ2 (4) = 12.30, p <.05). Results from pairwise comparisons revealed that children in the Dysregulated/High Maternal Report Reactors cluster differed significantly from children in Optimal Reactors cluster (χ2(1) = 4.22, p = .04, Hedge’s g = .78), such that there were more children in the nonexposed group than expected statistically and less children who had prenatal cocaine exposure than expected statistically in the Optimal Reactors cluster compared to the Dysregulated/High Maternal Report Reactors cluster. Furthermore, pairwise comparisons also indicated that the Optimal Reactors cluster differed significantly from the Dysregulated/Low Maternal Report Reactors cluster (χ2(1) = 11.15, p = .001, Hedge’s g = 1.03), such that there were less children who had prenatal cocaine exposure than expected statistically and more children in the nonexposed group than expected statistically in the Optimal Reactors cluster compared to the Dysregulated/Low Maternal Report Reactors cluster.
A multinomial logistic regression was conducted to determine whether cluster membership differed as a function of prenatal cocaine exposure after statistically accounting for the effects of gestational age, birth weight, prenatal alcohol, cigarette, and marijuana use. The Optimal Reactors cluster was chosen as the referent group. Results of the analysis indicated that the overall model was significant (LR χ2 = 33.10, p <.001). Specifically, prenatal cocaine exposure was associated with cluster membership (b = 1.89, Wald = 5.99, p = .014), such that infants prenatally exposed to cocaine had a 6.64 fold increase in the odds of being in the Dysregulated/Low Maternal Report Reactors cluster over being in the Optimal Reactors cluster. In other words, those who were exposed to cocaine prenatally were more likely to be in the Dysregulated/Low Maternal Report Reactors cluster rather than in the Optimal Reactors cluster, after accounting for the effects of gestational age, birth weight, and prenatal alcohol, cigarette, and marijuana use.
Clusters were then compared on measures of maternal prenatal alcohol, cigarette, and marijuana use. Results from this MANOVA indicated that there were no significant differences (Wilks’ λ = .89, F(12,278.10) = 1.05, p > .05, η2 = .04).
Differences on measures of maternal postnatal substance use (i.e., number of cigarettes, joints, drinks, and number of days used cocaine) among the clusters were then assessed. Results from this MANOVA were not statistically significant (Wilks’ λ = .83, F(16,321.42) = 1.23, p > .05, η2 = .04).
Maternal Negative Affect
The clusters were compared on maternal negative affect (i.e., depression and anxiety). Results demonstrated a significant multivariate effect (Wilks’ λ = .85, F(8,214) = 2.32, p < .05, η2 = .08). Follow-up univariate analyses revealed that the clusters differed with respect to maternal depression (F(4,108) = 2.67, p <.05, η2 = .09), such that mothers in the High Reactors cluster reported significantly higher levels of maternal depression compared to mothers in the Dysregulated/Low Maternal Report Reactors cluster.
Infant Birth Outcomes
MANOVAs were also used to assess differences among the clusters on birth outcomes, (see Table 4). The MANOVA comparing the clusters with regard to birth outcomes (i.e., gestational age, birth weight, head circumference, and obstetrics complication score) was statistically significant (Wilks’ λ = .75, F(16,309.20) = 1.91, p < .05, η2 = .07). Follow-up univariate ANOVAs indicated a significant difference in birth weight (F(4,104) = 4.65, p <.01, η2 = .15) such that children in the Optimal Reactors cluster had significantly higher birth weights compared to the children in the Dysregulated/Low Maternal Report Reactors cluster, who reported the lowest birth weights. An ANCOVA was also conducted to determine whether the clusters differed with regard to birth weight after adjusting for gestational age. Results demonstrated statistically significant differences among the clusters (F(4,106) = 4.03, p <.01, η2 = .13), such that those in the Optimal Reactors cluster had significantly higher birth weights after adjusting for gestational age compared to Dysregulated/High Maternal Report Reactors and Dysregulated/Low Maternal Report Reactors. In addition, Low Behavioral Reactors and High Reactors had significantly higher birth weights compared to Dysregulated/Low Maternal Report Reactors. Finally, results from a Fisher’s Exact Test revealed that the clusters did not differ with respect to infants being small for their gestational age (Fisher’s Exact Test Statistic = 5.44, p > .05) and revealed that clusters also did not differ with respect to preterm births (i.e. <37 weeks; Fisher’s Exact Test Statistic = 1.57, p =.89)1.
Table 4.
Group Differences for Clusters
| Cluster 1 Dysregulated/High Maternal Report Reactors (n = 9) | Cluster 2 Low Behavioral Reactors (n=15) | Cluster3 High Reactors (n = 37) | Cluster 4 Optimal Reactors (n=31) | Cluster5 Dysregulated/Low Maternal Report Reactors (n = 21) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| M | SD | M | SD | M | SD | M | SD | M | SD | |
| Maternal Characteristics | ||||||||||
| Age (years) | 31.11 | 6.21 | 30.27 | 7.49 | 30.11 | 8.52 | 30.10 | 6.42 | 33.43 | 9.65 |
| Parity | 4.67 | 2.40 | 4.00 | 2.93 | 3.38 | 1.95 | 3.97 | 2.01 | 3.86 | 1.74 |
| Maternal Education (years) | 10.78 | 1.39 | 11.93 | 2.76 | 12.03 | 1.55 | 12.03 | 1.54 | 11.86 | 1.77 |
| Prenatal cocaine exposure (% exposed) | 67% | n/a | 47% | n/a | 49% | n/a | 29% | n/a | 76% | n/a |
| Prenatal # of cigarettes/week | 34.54 | 31.93 | 19.98 | 38.28 | 27.75 | 50.09 | 14.10 | 25.90 | 27.01 | 29.61 |
| Prenatal # of standard drinks/week | 2.33 | 6.44 | 5.31 | 12.35 | 2.88 | 8.86 | .24 | .76 | .75 | 2.06 |
| Prenatal # of joints/week | .56 | 1.12 | 2.35 | 5.27 | 1.19 | 3.33 | 1.91 | 9.37 | .98 | 2.32 |
| Postnatal # of days cocaine/week | 12.00 | 36.00 | 13.20 | 33.23 | 1.19 | 5.26 | .03 | .18 | 1.33 | 3.07 |
| Postnatal # of cigarettes/week | 7.11 | 8.25 | 4.13 | 5.81 | 3.54 | 5.23 | 3.55 | 5.94 | 6.19 | 9.57 |
| Postnatal # of standard drinks/week | 3.89 | 7.64 | 5.47 | 8.54 | 3.65 | 4.15 | 2.87 | 5.71 | 1.52 | 2.18 |
| Postnatal # of joints/week | 1.11 | 2.42 | 1.13 | 1.85 | .78 | 2.38 | .81 | 1.74 | 1.14 | 3.37 |
| Maternal Depression* | 2.69 | 1.01 | 2.49 | .59 | 2.58a | .68 | 2.28 | .39 | 2.17b | .27 |
| Maternal Anxiety | 2.56 | .78 | 2.34 | .45 | 2.41 | .73 | 2.37 | .51 | 2.13 | .29 |
| Maternal Negative Affect* | 4.19 | .95 | 4.53 | .84 | 4.44a | .74 | 4.50 | .76 | 4.86b | .27 |
| Perinatal Variables | ||||||||||
| Gestational Age (weeks) | 39.33 | 1.41 | 38.87 | 1.13 | 38.92 | 1.77 | 39.26 | 1.26 | 38.48 | 2.54 |
| Birth weight (grams)* | 3057.67 | 489.65 | 3296.07 | 532.58 | 3168.65 | 494.40 | 3391.84a | 511.18 | 2791.05b | 602.37 |
| Birth weight Adjusted for Gestational Age* | 3000.97c | 489.65 | 3291.85a | 532.58 | 3177.20a,d | 494.40 | 3346.99a | 511.18 | 2869.30b | 602.37 |
| Head Circumference (cm) at birth | 34.06 | .79 | 33.53 | 1.19 | 33.11 | 1.13 | 33.54 | 1.56 | 32.86 | 2.00 |
| Obstetrical Complications Score (OCS) | 85.67 | 19.98 | 94.00 | 23.67 | 90.61 | 15.55 | 99.30 | 20.95 | 89.86 | 16.62 |
| Small for Gestational Age (SGA) (% Yes) | 33.3% n = 3 |
n/a | 13.3% n = 2 |
n/a | 16.2% n = 6 |
n/a | 16.1% n = 5 |
n/a | 38.1% n = 8 |
n/a |
| Preterm Birth (< 37 weeks) (% Yes) | 11.1% n = 1 |
n/a | 0% n = 0 |
n/a | 8.1% n = 3 |
n/a | 3.2% n = 1 |
n/a | 19% n = 4 |
n/a |
|
| ||||||||||
| Infant Negative Affect | 4.42 | .92 | 4.42 | .74 | 4.61 | .69 | 4.50 | .78 | 4.75 | .74 |
Notes: High scores on OCS reflect more optimal obstetric scores. Means with different superscripts are significantly different from each other.
p < 0.05.
Mother-Infant Interactions
Finally, using one-way ANOVA, the differences among the clusters with regard to maternal and infant negative affect during free play interactions were assessed. Results demonstrated that there were no significant differences among the clusters in childrens’ display of negative affect during free play (i.e., F(4, 104) = .91, p >.05). However, there were significant differences with regard to maternal negative affect exhibited during freeplay (Welch(4,32.17) = 3.04, p <.05, η2 = .06), with mothers in the High Reactors cluster displaying higher levels of negative affect during freeplay than mothers in the Dysregulated/Low Maternal Report Reactors cluster.2
Discussion
The primary purpose of this study was to take a person-centered approach to explore the possibility that distinct profiles of reactivity could be identified in a sample of high risk infants recruited on the basis of prenatal cocaine exposure and a demographically similar group of control infants who were exposed to substances other than cocaine. The results of this study indicate that there are identifiable differences in profiles of reactivity among this group of high risk infants and suggest a variety of developmental influences on these individual differences.
Reactivity Profiles
By examining patterns of behavioral and physiological regulatory processes, we identified five reactivity profiles in 13 month old infants. The first cluster (Dysregulated/High Maternal Report Reactors) consisted of infants who had an increase in RSA during the frustration task. According to Polyvagal Theory (Porges, 1996), an optimal response to environmental challenge is to release the vagal “brake” resulting in the suppression of RSA. Thus, infants in this Dysregulated/High Maternal Report Reactors profile demonstrated a pattern of high nonoptimal physiological reactivity. These infants also displayed moderate behavioral reactivity during a laboratory challenge but were reported by caregivers to be highly reactive over a variety of situational contexts. Thus, this group appears to be characterized by over-arousal and is similar to profiles of children with high levels of reactivity and disruptive behavior (Degnan et al., 2008; Hill et al., 2006).
The second cluster demonstrated low levels of physiological reactivity and had considerably lower levels of behavioral reactivity (Low Behavioral Reactors). These infants took much longer to react negatively and had very little change in RSA during the frustration task. The low levels of physiological reactivity may indicate low autonomic arousal (Raine, 2002). This group is similar to others that have been characterized as being under-aroused (van Goozen et al., 2000), a pattern that has been associated with externalizing problems in older children (Murray & Kochanska, 2002; van Goozen et al., 2000).
The third cluster of infants had moderate physiological reactivity and high levels of observed and reported reactivity (High Reactors). Thus, they appeared to be easily negatively aroused in an observed laboratory paradigm as well as across a wide-range of situational contexts as reported by their caregivers. However, these infants were capable of some optimal physiological reactivity (suppression of RSA). This indicates that there may be a subsample of infants that are more reliant on physiological processes than behavioral processes for regulating their negative reactivity
Infants in the fourth cluster had high levels of optimal physiological reactivity (RSA suppression), low levels of reported reactivity and displayed moderate levels of behavioral reactivity during the frustration task (Optimal Reactors). Thus, this group appears to display appropriate levels of reactivity and is well-regulated. This pattern is consistent with profiles of moderate levels of reactivity and regulation identified in samples of nonexposed children (Wilson et al., 2009) which are associated with better adjustment relative to profiles of either over- or under-regulation (Eisenberg & Fabes, 1992).
Finally, the fifth cluster consisted of infants who had high levels of nonoptimal physiological reactivity (increase in RSA rather than the optimal suppression of RSA), low levels of reported reactivity and moderate levels of observed behavioral reactivity (Dysregulated/Low Maternal Report Reactors). Thus, there was a mismatch between the various indicators of reactivity for infants in this group. Although their autonomic nervous system indicated high levels of nonoptimal reactivity, their caregivers indicated that they had low levels of reactivity across a wide range of situational contexts. This pattern is consistent with children who have been described as over-controlled and have increased levels of adjustment and internalizing problems (e.g., Murray & Kochanska 2002; Rubin, Stewart, & Coplan, 1995; Wilson et al., 2009).
Profile Differences in Substance Exposure, Infant and Maternal Risks
The second goal of this study was to identify possible factors that differentiated the various profiles to elucidate individual differences in patterns of reactivity in substance-exposed infants. Findings indicated that both maternal and infant characteristics differed between regulatory profiles suggesting that there are a number of developmental influences on the development of reactivity among cocaine-exposed infants.
As expected, maternal cocaine use during pregnancy did differentiate the profiles. A higher number of cocaine-exposed infants than expected were found in both of the Dysregulated Profiles (Dysregulated/High Maternal Report Reactors and Dysregulated/Low Maternal Report Reactors) which is consistent with increasing findings of increased regulatory difficulties in cocaine-exposed infants (e.g., Bendersky & Lewis, 1998; Delaney-Black et al., 2000; Delaney-Black et al., 2004; Dennis et al., 2006; Eiden et al., 2009; Mayes et al., 1996). Furthermore, a higher number of nonexposed infants than expected were found in the Optimal Reactors group indicating that cocaine exposed infants were less likely to display this more optimal pattern of reactivity. It is important to note that several other studies have found that some cocaine-exposed infants may be less reactive than nonexposed infants (Alessandri et al., 1993, 1995; Lester et al., 1991; Molitor, Mayes & Ward, 2003), however, prenatal cocaine exposure was not associated with the profile of Low Reactors. Future studies should explore if variables that were not included in this study would differentiate this profile from others or if, over time, a unique developmental trajectory will emerge for this group.
Measures of fetal growth also differentiated among the profiles. Infants in the Dysregulated/Low Maternal Report Reactivity group had lower birthweights than infants in the Optimal Reactors group. Fetal growth measures such as birthweight and gestational age, have been linked to a number of negative consequences including altered autonomic activity (e.g., DiPietro, Porges, & Uhly, 1992; Krafchuk et al., 1983). Consequently, the combination of poor fetal growth and prenatal exposure to cocaine may increase the chances of developing a regulatory pattern of underarousal. While both the Dysregulated/High Maternal Report Reactors and the Dysregulated/Low Maternal Report Reactors were more likely to be exposed to cocaine, there were differences between these two groups in birthweight of the infants as well as in maternal reports of reactivity. The Dysregulated/Low Maternal Report Reactors had lower birthweight and moms saw them as less reactive, while the Dysregulated/High Maternal Report Reactors did not differ from any other group on birthweight and their moms reported them as highly reactive.
Finally, maternal depression and negative affect was higher among mothers of infants in the High Reactors group. Studies have reported that maternal substance use is often associated with symptoms of depression (Boyd, 1993; Eiden, Peterson, & Coleman, 1999; Luthar, Cushing, Merikangas, & Rounsaville, 1998; Singer et al., 1997). This aspect of psychological functioning has consistently been linked to nonoptimal mother-infant interactions among women who do not use cocaine during pregnancy (e.g., Dickstein et al., 1998; Field, 1992; Gelfand & Teti, 1990) as well as among mothers who use cocaine and other substances (e.g., Beckwith, Howard, Espinosa, & Tyler, 1999; Luthar et al., 1998; Singer et al., 1997). Specifically, depressed mothers are more likely to display flatter affect during mother-child interactions, provide less stimulation and be less responsive toward their infants (Cohn & Campbell, 1992; Jameson, Gelfan, Kulcsar, & Teti, 1997). This was supported in the current study by the findings that the mothers of infants in this group displayed higher levels of maternal negative affect during free play with their infants. These aspects of maternal parenting behavior play a critical role in helping children manage their arousal and keep distress within tolerable limits in infancy. If infant affective arousal in response to environmental challenge is met with maternal negative affect, this is likely to result in poorer infant affective regulation (Feldman et al., 1999; Schore, 1994; Calkins & Fox, 1992). Thus, the combination of higher infant negative reactivity, higher levels of maternal depression and increased maternal negative affect during mother-infant interaction may explain the difficulty these infants have with behavioral regulation.
Limitations
Although the present study contributes to the current literature by identifying distinct regulatory profiles among cocaine-exposed infants, some limitations need to be acknowledged. First, although care was taken in the present study to identify substance use in this sample, the accurate assessment of substance use is difficult. Pregnant women are often hesitant to divulge information regarding the use of substances during pregnancy, particularly of illicit substances such as cocaine. Furthermore, although hair analyses are widely used as measures of substance use, concerns about its accuracy with chemically-treated hair and in women with varying hair color has been reported (Gerstenberg et al., 1994; Jurado, Kintz, Menedez & Repetto, 1997; Nakahara, Takahashi & Kikura, 1995). However, research has indicated that typical hair procedures (shampoo, condition, sprays, mousses, gels, bleaching, perms, dyeing, etc.) do not have a significant impact on the quantitative findings of substance use (Hubbard, Wilkins & Rollins, 2000). Although, the level of drug detection in severely treated hair that has been damaged may be affected, damaged hair can be readily identified from wash ratios. To address the limitations associated with addressing prenatal substance use using self-report and hair analyses, multiple indices of illicit substance use were used including self-report using the reliable Timeline Followback Interview, as well as analysis of hair and urine samples. Each of these measures has its own limitations although, when used in combination, the likelihood of accurately identifying cocaine use is increased. There were, however, no biological markers used in this study for assessing prenatal alcohol consumption or cigarette smoking. It is possible that mothers may have misrepresented their use of these substances as a result of inaccurate memory or hesitance to divulge usage. This, combined with our exclusion criteria of infants identified as having fetal alcohol spectrum disorder may, in turn, explain our finding of no association between prenatal alcohol and cigarette exposure and physiological regulation. Second, our physiological assessment of regulation was limited to a task designed to elicit frustration. Other aspects of negative affect such as fear were not assessed. Future studies should assess behavior/physiology correlates of regulation during a variety of tasks designed to elicit negative affect. The final limitation is related to this study’s reliance on RSA to index physiological regulation. Although the parasympathetic branch of the autonomic nervous system does play in important role in the physiological regulation of stress, it does not index the role of the sympathetic-adrenal-medullary system which is also critical to the stress response (Bernston, Cacioppo & Quigley, 1994). Future studies should, therefore, include measures of sympathetic nervous system activity when exploring regulatory profiles among exposed children. Finally, the Dysregulated/High Maternal Report Reactors Cluster only included nine infants, thus caution should be exercised when assessing group differences.
Conclusions
In conclusion, the findings of this study indicated that there are distinct regulatory profiles among a group of high risk children recruited on the basis of prenatal cocaine exposure and a demographically similar control group. These patterns are similar to patterns found in low risk samples of children and both maternal and infant characteristics can differentiate membership in these groups. It is not clear, however, if these profiles would persist beyond infancy into the preschool or school-aged years. Although studies with low risk infants have demonstrated substantial stability in temperament profiles and in profiles of disruptive behavior during infancy and early childhood (Degnan et al., 2008; Janson & Mathiesen, 2008) and frustration reactivity is thought to be stable during childhood, it is not clear if this type of stability would be found among high-risk substance-exposed children. Subsequently, future studies should explore whether there is stability in regulatory profiles across infancy and into childhood. Furthermore, numerous studies have identified distinct patterns of regulatory processes that differentiate children with internalizing and externalizing disorders. For example, studies have indicated that a combination of higher reactivity to frustration and lower regulation is associated with externalizing behavior problems (Diener & Kim, 2004; Eisenberg et al., 2000; Stifter, Spinrad, & Braungart-Rieker, 1999). In fact, Janson and Mathieson (2008) argue that studies should explore the association between temperamental profiles and internalizing/externalizing behavior problems in high-risk samples. As such, future studies should explore whether regulatory profiles among these high risk children predict externalizing or internalizing disorders in childhood.
Highlights.
There are distinct profiles of reactivity among cocaine-exposed infants.
Maternal behavior, cocaine use and psychopathology differentiate profiles.
Birthweight also differentiates these profiles.
Acknowledgments
The authors thank parents and infants who participated in this study and the research staff who were responsible for conducting numerous assessments with these families. Special thanks to Drs. Amol Lele and Luther Robinson for collaboration on data collection at Women and Children’s Hospital of Buffalo, and to Dr. Michael Ray for his collaboration on data collection at Sisters of Charity Hospital of Buffalo. This study was made possible by a grant from NIDA (R01 DA 013190).
Footnotes
Fisher’s exact test was used rather than the typical chi square test because there were expected counts less than 5 present.
Given that the assumption of homogeneity of variance was untenable (i.e., F(4, 104) = 5.05, p = .001), the Welch statistic, which is a robust test of equality of means, was utilized to determine whether the groups differed significantly from one another. Further, child and maternal negative affect during freeplay were transformed prior to analyses using reflection and a log10 transformation, which rendered their distributions within the range of normality.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aksan N, Goldsmith HH, Smider NA, Essex MJ, Clark R, Hyde JS, et al. Drivatio and prediction of temperamental types among preschoolers. Developmental Psychology. 1999;35:958–971. doi: 10.1037//0012-1649.35.4.958. [DOI] [PubMed] [Google Scholar]
- Alessandri SM, Sullivan MW, Bendersky M, Lewis M. Temperament in cocaine- exposed infants. In: Lewis M, Bendersky M, editors. Mothers, babies and cocaine: The role of toxins in development. Hillsdale, NJ: Erlbaum; 1995. pp. 273–286. [Google Scholar]
- Alessandri SM, Sullivan MW, Imaizumi S, Lewis M. Learning and emotional responsivity in cocaine-exposed infants. Developmental Psychology. 1993;29:989–997. [Google Scholar]
- Asendorpf JB. Head-to-head comparison of the predictive validity of personality types and dimensions. European Journal of Personality. 2003;17:327–346. [Google Scholar]
- Asendorpf JB, Borkeneau P, Ostendorf F, Van Aken MAG. Carving personality description at its joints: Confirmation of three replicable personality prototypes for both children and adults. European Journal of Personality. 2001;15:169–198. [Google Scholar]
- Azuma SD, Chasnoff IJ. Outcome of children prenatally exposed to cocaine and other drugs: A path analysis of three-year data. Pediatrics. 1993;92:396–402. [PubMed] [Google Scholar]
- Baumgartner WA, Hill VA, Blahd WH. Hair analysis for drugs of abuse. 1989. [Google Scholar]
- Bateman DA, Chiriboga CA. Dose response effect of cocaine on newborn head circumference. Pediatrics. 2000;106:E33. doi: 10.1542/peds.106.3.e33. [DOI] [PubMed] [Google Scholar]
- Behnke M, Eyler FD, Warner TD, Garvan CW, Hou W, Wobie K. Outcome from a prospective, longitudinal study of prenatal cocaine use: Preschool development at 3 years of age. Journal of Pediatric Psychology. 2006;31:41–49. doi: 10.1093/jpepsy/jsj027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP. Vagal tone, development, and Gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Beckwith L, Howard J, Espinosa M, Tyler R. Psychopathology, mother-child interaction, and infant development: Substance-abusing mothers and their offspring. Development & Psychopathology. 1999;11:715–725. doi: 10.1017/s095457949900228x. [DOI] [PubMed] [Google Scholar]
- Bendersky M, Bennett D, Lewis M. Aggression at age five as a function of prenatal exposure to cocaine, gender and environmental risk. Journal of Pediatric Psychology. 2006;31:71–84. doi: 10.1093/jpepsy/jsj025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendersky M, Gambini G, Lastella A, Bennett DS, Lewis M. Inhibitory motor control at five years as a function of prenatal cocaine exposure. Annals of the New York Academy of Sciences. 2003;24:345–351. doi: 10.1097/00004703-200310000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendersky M, Lewis M. Arousal modulation in cocaine-exposed infants. Developmental Psychology. 1998;34:555–564. [PMC free article] [PubMed] [Google Scholar]
- Bergman LR, Magnusson D. A person-oriented approach in research on developmental psychopathology. Development & Psychopathology. 1997;9:291–319. doi: 10.1017/s095457949700206x. [DOI] [PubMed] [Google Scholar]
- Bergman LR, Magnusson D, El-Khouri B. Studying individual development in an interindividual context: A person-oriented approach. In: Magnusson D, editor. Paths through life. Vol. 4. Mahwah, NJ: Erlbaum; 2003. [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Autonomic cardiac control. I. Estimation and validation from pharmacological blockades. Psychophysiology. 1994;31:572–585. doi: 10.1111/j.1469-8986.1994.tb02350.x. [DOI] [PubMed] [Google Scholar]
- Bornstein M, Suess P. Physiological self-regulation and information-processing in infancy: Cardiac vagal tone and habituation. Child Development. 2000;71:273–287. doi: 10.1111/1467-8624.00143. [DOI] [PubMed] [Google Scholar]
- Boyd CJ. The antecendent of women’s crack cocaine abuse: Family substance abuse, sexual abuse, depression and illicit drug use. Journal of Substance Abuse Treatment. 1993;10:433–438. doi: 10.1016/0740-5472(93)90002-j. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Copeland AL, Saper ZL. Programmed therapeutic messages as a smoking treatment adjunct: Reducing the impact of negative affect. Health Psychology. 1995;14:41–47. doi: 10.1037//0278-6133.14.1.41. [DOI] [PubMed] [Google Scholar]
- Brown RA, Burges ES, Sales SD, Whitely JA, Evans DM, Miller I. Reliability and validity of a smoking timeline followback interview. Psychology of Addictive Behaviors. 1998;12:101–112. [Google Scholar]
- Buss KA, Davidson RJ, Kalin NH, Goldsmith HH. Context specific freezing and associated physiological reactivity as a dysregulated fear response. Developmental Psychology. 2004;40:583–594. doi: 10.1037/0012-1649.40.4.583. [DOI] [PubMed] [Google Scholar]
- Busseri M, Sadava SW, Molnar DS, DeCourville NH. A person-centered approach to subjective well-being. Journal of Happiness Studies. 2009;10:161–181. [Google Scholar]
- Calkins SD. Cardiac vagal tone indices of temperamental reactivity and behavioral regulation in young children. Developmental Psychobiology. 1997;31:125–142. doi: 10.1002/(sici)1098-2302(199709)31:2<125::aid-dev5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Fox NA. The relations among infant temperament, security of attachment and behavioral inhibition at 24 months. Child Development. 1992;63:1456–1472. [PubMed] [Google Scholar]
- Caspi A, Silva PA. Temperamental qualities at age three predict personality traits in young adulthood: Longitudinal evidence from a birth cohort. Child Development. 1995;66:486–498. doi: 10.1111/j.1467-8624.1995.tb00885.x. [DOI] [PubMed] [Google Scholar]
- Chasnoff IJ, Griffith DR, MacGregor S, Dirkes K, Burns KA. Temporal patterns of cocaine use in pregnancy. Perinatal outcome. Journal of the American Medical Association. 1989;261:1741–1744. [PubMed] [Google Scholar]
- Clark R, Musick J, Scott F, Klehr K. The mothers’ project rating scale of mother-child interaction. 1980 Unpublished manuscript. [Google Scholar]
- Clark R. The parent-child early relational assessment: A factorial validity study. Educational Psychology Measurement. 1999;59:821–846. [Google Scholar]
- Cohn JF, Campbell SB. Influence of maternal depression on infant affect regulation. In: Cicchetti D, Toth SL, editors. Developmental perspectives on depression. 4. Rochester, NY: University of Rochester Press; 1992. pp. 103–130. [Google Scholar]
- Coles CD, Platzman KA, Smith I, James M, Falek A. Effects of cocaine and alcohol use in pregnancy on neonatal growth and neurobehavioral status. Neurotoxicology and Teratology. 1992;14:23–33. doi: 10.1016/0892-0362(92)90025-6. [DOI] [PubMed] [Google Scholar]
- Costa PT, Herbst JH, McCrae RR, Samuels J, Ozer DJ. The replicability and utility of three personality types. European Journal of Personality. 2002;16:S73–S87. [Google Scholar]
- DeGangi GA, DiPietro JA, Greenspan SI, Porges SW. Psychophysiological characteristics of the regulatory disordered infant. Infant Behavior and Development. 1991;14:37–50. [Google Scholar]
- Degnan KA, Calkins SD, Keane SP, Hill-Soderlund AL. Profiles of disruptive behavior across early childhood: Contributions of frustration reactivity, physiological regulation, and maternal behavior. Child Development. 2008;79:1357–1376. doi: 10.1111/j.1467-8624.2008.01193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney-Black V, Covington C, Templin T, Agera J, Nordstrom-Klee B, Martier S, et al. Teacher-assessed behavior of children prenatally exposed to cocaine. Pediatrics. 2000;106:782–791. doi: 10.1542/peds.106.4.782. [DOI] [PubMed] [Google Scholar]
- Delaney-Black V, Covington C, Templin T, Kershaw T, Nordstrom-Klee B, Ager J, Clark N, Surendran A, Martier S, Sokol RJ. Expressive language development of children exposed to cocaine prenatally: Literature review and report of a prospective study. Journal of Communicative Disorders. 2000;33:463–481. doi: 10.1016/s0021-9924(00)00033-2. [DOI] [PubMed] [Google Scholar]
- Dennis T, Bendersky M, Ramsay D, Lewis M. Reactivity and regulation in children prenatally exposed to cocaine. Developmental Psychology. 2006;42:688–697. doi: 10.1037/0012-1649.42.4.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis LE. The Brief Symptom Inventory (BSI) administration, scoring, and procedures manual. Minneapolis, MN: National Computer Systems; 1993. [Google Scholar]
- Dickstein S, Seifer R, Hayden LC, Schiller M, Sameroff AJ, Keitner G, Miller I, Rasmussen S, Matzko M, Magee KD. Levels of family assessment: II. Impact of maternal psychopathology on family functioning. Journal of Family Psychology. 1998;12:23–40. [Google Scholar]
- Diener M, Kim DY. Maternal and Child Predictors of Children’s Social Competence. Journal of Applied Developmental Psychology. 2004;25:3–24. [Google Scholar]
- DiPietro JA, Larson SK, Porges SW. Behavioral and heart-rate pattern differences between breast-fed and bottle-fed neonates. Developmental Psychology. 1987;23:467–474. [Google Scholar]
- DiPietro JA, Porges SW, Uhly B. Reactivity and developmental competence in preterm and full-term infants. Developmental Psychology. 1992;28:1–11. [Google Scholar]
- Eiden RD, Lewis A, Croff S, Young E. Maternal cocaine use and infant behavior. Infancy. 2002;3:77–96. [Google Scholar]
- Eiden RD, McAuliffe S, Kachadourian L, Coles CD, Colder C, Schuetze P. Effects of prenatal cocaine exposure on infant reactivity and regulation. Neurotoxicology and Teratology. 2009;31:60–68. doi: 10.1016/j.ntt.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden RD, Peterson M, Coleman T. Maternal cocaine use and the caregiving environment during early childhood. Psychology of Addictive Behaviors. 1999;13:293–302. [Google Scholar]
- Eiden RD, Stevens A, Schuetze P, Dombkowski LE. A conceptual model for maternal behavior among polydrug cocaine-using mothers: The role of postnatal cocaine use and maternal depression. Psychology of Addictive Behaviors. 2006;20(1):1–10. doi: 10.1037/0893-164X.20.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA. Emotion, regulation, and the development of social competence. Emotion and social behavior. In: Clark MS, editor. Review of personality and social psychology. Vol. 14. Thousand Oaks, CA: Sage; 1992. pp. 119–150. [Google Scholar]
- Eisenberg N, Fabes RA, Guthrie IK, Reiser M. Dispositional emotionality and regulation: Their role in predicting quality of social functioning. Journal of Personality and Social Psychology. 2000;78:136–157. doi: 10.1037//0022-3514.78.1.136. [DOI] [PubMed] [Google Scholar]
- Feldman R, Greenbaum CW, Yirmiya N. Mother-infant affect synchrony as an antecedent of the emergence of self-control. Developmental Psychology. 1999;35:223–231. doi: 10.1037//0012-1649.35.1.223. [DOI] [PubMed] [Google Scholar]
- Field TM. Interventions in early infancy. Infant Mental Health Journal. 1992;13:329–336. [Google Scholar]
- Fried PA, Makin JE. Neonatal behavioural correlates of prenatal exposure to marihuana, cigarettes and alcohol in a low risk population. Neurotoxicology & Teratology. 1987;9:1–7. doi: 10.1016/0892-0362(87)90062-6. [DOI] [PubMed] [Google Scholar]
- Fried PA, Watkinson B, Dillon RF. Neonatal neurological status in a low-risk population after prenatal exposure to cigarettes, marijuana, and alcohol. Journal of Developmental and Behavioral Pediatrics. 1987;8:318–326. [PubMed] [Google Scholar]
- Fox NA. Psychophysiological correlates of emotional reactivity during the first year of life. Developmental Psychology. 1989;25:364–372. [Google Scholar]
- Garstein MA, Rothbart MK. Studying infant temperament via a revision of the Infant Behaviour Questionnaire. Journal of Infant Behavior and Development. 2003;26:64–86. [Google Scholar]
- Gawin FH, Ellinwood EH. Cocaine and other stimulants: Actions, abuse and treatment. New England Journal of Medicine. 1988;318:1173–1182. doi: 10.1056/NEJM198805053181806. [DOI] [PubMed] [Google Scholar]
- Gelfand DM, Teti DM. The effects of maternal depression on children. Clinical Psychology Review. 1990;10:329–353. [Google Scholar]
- Gerstenberg B, Schepers G, Voncken P, Volkel H. Nicotine and cotinine accumulation in pigmented and unpigmented rat hair. Drug Metabalites and Disposition. 1995;23:143–148. [PubMed] [Google Scholar]
- Goldsmith HH, Rothbart MK. Contemporary instruments for assessing early temperament by questionnaire and in the laboratory. Explorations in temperament: International perspectives on theory and measurement. In: Strelau J, Angleitner A, editors. Perspectives on individual differences. New York: Plenum; 1991. pp. 249–272. [Google Scholar]
- Goldsmith HH, Rothbart MK. Laboratory Temperament Assessment Battery (Technical Manual) University of Wisconsin-Madison; USA: 1999. [Google Scholar]
- Grossman P. Respiration, stress, and cardiovascular function. Psychophysiology. 1983;20:284–299. doi: 10.1111/j.1469-8986.1983.tb02156.x. [DOI] [PubMed] [Google Scholar]
- Grossman P, Karemaker J, Wieling W. Prediction of topic parasympathetic cardiac control using respiratory sinus arrhythmia: The need for respiratory control. Psychophysiology. 1991;28:201–216. doi: 10.1111/j.1469-8986.1991.tb00412.x. [DOI] [PubMed] [Google Scholar]
- Gunnar MR. Human developmental psychoneur- oendocrinology: A review of research on neuroendo- crine responses to challenge and threat in infancy and childhood. In: Lamb ME, Brown AL, Rogoff B, editors. Advances in developmental psychology. Vol. 4. Hillsdale, NJ: Erlbaum; 1986. pp. 51–103. [Google Scholar]
- Gunnar MR. Studies of the human infant’s adrenocortical response to potentially stressful events. New directions for child development: No 45. In: Lewis M, Worobey J, editors. Infant stress and coping. San Francisco; Jossey-Bass: 1989. pp. 3–18. [DOI] [PubMed] [Google Scholar]
- Handler A, Kistin N, Davis F, Ferre C. Cocaine use during pregnancy: Perinatal outcomes. American Journal of Epidemiology. 1991;133:818–825. doi: 10.1093/oxfordjournals.aje.a115961. [DOI] [PubMed] [Google Scholar]
- Hart D, Atkins R, Fegley S. Personality and development in childhood: A person-centered approach. Monographs of the Society for Research in Child Development. 2003;68(1) Serial No. 272. [PubMed] [Google Scholar]
- Heffelfinger AK, Craft S, White DA, Shyken J. Visual attention in preschool children prenatally exposed to cocaine: Implications for behavioral regulation. Journal of the International Neuropsychological Society. 2002;8:12–21. [PubMed] [Google Scholar]
- Hill AL, Degnan KA, Calkins SD, Keane SP. Profiles of externalizing behavior problems for boys and girls across preschool: The roles of emotional regulation and inattention. Developmental Psychology. 2006;42:913–928. doi: 10.1037/0012-1649.42.5.913. [DOI] [PubMed] [Google Scholar]
- Hubbard DL, Wilkins DG, Rollins DE. The incorporation of cocaine and metabolites into hair: Effects of dose and hair pigmentation. Drug Metabolites and Disposition. 2000;28:1464–1469. [PubMed] [Google Scholar]
- Jacobson SW, Bihun JT, Chiodo LM. Effects of prenatal alcohol and cocaine exposure on infant cortisol levels. Development & Psychopathology. 1999;11:195–208. doi: 10.1017/s0954579499002011. [DOI] [PubMed] [Google Scholar]
- James Long Company. IBI Analysis System reference guide. Caroga, NY: Author; 1999. [Google Scholar]
- Jameson PB, Gelfand D, Kulcsar E, Teti DM. Mother-toddler interaction patterns associated with maternal depression. Developmental Psychopathology. 1997;9:557–590/. [PubMed] [Google Scholar]
- Janson H, Mathieson KS. Temperament profiles from infancy to middle childhood: Development and associations with behavior problems. Developmental Psychology. 2008;44:1314–1328. doi: 10.1037/a0012713. [DOI] [PubMed] [Google Scholar]
- Jurado C, Kintz P, Menendez M, Repetto M. Influence of the cosmetic treatment of hair on drug concentrations. International Journal of Legal Medicine. 1997;110:159–163. doi: 10.1007/s004140050056. [DOI] [PubMed] [Google Scholar]
- Karmel BZ, Gardner JM. PCE effects on arousal modulated attention during the neonatal period. Developmental Psychobiology. 1996;29:463–480. doi: 10.1002/(SICI)1098-2302(199607)29:5<463::AID-DEV5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Kopp CB. Regulation of distress and negative emotions: A developmental view. Developmental Psychology. 1989;25:343–354. [Google Scholar]
- Krafchuk E, Tronick E, Clifton R. Behavioral an cardiac responses to sound in preterm infants varying in risk status and a hypothesis of their paradoxical eactivity. In: Field TM, Sostek AM, editors. Infants born at risk: Physiological and perceptual processes. Grune & Stratton; 1983. pp. 99–128. [Google Scholar]
- Lester BM, Corwin MJ, Sepkoski C, Seifer R, Peucker M, McLaughlin S, Golub HL. Neurobehavioral syndromes in cocaine-exposed newborn infants. Child Development. 1991;62:694–705. doi: 10.1111/j.1467-8624.1991.tb01563.x. [DOI] [PubMed] [Google Scholar]
- Lester BM, LaGasse L, Brunner S. Database of studies on prenatal cocaine exposure and child outcome. Journal of Drug Issues. 1997;27:487–499. [Google Scholar]
- Lewis BA, Singer LT, Short EJ, Minnes S, Arendt R, Weishampel P, et al. Four-year language outcomes of children exposed to cocaine in utero. Neurotoxicology & Teratology. 2004;26:617–627. doi: 10.1016/j.ntt.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Littman A, Parmelee B. Medical correlation of infant development. Pediatrics. 1978;61:474. doi: 10.1542/peds.61.3.470. [DOI] [PubMed] [Google Scholar]
- Luthar SS, Cushing G, Merikangas KR, Rounsaville BJ. Multiple jeopardy: Risk and protective factors among addicted mothers’ offspring. Development & Psychopathology. 1998;10:117–136. doi: 10.1017/s0954579498001333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magura S, Freeman RC, Siddiqi Q, Lipton DS. The validity of hair analysis for detecting cocaine and heroin use among addicts. International Journal of the Addictions. 1992;27:51–69. doi: 10.3109/10826089109063462. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf SC, McHale JL, Diener M, Godstein LH, Lehn L. Infant attachment: Contributions of infant temperament and maternal characteristics. Infant Behavior& Development. 2000;23:175–196. [Google Scholar]
- Mayes LC. A behavioral teratogenic model of the impact of prenatal cocaine exposure on arousal regulatory systems. Neurotoxicology & Teratology. 2002;24:385–395. doi: 10.1016/s0892-0362(02)00200-3. [DOI] [PubMed] [Google Scholar]
- Mayes LC, Bornstein MH, Chawarska K, Granger RH. Impaired regulation of arousal in three-month-old infants exposed to cocaine and other drugs. Developmental Psychopathology. 1996;8:29–42. [Google Scholar]
- Mayes LC, Grillon R, Granger R, Schottenfeld R. In: Cocaine: effects on the developing brain. Harvey JA, Kosofsky BE, editors. New York: New York Academy of Sciences; 1998. pp. 126–143. [Google Scholar]
- Molitor A, Mayes LC, Ward A. Emotion regulation behavior during a separation procedure in 18-month-old children of mothers using cocaine and other drugs. Development & Psychopathology. 2003;15:39–54. doi: 10.1017/s0954579403000038. [DOI] [PubMed] [Google Scholar]
- Murray KT, Kochanska G. Effortful control: Factor structure and relation to externalizing and internalizing behaviors. Journal of Abnormal Child Psychology. 2002;30:503–514. doi: 10.1023/a:1019821031523. [DOI] [PubMed] [Google Scholar]
- Nakahara Y, Takahashi K, Kikura R. Hair analysis for drugs of abuse. X. Effect of physicochemical properties of drugs on the incorporation rates into hair. Biological Pharmacology Bulletin. 1995;18:123–1227. doi: 10.1248/bpb.18.1223. [DOI] [PubMed] [Google Scholar]
- Nassogne MC, Evrard P, Courtoy PJ. Selective direct toxicity of cocaine on fetal mouse neurons. Teratogenic implications of neurite and apoptotic neuronal loss. Cocaine: Effects on the developing brain. In: Harvey JA, Kosofsky BE, editors. Annals of the New York Academy of Sciences. Vol. 846. New York: NY Academy of Sciences; 1998. pp. 51–68. [PubMed] [Google Scholar]
- Platzman KA, Coles CD, Lynch ME, Bard KA, Brown JV. Assessment of the caregiving environment and infant functioning in polydrug families: Use of a structured clinical interview. Infant Mental Health Journal. 2001;22(3):351–373. [Google Scholar]
- Porges SW. Respiratory sinus arrhythmia: Physiological basis, quantitative methods, and clinical implications. In: Grossman P, Janssen K, Vaitl D, editors. Cardiorespiratory and cardiosomatic psychophysiology. New York: Plenum Press; 1986. pp. 101–115. [Google Scholar]
- Porges SW. Vagal tone: An autonomic mediator of affect. In: Garber JA, Dodge KA, editors. The Development of Affect Regulation and Dysregulation. New York: Cambridge University Press; 1991. pp. 111–128. [Google Scholar]
- Porges SW. Physiological regulation in high-risk infants: A model for assessment and potential intervention. Development & Psychopathology. 1996;8:43–58. [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Portales L, Greenspan S. Infant regulation of the vagal brake predicts child behavior problems: A psychobiological models of social behavior. Developmental Psychobiology. 1996;29:697–712. doi: 10.1002/(SICI)1098-2302(199612)29:8<697::AID-DEV5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Raine A. Biosocial studies of antisocial and violent behavior in children and adults: A review. Journal of Abnormal Child Psychology. 2002;30:311–326. doi: 10.1023/a:1015754122318. [DOI] [PubMed] [Google Scholar]
- Regalado MG, Schechtman VL, Del Angel AP, Bean XD. Sleep disorganization in cocaine-exposed neonates. Infant Behavior and Development. 1995;18:319–327. [Google Scholar]
- Regalado MG, Schechtman VL, Del Angel AP, Bean XD. Cardiac and respiratory patterns during sleep in cocaine-exposed neonates. Early Human Development. 1996;44:187–200. doi: 10.1016/0378-3782(95)01708-9. [DOI] [PubMed] [Google Scholar]
- Regalado MG, Schechtman VL, Khoo MCK, Bean XD. Spectral analysis of heart rate variability and respiration during sleep in cocaine-exposed neonates. Clinical Physiology. 2001;21:428–436. doi: 10.1046/j.1365-2281.2001.00353.x. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Arousal systems and attentional processes. Biological Psychology. 1997;45:57– 71. doi: 10.1016/s0301-0511(96)05222-2. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Bates JE. Temperament. In: Damon W, Eisenberg N, editors. Handbook of child psychology: Vol. 3. Social, emotional and personality development. 5. Hoboken, NJ: Wiley; 1998. pp. 105–176. [Google Scholar]
- Rothbart MK, Derryberry . Development of individual differences in temperament. In: Lamb M, Brown A, editors. Advances in Developmental Psychology. Vol. 1. Hillsdale, NJ: Erlbaum; 1981. pp. 37–86. [Google Scholar]
- Rubin KH, Stewart SL, Coplan RJ. Social withdrawal in childhood: Conceptual and empirical perspectives. In: Ollendick TH, Prinz RJ, editors. Advances in clinical child psychology. Vol. 17. New York: Plenum Publishing; 1995. pp. 157–196. [Google Scholar]
- Schore AN. Affect regulation and the origin of self: The neurobiology of emotional development. Hillsdale: NJ: Erlbaum Associates, Inc; 1994. [Google Scholar]
- Schuetze P, Eiden RD. The association between maternal smoking and secondhand exposure and autonomic functioning at 2–4 weeks of age. Infant Behavior & Development. 2006;29:32–43. doi: 10.1016/j.infbeh.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Schuetze P, Eiden RD, Coles CD. Prenatal cocaine use and other substance exposure: Effects on infant autonomic regulation at 7-months of age. Developmental Psychobiology. 2007;49:276–289. doi: 10.1002/dev.20215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetze P, Eiden RD, Danielewicz S. The association between prenatal cocaine exposure and physiological regulation at 13 months of age. Journal of Child Psychology and Psychiatry. 2009;50:1401–1409. doi: 10.1111/j.1469-7610.2009.02165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetze P, Lopez F, Granger DA, Eiden RD. The association between prenatal cigarette exposure and cortisol reactivity at 7 months of age. Developmental Psychobiology. 2008;50:819–834. doi: 10.1002/dev.20334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifer R, Sameroff AJ, Barrett LC, Krafchuk E. Infant temperament measured by multiple observations and mother-report. Child Development. 1994;65:1478–1490. doi: 10.1111/j.1467-8624.1994.tb00830.x. [DOI] [PubMed] [Google Scholar]
- Silvestri JM, Long JM, Weese-Mayer DE, Barkov GA. Effect of prenatal cocaine on respiration, heart rate and sudden infant death syndrome. Pediatric Pulmonology. 1991;11:328–334. doi: 10.1002/ppul.1950110409. [DOI] [PubMed] [Google Scholar]
- Singer L, Arendt R, Minnes S, Farkas K, Yamashita T, Kliegman R. Increased psychological distress in post-partum, cocaine-using mothers. Journal of Substance Abuse. 1995;7:165–174. doi: 10.1016/0899-3289(95)90002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer LT, Arendt R, Farkas K, Minnes S, Huang J, Yamashita T. The relationship of prenatal cocaine exposure and maternal psychological distress to child development. Development & Psychopathology. 1997;9:473–489. doi: 10.1017/s0954579497001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell MB, Sobell LC, Klajner F, Pavan D, Basian E. The reliability of a timeline method for assessing normal drinker college students’ recent drinking history. Addictive Behaviors. 1986;11:149–161. doi: 10.1016/0306-4603(86)90040-7. [DOI] [PubMed] [Google Scholar]
- Stevens J. Applied Multivariate Statistics for the Social Sciences. Lawrence Erlbaum Associates; Hillsdale, NJ: 1986. [Google Scholar]
- Stifter CA, Braungart JM. The regulation of negative reactivity in infancy: Function and development. Developmental Psychology. 1995;31:448–455. [Google Scholar]
- Stifter CA, Corey JM. Vagal regulation and observed social behavior in infancy 2001 [Google Scholar]
- Stifter CA, Fox NA. Infant reactivity: Physiological correlates of newborn and 5- month temperament. Developmental Psychology. 1990;26:582–588. [Google Scholar]
- Stifter CA, Spinrad TL, Braungart-Rieker JM. Toward a developmental model of child compliance: The role of emotion regulation in infancy. Child Development. 1999;70:21–32. doi: 10.1111/1467-8624.00003. [DOI] [PubMed] [Google Scholar]
- Streissguth AP. Intrauterine alcohol and nicotine exposure: Attention and reaction time in 4- year-old children. Developmental Psychology. 1984;20:533–541. [Google Scholar]
- Suess PA, Porges SW, Plude DJ. Cardiac vagal tone and sustained attention in school- aged children. Psychophysiology. 1994;31:17–22. doi: 10.1111/j.1469-8986.1994.tb01020.x. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. 4. Heedham Heights, MA: Allyn & Bacon; 2001. [Google Scholar]
- Tucker DM, Williamson PA. Asymmetric neural control systems in human self-regulation. Psychological Review. 1984;91:185–215. [PubMed] [Google Scholar]
- Ungerer JA, Dolby R, Waters B, Barnett B, Kelk N, Lewin V. The early development of empathy: Self-regulation and individual differences in the first year. Motivation and Emotion. 1990;14(2):93–106. [Google Scholar]
- Van Goozen SH, van den Ban E, Matthys W, Cohen-Kettenis PT, Thijssen JH, van Engeland H. Hypothalamic-pituitary-adrenal axis and autonomic nervous system activity in disruptive children and matched controls. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:1438–1445. doi: 10.1097/00004583-200011000-00019. [DOI] [PubMed] [Google Scholar]
- Wilson AC, Lengua LJ, Tininenko J, Taylor A, Trancik A. Physiological profiles during delay of gratification: Associations with emotionality, self-regulation, and adjustment problems. Journal of Applied Developmental Psychology. 2009;30:780–790. doi: 10.1016/j.appdev.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods NS, Eyler FD, Behnke M, Conlon M. Cocaine use during pregnancy: Maternal depressive symptoms and infant neurobehavior over the first month. Infant Behavior & Development. 1993;16:83–98. [Google Scholar]