Abstract
Objective
High doses or prolonged exposure to ketamine increase neuronal apoptosis in the developing brain, although effects on neural stem progenitor cells (NSPCs) remain unexplored. This study investigated dose- and time- dependent responses to ketamine on cell death and neurogenesis in cultured rat fetal cortical NSPCs.
Design
Laboratory-based study
Setting
University research laboratory
Subject
Sprague-Dawley (SD) rats
Interventions
NSPCs were isolated from the cortex of SD rat fetuses on embryonic day 17 (E17). In dose-response experiments, cultured NSPCs were exposed to different concentrations of ketamine (0–100 μM) for 24 hours. In time-course experiments, NSPC cultures were exposed to 10 μM ketamine for different durations (0–48 hours).
Measurements and Main Results
Apoptosis and necrosis in NSPCs were assessed using activated caspase-3 immunostaining and lactate dehydrogenase (LDH) assays, respectively. Proliferative changes in NSPCs were detected using Bromo-deoxyuridine (BrdU) incorporation and Ki67 immunostaining. Neuronal differentiation was assessed using Tuj-1 immunostaining. Cultured NSPCs were resistant to apoptosis and necrosis following all concentrations and durations of ketamine exposure tested. Ketamine inhibited proliferation, with decreased numbers of BrdU-positive cells following ketamine exposure to 100 μM for 24 hours (P<0.005) or 10 μM for 48 hours (P<0.01), and reduced numbers of Ki67-positive cells following exposure to ketamine concentration higher than 10 μM for 24 hours (P<0.001) or at 10 μM for 48 hours (P<0.01). Ketamine enhanced neuronal differentiation, with all ketamine concentrations increasing Tuj-1-positive neurons (P<0.001) after 24-hours of exposure. This also occurred with all exposures to 10 μM ketamine for longer than 8 hours (P<0.001).
Conclusions
Clinically relevant concentrations of ketamine do not induce cell death in NSPCs via apoptosis or necrosis. Ketamine alters the proliferation and increases the neuronal differentiation of NSPCs isolated from the rat neocortex. These studies imply that ketamine exposure during fetal or neonatal life may alter neurogenesis and subsequent brain development.
Keywords: ketamine, NMDA receptors, neural stem cells, apoptosis, necrosis, neurogenesis
Introduction
Ketamine, a non-competitive N-methyl-D-aspartate receptor (NMDAR) antagonist, is used routinely as an anesthetic, analgesic, and sedative due to its wide therapeutic index and favorable hemodynamic effects (1). The safety of ketamine use in pediatrics was questioned (2), because animal studies showed that repeated doses of ketamine induced neuronal apoptosis in the developing brain (3–9). Neural stem progenitor cells (NSPCs), as precursors of mature neurons and glia, play a critical role in normal brain development and have different biological characteristics from differentiated neurons and glia. For example, physiological apoptosis in NSPCs and neurons is morphologically indistinguishable, but its underlying mechanisms appear to be different (10). To further evaluate the neurotoxic effects of ketamine on brain development, we explored the effects of ketamine on NSPCs from the rat fetal cortex.
Neurogenesis from NSPCs includes two processes: proliferation and differentiation. Activation of glutamate receptors can change the proliferation of neural stem cells (NSCs) or neural progenitor cells (NPCs), although the detailed mechanisms remain unclear (11–19). Some studies reported that NMDAR activation increases the proliferation of NPCs (11, 14–18), whereas other studies reported that NMDA reduces proliferation and inhibits neurosphere formation (12, 13, 19).
NSPCs have the capacity of differentiating into neurons, astrocytes, and oligodendrocytes. Any factor interfering with neuronal differentiation may result in abnormalities in neuronal number, migration, synaptogenesis and plasticity, thus altering structures in the developing brain. Repression of the calcium signaling mediated by glutamate receptors was associated with increased neuronal differentiation in human NPCs (20, 21). NMDARs, as ligand-gated calcium channels, are involved in regulating neuronal differentiation of NSPCs (22, 23), although it remains unclear if NMDAR activation promotes or inhibits the neuronal differentiation of NSPCs. In this study, we assessed the dose- and time-dependent effects of ketamine on proliferation and neuronal differentiation of cultured NSPCs isolated from the rat fetal cortex.
Materials and Methods
Animals
All animal use procedures were approved by the IACUC (Institutional Animal Care and Use Committee) of the University of Tennessee Health Science Center. Timed-pregnant Sprague-Dawley rats (Charles River Inc.) were housed at 24°C on a 12:12 hours light:dark cycle with free access to food and water.
NSPCs isolation and cultures
NSPCs isolation procedures were completed as reported previously(24). Briefly, the neocortex from E17 rats was dissected, collected and cut into small pieces for digestion in Accutase™ at 37°C; dissociated cells were rinsed and centrifuged at 120 × g for 5 min. Cell pellets were gently resuspended in proliferation medium DMEM/F12 medium (Invitrogen) containing 20 ng/ml each of epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF). Sieved cell suspensions were mixed with Percoll™ centrifugation medium, centrifuged at 21000×G for 30min, achieving separation into two cellular layers. Cells from the lower layer (NSPCs) were rinsed and suspended in proliferation medium. Isolated cells were seeded on poly-L-lysine (PLL) pre-coated cell culture dishes, plates, or glass coverslips at a density appropriate for the surface area. Seeded cells were cultured in 5% CO2, 100% humidity at 37°C. In each dish, half of the culture medium was replaced every three days. Adherent cells were passaged using Accutase™. Cultures at passage 1/2 were used for all in vitro experiments.
Cell treatments
In the dose-dependent assays, cultures were exposed to ketamine (Ketaset® Pfizer Inc., Fort Dodge, USA) at the following concentrations: 0 as control, 1, 10, 20, 50, and 100 μM for 24 hours. In time-course assays, cultures were exposed to 10 μM of ketamine for different durations: 0 as control,½, 1, 2, 4, 6, 8, 10, 12, 18, 24, and 48 hours. In the proliferation assays, cultures were exposed to 5-bromo-2′-deoxyuridine (BrdU, 10 μM) for 24 hours. In the differentiation tests, culture media were replaced with a differentiation medium containing 1% fetal bovine serum (FBS) after different ketamine treatments. After allowing a 3-week differentiation phase, cultures were fixed for immunostaining.
Immunofluorescent staining
Treated cells were fixed in 4% paraformaldehyde (PFA), and rehydrated in phosphate buffered saline (PBS). Cells were incubated in blocking solution (5% normal goat serum in PBS) at room temperature. Cells were incubated with primary antibodies (Table 1) at 4°C overnight, then washed in PBS containing 0.1% Tween 20, followed by incubation with diluted Alexa Fluor® secondary antibodies (Table 1) for 1 hour at room temperature. For nuclear staining, cells were incubated with 1 μg/ml DAPI for 10min at room temperature. Finally, the cells were washed with PBS and mounted on glass slides using aqueous mounting media. Negative controls were the cells incubated without any primary antibody.
Table 1.
Primary and Secondary Antibodies
| Antibody name | Specificity | Host species | Dilution rates | Company |
|---|---|---|---|---|
| Nestin | Neural stem cells | rabbit | 1:200 | Millipore |
| Musashi-1 | Neural precursor cells | rabbit | 1:200 | Millipore |
| Tuj-1 | Newborn neurons | mouse | 1:1000 | Millipore |
| GFAP | Astrocytes | rabbit | 1:1000 | Millipore |
| Active caspase-3 | Apoptotic cells | rabbit | 1:10 | Millipore |
| BrdU | Newly-generated cells | mouse | 1:1000 | Millipore |
| Ki67 | Proliferative cells | rabbit | 1:1000 | Millipore |
| NR1 | NMDA receptor subunit 1 | rabbit | 1:200 | Cell Signaling |
| NR2A | NMDA receptor subunit 2A | rabbit | 1:200 | Millipore |
| NR2B | NMDA receptor subunit 2B | rabbit | 1:200 | Millipore |
| rabbit IgG Alexa-594 | Specific to rabbit primary antibodies | goat | 1:1000 | Invitrogen |
| mouse IgG Alexa-488 | Specific to mouse primary antibodies | goat | 1:1000 | Invitrogen |
Cell death assays
We utilized activated caspase-3 staining to detect apoptotic cells in the ketamine-treated NSPCs. Ketamine exposed cultures were fixed and stained with active caspase-3 antibody and DAPI as described above. Caspase-3-positive (caspase-3+) and DAPI+ cells were quantified. The percentage of caspase-3+ cells in the total DAPI+ cells represents the rate of apoptosis in NSPCs. Assays of lactate dehydrogenase (LDH) were performed (CytoTox 96 Non-Radioactive Cytotoxicity Assay Kit, Promega) to assess any necrotic changes (cell damage or lysis) in the NSPCs exposed to ketamine. All experimental procedures were performed based on manufacturer’s protocols. Absorbance values were recorded at 490 nm.
Proliferation assays
BrdU, a thymidine analogue, can be incorporated into newly synthesized DNA in the S-Phase of the cell cycle. Ki67, an endogenous marker, is expressed in the nucleus during all phases of the cell cycle. Thus, BrdU staining identifies newly generated cells, whereas Ki67 staining represents all proliferating cells. To detect the direct effects of BrdU on the proliferation of NSPCs, we added 10 μM of BrdU to the culture media for 0–48 hours of exposure. Exposure to 10 μM BrdU for up to 24 hours did not induce cell death in NSPCs; also reported by others(25, 26). Treated cells were stained with BrdU, Ki67 antibodies, and DAPI based on the protocols described previously. The numbers of BrdU+, Ki67+ and DAPI+ cells were counted. The percentages of BrdU+ in DAPI+-cells, Ki67+ in DAPI+-cells, and BrdU+ in Ki67+- cells were calculated to represent the rates of newly-generated cells, all proliferating cells, and newly-generated cells among the proliferating cells, respectively.
Neuronal differentiation assays
Cultures were exposed to different concentrations of ketamine (0–100 μM) in the differentiation medium (1% FBS without bFGF or EGF) for 24 hours, or to 10 μM ketamine for different durations (0–48 hours). Following these treatments, culture media containing ketamine were removed and replaced with fresh differentiation media. Culture media were changed every three days. After 3 weeks, cells were fixed and stained using Tuj-1 antibody and DAPI based on the protocols described above. The percentage of Tuj-1+ cells in DAPI+ cells was represented the rate of neuronal differentiation of NSPCs.
Image capture and cell counting
A protocol-naive person collected the images from the coded samples to minimize potential observer bias. From each sample, ten visual fields at the magnification of 200X were randomly selected for image capture. All images were captured using an Olympus BX60 fluorescence microscope equipped with a Hamamatsu C4742-95 camera, using the Hamamatsu Imaging Software (HCImage 2.1 Live Version). Cells in each visual field were counted in a blinded manner. The total number of cells was based on DAPI+ cells. The percentage of a cell type per image field formed the data set (n=10) in each independent experiment. All studies were repeated in three independent experiments.
Statistical Analyses
Statistical analyses were performed using Excel 2007 and GraphPad InStat3. Independent samples of cultured NSPCs were used for observations made at each of the time points or drug concentrations used in our experiments. We used the Kolmogorov-Smirnov test for normal distribution and Levene’s test for homogeneity before performing a one-way analysis of variance (ANOVA) on each dataset. If ANOVA was significant, post hoc t-tests were performed at each time point to investigate any differences between the parallel control and ketamine groups, followed by the Bonferroni correction for multiple comparisons. In the case of non-normal distribution, nonparametric tests were used (Kruskal-Wallis ANOVA and post hoc Mann-Whitney U tests). Statistical significance was set up at P<0.05. All data were expressed as the mean ± standard error.
Results
Isolation and identification of NSPC cultures
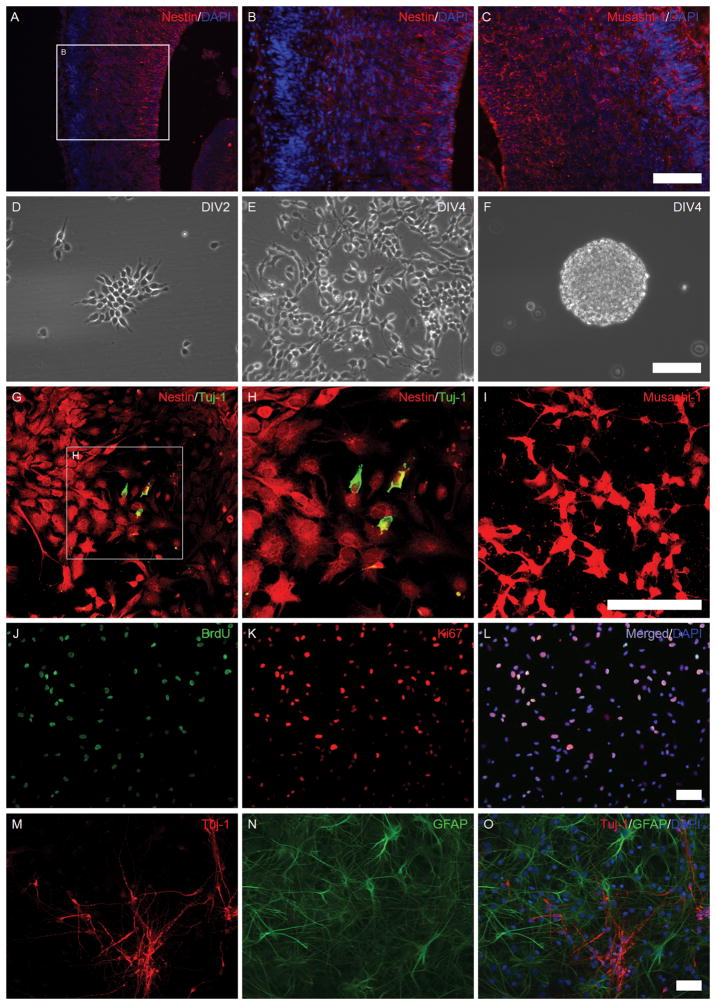
Two characteristic markers (Nestin and Musashi-1) were used to identify NSPCs in the fetal cortex(Figure 1A–C), consistent with the well-known cellular composition of fetal rat brains. When NSPCs were isolated, plated in pre-coated culture dishes, they grew to an adherent monolayer by 4 days in vitro (DIV 4) (Figure 1D–E). When seeded in a non-coated culture dish, NSPCs grew into neurospheres by DIV4-5 (Figure 1F). Tuj-1, Nestin and Musashi-1 were employed to identify the cultures as NSPCs. All stained cells were Nestin+ (Figure 1G–H) and Musashi-1+ (Figure 1I). A few cells with Nestin+/Tuj-1+ staining (Figure 1G–H) were characteristic of neuronal progenitor cells.
Figure 1. The isolation and identification of NSPCs from the fetal brain.
Images A–C: rat fetal brain sections were immunostained using anti-Nestin (red), anti-Musashi-1 (red), and DAPI (blue). Images D–F: in vitro cultured NSPCs; Images D–E: adherent monolayer NSPC cultures at days 2–4 in vitro; Image F: NSPC neurospheres in a non-coated culture dish. Images G–I: the expression of Nestin, Tuj-1, and Musashi-1 in cultured NSPCs (confocal microscopy); Images G–H: Nestin (red) and Tuj-1 (green); Image I: Musashi-1 (red). Images J–L: The proliferative potency of NSPCs; Image J: BrdU (green); Image K: Ki67 (red); Image L: a merged picture of image J, K, and DAPI (blue). Images M–O: Differentiation potency of NSPCs; Image M: Tuj-1 (red), a marker for newborn neurons; Image N: GFAP (green), a marker for astrocytes; Image O: a merged picture of Images M, N, and DAPI (blue). (Scale bars: 50 μm).
The abilities of proliferation and multi-potent differentiation were also assessed in these cultures. In proliferation tests, almost all cells were Ki67+ among which some were BrdU+ (Figure 1J–L), indicating that obtained cultures possessed the ability of self-renewal. When NSPC cultures were grown in spontaneous differentiation medium for 3 weeks, differentiated cells were Tuj-1+ (nascent neurons) or GFAP+ (astrocytes), and no overlap of Tuj-1+ and GFAP+ staining was observed (Figure 1M–O), indicating the cultures had the capacity for multi-potent differentiation.
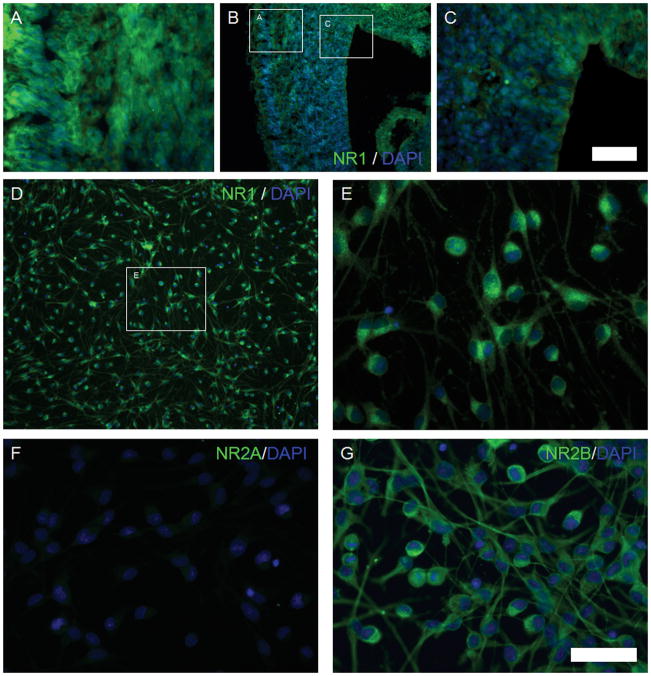
NMDAR subunits were detected in the fetal brain sections and cultured NSPCs. NMDAR 1 (NR1) was expressed in the entire embryonic brain (Figure 2A–C) and in cultured NSPCs (Figure 2D–E). Additionally, NR1 expression in the cortical plate (CP, Figure 2A) was higher than in the ventricular zone (VZ) and subventricular zone (SVZ, Figure 2C). NMDAR 2B (NR2B), specific to the developing brain, was expressed in NSPCs (Figure 2G), whereas the expression of NR2A, characteristic of the adult brain, was weak in the fetal cortex (Figure 2F). Although expression of the subunits indicates that ketamine may induce some responses in NSPCs, it is unclear if these subunits are assembled or functional as NMDARs.
Figure 2. The expression of NMDA receptors in cultured NSPCs from rat fetal cortex.
Images A–C: the expression of NR1 in the rat fetal brain, NR1 (green) and DAPI (blue). Image A: cortical plate (400X magnification); Image B: entire neocortex (100X magnification); Image C: VZ and SVZ regions (400X magnification). Images D and E: the expression of NR1 (green) in cultured NSPCs; Image E is a magnified picture of the inset of Image D. Images F and G: the expression of NR2A and NR2B in cultured NSPCs. Image F: NR2A (green) and DAPI (blue); Image G: NR2B (green) and DAPI (blue). (Scale bars: 50 μm)
NSPCs are resistant to ketamine-induced cell death
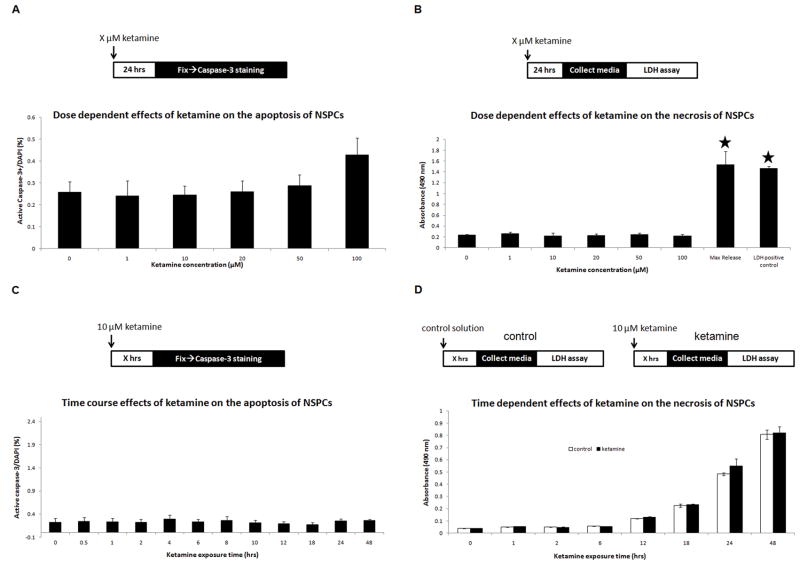
In apoptosis assays, we measured activated caspase-3 cells in NSPCs exposed to different ketamine concentrations: 0, 1, 10, 20, 50, and 100 μM for 24 hours. No significant differences occurred in caspase-3+ cells between the control and ketamine groups (ANOVA, P=0.181, n=10), indicating no dose-dependent effects of ketamine on apoptosis in NSPCs (Figure 3A). These results suggest that cultured NSPCs are resistant to ketamine-induced apoptosis, at clinically relevant concentrations for 24 hours(8).
Figure 3. Effects of ketamine on apoptosis and necrosis in cultured NSPCs.
Panel A: dose-dependent responses of ketamine on apoptosis in cultured NSPCs. Ketamine exposure concentrations: X = 0, 1, 10, 20, 50, and 100 μM. Panel B: dose-dependent responses of ketamine (X = 0, 1, 10, 20, 50, or 100 μM) on necrosis in cultured NSPCs. Maximal release, cells were treated by Triton X-100; LDHpositive control, provided by the LDH assay kit. Absorbance values were measured at 490 nm. Stars represent significant differences compared to the control and all ketamine treatment groups. Panel C: time-dependent effects of ketamine on apoptosis of cultured NSPCs, as measured by percentage of active caspase-3+ cells in DAPI+ cells. Ketamine exposure duration: X = 0, 0.5, 1, 2, 4, 6, 8, 10, 12, 18, 24, and 48 hours. Panel D: time-dependent effects of ketamine on necrosis of cultured NSPCs using LDH assays. NSPC cultures were exposed to 10 μM of ketamine (black columns) for different durations (X = 0, 1, 2, 6, 12, 18, 24, and 48 hours) with parallel controls (white columns). No significant difference was found.
LDH assays were utilized to detect necrotic changes in ketamine-exposed cultured NSPCs. Although LDH release also occurs at a late stage of apoptosis, this release is insignificant compared to the LDH release from necrosis. Therefore, LDH assays represent the necrotic changes in NSPCs exposed to ketamine. In this study, no tested concentrations of ketamine elevated LDH levels in the culture media (ANOVA, P=0.57). In all ketamine groups, LDH release was significantly lower than in the maximal release group (NSPCs treated with Triton X-100) and the LDH positive control (bovine LDH) (ANOVA, P<0.0001) (Figure 3B). These data suggest that ketamine does not induce cell death in NSPCs.
In time-dependent apoptosis assays (0–48 hours), no significant changes in apoptosis occurred in cultured NSPCs following exposure to 10 μM ketamine for upto 48 hours, as compared to control (ANOVA, P=0.989) (Figure 3C). All durations of exposure showed low apoptotic rates (less than 0.3%). In the LDH detection of necrosis, NSPC cultures were exposed to 10 μM of ketamine for different durations (0, 1, 2, 6, 12, 18, 24, and 48 hours) while parallel controls without ketamine exposure were setup for comparisons at each duration. Paired t-tests indicated no significant difference in LDH release between the parallel controls and ketamine treatment groups at each time point (Figure 3D). Taken together, these data suggest that ketamine does not time-dependently induce cell death in NSPCs.
Ketamine inhibits the proliferation of NSPCs
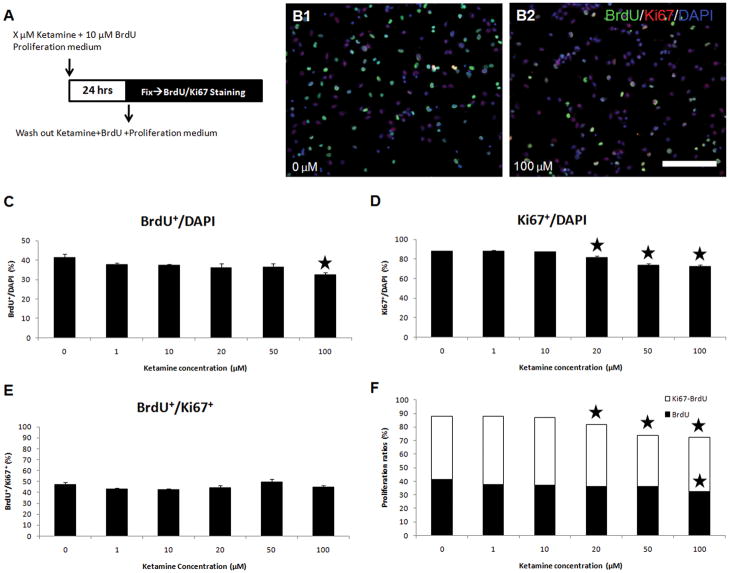
In dose-response assays, we found that the percentage of BrdU+/DAPI+ cells in cultured NSPCs exposed to 100 μM of ketamine decreased to 32.3±1.4%, compared to the controls at 41.5±1.6% (ANOVA, P=0.0036; Bonferroni, P<0.001) (Figure 4C). Two typical pictures are shown in Figure 4B1–B2. Ketamine at 20, 50, and 100 μM reduced the percentages of Ki67+/DAPI+ cells compared with the control (ANOVA, P<0.0001; Bonferroni, P<0.001). No significant changes occurred in the percentage of Ki67+/DAPI+ cells at low or clinically relevant concentrations of ketamine (1, 10 μM) (Figure 4D). No significant changes occurred in the percentage of BrdU+/Ki67+ cells compared to the controls at any of the ketamine concentrations used (Figure 4E).
Figure 4. Dose-dependent effects of ketamine on the proliferation of NPSCs.
Panel A: Ketamine (concentrations: X = 0, 1, 10, 20, 50, or 100 μM) and BrdU were added to culture media for 24 hours, then cells were fixed and stained with anti-BrdU and anti-Ki67 antibodies. Panel B: two typical pictures showing the difference of BrdU and Ki67 staining between the control (B1) and 100 μM ketamine (B2) groups. In the images, green or white points are BrdU+ cells; purple points are Ki67+ and BrdU−. (Scale bar: 50 μm) Panels C–F: dose-dependent effects of ketamine on the proliferation of NSPCs. Panel C: the percentage of BrdU+ cells in total cells (DAPI+) is plotted versus different concentrations of ketamine. Panel D: the percentage of Ki67+ cells in the total cells (DAPI+) exposed to ketamine. Panel E: the percentage of BrdU+ cells in Ki67+ cells represents the percentage of proliferating cells to the cells having proliferative capacity. Panel F: a combined graph of graphs C and D. (stars represent significant differences ketamine vs. control).
In the BrdU incorporation assays, the number of newly-generated cells (BrdU+/DAPI+) following BrdU exposure for 24 hours was reduced by approximately 9% at 100 μM ketamine. In Ki67 staining, proliferative cells (Ki67+/DAPI+) were also reduced (6.4% for 20 μM, 14.1% for 50 μM, and 15.8% for 100 μM ketamine) compared to the control. These results imply that ketamine inhibits the proliferation of cultured NSPCs in a dose-dependent manner (Figure 4F).
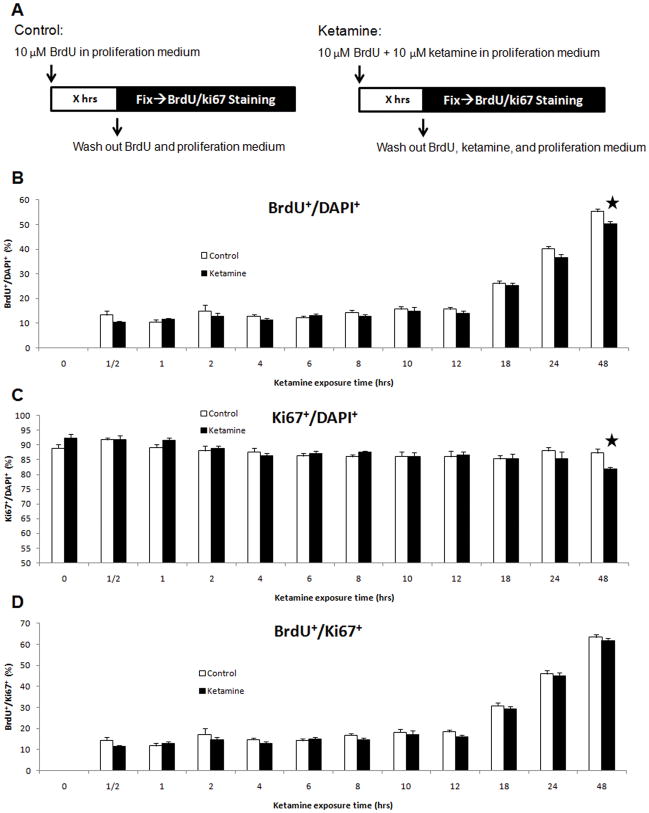
In the time-dependent assays, parallel controls were included for each duration of ketamine exposure (Figure 5A). Only long-term exposure (48 hours) to 10 μM of ketamine reduced the percentages of BrdU+/DAPI+ (t-tests, P<0.01) and Ki67+/DAPI+ (t-tests, P<0.01) cells compared to controls (Figures 5B–C). No significant changes occurred for the percentages of BrdU+/Ki67+ cells at any duration tested (Figure 5D). These data indicate that only long-term exposures to a clinically relevant concentration of ketamine can significantly inhibit the proliferation of NSPCs.
Figure 5. Time-dependent effects of ketamine on the proliferation of NSPCs.
Panel A: 10 μM of ketamine was added into cultures for different durations (X = 0,½, 1, 2, 4, 6, 8, 10, 12, 18, 24, and 48 hours) with parallel controls at each time point. Cultures were fixed and stained with anti-BrdU, anti-Ki67 antibodies and DAPI. Panels B–D: time-course response of ketamine on proliferation of cultured NSPCs. Panel B: the percentage of BrdU+ cells in total cells (DAPI+) vs. ketamine exposure time. Panel C: percentage of Ki67+ cells in the total cells (DAPI+) vs. ketamine exposure time. Panel D: the ratio of BrdU+ cells to Ki67+ cells vs. ketamine exposure time. (Stars represent the significant differences ketamine vs. control)
Ketamine enhances the neuronal differentiation of NSPCs
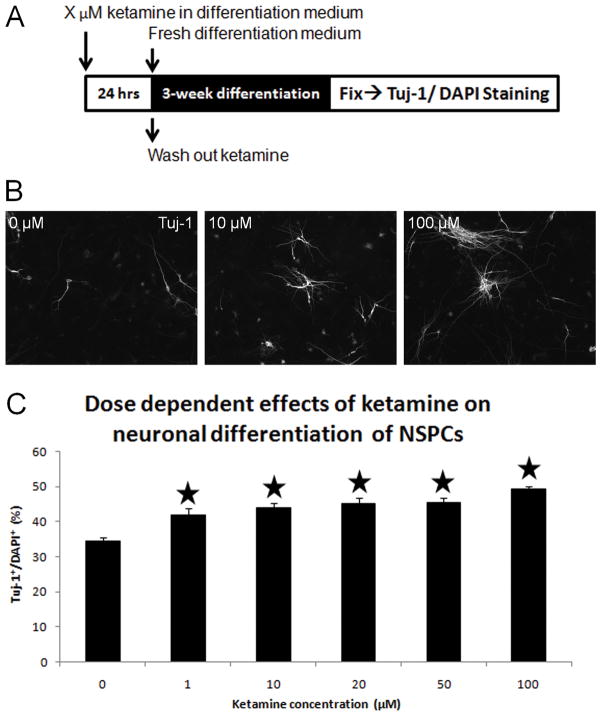
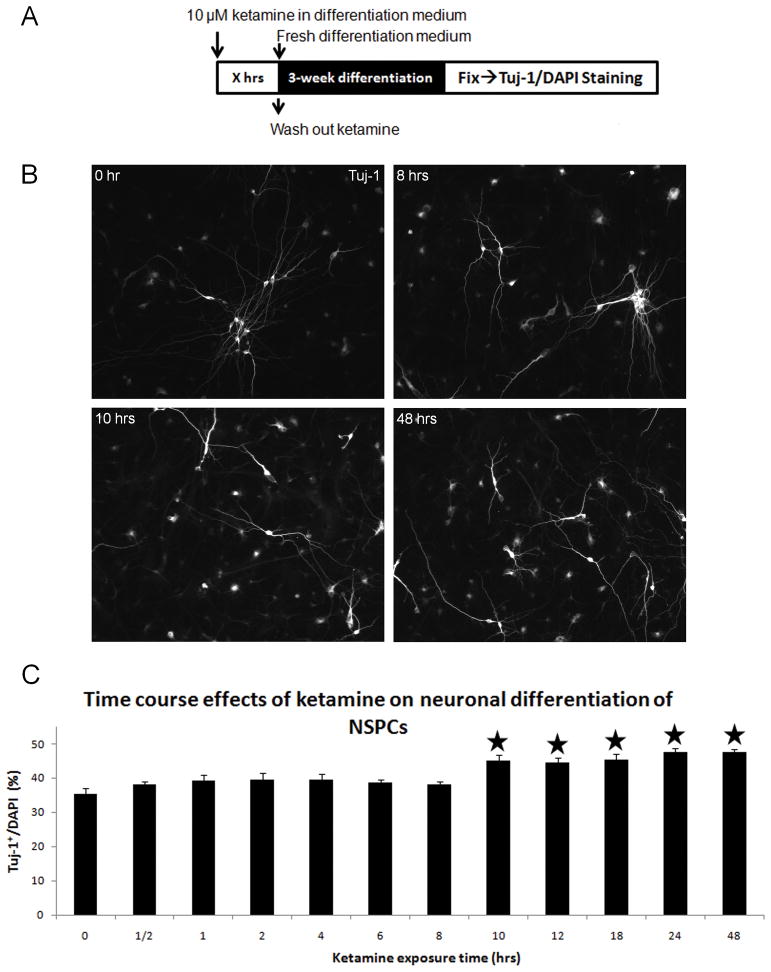
Neuronal differentiation of cultured NSPCs exposed to different concentrations of ketamine was detected using Tuj-1 staining. Without ketamine exposure, 34.3±4.1% of control NSPCs differentiated into newborn neurons (Tuj-1+). Compared with controls, all tested concentrations of ketamine significantly increased the percentages of Tuj-1+/DAPI+ cells (ANOVA, P<0.0001; Bonferroni, P<0.05) (Figure 6C). Typical Tuj-1 staining images of the 0, 10 and 100 μM ketamine groups are presented in Figure 6B, showing increases in Tuj-1+ staining in the 10 and 100 μM groups. In the time-dependent assays, short-term ketamine exposure did not induce any increase of neuronal differentiation in cultured NSPCs, whereas the long-term exposures (10 hours or longer) significantly enhanced differentiation of cultured NSPCs into neurons (ANOVA, P<0.0001; Bonferroni, P<0.01) (Figure 7). These data indicate that ketamine promotes neuronal differentiation of cultured NSPCs in time- and dose-dependent manners.
Figure 6. Dose-dependent effects of ketamine on neuronal differentiation of NPSCs.
Panel A: dose-dependent effects of ketamine on neuronal differentiation of NSPCs exposed to ketamine (concentration: X = 0, 1, 10, 20, 50, and 100 μM) over a 3-week spontaneous differentiation phase. Panel B: Tuj-1 staining of differentiated NSPCs exposed to 0, 10, and 100 μM of ketamine for 24 hours. Panel C: changes in the percentage of the Tuj-1+ cells in total cells (DAPI+) vs. ketamine concentrations. (Stars represent the significant differences ketamine vs. control)
Figure 7. Time-dependent effects of ketamine on neuronal differentiation of NSPCs.
Panel A: NSPC cultures were exposed to 10 μM of ketamine for different durations (X = 0, ½, 1, 2, 4, 6, 8, 10, 12, 18, 24, and 48 hours). Panel B: Tuj-1 staining of differentiated NSPCs exposed to 10 μM of ketamine for 0, 8, 10, and 48 hours. Panel C: time-dependent effects of ketamine on neuronal differentiation of NSPCs. (Stars represent the significant differences ketamine vs. control)
Discussion
Ketamine sedation and anesthesia are widely used for children and infants, including preterm neonates (27, 28). Developmental changes occurring at these stages may involve mature neurons and NSPCs. In vivo and in vitro animal studies have reported ketamine-induced neuronal apoptosis in the newborn brain (5, 8, 29), with specific brain regions being highly vulnerable to NMDAR blockade in early development (3). Prolonged ketamine anesthesia (24 hours) in newborn monkeys was also associated with long-term cognitive deficits (30). However, neurotoxic effects of ketamine exposure in neurons cannot be extrapolated to NSPCs, because the mechanisms regulating cell signaling, survival and cell death are significantly different between neurons and their progenitors (10, 31, 32).
In contrast to previous findings, we found that cultured NSPCs were resistant to apoptotic and necrotic cell death across a broad range of concentrations and durations of ketamine exposure. Prolonged exposure (48 hours) and high concentrations (20, 50, 100μM) of ketamine significantly inhibited the proliferation of NSPCs. Ketamine exposures also enhanced neuronal differentiation at all concentrations tested for 24 hours (1–100μM) and at clinically relevant concentrations (10μM) for 10 hours or longer.
One explanation for the resistance of NSPCs to ketamine-induced cell death (necrosis or apoptosis) may be the lack of functional NMDARs. Glutamate receptor stimulation does not induce Ca2+ influx into neural stem cells, suggesting a lack of functional NMDA activity (33). In neural precursor cells of the postnatal olfactory bulb, increased calcium permeability was mediated by AMPA receptors not NMDA receptors, although high doses of glutamate could induce intracellular calcium currents (34). Despite the robust expression of NMDAR subunits, functional NMDARs in NSPCs are not expressed until a later stage of neuronal commitment or initiation of synaptic communication (35–37). Thus, ketamine exposure may not lead to neurotoxicity in cultured NSPCs possibly due to the absence of functional NMDARs.
The neurogenesis of NSPCs plays a pivotal role in early neural development (38). Any factors disturbing the precisely controlled and highly regulated cellular events during neurogenesis could result in abnormalities in brain structure and function (39). Exposing NSPCs to high concentrations of ketamine (20, 50, 100 μM) for 24 hours or therapeutic concentrations of ketamine (10 μM) for 48 hours notably inhibited their proliferation rates. Consistent with our findings, a recent study reported that high-dose S(+) ketamine dose-dependently blocked proliferation in the post-ischemic rat brain (40). Another in vitro study also found that proliferation of NSPCs in cultured neurospheres was grossly inhibited by MK-801, an NMDAR antagonist like ketamine (16).
The role of NMDARs in hippocampal neurogenesis in the normal or post-injured animal models remains controversial (12, 41–43), partly because of the different models used. Previous studies focused on neurogenesis in the adult hippocampus, which may present different biological properties from those of cortical fetal NSPCs. Also, those studies used MK-801, which is a more specific NMDAR antagonist than ketamine. Ketamine interacts with many other receptors, such as dopaminergic, serotonergic, and other receptors (44). Neuronal proliferation in the hippocampus may also be affected by NMDA blockade-induced potential changes in the local microenvironments, including cytokine secretion, activation or deactivation of neurons and astrocytes (45).
NMDAR activation promotes the neuronal differentiation of NPCs (11, 19, 46, 47), whereas we found that ketamine-induced NMDA blockade also promoted the neuronal differentiation of cultured NSPCs. In our experiments, ketamine may have induced the expression of NMDA receptor subunits in NSPCs exposed for 24 hours. Previous studies have also reported that ketamine induces expression of the NR1 subunit in neurons(8). As neuronal differentiation proceeds, we posit that ketamine may upregulate the functional NMDARs formed, thus enabling increased calcium influx (37) and promoting the neuronal differentiation of NPSCs (48). We also found that 10-hour or longer exposures to 10 μM ketamine resulted in significantly increased neuronal differentiation, thereby potentially creating the conditions for synaptogenesis and NMDAR-mediated calcium influx. Further studies will examine the expression and functional activity of NMDARs in the cultured NSPCs exposed to ketamine.
The consequences of ketamine-induced changes in neurogenesis in the developing brain are speculative. First, since proliferation of NSPCs in the VZ is inhibited, this could reduce the cellularity of the developing brain, leading to a paucity of the neurons/glia required for normal brain development. Second, earlier differentiation of NSPCs during neuronal migration may result in abnormally positioned neurons within the cortical layers. Finally, ketamine exposure in utero could disturb the internal structure of the cortex and the formation of neuronal circuits, possibly leading to altered brain functions. Syndromes associated with the migration arrest of immature neurons are associated with seizures, cognitive deficits, and other clinical features (49, 50).
A pharmacological model for schizophrenia based on NMDA receptor hypofunction was noted following acute (51, 52) or chronic (53–55) administration of NMDA receptor antagonists (phencyclidine, ketamine, MK-801). As noted in this study, the proliferation of NSPCs is also reduced in schizophrenia (56). However, the validity of the NMDA hypofunction theory has been questioned as a model for human schizophrenia (51, 57). For example, ketamine exposure fails to match the cognitive and mood dysfunctions accompanying schizophrenia (57). Additionally, since schizophrenia tends to not manifest until adolescence it is difficult to assess whether reduced numbers of embryonic or newborn NSPCs would predispose an individual to manifest schizophrenia in the future. We propose in vivo studies to investigate these ketamine-induced changes in neurogenesis and their functional consequences.
Human NSPCs exist throughout the entire life span. However, adult NPCs have different biological characteristics from fetal NSPCs, such as proliferation capacity and differentiation direction. In the adult brain, it is likely more NPCs would differentiate into glia to repair and protect damaged brain regions(58). Our NSPCs were isolated from the VZ and SVZ of rat fetal cortices. In the adult, NPCs are only located at the SVZ and the VZ is absent after brain development. Therefore, we cannot extrapolate our findings from fetal cortical NSPCs to adult cortical NPCs. These findings imply, however, that ketamine could also affect the neurogenesis of adult NPCs in the post-traumatic brain. Assuming that ketamine leads to similar changes in adult NPCs as with fetal NSPCs, ketamine exposure during neurosurgical anesthesia or other neurological illnesses could potentially damage the repair mechanisms operating in the mature brain.
This study successfully utilized cultured fetal NSPCs as a research tool for evaluating the developmental neurotoxicity of ketamine. Ketamine does not induce cell death in cultured NSPCs but inhibits the proliferation and enhances the neuronal differentiation of NSPCs in a dose- and time-dependent manner. The developmental neurotoxicity of other anesthetics, sedatives, and analgesics, or various drug combinations can be investigated using a similar approach. Future studies will be conducted to confirm these findings using in vivo rodent or nonhuman primate models, and even cultured human NSPCs, and to investigate the underlying cellular mechanisms.
Acknowledgments
This work is attributed to the Department of Pediatrics, University of Tennessee Health Science Center.
This work was support by the funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) via U10-HD50009, the Oakley Endowed Chair of Critical Care Medicine, Little Rock, AR, St. Jude Chair of Critical Care Medicine, Memphis, TN and the Arkansas Children’s Hospital Student and Clinical Staff Research Grant, Little Rock, AR.
Footnotes
The authors have not disclosed any potential conflicts of interest
References
- 1.Sinner B, Graf BM. Ketamine. Handb Exp Pharmacol. 2008;182:313–333. doi: 10.1007/978-3-540-74806-9_15. [DOI] [PubMed] [Google Scholar]
- 2.Rappaport B, Mellon RD, Simone A, et al. Defining safe use of anesthesia in children. N Engl J Med. 2011;364(15):1387–1390. doi: 10.1056/NEJMp1102155. [DOI] [PubMed] [Google Scholar]
- 3.Ikonomidou C, Bosch F, Miksa M, et al. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283(5398):70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- 4.Scallet AC, Schmued LC, Slikker W, Jr, et al. Developmental neurotoxicity of ketamine: morphometric confirmation, exposure parameters, and multiple fluorescent labeling of apoptotic neurons. Toxicol Sci. 2004;81(2):364–370. doi: 10.1093/toxsci/kfh224. [DOI] [PubMed] [Google Scholar]
- 5.Slikker W, Jr, Zou X, Hotchkiss CE, et al. Ketamine-induced neuronal cell death in the perinatal rhesus monkey. Toxicol Sci. 2007;98(1):145–158. doi: 10.1093/toxsci/kfm084. [DOI] [PubMed] [Google Scholar]
- 6.Takadera T, Ishida A, Ohyashiki T. Ketamine-induced apoptosis in cultured rat cortical neurons. Toxicol Appl Pharmacol. 2006;210(1–2):100–107. doi: 10.1016/j.taap.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Walker SM, Westin BD, Deumens R, et al. Effects of intrathecal ketamine in the neonatal rat: evaluation of apoptosis and long-term functional outcome. Anesthesiology. 2010;113(1):147–159. doi: 10.1097/ALN.0b013e3181dcd71c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang C, Sadovova N, Fu X, et al. The role of the N-methyl-D-aspartate receptor in ketamine-induced apoptosis in rat forebrain culture. Neuroscience. 2005;132(4):967–977. doi: 10.1016/j.neuroscience.2005.01.053. [DOI] [PubMed] [Google Scholar]
- 9.Zou X, Patterson TA, Divine RL, et al. Prolonged exposure to ketamine increases neurodegeneration in the developing monkey brain. Int J Dev Neurosci. 2009;27(7):727–731. doi: 10.1016/j.ijdevneu.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Lindsten T, Golden JA, Zong WX, et al. The proapoptotic activities of Bax and Bak limit the size of the neural stem cell pool. J Neurosci. 2003;23(35):11112–11119. doi: 10.1523/JNEUROSCI.23-35-11112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joo JY, Kim BW, Lee JS, et al. Activation of NMDA receptors increases proliferation and differentiation of hippocampal neural progenitor cells. J Cell Sci. 2007;120(Pt 8):1358–1370. doi: 10.1242/jcs.002154. [DOI] [PubMed] [Google Scholar]
- 12.Kitayama T, Yoneyama M, Tamaki K, et al. Regulation of neuronal differentiation by N-methyl-D-aspartate receptors expressed in neural progenitor cells isolated from adult mouse hippocampus. J Neurosci Res. 2004;76(5):599–612. doi: 10.1002/jnr.20095. [DOI] [PubMed] [Google Scholar]
- 13.Kitayama T, Yoneyama M, Yoneda Y. Possible regulation by N-methyl-d-aspartate receptors of proliferative progenitor cells expressed in adult mouse hippocampal dentate gyrus. J Neurochem. 2003;84(4):767–780. doi: 10.1046/j.1471-4159.2003.01567.x. [DOI] [PubMed] [Google Scholar]
- 14.Luk KC, Kennedy TE, Sadikot AF. Glutamate promotes proliferation of striatal neuronal progenitors by an NMDA receptor-mediated mechanism. J Neurosci. 2003;23(6):2239–2250. doi: 10.1523/JNEUROSCI.23-06-02239.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luk KC, Sadikot AF. Glutamate and regulation of proliferation in the developing mammalian telencephalon. Dev Neurosci. 2004;26(2–4):218–228. doi: 10.1159/000082139. [DOI] [PubMed] [Google Scholar]
- 16.Mochizuki N, Takagi N, Kurokawa K, et al. Effect of NMDA receptor antagonist on proliferation of neurospheres from embryonic brain. Neurosci Lett. 2007;417(2):143–148. doi: 10.1016/j.neulet.2007.02.066. [DOI] [PubMed] [Google Scholar]
- 17.Sah DW, Ray J, Gage FH. Bipotent progenitor cell lines from the human CNS. Nat Biotechnol. 1997;15(6):574–580. doi: 10.1038/nbt0697-574. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki M, Nelson AD, Eickstaedt JB, et al. Glutamate enhances proliferation and neurogenesis in human neural progenitor cell cultures derived from the fetal cortex. Eur J Neurosci. 2006;24(3):645–653. doi: 10.1111/j.1460-9568.2006.04957.x. [DOI] [PubMed] [Google Scholar]
- 19.Yoneyama M, Nakamichi N, Fukui M, et al. Promotion of neuronal differentiation through activation of N-methyl-D-aspartate receptors transiently expressed by undifferentiated neural progenitor cells in fetal rat neocortex. J Neurosci Res. 2008;86(11):2392–2402. doi: 10.1002/jnr.21696. [DOI] [PubMed] [Google Scholar]
- 20.Feliciano DM, Edelman AM. Repression of Ca2+/calmodulin-dependent protein kinase IV signaling accelerates retinoic acid-induced differentiation of human neuroblastoma cells. J Biol Chem. 2009;284(39):26466–26481. doi: 10.1074/jbc.M109.027680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitney NP, Peng H, Erdmann NB, et al. Calcium-permeable AMPA receptors containing Q/R-unedited GluR2 direct human neural progenitor cell differentiation to neurons. FASEB J. 2008;22(8):2888–2900. doi: 10.1096/fj.07-104661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aruffo C, Ferszt R, Hildebrandt AG, et al. Low doses of L-monosodium glutamate promote neuronal growth and differentiation in vitro. Dev Neurosci. 1987;9(4):228–239. doi: 10.1159/000111625. [DOI] [PubMed] [Google Scholar]
- 23.Bading H, Segal MM, Sucher NJ, et al. N-methyl-D-aspartate receptors are critical for mediating the effects of glutamate on intracellular calcium concentration and immediate early gene expression in cultured hippocampal neurons. Neuroscience. 1995;64(3):653–664. doi: 10.1016/0306-4522(94)00462-e. [DOI] [PubMed] [Google Scholar]
- 24.Palmer TD, Markakis EA, Willhoite AR, et al. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J Neurosci. 1999;19(19):8487–8497. doi: 10.1523/JNEUROSCI.19-19-08487.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuwagata M, Ogawa T, Nagata T, et al. The evaluation of early embryonic neurogenesis after exposure to the genotoxic agent 5-bromo-2′-deoxyuridine in mice. Neurotoxicology. 2007;28(4):780–789. doi: 10.1016/j.neuro.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 26.Taupin P. BrdU immunohistochemistry for studying adult neurogenesis: paradigms, pitfalls, limitations, and validation. Brain Res Rev. 2007;53(1):198–214. doi: 10.1016/j.brainresrev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Tobias JD, Martin LD, Wetzel RC. Ketamine by continuous infusion for sedation in the pediatric intensive care unit. Crit Care Med. 1990;18(8):819–821. doi: 10.1097/00003246-199008000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Garcia Guerra G, Robertson CM, Alton GY, et al. Neurodevelopmental outcome following exposure to sedative and analgesic drugs for complex cardiac surgery in infancy(*) Paediatr Anaesth. 2011;21(9):932–941. doi: 10.1111/j.1460-9592.2011.03581.x. [DOI] [PubMed] [Google Scholar]
- 29.Zou X, Patterson TA, Sadovova N, et al. Potential neurotoxicity of ketamine in the developing rat brain. Toxicol Sci. 2009;108(1):149–158. doi: 10.1093/toxsci/kfn270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paule MG, Li M, Allen RR, et al. Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicol Teratol. 2011;33(2):220–230. doi: 10.1016/j.ntt.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsieh WY, Hsieh YL, Liu DD, et al. Neural progenitor cells resist excitatory amino acid-induced neurotoxicity. J Neurosci Res. 2003;71(2):272–278. doi: 10.1002/jnr.10476. [DOI] [PubMed] [Google Scholar]
- 32.Brazel CY, Nunez JL, Yang Z, et al. Glutamate enhances survival and proliferation of neural progenitors derived from the subventricular zone. Neuroscience. 2005;131(1):55–65. doi: 10.1016/j.neuroscience.2004.10.038. [DOI] [PubMed] [Google Scholar]
- 33.Ono T, Hashimoto E, Ukai W, et al. The role of neural stem cells for in vitro models of schizophrenia: neuroprotection via Akt/ERK signal regulation. Schizophr Res. 2010;122(1–3):239–247. doi: 10.1016/j.schres.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 34.Darcy DP, Isaacson JS. Calcium-permeable AMPA receptors mediate glutamatergic signaling in neural precursor cells of the postnatal olfactory bulb. J Neurophysiol. 2010;103(3):1431–1437. doi: 10.1152/jn.00821.2009. [DOI] [PubMed] [Google Scholar]
- 35.Jelitai M, Schlett K, Varju P, et al. Regulated appearance of NMDA receptor subunits and channel functions during in vitro neuronal differentiation. J Neurobiol. 2002;51(1):54–65. doi: 10.1002/neu.10049. [DOI] [PubMed] [Google Scholar]
- 36.Varju P, Schlett K, Eisel U, et al. Schedule of NMDA receptor subunit expression and functional channel formation in the course of in vitro-induced neurogenesis. J Neurochem. 2001;77(6):1444–1456. doi: 10.1046/j.1471-4159.2001.00352.x. [DOI] [PubMed] [Google Scholar]
- 37.Karkkainen V, Louhivuori V, Castren ML, et al. Neurotransmitter responsiveness during early maturation of neural progenitor cells. Differentiation. 2009;77(2):188–198. doi: 10.1016/j.diff.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Ferguson KL, Slack RS. Growth factors: can they promote neurogenesis? Trends Neurosci. 2003;26(6):283–285. doi: 10.1016/S0166-2236(03)00100-0. [DOI] [PubMed] [Google Scholar]
- 39.Breier JM, Gassmann K, Kayser R, et al. Neural progenitor cells as models for high-throughput screens of developmental neurotoxicity: state of the science. Neurotoxicol Teratol. 2010;32(1):4–15. doi: 10.1016/j.ntt.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 40.Winkelheide U, Lasarzik I, Kaeppel B, et al. Dose-dependent effect of S(+) ketamine on post-ischemic endogenous neurogenesis in rats. Acta Anaesthesiol Scand. 2009;53(4):528–533. doi: 10.1111/j.1399-6576.2009.01905.x. [DOI] [PubMed] [Google Scholar]
- 41.Kluska MM, Witte OW, Bolz J, et al. Neurogenesis in the adult dentate gyrus after cortical infarcts: effects of infarct location, N-methyl-D-aspartate receptor blockade and anti-inflammatory treatment. Neuroscience. 2005;135(3):723–735. doi: 10.1016/j.neuroscience.2005.06.082. [DOI] [PubMed] [Google Scholar]
- 42.Tamaki K, Yamada K, Nakamichi N, et al. Transient suppression of progenitor cell proliferation through NMDA receptors in hippocampal dentate gyrus of mice with traumatic stress experience. J Neurochem. 2008;105(5):1642–1655. doi: 10.1111/j.1471-4159.2008.05253.x. [DOI] [PubMed] [Google Scholar]
- 43.Yanamoto H, Miyamoto S, Tohnai N, et al. Induced spreading depression activates persistent neurogenesis in the subventricular zone, generating cells with markers for divided and early committed neurons in the caudate putamen and cortex. Stroke. 2005;36(7):1544–1550. doi: 10.1161/01.STR.0000169903.09253.c7. [DOI] [PubMed] [Google Scholar]
- 44.Chizh BA. Low dose ketamine: a therapeutic and research tool to explore N-methyl-D-aspartate (NMDA) receptor-mediated plasticity in pain pathways. J Psychopharmacol. 2007;21(3):259–271. doi: 10.1177/0269881105062484. [DOI] [PubMed] [Google Scholar]
- 45.Jander S, Schroeter M, Stoll G. Role of NMDA receptor signaling in the regulation of inflammatory gene expression after focal brain ischemia. J Neuroimmunol. 2000;109(2):181–187. doi: 10.1016/s0165-5728(00)00317-9. [DOI] [PubMed] [Google Scholar]
- 46.Deisseroth K, Singla S, Toda H, et al. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42(4):535–552. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- 47.Pearce IA, Cambray-Deakin MA, Burgoyne RD. Glutamate acting on NMDA receptors stimulates neurite outgrowth from cerebellar granule cells. FEBS Lett. 1987;223(1):143–147. doi: 10.1016/0014-5793(87)80525-2. [DOI] [PubMed] [Google Scholar]
- 48.Muth-Kohne E, Pachernegg S, Karus M, et al. Expression of NMDA receptors and Ca2+-impermeable AMPA receptors requires neuronal differentiation and allows discrimination between two different types of neural stem cells. Cell Physiol Biochem. 2010;26(6):935–946. doi: 10.1159/000324002. [DOI] [PubMed] [Google Scholar]
- 49.Noctor SC, Martinez-Cerdeno V, Ivic L, et al. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7(2):136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- 50.Noctor SC, Palmer SL, Hasling T, et al. Interference with the development of early generated neocortex results in disruption of radial glia and abnormal formation of neocortical layers. Cerebral Cortex. 1999;9(2):121–136. doi: 10.1093/cercor/9.2.121. [DOI] [PubMed] [Google Scholar]
- 51.Adell A, Jimenez-Sanchez L, Lopez-Gil X, et al. Is the Acute NMDA Receptor Hypofunction a Valid Model of Schizophrenia? Schizophr Bull. 2012;38(1):9–14. doi: 10.1093/schbul/sbr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Radant AD, Bowdle TA, Cowley DS, et al. Does ketamine-mediated N-methyl-D-aspartate receptor antagonism cause schizophrenia-like oculomotor abnormalities? Neuropsychopharmacology. 1998;19(5):434–444. doi: 10.1016/S0893-133X(98)00030-X. [DOI] [PubMed] [Google Scholar]
- 53.Rujescu D, Bender A, Keck M, et al. A pharmacological model for psychosis based on N-methyl-D-aspartate receptor hypofunction: molecular, cellular, functional and behavioral abnormalities. Biol Psychiatry. 2006;59(8):721–729. doi: 10.1016/j.biopsych.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 54.Baier PC, Blume A, Koch J, et al. Early postnatal depletion of NMDA receptor development affects behaviour and NMDA receptor expression until later adulthood in rats--a possible model for schizophrenia. Behav Brain Res. 2009;205(1):96–101. doi: 10.1016/j.bbr.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 55.Farber NB, Wozniak DF, Price MT, et al. Age-specific neurotoxicity in the rat associated with NMDA receptor blockade: potential relevance to schizophrenia? Biol Psychiatry. 1995;38(12):788–796. doi: 10.1016/0006-3223(95)00046-1. [DOI] [PubMed] [Google Scholar]
- 56.Reif A, Fritzen S, Finger M, et al. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry. 2006;11(5):514–522. doi: 10.1038/sj.mp.4001791. [DOI] [PubMed] [Google Scholar]
- 57.Gilmour G, Dix S, Fellini L, et al. NMDA receptors, cognition and schizophrenia - Testing the validity of the NMDA receptor hypofunction hypothesis. Neuropharmacology. 2012;62(3):1401–1412. doi: 10.1016/j.neuropharm.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 58.Kazanis I. Can adult neural stem cells create new brains? Plasticity in the adult Mammalian neurogenic niches: realities and expectations in the era of regenerative biology. Neuroscientist. 2012;18(1):15–27. doi: 10.1177/1073858410390379. [DOI] [PubMed] [Google Scholar]