Abstract
Neural development in metazoans is characterized by the establishment of initial process tracts by pioneer axons and the subsequent extension of follower axons along these pioneer processes. Mechanisms governing the fidelity of follower extension along pioneered routes are largely unknown. In C. elegans, formation of the right angle-shaped lumbar commissure connecting the lumbar and preanal ganglia is an example of pioneer/follower dynamics. We find that the dystroglycan ortholog DGN-1 mediates the fidelity of follower lumbar commissure axon extension along the pioneer axon route. In dgn-1 mutants, the axon of the pioneer PVQ neuron faithfully establishes the lumbar commissure, but axons of follower lumbar neurons, such as PVC, frequently bypass the lumbar commissure and extend along an oblique trajectory directly toward the preanal ganglion. In contrast, disruption of the UNC-6/netrin guidance pathway principally perturbs PVQ ventral guidance to pioneer the lumbar commissure. Loss of DGN-1 in unc-6 mutants has a quantitatively similar effect on follower axon guidance regardless of PVQ axon route, indicating that DGN-1 does not mediate follower/pioneer adhesion. Instead, DGN-1 appears to block premature responsiveness of follower axons to a preanal ganglion-directed guidance cue which mediates ventral-to-anterior reorientation of lumbar commissure axons. Deletion analysis shows that only the most N-terminal DGN-1 domain is required for these activities. These studies suggest that dystroglycan modulation of growth cone responsiveness to conflicting guidance cues is important for restricting follower axon extension to the tracts laid down by pioneers.
INTRODUCTION
In both ecdysozoans and vertebrates, axon tract formation is characterized by two common features. First, pioneer neurons lay down initial axon tracts in an orthogonal scaffold by interpreting guidance cues in the environment to orient extension of their axons with respect to the body axes and to execute turns. Second, later-arising follower axons fasciculate with and extend along the pioneer tracts to navigate to their own target areas (Durbin, 1987; Harrison, 1910; Mastick and Easter, 1996; Thomas et al., 1984; Wilson et al., 1990). This temporal distinction between pioneer and follower is reinforced by morphological differences between the growth cones of the two classes, with pioneer axons displaying greater specialization and more filopodia (Kim et al., 1991; Lopresti et al., 1973; Nordlander, 1987; Yaginuma et al., 1991). These differences led to the proposal that pioneer axons serve as pathfinders which form a “labeled pathway” to direct follower guidance (Raper et al., 1984).
However, the dependence of follower axons on pioneer axons for proper navigation is variable. In some instances follower axons frequently stall at turning points or obstacles in the absence of pioneer axons (Durbin, 1987; Ghosh et al., 1990; Klose and Bentley, 1989; Raper et al., 1984). However, many examples demonstrate that follower axons can be capable of navigating to their targets in the absence of normal pioneer tracts (Bak and Fraser, 2003; Chitnis and Kuwada, 1991; Keshishian and Bentley, 1983). An interesting system illustrating both behaviors is provided by metathoracic limb development in the grasshopper, where the pioneer Ti1 axon is required for extension of SGO axons across the tibia-femur boundary, but is dispensable for extension of axons arising in leg segments proximal to the boundary but following the same route as the Ti1 axon (Keshishian and Bentley, 1983; Klose and Bentley, 1989). These observations suggest that follower axons may in general be competent to respond to the same guidance cues utilized by pioneer axons to navigate across untrammeled ground, but that in challenging environments follower axons may be constrained to display strong reliance on the pioneer axon to ensure fidelity of the route taken. This model leads to the prediction that specific mechanisms exist to modulate the ability of follower axons to respond to primary guidance cues independently of pioneer axons.
In C. elegans, an example of a pioneer-follower relationship is the development of the lumbar commissures, bilateral axon tracts through which several neurons in the bilateral posterior lumbar ganglia project axons ventrally and anteriorly into the preanal ganglion and ventral nerve cord (Hall and Russell, 1991; White et al., 1986). The PVQ neurons pioneer the lumbar commissures and are important in extension of follower lumbar axons through the commissures (Durbin, 1987). Proper guidance of axons through the lumbar commissure requires the guidance cue UNC-6/netrin, although the relationship of netrin signaling to PVQ pioneering is unclear (Ren et al., 1999). Here, we extend these studies by showing that the C. elegans dystroglycan ortholog DGN-1 plays an important role in follower axon guidance through the lumbar commissure.
In vertebrates, dystroglycan (DG) functions as a key basement membrane receptor in skeletal muscle and in glia. Dystroglycan binds several widely-expressed basement membrane components such as laminin α chains, perlecan and agrin (Michele and Campbell, 2003). Genetic ablation of dystroglycan in muscle leads to muscular dystrophy (Cohn et al., 2002). Loss of glial dystroglycan results in disruption of the pial basement membrane and of neural migrations in the brain (Moore et al., 2002; Satz et al., 2010), and defects in myelination and nodal architecture in peripheral nerves (Saito et al., 2003). Dystroglycan also plays an important role in polarization of some epithelial cells in response to the basement membrane (Muschler et al., 2002). In central nervous system neurons, dystroglycan localizes to postsynaptic specializations and plays a role in synaptic plasticity (Satz et al., 2010; Zaccaria et al., 2001), possibly through interaction with the neuronal surface protein neurexin (Sugita et al., 2001).
In C. elegans, DGN-1 is expressed in epithelia and neurons, and dgn-1 null mutants display defects in epithelial morphogenesis, motoneuron axon guidance and neuroanatomic maintenance (Johnson et al., 2006; Johnson and Kramer, submitted). We report here that DGN-1/dystroglycan functions in lumbar commissure formation to constrain follower axon guidance to the trajectory taken by the pioneer PVQ axon. DGN-1 does not appear to mediate the adhesion of pioneer and follower axons, but rather suppresses the ability of follower axons to respond prematurely to turning cues in a pioneer-independent manner.
METHODS
Strains used
Culture and manipulation C. elegans were performed according to standard methods (Brenner, 1974). All strains were maintained at 20°C for phenotype analysis. The wild-type strain N2 var. Bristol and the following reference null mutant alleles were used: unc-40(e1430) I; lin-44(n1792) I; cwn-1(ok546) II; unc-5(e53) IV; egl-20(n585); cwn-2(ok895) IV; sax-3(ky123) X; unc-6(ev400) X; dgn-1(cg121) X; and slt-1(eh15) X. The dgn-1(cg121) allele was maintained as a heterozygote balanced by the visible marker qIs54 [myo-2p::GFP, pes-10p::GFP, gut promoter::GFP] X as previously described (Johnson et al., 2006). The following reporter transgenes were used for phenotype analysis of the indicated neuron(s): ALM and PLM: zdIs5[mec-4p::GFP] I; DA9: trIs30[him-4p::Mb::YFP; hmr-1bp::DsRed2; unc-129snp::DsRed2] I; PHA, PHB and PHC: inIs179[ida-1p::GFP] II; PVQ: hdIs26[odr-2p::CFP, sra-6p::DsRed2] III; PVC: rhIs4[glr-1p::GFP, dpy-20(+)] III; PVT: otIs7[zig-2p::GFP] III; PQR: adEx1295[gcy-32p::GFP, lin-15(+)]. All mutant and transgenic marker stains except for dgn-1(cg121) were obtained from the Caenorhabditis Genetics Center (Univ. of Minnesota).
dgn-1(+) and deletion mutant transgenes
The following heterologous promoter regions were used (basepair numbers are relative to ATG start codon as identified in Wormbase): unc-119p, −1671 bp to −6 bp (Altun-Gultekin et al., 2001; Hardin et al., 2008; Maduro and Pilgrim, 1995); unc-33p, −2746 bp (Altun-Gultekin et al., 2001); unc-14p, −1398 bp (Ogura et al., 1997); rgef-1p, −3440 bp (Altun-Gultekin et al., 2001); aex-3p, −1320 bp (Iwasaki et al., 1997); rab-3p, −1343 bp (Nonet et al., 1997); dpy-7p, −303 bp (Gilleard et al., 1997); lin-26FGHip, −4000 bp (Landmann et al., 2004); ajm-1p, −6325 bp to −830 bp of ajm-1e isoform ATG (Hardin et al., 2008; Koppen et al., 2001). Transgene constructs of dgn-1(+) and dgn-1 deletion mutants under heterologous promoters were generated in the plasmid pBJ230, derived from pPD30.38 by replacing the unc-54 promoter region (nt 25-985 of pPD30.38) with tandem NotI and AscI sites by mutagenic PCR using Phu polymerase (Finnzyme). Heterologous promoter regions were amplified from N2 genomic DNA or from plasmid pCB101.2 (rgef-1p) with Phu polymerase using primers that introduced 5′ NotI and 3′ AscI sites, A/T-cloned into pGEM-T (Promega), and subcloned as NotI-AscI fragments into NotI/AscI-digested pBJ230. The coding region of the dgn-1(+) genomic DNA was amplified from plasmid pJK600 with Phu polymerase using sense primer NheIdgn1F (5′-GCTAGCATGCGTCTCATTTTCCTGGT-3′), introducing a 5′ NheI site immediately before the ATG start codon in exon 2, and antisense primer KpnIdgn1R (5′-GGTACCTTAAGGAGGAATGAATGGAG-3′), introducing a 3′ KpnI sites immediately after the TAA stop codon in exon 6. This PCR product was A/T-cloned into pGEM-T and subcloned as a NheI-KpnI fragment into pBJ230-derived plasmids carrying heterologous promoters.
Deletion mutations of dgn-1 were generated in pBJ195, a plasmid containing the coding region of dgn-1(+), by mutagenic PCR using Phu polymerase. For DGN-1pat-3TM, mutagenic primers were used which replaced the coding sequence for the DGN-1 transmembrane domain (aa 474-496) with the coding sequence for the PAT-3 transmembrane domain (aa 738-760 of PAT-3). Deletion mutant coding regions flanked by 5′ NheI and 3′ KpnI sites were amplified using Phu polymerase and subcloned as NheI-KpnI fragments into pBJ236, a pBJ230-derived plasmid carrying unc-119p. The same sense primer Nhedgn1F was used for all deletions. The following antisense primers were used: for DGN-1ΔCyto and DGN-1ΔCoreΔCyto, KpnIdgn1delR (5′-AAGGTACCTTATTTCTTGATACAAGCAC-3′); for DGN-1Nterm, KpnIdgn1delTMcytoR (5′-AAGGTACCTTAGTCAGCTTCTTGAATTGA-3′); for all other constructs, primer KpnIdgn1R. The coding regions of wild-type and deletion mutant dgn-1 constructs were verified by DNA sequencing.
Transgene plasmid were introduced by germline injection (Mello et al., 1991) into N2 animals, and stable extrachromosomal arrays were mated into the dgn-1(cg121) background. Transgene plasmids were injected at 25 μg/ml with 75 μg/ml pRF4 (Mello et al., 1991) and 10μg/ml pPD122.45 [myo-2p::GFP-NLS(nuclear localization signal)] as visible co-injection markers.
Phenotype analysis
Trajectories of PVQ and PVC axons between the lumbar ganglion and the preanal ganglion were scored in young adult animals carrying hdIs26 rhIs4 (red PVQ, green PVC) using a Zeiss Axiophot microscope equipped for epifluorescence. PVQ and PVC trajectories were scored as “lumbar commissure” (distinct right angle trajectory near the anus, with discrete ventral and anterior legs leading into the preanal ganglion), “oblique” (direct trajectory to preanal ganglion with no distinct ventral and anterior legs) or “lateral” (lateral and anterior trajectory, no entry into preanal ganglion). PVQ and PVC axons were also assessed for possible defects in anterior guidance in the lumbar commissure: posterior-directed axon (i.e., reversal of direction), extra posterior-directed axon branch, or early termination of axon extension. Animals were scored only if both PVQ and PVC axons trajectories on each side could be assessed, in order to compare ipsilateral and contralateral PVQ/PVC pairs. Guidance of PHA/B/C (inIs179), PQR (adEx1295) and DA9 (trIs30) axons were scored similarly using the indicated markers, except that for PHA/B/C the identity of a misguided axon could not be unambiguously determined, so the PHA/B/C set on a side was scored as misguided if any of the three axons were misguided. ALM and PLM axons were scored as “lateral” (axon extended anteriorly along lateral surface) or “ventralized” (axon initially extended anteriorly but diverted ventrally into ventral nerve cord) using young adult animals carrying the zdIs5 marker observed on a Leica MZ16 dissecting microscope equipped for epifluorescence.
RESULTS
Defects in guidance of follower lumbar commissure axons in dgn-1 null mutants
The bilateral lumbar ganglia of C. elegans lie in lateral positions dorsal to the rectum/anus (Hall and Russell, 1991; White et al., 1986). Several lumbar neurons, such as PVQ and PVC, project axons into the preanal ganglion and ventral nerve cord (VNC) through the bilateral lumbar commissures, which display a characteristic right angle shape with distinct ventral- and anterior-directed legs (Fig. 1A, top panel). The PVQ axons pioneer the guidance of follower lumbar axons through the lumbar commissures. Independently, the preanal ganglion motoneurons DA8 and DA9 pioneer the left and right lumbar commissures, respectively, projecting axons into the dorsal nerve cord (Durbin, 1987).
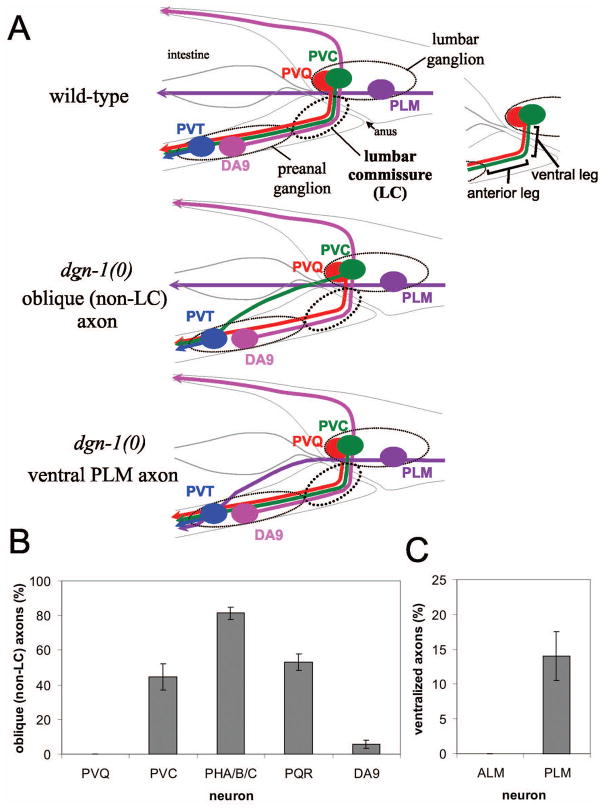
Figure 1. Defects in lumbar ganglion neural guidance in dgn-1 mutants.
(A) Several lumbar ganglion neurons, including PVQ (red) and PVC (green), project axons into the preanal ganglion through the lumbar commissure (LC), which has distinct ventral and anterior legs. The PLM (purple) axon in contrast projects anteriorly at a lateral position. The preanal ganglion neurons DA8 (not shown) and DA9 (pink) project axons dorsally through the left and right lumbar commissures, respectively; for convenience, DA9 is shown on the left in this schematic (upper panel). In dgn-1 null mutants, follower LC axons such as PVC sometimes fail to enter the lumbar commissure, instead taking an oblique route toward the preanal ganglion (middle panel), and PLM axons are occasionally directed ventrally toward the preanal ganglion (lower panel). (B) Oblique (non-LC) defects of specific lumbar commissure axons in dgn-1(cg121) null mutants is shown. For PHA/B/C, one or more of three axons per side could be defective (see Materials and Methods). (C) Ventralization of PLM axons in dgn-1(cg121). In B and C, percent of axons with guidance defects and the standard error of the proportion (error bars) is shown; N=100–232 axons scored for each neuron type.
Null mutants of dgn-1 display a defect in the guidance of several follower lumbar commissure axons (PVC, PHA/B/C and PQR) in which approximately 40% or more of axons bypass the lumbar commissure and instead follow oblique paths directly toward the preanal ganglion (Fig. 1A, middle panel; Fig. 2A–K). In contrast, the PVQ pioneer axons in dgn-1(cg121) animals always execute the right angle ventral-to-anterior turn characteristic of the lumbar commissure (Figs. 1B and 2J). Similarly, the DA9 axon almost always extends through the right lumbar commissure in dgn-1 mutants (Fig. 1B). Thus, pioneer axon guidance through the lumbar commissures is largely normal in dgn-1(cg121) mutants, whereas the fidelity of follower axon migration is compromised.
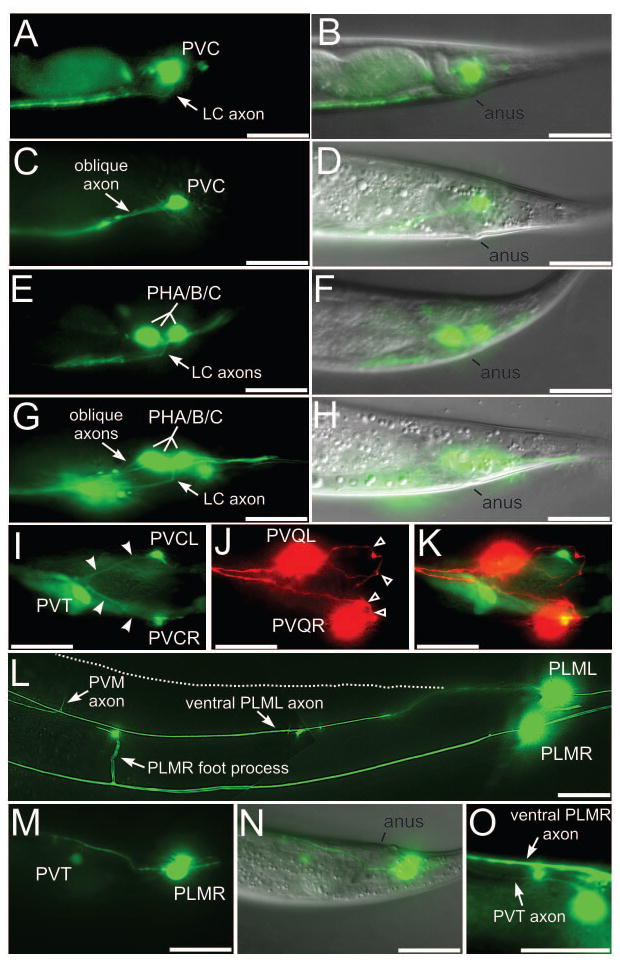
Figure 2. Axon guidance defects in lumbar neurons of dgn-1 mutants.
(A–D) Lateral views of lumbar commissure PVC (A) or PHA/B/C (E) axons in wild-type animals, and of oblique PVC (C) or PHA/B/C (G) axon trajectories in dgn-1(cg121) animals. One of the three PHA/B/C axons extends normally through the lumbar commissure in G. Overlays with the corresponding DIC images in (B,D,F,H) marking position of the anus. (I–K) Ventral view of a dgn-1(cg121) animal carrying markers for PVC (green, I), PVQ (red, J) and the preanal ganglion neuron PVT (green, I). Oblique PVC axons (I, filled arrowheads) extend directly toward the preanal ganglion, marked by PVT. The pioneer PVQ axons always traverse the lumbar commissure (J, open arrowheads) in dgn-1 mutants. K, overlay of I and J. In dgn-1(cg121), lumbar neuron cell bodies can be displaced anterior of their normal position, e.g. PVQL in J. (L) PLML axon in dgn-1(cg121) misrouted into the ventral nerve cord (marked by the entering PVM axon and the ventral cord foot process of PLMR). Normal lateral course of PLML is indicated (dotted line). In contrast the PLMR axon follows the normal lateral trajectory. (M–O) Subventral view of a dgn-1(cg121) animal carrying markers for PLM and PVT (green, M) and overlay with the DIC image (N), showing the misguided PLMR axon entering the ventral cord in the preanal ganglion region between PVT and the anus. In a different focal plane (O), the PVT and PLMR axons can be seen running parallel in the ventral cord. Bars: 20 μm.
We note a distinct lumbar neuron phenotype of dgn-1 mutants in which the cell bodies of some lumbar neurons are displaced anterior of their normal positions (e.g., PVQL in Fig. 2J) due to a failure of positional maintenance during late embryonic and larval stages. Lumbar neuron cell bodies are positioned normally in 3-fold dgn-1 mutant embryos. The displacement defects thus accumulate after axon guidance through the lumbar commissure is complete (Durbin, 1987). Moreover, PVQ cell bodies show a high penetrance of displacement (~65% by adult stage) despite the fact that their axons always utilize the lumbar commissure, indicating that cell body displacement and axon guidance defects are independent. This cell body displacement phenotype will be described in a separate report (Johnson and Kramer, submitted).
Ventralization of PLM axons in dgn-1 null mutants
The bilateral PLM mechanosensory neurons lie in the lumbar ganglia and extend axons anteriorly at a lateral level (Fig. 1A, top panel) along the dorsal edges of the ventral muscle quadrants (White et al., 1986). In dgn-1(cg121) mutants, 14% of PLM axons are misguided into the ventral nerve cord (Fig. 1A, lower panel; Fig. 1C; Fig. 2L–O). Ventrally misguided PLM axons enter the VNC anterior to the anus and at or posterior to the most anterior preanal ganglion neuron PVT (identified by the otIs7 marker) in 97% (N=30) of cases, indicating that ventralized PLM axons are targeted toward the preanal ganglion. In contrast, the axons of the non-lumbar ALM mechanosensory neurons extend anteriorly at a normal lateral position in dgn-1 null animals. (Fig. 1C). Thus, dgn-1 mutants display abnormal axon trajectories toward the preanal ganglion even in lumbar ganglion neurons which do not normally transit the lumbar commissure or enter the preanal ganglion.
dgn-1 is expressed transiently in lumbar ganglion neurons during embryogenesis
We have previously reported that dgn-1 is expressed broadly in ectodermal cells in pre-morphological embryos (Johnson et al., 2006). We further observe that dgn-1 is expressed in lumbar ganglion neurons by the comma stage of embryogenesis (Fig. 3A–D), just before the beginning of lumbar axogenesis (Durbin, 1987). Embryonic expression of dgn-1 in lumbar neurons is transient, although residual expression can be seen in some lumbar neurons in early larvae (Fig. 3E,F), by which time embryonic stage axon extension has concluded.
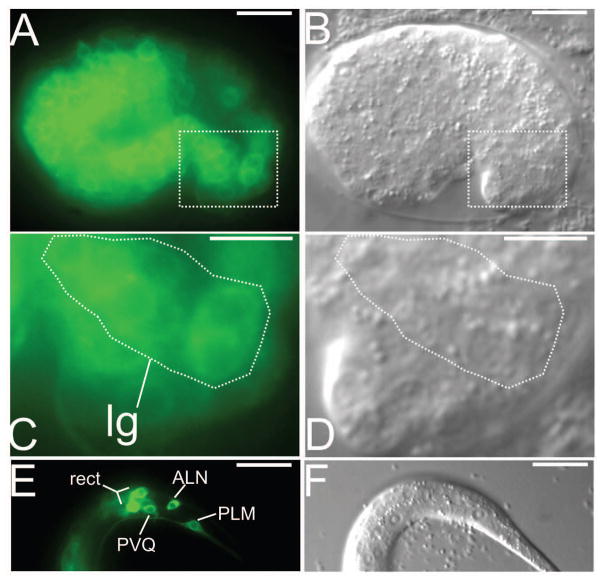
Figure 3. Embryonic expression of dgn-1 in lumbar ganglion neurons.
(A) In comma stage embryos, a dgn-1p::GFP transcriptional reporter is expressed in several lumbar ganglion (lg) neurons. (B) Overlay of A with corresponding differential interference contrast (DIC) image. (C,D) Enlargement of boxed regions in A,B. The lumbar ganglion neurons are the most posterior neurons (identified by a stippled appearance under DIC) in the embryo. (E) Expression of dgn-1p::GFP can still be observed in early L1 stage animals in some lumbar neurons (PVQ, PLM, ALN) as well as in the rectal gland cells (rect). (F) DIC image of E. Bar: 20 μm (A,B,E,F) or 10 μm (C,D).
Neuroblast/early neuronal role for DGN-1 in lumbar ganglion axon guidance
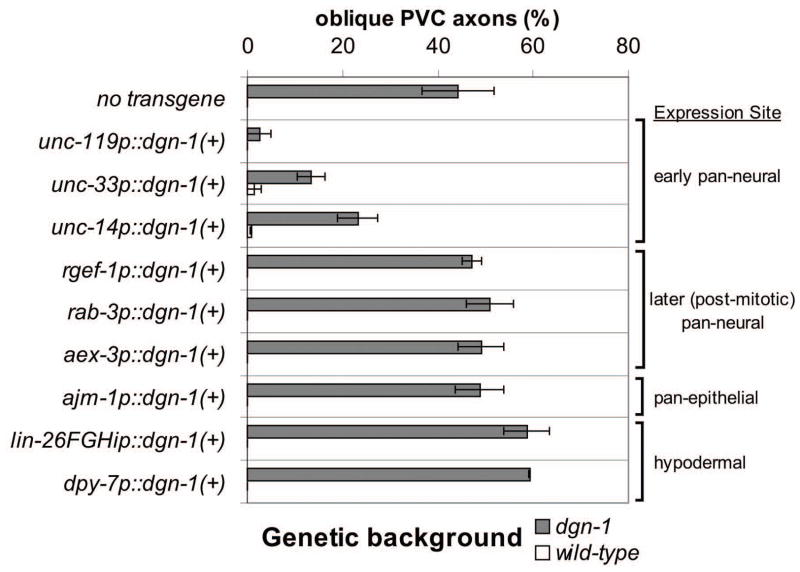
Expression of dgn-1 in lumbar neurons during axon extension suggests that DGN-1 function is required in these neurons. However, axons in C. elegans extend between hypodermal (epidermal) cells and their subjacent basement membrane (White et al., 1986), and expression of dgn-1 is also observed in hypodermal cells (Johnson et al., 2006). We re-expressed the coding region of dgn-1(+) under the regulatory regions (“promoters”) of various neurally- or hypodermally-expressed genes in dgn-1(cg121) animals to determine the site of DGN-1 function. DGN-1 rescues PVC axon guidance when expressed under the promoters of multiple genes (unc-119, unc-33, unc-14) which are expressed broadly in neuroblasts and post-mitotic neurons and whose functions are required for axogenesis (Fig. 4). Transgenic dgn-1 mutants carrying these constructs show significantly lower lumbar commissure guidance defects for PVC (p<0.0005 for unc-119, unc-33 or unc-14 promoter constructs; two-tailed Z test). DGN-1 expression under these promoters also rescues PLM axon guidance (not shown). No significant reduction of PVC guidance defects is seen when re-expressing DGN-1 under promoters of genes (rgef-1, aex-3, and rab-3) showing broad neural expression later in post-mitotic neurons and which are involved in synaptic function in mature neurons (Fig. 4; p > 0.10 for rgef-1, aex-3, or rab-3 promoter constructs). A caveat of these studies is that the early-expressing neuroblast/neural promoters such as unc-119p which drive rescuing expression of dgn-1(+) are not exclusively active in neural lineage cells in early embryos, but can also be active in hypodermal lineage cells (Hardin et al., 2008). However, re-expression of DGN-1 under hypodermis-specific promoters (dpy-7p, lin-26FGHip) or broadly expressed epithelial promoters (ajm-1p) fails to rescue (Fig. 4). Transgenic dgn-1 mutants carrying the ajm-1p construct do not show significantly lower lumbar commissure guidance defects for PVC (p>0.71), while transgenic dgn-1 mutants carrying the dpy-7p or lin-26FGHip constructs show slightly greater PVC guidance defects (p<0.0027 for either promoter construct). These three hypodermal or pan-epithelial promoters are active in hypodermal lineage cells in pre-morphological (<400 min after first cleavage) embryos (lin-26FGHip) or in comma stage (~400 min) embryos (dpy-7p, ajm-1p), well before lumbar axogenesis is occurring (~500 min). We conclude that DGN-1 function is required in neuroblasts or early in post-mitotic neurons, by the start of axogenesis, to ensure correct axon guidance.
Figure 4. Rescue of lumbar commissure guidance in dgn-1 mutants by early neural expression of dgn-1(+).
PVC oblique guidance defects were scored in dgn-1(cg121) or wild-type animals carrying an extrachromosomal array containing dgn-1(+) transgenes driven by the indicated heterologous promoters. Average and range (error bars) of two independent extrachromosomal array lines is shown. N=100–138 axons scored for each transgene line in each genetic background. Some averages or ranges are 0 and no bar is visible.
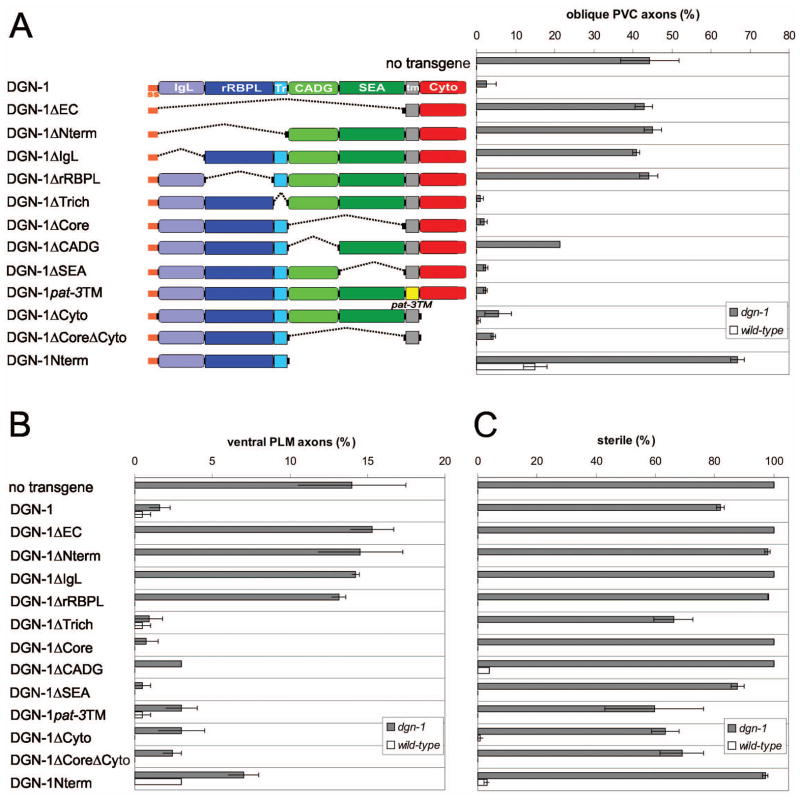
DGN-1 function in lumbar neuron guidance requires a membrane-anchored N-terminal domain
Like vertebrate dystroglycan, DGN-1 contains a central DG (dystroglycan) core motif composed of an amino-terminal cadherin-repeat like CADG domain (Dickens et al., 2002) and a carboxy-terminal SEA domain (Akhavan et al., 2008). In both vertebrate dystroglycan and DGN-1, the DG core is flanked by an amino-terminal region comprised of immunoglobulin-like (Ig-like) and ribosomal RNA binding protein-like (rRBP-like) domains (Bozic et al., 2004), and carboxy-terminal transmembrane and cytoplasmic domains (Johnson et al., 2006). Transgenic expression of deletion mutants of DGN-1 under the unc-119 promoter in dgn-1(cg121) animals reveals that robust DGN-1 function in lumbar axon guidance requires an intact, membrane-anchored N-terminal domain (Fig. 5). Transgenic constructs expressing intact DGN-1 or DGN-1 deletions retaining both an intact N-terminal domain and a transmembrane anchor significantly reduce the level of PVC guidance defects in dgn-1 mutants (p<0.0015 for any such construct). Transgenic constructs expressing DGN-1 deletions lacking an intact N-terminal domain do not show a significant reduction in PVC guidance defects in dgn-1 mutants (p>0.36 for any such construct). A secreted N-terminal domain (DGN-1Nterm) lacking a transmembrane anchor also fails to rescue and in fact shows enhanced PVC guidance defects in dgn-1 mutants (p<0.0002) and in wild-type animals. Similar results are seen for rescue of PLM guidance defects (Fig. 5), although intriguingly the secreted N-terminal domain shows marginal but not statistically significant (p>0.05) rescue of PLM guidance, suggesting PLM guidance may not strictly require membrane anchorage of DGN-1. A minimal construct retaining only the N-terminal domain and the transmembrane domain (DGN-1ΔCoreΔCyto) affords almost complete rescue of both PVC and PLM guidance. This construct also contains a short threonine-rich region of DGN-1 at the end of the N-terminal domain (Johnson et al., 2006); however, the T-rich region per se is not required. The primary sequence of the transmembrane domain is not essential, since replacement of the native DGN-1 transmembrane domain with that of PAT-3/β–integrin (Gettner et al., 1995) affords robust rescue.
Figure 5. Function of DGN-1 structural domains in axon guidance and gonadogenesis.
A series of DGN-1 domain deletion mutants (A, left panel) was expressed transgenically in dgn-1(cg121) or wild-type animals under the control of the early neural promoter unc-119p. Transgenic animals were scored for oblique PVC axons (A, right panel), ventralized PLM axons (B), and sterility (C). Average and range (error bars) of two independent extrachromosomal array lines is indicated. N=100–150 axons (A,B) or N=50–75 animals (C) scored for each transgene line in each genetic background.
Surprisingly, we observe that unc-119-driven DGN-1 expression partially rescues the defect in gonad formation and consequent sterility of dgn-1(cg121) animals previously described (Johnson et al., 2006). In contrast to axon guidance, DGN-1 function in gonad formation requires both the N-terminal region and the CADG domain of the DG core region, in the context of a membrane-anchored protein (Fig. 5C). Partial rescue of fertility is also observed when wild-type DGN-1 expression is driven by other early pan-neural promoters (unc-33, unc-14) but not with later pan-neural or with hypodermal/epithelial promoters (not shown). These promoters likely drive transient expression of DGN-1 in some cell type in early embryos relevant to gonad formation, most likely the somatic gonad precursors Z1 and Z4 in which dgn-1(+) is normally expressed (Johnson et al., 2006). DGN-1 function may be required early in these cells, or perdurance of transiently expressed DGN-1 may mediate weak rescue of gonadogenesis/sterility later in embryogenesis. The unc-119 promoter is known to drive early embryonic expression in non-neural cells (Hardin et al., 2008), and both unc-33 and unc-14 have roles in some non-neural cells (Branda and Stern, 2000). The fact that rescue of sterility depends on elements of both the N-terminal and DG core regions, whereas rescue of axon guidance requires only the N-terminal domain, indicates the existence of tissue-specific interaction partners of dystroglycan in C. elegans.
Distinct roles for DGN-1 and the netrin pathway in lumbar commissure guidance
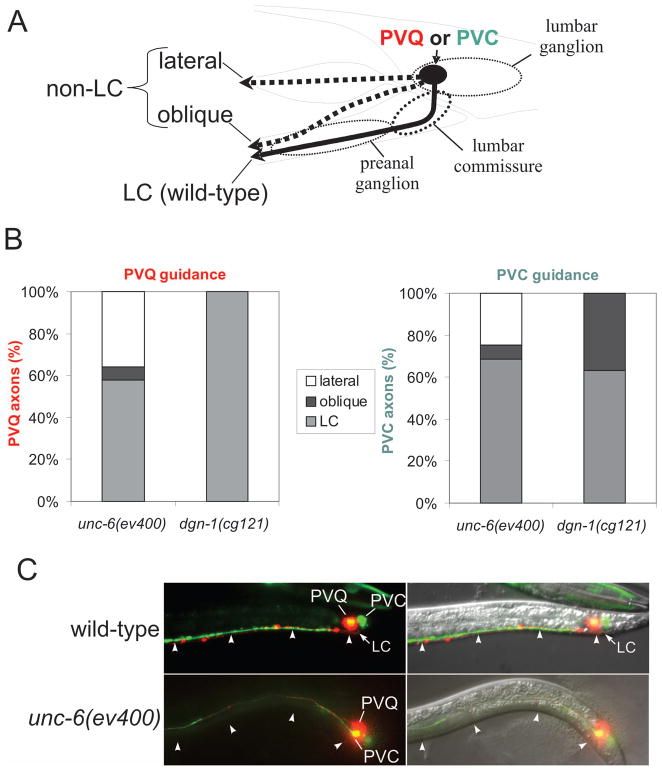
The UNC-6/netrin dorsal/ventral (D/V) guidance system has been implicated previously in lumbar commissure guidance (Ren et al., 1999). In unc-6(ev400) null mutants, defects in lumbar commissure guidance are seen in both the pioneer PVQ axon and the follower PVC axon (Fig. 6). Few of the misguided PVQ (14%, N=84) or PVC (22.2%, N=63) axons in unc-6 mutants migrate along an oblique course to the preanal ganglion; most misguided lumbar axons in unc-6(ev400) instead show complete loss of ventral guidance, migrating anteriorly at a lateral position. In contrast, in dgn-1 null mutants PVQ axons always utilize the lumbar commissure, and all misguided PVC axons follow an oblique trajectory (Fig. 6). The unc-40(e1430) mutant, lacking the attractive netrin receptor UNC-40/DCC, shows lumbar commissure guidance defects of PVQ and PVC similar to those in unc-6(ev400) animals, whereas unc-5(e53) mutants lacking the repulsive netrin receptor UNC-5 show no defects in lumbar commissure guidance of PVQ or PVC (Table 1).
Figure 6. Distinct lumbar guidance defects in unc-6/netrin and dgn-1/dystroglycan mutants.
(A) PVQ and PVC axons were scored for normal transit of the lumbar commissure (LC) or failure to transit the lumbar commissure by either following an oblique trajectory toward the preanal ganglion or by extending at a lateral position and failing to enter the preanal ganglion. (B) Distribution of PVQ and PVC axon trajectories in unc-6/netrin (N=200 axons for each neuron) and dgn-1/dystroglycan mutants (N=212 axons for each neuron). (C) Example of lateralized PVQ and PVC axons in unc-6/netrin mutants. In wild-type animals PVQ (red) and PVC (green) axons extend ventrally through the lumbar commissure (LC) and anteriorly along the ventral nerve cord at the ventral surface (arrowheads). In unc-6(ev400) netrin mutants, ventral guidance of PVQ and PVC axons sometimes fails completely, and axons extend directly anterior at a lateral position, away from the ventral surface (arrowheads).
Table 1.
Defects of trajectory of PVQ and PVC axons in guidance mutants.
| PVQa
|
PVCa
|
PVQ/PVCa
|
Nd
|
|||||
|---|---|---|---|---|---|---|---|---|
| total non-LCb
|
lateralb
|
obliqueb
|
total non-LCb
|
lateralb
|
obliqueb
|
A/P LC defectsc
|
||
| dgn-1 and D/V guidance factors | ||||||||
| dgn-1(cg121) | 0 | 0 | 0 | 36.8 | 0 | 36.8 | 0 | 212 |
| unc-6(ev400) dgn-1(cg121) | 61 | 21.5 | 39.5 | 68 | 12.5 | 55.5 | 0 | 200 |
| unc-6(ev400) | 42 | 36 | 6 | 31.5 | 24.5 | 7 | 0 | 200 |
| unc-40(e1430) | 32.4 | 30.3 | 2.1 | 14.1 | 11.1 | 3 | 0 | 234 |
| unc-5(e53) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 238 |
| sax-3(ky123) | 1.8 | 1.5 | 0.3 | 1.4 | 1.2 | 0.2 | 0 | 1448 |
| slt-1(eh15) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 420 |
| unc-6(ev400) slt-1(eh15) | 69.8 | 65.3 | 4.5 | 59.4 | 53 | 6.4 | 0 | 308 |
| unc-40(e1430); slt-1(eh15) | 48.3 | 45.3 | 3 | 20.8 | 16.1 | 4.7 | 0 | 236 |
| Wnts | ||||||||
| lin-44(n1792) | 0.5 | 0 | 0.5 | 0.5 | 0 | 0.5 | 0 | 208 |
| cwn-1(ok546) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 222 |
| egl-20(e585) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 216 |
| cwn-2(ok895) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 240 |
| lin-44; cwn-1 | 1.8 | 0 | 1.8 | 1.8 | 0 | 1.8 | 0 | 226 |
| lin-44; egl-20 | 0.9 | 0 | 0.9 | 0.9 | 0 | 0.9 | 0 | 234 |
| lin-44; cwn-2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 212 |
| cwn-1; egl-20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 216 |
| cwn-1; cwn-2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 236 |
| lin-44; cwn-1; egl-20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 152 |
| lin-44; cwn-1; cwn-2 | 0.4 | 0 | 0.4 | 0.4 | 0 | 0.4 | 0 | 236 |
Numbers designate percent of axons showing indicated defects.
PVQ and PVC axon trajectories scored for erroneous non-entry into the lumbar commissure (LC). Lateral: no ventral extension, axon extended anterior. Oblique: axon extended obliquely into preanal ganglion. Total non-LC: lateral + oblique.
PVQ and PVC axons scored for erroneous posterior turning or ectopic posterior branches along ventral midline.
Number of PVQ/PVC axon pairs scored.
In unc-6 null mutants only about 30–40% of PVQ or PVC axons fail to migrate ventrally into the lumbar commissure, suggesting the involvement of other D/V guidance pathways. The Slit/Robo pathway constitutes the second major D/V guidance system in C. elegans (Hao et al., 2001; Killeen and Sybingco, 2008). Slit null mutants, slt-1(eh15), show no defects in lumbar guidance of PVQ or PVC, although sax-3(ky123) animals lacking SAX-3/Robo show low penetrance defects (Table 1), suggesting a weak involvement of the Slit/Robo pathway in lumbar commissure guidance. Both unc-6(ev400) slt-1(eh15) and unc-40(e1430);slt-1(eh15) double mutants show increases in lumbar commissure guidance defects of both PVQ and PVC (Table 1), indicating that the Slit/Robo pathway plays a parallel, supportive role to the Netrin/DCC pathway. In all of these D/V guidance mutants, the majority of misguided axons display complete failure of ventral guidance, extending laterally rather than taking an oblique path to the preanal ganglion as seen in dgn-1 mutants (Table 1).
Pioneer-dependence of follower axon guidance in the lumbar commissure is largely independent of netrin
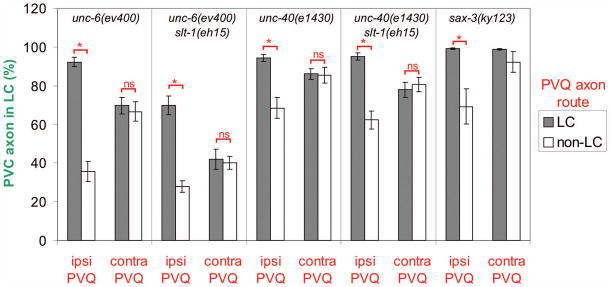
We analyzed the correlation of defects in pioneer PVQ and follower PVC guidance in the same animal using differential cell markers (Fig. 7). In unc-6(ev400) the PVC axon transits the lumbar commissure in only 35.7% (N=84) of cases where the ipsilateral PVQ axon fails to extend through the lumbar commissure, but does so in 92.2% (N=116) of cases where the ipsilateral PVQ axon migrated through the lumbar commissure. Thus, lumbar commissure guidance of the PVC axon is almost normal when that of the ipsilateral PVQ is normal, even in the absence of netrin. The fidelity of lumbar commissure guidance of PVC shows no dependence on the route of the contralateral PVQ axon, indicating that the PVQ pioneer facilitates follower axon guidance via a short-range mechanism. The contingency of lumbar commissure guidance of PVC on ipsilateral but not contralateral PVQ trajectory is also observed in unc-40(e1430), sax-3(ky123), unc-6(ev400) slt-1(eh15), and unc-40(e1430);slt-1(eh15) mutants (Fig. 7). Even in unc-6(ev400) slt-1(eh15), the PVC axon transits the lumbar commissure in 69.9% (N=93) of cases where the ipsilateral PVQ axon did so, despite the loss of both netrin and Slit guidance pathways.
Figure 7. Ipsilateral PVQ pioneer axon route is the major determinant of follower axon trajectory.
PVC axon route (LC or non-LC as in Fig. 6A) in netrin and Slit pathway mutants was correlated with the route taken by either the ipsilateral (ipsi PVQ) or the contralateral (contra PVQ) PVQ axon. Data analyzed by a two-tailed Z test: *, p < 0.002; ns, not significant. N=76–215 ipsilateral or contralateral PVC/PVQ pairs scored for either PVQ route (LC or non-LC) in each mutant background, except for sax-3(ky123), where N=1422 (LC PVQ) or N=26 (non-LC PVQ) ipsilateral or contralateral PVC/PVQ pairs. PVC vs. contralateral PVQ data for sax-3(ky123) could not be analyzed for significance because the small absolute number of non-LC PVC observed (2/26) fell below the recommended threshold for Z test application.
The correlation of PVQ and PVC axon trajectories persists in laterally misguided axons in unc-6 mutants. We examined unc-6(ev400) animals doubly marked for PVQ and PVC or for PVQ and PLM, for cases in which PVQ axons extended laterally. When the ipsilateral PVC is also misguided along a lateral trajectory, the misguided PVQ and PVC axons extend adjacent to one another at the light microscope level in 90% (N=50) of cases. In contrast only 2% (N=50) of PLM axons, which extend laterally even in unc-6(ev400) mutants, run adjacent to a lateral PVQ axon on the same side of the animal. This observation suggests that PVQ produces a cell surface or pericellular factor that specifically adheres to or attracts follower lumbar commissure axons like PVC, but not axons like PLM, which do not extend through the lumbar commissure.
DGN-1 functions in both pioneer-dependent and – independent lumbar commissure guidance
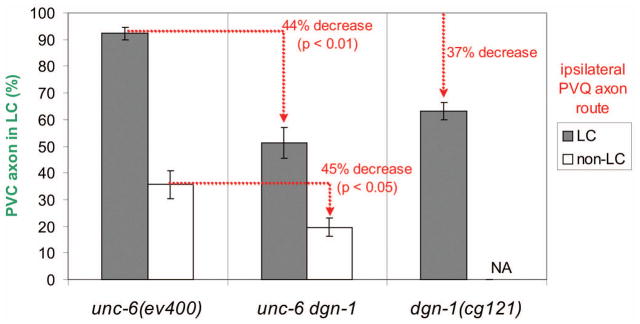
We examined the correlation of PVQ and PVC guidance in unc-6(ev400) dgn-1(cg121) double mutants (Fig. 8). Unlike in dgn-1(cg121) alone, the netrin mutant background presents cases in which the PVQ pioneer is misguided, allowing us to assess the effect of loss of DGN-1 on PVQ-dependent and – independent lumbar commissure guidance of PVC. If DGN-1 mediates PVQ-dependence of PVC guidance (e.g., via adhesion), we would expect loss of DGN-1 to reduce the fidelity of lumbar commissure guidance of PVC only when the ipsilateral PVQ transits the lumbar commissure. Indeed, in cases involving a lateralized PVQ, one might expect an improvement in the fidelity of lumbar commissure guidance of the ipsilateral PVC, through loss of a putative DGN-1 mediated adhesion to the misguided PVQ. However, we find that loss of dgn-1 function in the unc-6(ev400) background reduces the fidelity of lumbar commissure guidance of PVC by about 45% regardless of the ipsilateral PVQ route, an effect comparable to that of the dgn-1 mutation alone in an otherwise wild-type background. Moreover, the fidelity of lumbar commissure guidance of the PVC axon is still dependent on the route of the ipsilateral PVQ axon even in the absence of both netrin and DGN-1, indicating that these factors do not act in parallel in PVQ-dependent PVC guidance. Thus, DGN-1 does not appear to act as the mediator of PVQ dependence of follower axon guidance but instead helps ensure that follower lumbar axons execute the same lumbar commissure navigation program as the PVQ pioneer.
Figure 8. DGN-1 is required for both PVQ-dependent and PVQ-independent guidance of follower lumbar axons.
The fidelity of lumbar commissure guidance of PVC follower axons was correlated with the route (LC or non-LC) of the ipsilateral PVQ axon in unc-6(ev400), dgn-1(cg121) and unc-6 dgn-1 double mutants. Loss of dgn-1 in the unc-6 background reduces the fidelity of PVC guidance to a similar extent regardless of ipsilateral PVQ route. Average and standard error of the proportion (error bars) is shown. N= 78–212 ipsilateral PVC/PVQ pairs scored for either PVQ route (LC or non-LC) in each mutant background. NA, not applicable: dgn-1(cg121) shows no non-LC trajectories for PVQ.
Although the unc-6(ev400) dgn-1(cg121) double mutant shows an additive effect on PVC guidance, it displays an unexpected synergistic effect on PVQ guidance. Lumbar commissure guidance of the PVQ axon is normal in dgn-1(cg121). In unc-6(ev400) mutants 42% (N=200) of PVQ axons show defective lumbar commissure guidance, with only 14% (N=84) of misguided PVQ axons taking an oblique path to the preanal ganglion (Table 1). In contrast, in unc-6(ev400) dgn-1(cg121) double mutants 61% (N=200) of PVQ axons show defective lumbar commissure guidance, with 64.8% (N=122) of misguided PVQ axons taking an oblique path to the pre-anal ganglion. Thus, in the absence of netrin, PVQ shows similar defects due to loss of DGN-1 as seen for follower lumbar axons in an otherwise wild-type background, suggesting that strong netrin responsiveness by PVQ may override the need for dystroglycan function in the pioneer. The unc-6(ev400) dgn-1(cg121) double mutant also shows a lower level of lateral PVQ axons compared to unc-6(ev400), 21.5% (N=200) versus 36% (N=200), implying that DGN-1 may suppress attraction of the PVQ axon ventrally toward the preanal ganglion even in the absence of netrin (Table 1).
Role of Wnt signaling in lumbar commissure guidance
The characteristic sharp anterior turn in lumbar commissure axons (Fig. 1A, top panel) suggests that axons migrating through the commissure switch from responding to D/V-oriented netrin and Slit signals to respond to an unknown anterior/posterior (A/P) guidance cue. Wnt proteins direct many neural cell and axon migrations along the A/P axis in C. elegans (Silhankova and Korswagen, 2007). We examined PVQ and PVC in Wnt mutants for reversal or termination of anterior leg guidance in the lumbar commissure and for ectopic posterior-directed processes, phenotypes consistent with a possible role in A/P guidance of lumbar commissure axons reaching the ventral midline. We found no defects in anterior-leg lumbar commissure guidance in single, double or triple Wnt mutants (Table 1). We did however observe premature termination of PVQ axons near the back bulb of the pharynx in ~13–19% of cwn-2 mutants and ~55–59% of cwn-1; cwn-2 double mutants (data not shown). The cwn-2 gene is expressed strongly in the posterior pharynx (Seong Hoon Kang and JMK, unpublished), consistent with a role for cwn-2 in local guidance of PVQ near the pharynx. Thus, A/P guidance of lumbar axons in the ventral nerve cord may be controlled by Wnt signaling on a regional basis, but the anterior turn of axons within the lumbar commissure appears to be independent of Wnt signaling.
DISCUSSION
Mechanisms of axon guidance through the lumber commissure
Our observations suggest that lumbar axon guidance through the lumbar commissure is governed by two competing signals: a ventral-directing signal provided by the netrin/DCC and Slit/Robo pathways, and a preanal ganglion-directing signal presumably produced by neurons in the preanal ganglion (Fig. 9). The nature of the preanal ganglion signal is unclear, although Wnt signaling is unlikely to play a role. The pioneer PVQ axon is the principal target of netrin/DCC and Slit/Robo mediated guidance in the ventral leg of the lumbar commissure. Strong responsiveness of the PVQ axon to netrin and suppression of premature responsiveness to the preanal ganglion signal combine to ensure that the PVQ axon extends toward the ventral midline before turning anterior toward the preanal ganglion. In contrast, netrin dependence of follower lumbar axons is mainly indirect, through follower adhesion/attraction to the PVQ axon. During anterior leg guidance, follower axons can evidently respond independently to the preanal ganglion-directing signal, since in netrin pathway mutants PVC follower axons entering the lumbar commissure without the ipsilateral PVQ axon present still turn anteriorly. Thus, follower axons must still require suppression of premature responsiveness to the preanal ganglion-directing cue to allow faithful tracking along the pioneer PVQ route.
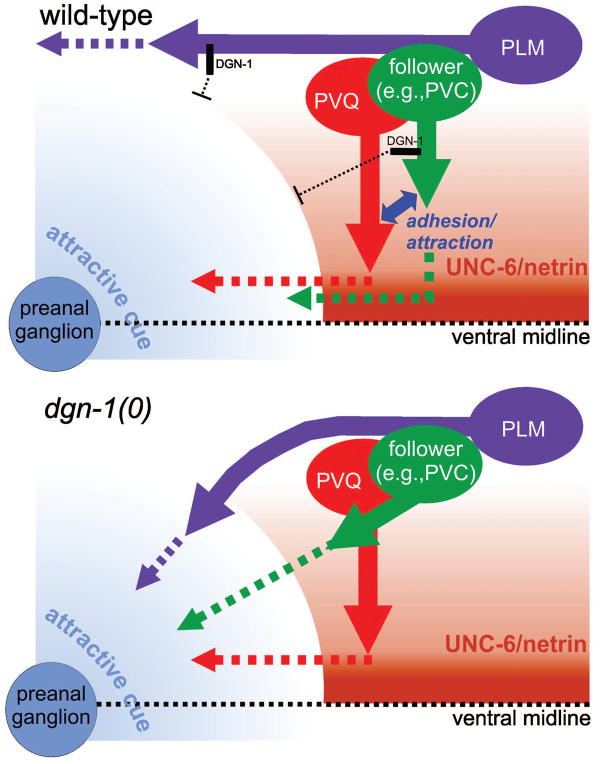
Figure 9. DGN-1/dystroglycan suppresses premature or abnormal response of lumbar axons to a local guidance cue.
In wild-type animals (upper panel), UNC-6/netrin signaling directs PVQ axon extension ventrally to the midline, where a local guidance cue, probably produced by the preanal ganglion, promotes anterior turning of the PVQ growth cone. In follower lumbar neurons, DGN-1 acts to block premature response to the preanal ganglion cue, allowing the follower axons to track along the PVQ pioneer through an adhesive or short-range attractive interaction. In the absence of DGN-1 (lower panel), follower lumbar axons polarize obliquely toward the preanal ganglion cue rather than tracking along PVQ. DGN-1 similarly blocks abnormal response of the PLM axon to the same attractive preanal ganglion signal, allowing it to maintain a lateral position.
DGN-1 appears to play a key role in suppressing orientation toward this putative preanal ganglion-directing guidance factor and thus in modulating the relative responsiveness of lumbar axons to competing D/V and A/P guidance signals. In the absence of DGN-1, follower lumbar axons prematurely begin A/P migration before completing D/V migration, resulting in oblique follower axon trajectories leading to the preanal ganglion. Similar oblique trajectories have been observed during the first turn of the distal tip cell (DTC) of the gonad when precocious expression of UNC-5 from transgenes leads to premature dorsal turning of the DTC away from the ventral midline before the completion of gonad arm extension along the A/P axis of the ventral body wall (Su et al., 2000). The abnormal guidance of PLM axons toward the preanal ganglion in dgn-1 mutants suggests that DGN-1 also suppresses undesired responsiveness of the PLM axon to this signal in order to maintain its lateral position. Confirmation of this model will require identification of the putative preanal ganglion-derived guidance cue and correlation of its expression relative to other known guidance cues and with the timing of lumbar axon extension. We note that our data on the role of dgn-1 in follower axon guidance are also consistent with an alternative to the model in Fig. 9, one in which dgn-1 functions in the preanal ganglion to suppress expression or activity of the putative attractive cue for lumbar axons.
Our model for a preanal ganglion role (Fig. 9) is derived from the sharp bend in axon trajectories in the lumbar commissure in wild-type larval/adult stage animals, and from the correlation of axon trajectories in mutant animals with the expected position of preanal ganglion cells during lumbar axogenesis based on their positions in larva/adult stages. Examination of the annotated serial sections of embryos undergoing lumbar axogenesis used by Durbin in his analysis of lumbar commissure formation (Durbin, 1987), available online at Wormatlas (www.wormatlas.org), confirm that the sharp transition between the ventral and anterior legs of the lumbar commissure is evident both at ~500 min of embryonic development, when only the pioneer PVQ and DA8/9 axons are present, and at ~515 min, by which time the follower lumbar axons have descended through the lumbar commissure and entered the preanal ganglion. The position of preanal ganglion cells in these serial sections is also consistent with our model. At ~500 min, the posteriormost cell bodies of the preanal ganglion (DA8 and DA9) abut the location of the bend in the lumbar commissure, but the anteriormost cell body, PVT, is positioned approximately two cell diameters forward of the bend. By ~515 min, even the DA8/9 cell bodies are positioned significantly anterior of the bend, with the cell bodies of certain rectal epithelial cells interposed. We thus feel confident that the preanal ganglion cell body and lumbar axon positions seen in post-embryonic stages reflect their locations during lumbar axogenesis, and are not significantly distorted by elongation of the embryo or development of the rectum and anus.
PVQ dependence of follower axon guidance
Our findings are consistent with the interpretation that the PVQ pioneer greatly facilitates but is not stringently required for lumbar commissure navigation by follower lumbar axons. PVQ expresses unc-6 around the time of lumbar axogenesis, leading Wadsworth and colleagues to propose that PVQ-produced netrin mediates the dependence of follower lumbar axons on PVQ (Ren et al., 1999). Our results indicate that netrin expression by PVQ cannot be the major mediator of follower axon guidance, since follower axon trajectory still depends strongly on ipsilateral PVQ route even in unc-6 null mutants. The nature of the PVQ-mediated guidance signal remains to be identified. One candidate is the putative cell adhesion molecule FMI-1/flamingo, an atypical cadherin family member that mediates at least some pioneer-directed follower axon guidance events in the ventral nerve cord (Steimel et al., 2010). Alternatively, PVQ may produce a pericellular factor that acts over a short range to attract follower axons.
Role of the preanal ganglion in patterning of the posterior nervous system
Our observations support a role for the preanal ganglion in organization of the posterior nervous system of C. elegans. The preanal ganglion is a key center for organizing the VNC. Studies of the developing nervous system by Durbin suggest that preanal ganglion neurons serve as guideposts for specific decussation events that distribute axons entering from the lumbar commissure into the left and right fascicles of the VNC (Durbin, 1987). In addition, the PVT neuron in the preanal ganglion secretes immunoglobulin domain proteins that act to maintain fascicle organization and prevent axon displacement across the ventral midline due to mechanical stress (Aurelio et al., 2002; Benard et al., 2009). Factors produced by preanal ganglion neurons may also play a role in organizing the local trajectories of lumbar axons. Indeed, ablation of PVT indicates that this neuron plays a role in organizing the lumbar commissure (Ren et al., 1999).
DGN-1/dystroglycan in lumbar axon guidance
DGN-1 likely functions cell autonomously in lumbar neurons to suppress premature response to attractive cues from the preanal ganglion. However, our present data could also be explained by a role of DGN-1 in preanal ganglion neurons in blocking production of the attractive cue. The dgn-1 gene is expressed in the preanal PVP neurons throughout development (Johnson et al., 2006), and may be transiently expressed during embryogenesis in other preanal ganglion neurons. Further work will be needed to identify the mechanism of dystroglycan action and the nature of the attractive cue directing anterior turning of lumbar commissure axons.
DGN-1 function in lumbar axon guidance involves its interaction with extracellular factors since it requires the N-terminal domain of DGN-1. Intriguingly, the N-terminus of dystroglycan paralogs in C. elegans and other nematode species is highly variable (Johnson et al., 2006), suggesting a modular structure for these proteins in which the idiosyncratic N-terminus directs protein-specific interactions with extracellular partners. The DGN-1 N-terminus, however, is clearly homologous to the N-terminal region of vertebrate α-dystroglycan. This region of α-dystroglycan is required for binding of LARGE, a putative glycosyltransferase that generates an unidentified extension of O-mannose-linked glycans decorating the adjacent mucin-like region of α-dystroglycan, which mediates binding to laminin and other LG domain proteins (Kanagawa et al., 2004). However, we do not observe defects in lumbar axon guidance (or other dgn-1-like phenotypes) in lge-1(tm1051), a deletion mutant of C. elegans LARGE (unpublished). Following LARGE modification, the N-terminus of α-dystroglycan is cleaved by a furin-like protease (Kanagawa et al., 2004; Saito et al., 2008; Singh et al., 2004). The fate of the released N-terminus in vivo is not known, although it can be detected in human serum and cerebrospinal fluid (Saito et al., 2011; Saito et al., 2008). In vitro analysis of the N-terminal domain of mouse or human α-dystroglycan indicates that this region possesses a binding site for laminin and possibly for other extracellular matrix components (Bozic et al., 2004; Hall et al., 2003), although this finding is not supported by other studies (Kanagawa et al., 2004). The role of the N-terminal domain of α-dystroglycan, outside of LARGE binding, thus remains an intriguing question.
Identification of factors that bind to the N-terminal domain of DGN-1/dystroglycan will help to address the function of this domain in lumbar axon guidance. An intriguing possibility is that the N-terminal domain suppresses lumbar follower axon attraction to the preanal ganglion by interacting physically with an unidentified preanal ganglion-derived guidance cue or with the cell surface receptor for this putative cue. Analysis of the function of the N-terminal domain of DGN-1 in axon guidance should help to reveal novel functions of the homologous region of α-dystroglycan which may be important in human development and disease.
Implications for neuronal dystroglycan function in vertebrates
Dystroglycan in expressed in both glia and neurons in the mammalian brain. Glial dystroglycan plays a prominent role in stabilizing the pial basement membrane, and loss of its function results in type II (cobblestone) lissencephaly, glial/neuronal heterotopias and aberrant radial migration (Moore et al., 2002; Satz et al., 2010). In contrast, in the absence of neuronal dystroglycan the morphology of the brain is spared, but long term potentiation in the hippocampus is impaired (Satz et al., 2010), suggesting that neuronal dystroglycan is involved in synaptic plasticity. Dystroglycan localizes to postsynaptic specializations throughout the brain (Zaccaria et al., 2001). The mechanistic basis of neuronal dystroglycan function in synaptic plasticity is unclear, but may involve its ability to organize postsynaptic signaling complexes (Grady et al., 2000; Satz et al., 2010). Many axon guidance factors play additional roles in determining the location, morphology and strength of synaptic contacts (Shen and Cowan, 2010), however, suggesting an alternate hypothesis in which dystroglycan plays a role in the formation and/or maintenance of synapses related to its potential functions in axon guidance. Elucidation of the mechanisms by which DGN-1 controls specific axon guidance events in C. elegans may help to identify the pathways and partners involved in neuronal dystroglycan function in the mammalian brain.
Acknowledgments
We thank Seong Hoon Kang for assistance with the Wnt mutant strains, Claire Benard (Columbia University) for pCB101.2, and the Andrew Fire lab (Stanford University) for pPD30.38 and pPD122.45. Some strains used in this work were provided by the C. elegans Genetics Center which is funded by the NIH National Center for Research Resources (NCRR). This work was supported by NIH grant GM081775.
References
- Akhavan A, Crivelli SN, Singh M, Lingappa VR, Muschler JL. SEA domain proteolysis determines the functional composition of dystroglycan. FASEB J. 2008;22:612–21. doi: 10.1096/fj.07-8354com. [DOI] [PubMed] [Google Scholar]
- Altun-Gultekin Z, Andachi Y, Tsalik EL, Pilgrim D, Kohara Y, Hobert O. A regulatory cascade of three homeobox genes, ceh-10, ttx-3 and ceh-23, controls cell fate specification of a defined interneuron class in C. elegans. Development. 2001;128:1951–69. doi: 10.1242/dev.128.11.1951. [DOI] [PubMed] [Google Scholar]
- Aurelio O, Hall DH, Hobert O. Immunoglobulin-domain proteins required for maintenance of ventral nerve cord organization. Science. 2002;295:686–90. doi: 10.1126/science.1066642. [DOI] [PubMed] [Google Scholar]
- Bak M, Fraser SE. Axon fasciculation and differences in midline kinetics between pioneer and follower axons within commissural fascicles. Development. 2003;130:4999–5008. doi: 10.1242/dev.00713. [DOI] [PubMed] [Google Scholar]
- Benard C, Tjoe N, Boulin T, Recio J, Hobert O. The small, secreted immunoglobulin protein ZIG-3 maintains axon position in Caenorhabditis elegans. Genetics. 2009;183:917–27. doi: 10.1534/genetics.109.107441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozic D, Sciandra F, Lamba D, Brancaccio A. The structure of the N-terminal region of murine skeletal muscle alpha-dystroglycan discloses a modular architecture. J Biol Chem. 2004;279:44812–6. doi: 10.1074/jbc.C400353200. [DOI] [PubMed] [Google Scholar]
- Branda CS, Stern MJ. Mechanisms controlling sex myoblast migration in Caenorhabditis elegans hermaphrodites. Dev Biol. 2000;226:137–51. doi: 10.1006/dbio.2000.9853. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitnis AB, Kuwada JY. Elimination of a brain tract increases errors in pathfinding by follower growth cones in the zebrafish embryo. Neuron. 1991;7:277–85. doi: 10.1016/0896-6273(91)90266-3. [DOI] [PubMed] [Google Scholar]
- Cohn RD, Henry MD, Michele DE, Barresi R, Saito F, Moore SA, Flanagan JD, Skwarchuk MW, Robbins ME, Mendell JR, et al. Disruption of DAG1 in differentiated skeletal muscle reveals a role for dystroglycan in muscle regeneration. Cell. 2002;110:639–48. doi: 10.1016/s0092-8674(02)00907-8. [DOI] [PubMed] [Google Scholar]
- Dickens NJ, Beatson S, Ponting CP. Cadherin-like domains in alpha-dystroglycan, alpha/epsilon-sarcoglycan and yeast and bacterial proteins. Curr Biol. 2002;12:R197–9. doi: 10.1016/s0960-9822(02)00748-0. [DOI] [PubMed] [Google Scholar]
- Durbin RM. PhD diss. University of Cambridge; UK: 1987. Studies on the development and organisation of the nervous system of Caenorhabditis elegans. [Google Scholar]
- Gettner SN, Kenyon C, Reichardt LF. Characterization of beta pat-3 heterodimers, a family of essential integrin receptors in C. elegans. J Cell Biol. 1995;129:1127–41. doi: 10.1083/jcb.129.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Antonini A, McConnell SK, Shatz CJ. Requirement for subplate neurons in the formation of thalamocortical connections. Nature. 1990;347:179–81. doi: 10.1038/347179a0. [DOI] [PubMed] [Google Scholar]
- Gilleard JS, Barry JD, Johnstone IL. cis regulatory requirements for hypodermal cell-specific expression of the Caenorhabditis elegans cuticle collagen gene dpy-7. Mol Cell Biol. 1997;17:2301–11. doi: 10.1128/mcb.17.4.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady RM, Zhou H, Cunningham JM, Henry MD, Campbell KP, Sanes JR. Maturation and maintenance of the neuromuscular synapse: genetic evidence for roles of the dystrophin--glycoprotein complex. Neuron. 2000;25:279–93. doi: 10.1016/s0896-6273(00)80894-6. [DOI] [PubMed] [Google Scholar]
- Hall DH, Russell RL. The posterior nervous system of the nematode Caenorhabditis elegans: serial reconstruction of identified neurons and complete pattern of synaptic interactions. J Neurosci. 1991;11:1–22. doi: 10.1523/JNEUROSCI.11-01-00001.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall H, Bozic D, Michel K, Hubbell JA. N-terminal alpha-dystroglycan binds to different extracellular matrix molecules expressed in regenerating peripheral nerves in a protein-mediated manner and promotes neurite extension of PC12 cells. Mol Cell Neurosci. 2003;24:1062–73. doi: 10.1016/j.mcn.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Hao JC, Yu TW, Fujisawa K, Culotti JG, Gengyo-Ando K, Mitani S, Moulder G, Barstead R, Tessier-Lavigne M, Bargmann CI. C. elegans slit acts in midline, dorsal-ventral, and anterior-posterior guidance via the SAX-3/Robo receptor. Neuron. 2001;32:25–38. doi: 10.1016/s0896-6273(01)00448-2. [DOI] [PubMed] [Google Scholar]
- Hardin J, King R, Thomas-Virnig C, Raich WB. Zygotic loss of ZEN-4/MKLP1 results in disruption of epidermal morphogenesis in the C. elegans embryo. Dev Dyn. 2008;237:830–6. doi: 10.1002/dvdy.21455. [DOI] [PubMed] [Google Scholar]
- Harrison RG. The outgrowth of the nerve fiber as a mode of protoplasmic movement. J Exp Zool. 1910;9:787–846. doi: 10.1002/jez.1401420103. [DOI] [PubMed] [Google Scholar]
- Iwasaki K, Staunton J, Saifee O, Nonet M, Thomas JH. aex-3 encodes a novel regulator of presynaptic activity in C. elegans. Neuron. 1997;18:613–22. doi: 10.1016/s0896-6273(00)80302-5. [DOI] [PubMed] [Google Scholar]
- Johnson RP, Kang SH, Kramer JM. C. elegans dystroglycan DGN-1 functions in epithelia and neurons, but not muscle, and independently of dystrophin. Development. 2006;133:1911–21. doi: 10.1242/dev.02363. [DOI] [PubMed] [Google Scholar]
- Kanagawa M, Saito F, Kunz S, Yoshida-Moriguchi T, Barresi R, Kobayashi YM, Muschler J, Dumanski JP, Michele DE, Oldstone MB, et al. Molecular recognition by LARGE is essential for expression of functional dystroglycan. Cell. 2004;117:953–64. doi: 10.1016/j.cell.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Keshishian H, Bentley D. Embryogenesis of peripheral nerve pathways in grasshopper legs. III. Development without pioneer neurons. Dev Biol. 1983;96:116–24. doi: 10.1016/0012-1606(83)90316-0. [DOI] [PubMed] [Google Scholar]
- Killeen MT, Sybingco SS. Netrin, Slit and Wnt receptors allow axons to choose the axis of migration. Dev Biol. 2008;323:143–51. doi: 10.1016/j.ydbio.2008.08.027. [DOI] [PubMed] [Google Scholar]
- Kim GJ, Shatz CJ, McConnell SK. Morphology of pioneer and follower growth cones in the developing cerebral cortex. J Neurobiol. 1991;22:629–42. doi: 10.1002/neu.480220608. [DOI] [PubMed] [Google Scholar]
- Klose M, Bentley D. Transient pioneer neurons are essential for formation of an embryonic peripheral nerve. Science. 1989;245:982–4. doi: 10.1126/science.2772651. [DOI] [PubMed] [Google Scholar]
- Koppen M, Simske JS, Sims PA, Firestein BL, Hall DH, Radice AD, Rongo C, Hardin JD. Cooperative regulation of AJM-1 controls junctional integrity in Caenorhabditis elegans epithelia. Nat Cell Biol. 2001;3:983–91. doi: 10.1038/ncb1101-983. [DOI] [PubMed] [Google Scholar]
- Landmann F, Quintin S, Labouesse M. Multiple regulatory elements with spatially and temporally distinct activities control the expression of the epithelial differentiation gene lin-26 in C. elegans. Dev Biol. 2004;265:478–90. doi: 10.1016/j.ydbio.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Lopresti V, Macagno ER, Levinthal C. Structure and development of neuronal connections in isogenic organisms: cellular interactions in the development of the optic lamina of Daphnia. Proc Natl Acad Sci U S A. 1973;70:433–7. doi: 10.1073/pnas.70.2.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maduro M, Pilgrim D. Identification and cloning of unc-119, a gene expressed in the Caenorhabditis elegans nervous system. Genetics. 1995;141:977–88. doi: 10.1093/genetics/141.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastick GS, Easter SS., Jr Initial organization of neurons and tracts in the embryonic mouse fore- and midbrain. Dev Biol. 1996;173:79–94. doi: 10.1006/dbio.1996.0008. [DOI] [PubMed] [Google Scholar]
- Mello CC, Kramer JM, Stinchcomb D, Ambros V. Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 1991;10:3959–70. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michele DE, Campbell KP. Dystrophin-glycoprotein complex: post-translational processing and dystroglycan function. J Biol Chem. 2003;278:15457–60. doi: 10.1074/jbc.R200031200. [DOI] [PubMed] [Google Scholar]
- Moore SA, Saito F, Chen J, Michele DE, Henry MD, Messing A, Cohn RD, Ross-Barta SE, Westra S, Williamson RA, et al. Deletion of brain dystroglycan recapitulates aspects of congenital muscular dystrophy. Nature. 2002;418:422–5. doi: 10.1038/nature00838. [DOI] [PubMed] [Google Scholar]
- Muschler J, Levy D, Boudreau R, Henry M, Campbell K, Bissell MJ. A role for dystroglycan in epithelial polarization: loss of function in breast tumor cells. Cancer Res. 2002;62:7102–9. [PubMed] [Google Scholar]
- Nonet ML, Staunton JE, Kilgard MP, Fergestad T, Hartwieg E, Horvitz HR, Jorgensen EM, Meyer BJ. Caenorhabditis elegans rab-3 mutant synapses exhibit impaired function and are partially depleted of vesicles. J Neurosci. 1997;17:8061–73. doi: 10.1523/JNEUROSCI.17-21-08061.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordlander RH. Axonal growth cones in the developing amphibian spinal cord. J Comp Neurol. 1987;263:485–96. doi: 10.1002/cne.902630403. [DOI] [PubMed] [Google Scholar]
- Ogura K, Shirakawa M, Barnes TM, Hekimi S, Ohshima Y. The UNC-14 protein required for axonal elongation and guidance in Caenorhabditis elegans interacts with the serine/threonine kinase UNC-51. Genes Dev. 1997;11:1801–11. doi: 10.1101/gad.11.14.1801. [DOI] [PubMed] [Google Scholar]
- Raper JA, Bastiani MJ, Goodman CS. Pathfinding by neuronal growth cones in grasshopper embryos. IV. The effects of ablating the A and P axons upon the behavior of the G growth cone. J Neurosci. 1984;4:2329–45. doi: 10.1523/JNEUROSCI.04-09-02329.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren XC, Kim S, Fox E, Hedgecock EM, Wadsworth WG. Role of netrin UNC-6 in patterning the longitudinal nerves of Caenorhabditis elegans. J Neurobiol. 1999;39:107–18. [PubMed] [Google Scholar]
- Saito F, Moore SA, Barresi R, Henry MD, Messing A, Ross-Barta SE, Cohn RD, Williamson RA, Sluka KA, Sherman DL, et al. Unique role of dystroglycan in peripheral nerve myelination, nodal structure, and sodium channel stabilization. Neuron. 2003;38:747–58. doi: 10.1016/s0896-6273(03)00301-5. [DOI] [PubMed] [Google Scholar]
- Saito F, Saito-Arai Y, Nakamura-Okuma A, Ikeda M, Hagiwara H, Masaki T, Shimizu T, Matsumura K. Secretion of N-terminal domain of alpha-dystroglycan in cerebrospinal fluid. Biochem Biophys Res Commun. 2011;411:365–9. doi: 10.1016/j.bbrc.2011.06.150. [DOI] [PubMed] [Google Scholar]
- Saito F, Saito-Arai Y, Nakamura A, Shimizu T, Matsumura K. Processing and secretion of the N-terminal domain of alpha-dystroglycan in cell culture media. FEBS Lett. 2008;582:439–44. doi: 10.1016/j.febslet.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Satz JS, Ostendorf AP, Hou S, Turner A, Kusano H, Lee JC, Turk R, Nguyen H, Ross-Barta SE, Westra S, et al. Distinct functions of glial and neuronal dystroglycan in the developing and adult mouse brain. J Neurosci. 2010;30:14560–72. doi: 10.1523/JNEUROSCI.3247-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K, Cowan CW. Guidance molecules in synapse formation and plasticity. Cold Spring Harb Perspect Biol. 2010;2:a001842. doi: 10.1101/cshperspect.a001842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhankova M, Korswagen HC. Migration of neuronal cells along the anterior-posterior body axis of C. elegans: Wnts are in control. Curr Opin Genet Dev. 2007;17:320–5. doi: 10.1016/j.gde.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Singh J, Itahana Y, Knight-Krajewski S, Kanagawa M, Campbell KP, Bissell MJ, Muschler J. Proteolytic enzymes and altered glycosylation modulate dystroglycan function in carcinoma cells. Cancer Res. 2004;64:6152–9. doi: 10.1158/0008-5472.CAN-04-1638. [DOI] [PubMed] [Google Scholar]
- Steimel A, Wong L, Huarcaya Najarro E, Ackley BD, Garriga G, Hutter H. The Flamingo ortholog FMI-1 controls pioneer-dependent navigation of follower axons in C. elegans. Development. 2010;137:3663–73. doi: 10.1242/dev.054320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su M, Merz DC, Killeen MT, Zhou Y, Zheng H, Kramer JM, Hedgecock EM, Culotti JG. Regulation of the UNC-5 netrin receptor initiates the first reorientation of migrating distal tip cells in Caenorhabditis elegans. Development. 2000;127:585–94. doi: 10.1242/dev.127.3.585. [DOI] [PubMed] [Google Scholar]
- Sugita S, Saito F, Tang J, Satz J, Campbell K, Sudhof TC. A stoichiometric complex of neurexins and dystroglycan in brain. J Cell Biol. 2001;154:435–45. doi: 10.1083/jcb.200105003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JB, Bastiani MJ, Bate M, Goodman CS. From grasshopper to Drosophila: a common plan for neuronal development. Nature. 1984;310:203–7. doi: 10.1038/310203a0. [DOI] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- Wilson SW, Ross LS, Parrett T, Easter SS., Jr The development of a simple scaffold of axon tracts in the brain of the embryonic zebrafish, Brachydanio rerio. Development. 1990;108:121–45. doi: 10.1242/dev.108.1.121. [DOI] [PubMed] [Google Scholar]
- Yaginuma H, Homma S, Kunzi R, Oppenheim RW. Pathfinding by growth cones of commissural interneurons in the chick embryo spinal cord: a light and electron microscopic study. J Comp Neurol. 1991;304:78–102. doi: 10.1002/cne.903040107. [DOI] [PubMed] [Google Scholar]
- Zaccaria ML, Di Tommaso F, Brancaccio A, Paggi P, Petrucci TC. Dystroglycan distribution in adult mouse brain: a light and electron microscopy study. Neuroscience. 2001;104:311–24. doi: 10.1016/s0306-4522(01)00092-6. [DOI] [PubMed] [Google Scholar]