Abstract
Variations in the predominance of an object engaged in binocular rivalry may arise from variations in the durations of dominance phases, suppression phases, or both. Earlier work has shown that the predominance of a binocular rival target is enhanced if that target fits well—via common color, orientation, or motion—with its surrounding objects. In the present experiments, the global context outside of the region of rivalry was changed during rivalry, to learn whether contextual information alters the ability to detect changes in a suppressed target itself. Results indicate that context will maintain the dominance of a rival target, but will not encourage a suppressed target to escape from suppression. Evidently, the fate of the suppressed stimulus is determined by neural events distinct from those responsible for global organization during dominance. To reconcile diverse findings concerning rivalry, it may be important to distinguish between processes responsible for selection of one eye’s input for dominance from processes responsible for the implementation and maintenance of suppression.
1 Introduction
Vision can be confusing: when confronted with ambiguous optical information, human vision can lapse into a state of uncertainty wherein perceptual experience fluctuates repeatedly and unpredictably between multiple alternative visual interpretations. Familiar examples of this confused behavior include bistable perception associated with viewing the vase/face figure (Rubin 1921), the Necker cube (Necker 1832), and ambiguous optic flow specifying rotating objects (Wallach and O’Connell 1953). In addition to providing engaging demonstrations of the ‘constructive’ nature of perception, multi-stable perceptual phenomena represent a potentially powerful tool for studying the neural concomitants of visual awareness (Logothetis 1998)—fluctuations in perception in the absence of changing physical stimulation implicate changing neural activity at some stages of visual processing. In this paper, we exploit one well-known form of multistability—binocular rivalry—to learn how human vision exploits global context in the resolution of conflict.
When incompatible images are viewed dichoptically, those images resist binocular fusion and, instead, undergo alternating periods of dominance and suppression; this is the well-known phenomenon of binocular rivalry. Since the early work of Diaz-Caneja (1928; see Alais et al 2000 for a translation into English), it has been known that spatially distributed stimulus features can be simultaneously dominant in rivalry when those features together form a coherent, global form. Consider, for example, a recent study by Kovács et al (1996). They presented pairs of rival targets, each comprising red spots and green spots distributed throughout a relatively large expanse of the visual field; the green spots in one eye’s image were placed at the same location as red spots in the other eye’s image, and vice versa, thus producing the conditions for rivalry throughout the array. Because of the interocular distribution of colors, an overall impression of all green spots could only be formed by the simultaneous dominance of spatially distributed spots from the images in both eyes. In fact, the periods in which the perceived image comprised dots of a single color were more common than expected on the basis of independent simultaneous dominance of all spots of a given color. Features other than color, including shared motion and good continuation of form, can also promote the simultaneous dominance of widely separated stimulus elements (Alais and Blake 1998, 1999; Ngo et al 2000; Whittle et al 1968). Considered together, these results imply that dominance phases of spatially distributed rival features can become entrained when those features form a spatially coherent context.
In addition to encouraging dominance entrainment, spatial coherence can also boost the overall predominance of a rival target, where predominance refers to the total percentage of the entire viewing period that the target is dominant. How does this boost in predominance come about? It is important to keep in mind that a given target can enjoy enhanced predominance for any of several reasons: (a) it remains dominant for longer periods of time, on average; (b) it remains suppressed for briefer periods of time, on average; or (c) it remains dominant for longer durations and suppressed for shorter durations. Because binocular rivalry likely involves multiple neural operations distributed over different stages of visual processing (eg Blake 2001; Nguyen et al 2001; Ooi and He 1999), it is important to ask just how changes in predominance with context are produced, for an answer to that question can provide important guidelines in the search for the neural concomitants of binocular rivalry. Results from the experiments reported here support the conclusion that context influences predominance by affecting dominance phases of rivalry, not suppression phases.
2 Experiment 1: Local/global motion
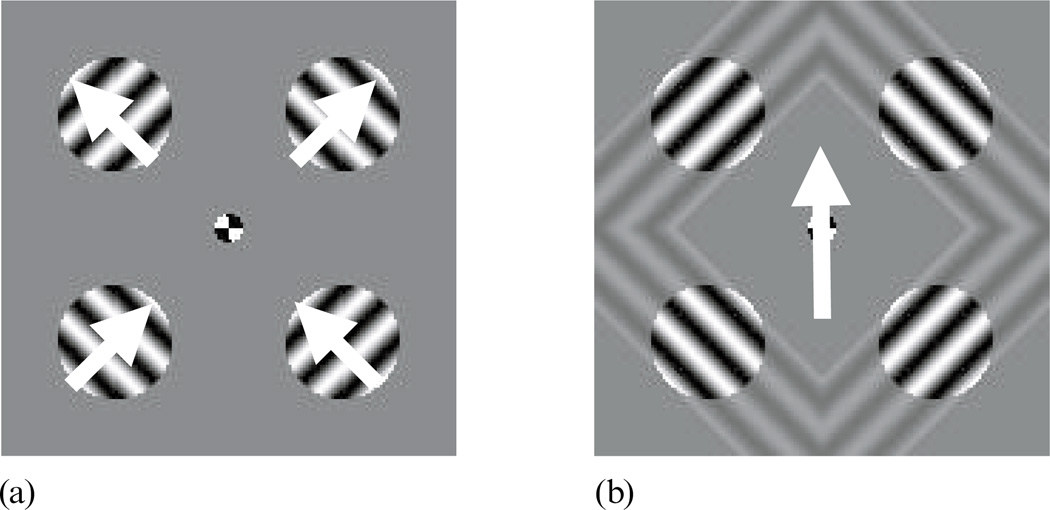
In this experiment, we document the effect of context on predominance using a multi-component motion display, and we then determine how changes in that display affect dominance and suppression durations. The display presented to one eye consisted of four sinusoidal gratings, each appearing within a circular aperture whose center was at one of the vertices of an imaginary square. The gratings in the upper-right and lower-left corner were oriented 458 counterclockwise from vertical, and the upper-left and lower-right gratings were oriented 458 clockwise from vertical. When the gratings in such a display drift in the directions indicated by the arrows in figure 1a, they fluctuate in perceived direction of motion, sometimes appearing to move in a direction orthogonal to their contour orientations (local motion) and at other times appearing to move upward (global motion) in a configuration resembling a diamond, as illustrated in figure 1b (Alais et al 1998; Lorenceau and Shiffrar 1992). It is important to note that the presence of all four gratings is crucial for perception of global motion; if just one is missing or drifting in an inappropriate direction, the sense of coherent motion is substantially reduced (Alais and Blake 1998).
Figure 1.
When four gratings drift in the directions indicated in (a), they intermittently appear to form a single, diamond-shaped figure drifting upward and seen through apertures, as depicted in (b).
Experiment 1 consisted of three phases, the first being a tracking task simply to confirm that global context (in this case, coherent motion among the four gratings in the ‘apertures’) boosts the predominance of a rival target (in this case, one of the four gratings) for our particular display configurations. In the second phase of the experiment, we established that coherent motion was reliably perceived when the four gratings simultaneously began moving in their respective directions. In the third phase of the experiment, we measured the effect of coherent motion onset on dominance durations and suppression durations of the rival grating.
2.1 Observers
Both authors (KS and RB) and another experienced psychophysical observer (SL) who was naïve to the hypotheses tested in the experiment served as observers in this experiment. All are male, have corrected-to-normal vision, and ranged in age from 34 to 55 years.
2.2 Apparatus and stimuli
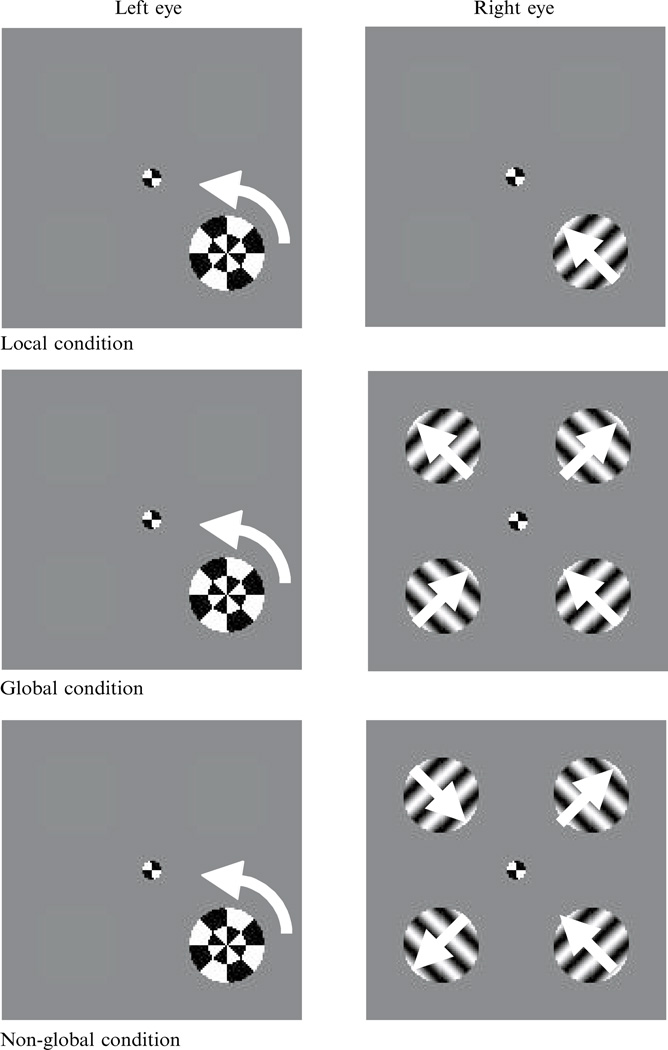
Stimuli were generated by an Apple 7600 Power PC computer and presented on an NEC MultiSync 21-inch monitor (85 Hz frame rate, 10246768 pixel resolution) with a linearized, gray-scale luminance output; the screen luminance provided the only light in the otherwise dark room. A mirror stereoscope was used to present displays to each eye separately, at a viewing distance of 89 cm. All stimuli were presented against a medium-gray (13.5 cd m−2) background. The fixation mark was a circle 0.35 deg in diameter, in which the upper-right and lower-left quadrant were black and the other two quadrants white (58.6 cd m−2). The image in each eye was surrounded by a square frame 4.4 deg on a side and 0.18 deg thick, with the frame consisting of alternating black and white (58.6 cd m−2) bands, each 0.49 deg long. The fixation mark and the checkered border served to promote stable binocular alignment. In the tracking and the rivalry duration tasks (the first and third parts of the experiment), the image presented to one eye contained a gray radial checkerboard with a contrast of 0.41, rotating counterclockwise at 0.36 revolutions s−1, imaged at the same spatial location as a grating presented to the other eye—these two dissimilar monocular targets engaged in unambiguous binocular rivalry. The image in the other eye contained either a single drifting grating (local motion), four drifting gratings that appeared to form a single diamond drifting upward (global motion), or four drifting gratings that moved in directions that did not engender the perception of coherent motion (non-global motion). The contrast of all gratings was 1.0 (the ‘trough’ portion of the grating being unmeasurably dark); the disparity in contrast allowed the rival gratings to predominate over the higher-energy checkerboard roughly half the time. The centers of the circular grating apertures—each of which had a diameter of 1.5 deg—were placed on the vertices of an imaginary square 1.6 deg on a side, and which was centered on the fixation mark. The gratings were 2.0 cycles deg−1 moving at 0.72 deg s−1. Because the second part of the experiment—the global-motion capture task—was not a rivalry task, the image presented to one eye was either the global or non-global motion configuration, but the image presented to the other eye contained only the fixation mark and surrounding frame. These displays are all depicted in figure 2.
Figure 2.
Displays for experiment 1. In the ‘local’ condition, a drifting grating in the display presented to one eye corresponded to a rotating radial checkerboard in the display presented to the other eye. In the ‘global’ condition, the target grating was accompanied by three gratings drifting in directions consistent with coherent global motion. In the ‘non-global’ condition, the accompanying gratings drifted in directions that could not be resolved into a single coherent pattern of motion.
2.3 Phase 1. Effect of context on rivalry predominance
2.3.1 Procedure
Observers pressed a key to begin each 60-s trial, during which they pressed and held one of two keys to indicate whether the target grating or the rotating checkerboard was exclusively dominant. (If either of the two keys was depressed at the end of the 60-s viewing period, the trial continued until that key was released.) Observers took a brief break after each trial. The three context conditions (local, global, and non-global motion) contained four repetitions of the tracking task, at all combinations of two eyes (grating contained in the right eye’s image and the checkerboard in the left eye’s image, or vice versa) and two checkerboard locations (upper-right or lower-left vertex), presented in random order. The target-grating predominance was calculated by dividing the amount of time it was visible in a single trial by the duration of that trial, then taking the average across the four trials in each condition.
2.3.2 Results and discussion
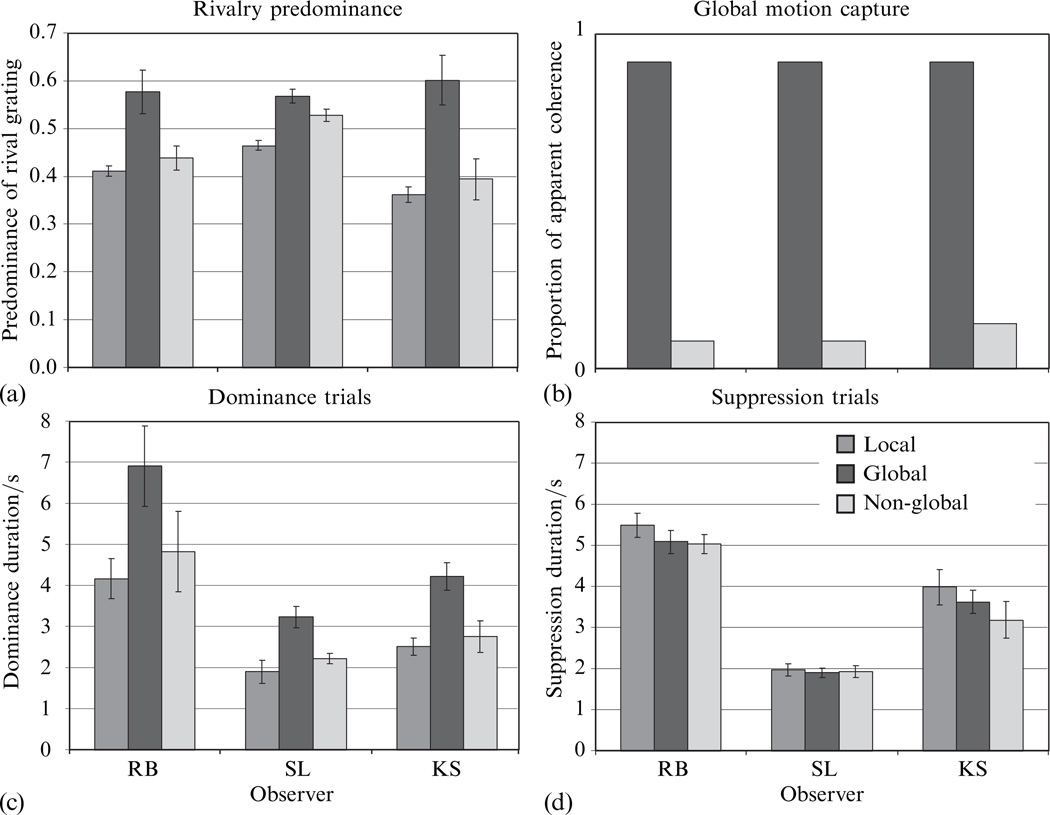
Because the outcome of the experiment was the same across different levels of the counterbalancing factors (quadrant and eye), the data were pooled across these factors; this was true for the data gathered in phases 2 and 3 as well. As can be seen in figure 3a, the predominance of the rival grating was enhanced when it appeared within a configuration that implied coherent, global motion—an effect that had been expected on the basis of earlier work (Alais and Blake 1998).
Figure 3.
Results from experiment 1. (a) In the tracking task, the target grating was more predominant in the global condition than either the non-global or local conditions. (b) In the global-motion-capture task, the drifting gratings appeared to be a single moving object only when the gratings drifted as in the ‘global’ condition, not the ‘non-global’ condition. (c) In dominance duration trials, the target grating remained dominant for a longer period of time in the global condition than in either the local or non-global conditions. (d) In suppression trials, the duration of suppression was no shorter in the global condition than in the other two conditions. The error bars represent ±1 SEM.
2.4 Phase 2. Global-motion capture
2.4.1 Procedure
The main part of the experiment (part 3) relies on the ability to immediately induce global coherent motion in a single grating. To validate our procedure, we presented an array of four gratings to one eye only, the other eye viewing an uncontoured field. Three of the four gratings were stationary, and the fourth drifted in a direction perpendicular to the orientation of its contours (local motion). Observers pressed a button which caused the other three gratings to begin drifting either in a configuration consistent with global upward motion or in a configuration inconsistent with global motion. At the end of a 1-s period, the gratings disappeared and observers indicated whether or not the initially local grating changed its apparent direction of motion (ie whether it remained local or was recruited into global motion) A total of 32 trials were administered, consisting of eight repetitions at each level of context (2) and eye (2), presented in random order.
2.4.2 Results and discussion
As documented by the results in figure 3b, a single grating drifting orthogonally to its contour appeared to move in a new direction when the three other gratings began to move in a configuration consistent with global motion upward. No such change in perceived direction of motion of the initially drifting grating was perceived, however, when the other three gratings began moving in a non-global configuration. These two findings—increased predominance and motion capture—lead to the question addressed by the main part of this experiment: does the introduction of global context prolong durations of dominance of a rival grating, abbreviate durations of suppression of that grating, or both?
2.5 Phase 3. Dominance/suppression
2.5.1 Procedure
At the beginning of each trial, observers viewed with one eye an array of four gratings: three stationary and one drifting grating that was engaged in rivalry with a rotating checkerboard in the image in the other eye. Observers pressed and held a key either when the rival grating was exclusively dominant or when the grating was completely suppressed. The authors ran the dominance trials in the first block and suppression trials in the second, whereas the naïve observer (SL) ran the two blocks in the opposite order. The key-press caused the three stationary gratings to begin drifting in the directions associated with either the global motion configuration or the non-global motion configuration. Observers released the key when the perceptual state of the rival grating changed (ie became suppressed during trials in which the button press coincided with the onset of dominance, or vice versa), at which point the checkerboard and grating(s) were removed from the display, leaving only the frames and fixation marks. Observers then pressed another key to display the elements for the following trial. Also included were control trials on which a single drifting grating simply continued drifting while the observer held the key until the rival state of that grating changed. Each block consisted of 72 trials: 3 repetitions of every combination of checkerboard location (4), eye to which the checkerboard was presented (2), and context (local, global, and non-global). The dependent variable was the duration of the dominance phase, or the suppression phase.
2.5.2 Results and discussion
The results from the measurements of dominance and suppression durations, shown in figures 3c and 3d, provide a clear answer to the question whether global context prolongs dominance, abbreviates suppression, or both: introduction of global context lengthens the average duration of dominance of the rival grating but has essentially no effect on the average duration of suppression of that grating. This pattern of results implies, then, that the increased predominance of a grating contained within a larger, global context (figure 3a) comes about only through a lengthening of its dominance durations. In this respect, global context operates quite differently from variations in stimulus strength of a rival target, which primarily affect suppression durations (Fox and Rasche 1969; Levelt 1965; Mueller and Blake 1989).
Now, one could argue that the effect of context on suppression is too subtle to be reflected in the successive durations of suppression. We find this argument unconvincing, since suppression durations do systematically vary with stimulus manipulations such as target contrast and contour density (Levelt 1965). Still, we felt it worthwhile to verify this conclusion using a more indirect, albeit potentially more sensitive index of suppression magnitude, an observer’s sensitivity to changes in a suppressed target (Blake and Fox 1974b; Fox and Check 1968; Freeman and Nguyen 2001; Norman et al 2000; O’Shea and Crassini 1981; Walker and Powell 1979). In the following experiment, we reversed the contrast polarity of a rival target during suppression, over a range of time courses. This change in the suppressed target occurred either in isolation or in concert with equivalent changes in neighboring but nonrivalling targets. Is change in the rival target easier to detect when it occurs in context?
3 Experiment 2: Context from shape-by-shading
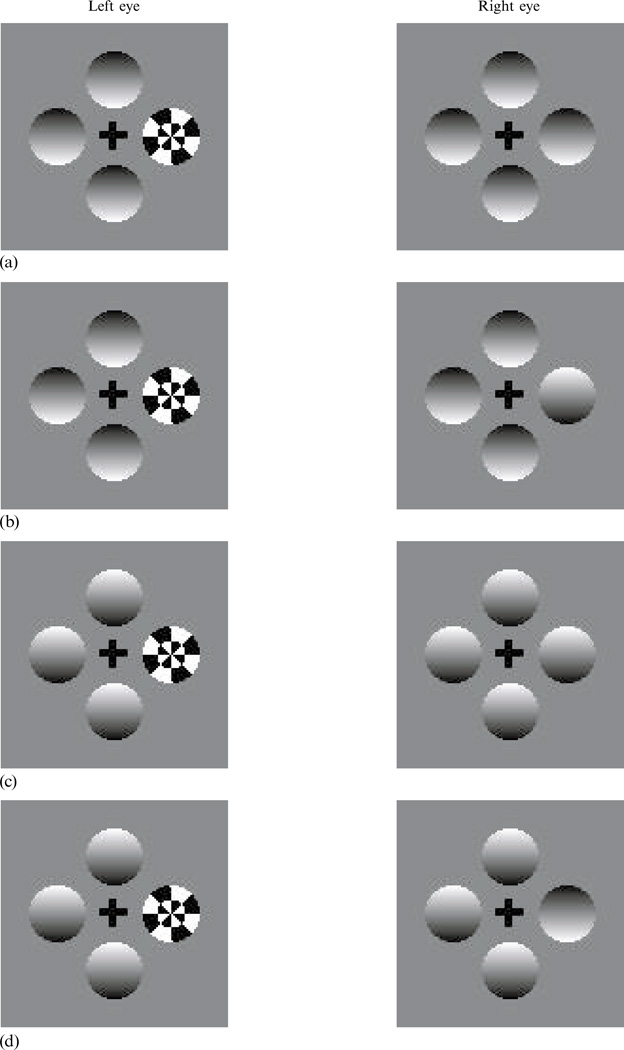
Consider the display in figure 4a. The right eye views a quartet of ‘dimples’ whose implied shading evokes the impression of convexity or concavity, and the left eye views the same array except for one region which is replaced with a radial grating. When one views these half-images through a stereoscope, the identical portions of the two images will be continuously visible, but the incompatible regions will rival: the right-hand dimple will appear and disappear over time as it rivals with the radial grating. In a pilot study, we confirmed that predominance of this rival dimple is enhanced by the presence of the context dimples, an effect similar to the one documented in figure 3a. We also confirmed that abruptly altering the contrast polarity of the rival dimple while it is suppressed triggers its return to dominance—the ‘new’ target becomes immediately visible; this observation is entirely consistent with earlier work (eg Walker and Powell 1979). Using figure 4a, readers may verify the potency of this kind of transient event first by fusing the right and left displays, then by flicking a finger in front of the rival dimple when it is suppressed.
Figure 4.
Visual displays for experiment 2. Represented here is an initial display for all four conditions (a), and a final display in conditions (ii) through (iv). (b) Condition (ii): the target dimple, but not the context dimples, reverses polarity. (c) Condition (iii): both the target and context dimples reverse polarity. (d) Condition (iv): the context dimples, but not the target dimple, reverse polarity.
But suppose the abrupt reversal in contrast polarity were to occur among the three ‘context’ dimples as well as the rival dimple itself. Does this global, contextual event enhance the escape of the rival dimple from suppression? Results from the following experiment show that it does not.
3.1 Observers
The same three individuals who took part in the previous experiment again served as observers in this experiment.
3.2 Apparatus and stimuli
The apparatus was identical to that in the previous experiment. The displays presented to both eyes contained a central fixation mark consisting of black vertical and horizontal bars 0.58 deg long by 0.081 deg wide surrounded by a white (58.6 cd m−2) square frame 4.7 deg on a side and 0.15 deg thick. In addition, the display presented to each eye contained either a radial checkerboard with a contrast of 0.82 or a ‘dimple’—ie a disc that appeared curved owing to a vertically oriented shading gradient produced by a linear ramp of luminance ranging from 2.04 to 37.2 cd m−2 (as in Kleffner and Ramachandran 1992)—with the dimple being centered on a point 1.16 deg to the right of the fixation mark. We will term this dimple the ‘target’ to distinguish it from the ‘context’ dimples which did not engage in rivalry. The context dimples were placed at the same distance from the fixation mark as the target, and at the other three cardinal directions, as in figure 4a.
3.3 Procedure
The contrast of the radial checkerboard flickered in counterphase at 5 Hz, to boost its potency as a rival target. On each trial of the detection task, observers viewed an array of four dimples, one of which rivaled with the checkerboard (figure 4a). When the checkerboard was exclusively dominant with no hint of the dimple, observers pressed a key, thereby initiating one of four conditions: (i) the display remained unchanged; (ii) the rival dimple, but not the context dimples, reversed polarity (figure 4b); (iii) all four dimples reversed polarity (figure 4c); or (iv) the three non-rival dimples, but not the rival dimple, reversed polarity (figure 4d). The duration over which the change in contrast polarity of the dimples occurred was varied from 250 to 1000 ms, in 250 ms steps. In each trial, the dimples and checkerboard disappeared 1250 ms after the initiating button-press. Each observer ran 2 blocks of 320 trials, each of which included 5 repetitions of each combination of condition (4), eye to which the checkerboard was presented (2), duration of polarity reversal (4), and polarity of rival dimple at the initiation of the trial (2: apparently convex or concave).
3.4 Results and discussion
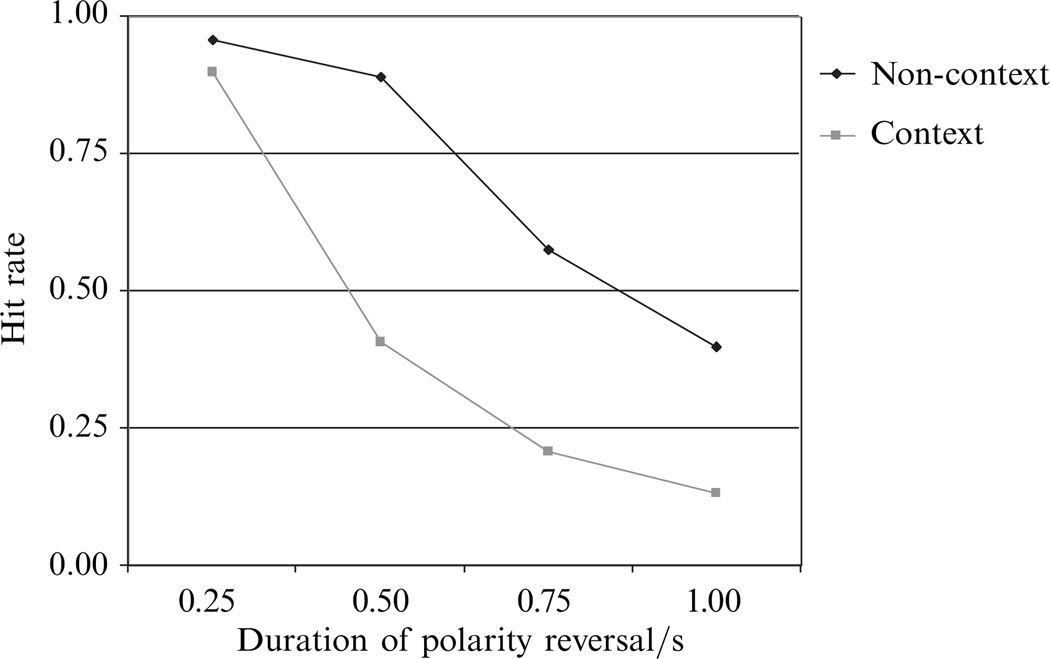
As in experiment 1, because there was no difference in performance between the two levels of the counterbalancing factor (rival target presented to the right or left eye), the reported data were pooled. On those trials where the rival dimple remained unchanged—conditions (i) and (iv)—observers almost never mistakenly said the dimple did change, ie the false-alarm rates for these two conditions were essentially zero. Of relevance for our purposes are the results plotted in figure 5, showing the hit rates for the two conditions in which the suppressed, rival dimple did change polarity, namely conditions (ii) (‘no context’) and (iii) (‘context’). In both conditions, the likelihood of detecting a change in the polarity of the rival dimple (hit rate) was highest when the polarity reversal was most abrupt, and fell as the polarity reversal was ever more gradual. This result is not at all surprising. For one thing, it is well established that abrupt transients are most effective in prematurely terminating suppression (Walker and Powell 1979), whereas gradual ones are not (Blake and Fox 1974b). For another, gradual changes in the contrast polarity of the rival target mean that the effective contrast of that target would be very low for an appreciable part of the trial duration. This, too, could adversely affect detection performance. What is perhaps surprising, however, is the effect of context: the hit rate was not higher in the context condition than in the non-context condition, meaning that concurrent changes to the surrounding dimples did not enhance the probability that changes to the rival dimple would terminate suppression. In fact, changes in the context dimples interfered with detection of an equivalent change in the rival dimple. Perhaps attentional resources are reflexively drawn to the context dimples when they change, thereby adversely affecting detection of the change in the rival dimple (Ooi and He 1999). Whatever the cause, it remains abundantly clear that the suppressed rival target is not benefiting from the context provided by the presence of other, nearby targets—a conclusion in line with the results of experiment 1.
Figure 5.
Results from experiment 2. Polarity reversals were easier to detect in the non-context condition than the context condition, meaning that concurrent changes in the context dimples did not help the target dimple break suppression.
4 General discussion
In the competition for perceptual dominance that characterizes binocular rivalry, a competing target can benefit from the company it keeps if, together with its companions, that competing target forms a coherent global figure. This observation, together with other work showing that dominance phases of spatially extended rival targets can become entrained (eg Diaz-Caneja 1928; Kovács et al 1996), underscores the role of global context in the resolution of local competition during rivalry. The present study goes one step further by showing that the effect of context on rivalry predominance operates primarily by lengthening dominance durations, not by abbreviating suppression durations. This finding has some potentially important implications.
First, the effect of context on dominance phases may explain how spatially distributed rival targets become perceptually entrained (Kovács et al 1996). Local eye-based ‘zones’ of rivalry may initially become dominant more or less independently, but as multiple zones portraying spatially coherent features become dominant simultaneously they mutually reinforce one another, lengthening their dominance durations and thereby automatically synchronizing their phases. This mutual reinforcement could even occur when spatially distributed, coherent features were distributed between the views presented to the two eyes, thereby promoting global coherence interocularly as Kovács et al described.
Turning to another implication of the present results, the differential effects of context on dominance and suppression serve as a reminder that rivalry is not an omnibus process mediated by a single neural mechanism, a point articulated by Fox (1991) among others. Instead, rivalry probably entails multiple, distributed neural operations, including those promoting dominance, those implementing suppression, and those responsible for alternations in dominance. The idea that rivalry involves multiple neural operations may help us reconcile diverse findings from brain imaging studies of humans experiencing rivalry (Lumer et al 1998; Polonsky et al 2000; Tong and Engel 2001; Tong et al 1998; Tononi et al 1998) and single-unit studies from awake, behaving monkeys experiencing rivalry (Logothetis 1998). Both lines of evidence indicate that neural concomitants of rivalry are detectable at early stages of visual processing, with those neural concomitants becoming more pronounced at higher stages. An emerging view, then, is that rivalry has no single ‘locus’ but, instead, is the perceptual outcome culminating from a cascade of neural events occurring throughout the visual pathways (Nguyen et al 2001). And, according to the present results, those distributed neural events may be subserving different aspects of rivalry. Why do we say this?
During binocular rivalry, neural information associated with the currently dominant stimulus flows throughout the entire visual hierarchy, engaging the same neural operations as those activated during non-rival stimulation. Depending on the particulars of the dominant rival target, those operations could include visual grouping, attentional modulation, and whatever affective processes might be triggered by a visual object or event. It is not surprising, then, that these very factors—spatial configuration (eg Whittle et al 1968), attention (Ooi and He 1999), affective content (eg Kohn 1960)—have been shown to influence rivalry predominance. For the dominant stimulus, vision proceeds as it normally would. But what about a rival target during suppression? The present study reveals that a suppressed target, unlike a dominant one, no longer enjoys any benefit from the larger context in which it is embedded; a suppressed rival target behaves like a lonely competitor, isolated temporarily from its supporting context and relying, therefore, on its own strength to overcome its temporary loss of visibility. The inability of context to counteract suppression suggests that whatever neural processes are amplifying the salience of a dominant target are not engaged during suppression. Perhaps, in other words, neural information about a suppressed target is lost relatively early in visual processing, before that information can engage mechanisms supporting grouping, attention, and other ‘top – down’ processes (but not so early that it interferes with the build-up of several visual adaptation aftereffects—Blake and Fox 1974a; Lehmkuhle and Fox 1974; Wade and Wenderoth 1978). This interpretation casts a new light on the enduring controversy over whether rivalry is an ‘early’ or a ‘late’ process, a controversy that dates back to the 19th century (Walker 1978). According to the view advanced here and elaborated elsewhere (Blake 2001, rivalry involves both early and late processes, with the two operating differentially to effect dominance and suppression phases of rivalry.
Acknowledgments
This work was supported by a research grant (EY013358) and a training grant (EY07135) from the National Institutes of Health.
References
- Alais D, Blake R. Interactions between global motion and local binocular rivalry. Vision Research. 1998;38:637–644. doi: 10.1016/s0042-6989(97)00190-9. [DOI] [PubMed] [Google Scholar]
- Alais D, Blake R. Grouping visual features during binocular rivalry. Vision Research. 1999;39:4341–4353. doi: 10.1016/s0042-6989(99)00146-7. [DOI] [PubMed] [Google Scholar]
- Alais D, O’Shea RP, Mesana-Alais C, Wilson I G. On binocular alternation. Perception. 2000;29:1437–1445. doi: 10.1068/p3017. [DOI] [PubMed] [Google Scholar]
- Alais D, Smagt MJ van der, Berg AV van den, Grind WA van de. Local and global factors affecting the coherent motion of gratings presented in multiple apertures. Vision Research. 1998;38:1581–1591. doi: 10.1016/s0042-6989(97)00331-3. [DOI] [PubMed] [Google Scholar]
- Blake R. Primer on binocular rivalry, including controversial issues. Mind and Brain. 2001;2:5–38. [Google Scholar]
- Blake R, Fox R. Adaptation to invisible gratings and the site of binocular rivalry suppression. Nature. 1974a;249:488–490. doi: 10.1038/249488a0. [DOI] [PubMed] [Google Scholar]
- Blake R, Fox R. Binocular rivalry suppression: Insensitive to spatial frequency and orientation change. Vision Research. 1974b;14:687–692. doi: 10.1016/0042-6989(74)90065-0. [DOI] [PubMed] [Google Scholar]
- Diaz-Caneja E. Sur l’alternance binoculaire. Annales d’Oculistique. 1928:721–731. [Google Scholar]
- Fox R. Binocular Vision Ed. D M Regan, volume 9 of Vision and Visual Dysfunction Ed. J R Cronly-Dillon. London: Macmillan; 1991. Binocular rivalry; pp. 93–110. [Google Scholar]
- Fox R, Check R. Detection of motion during binocular rivalry suppression. Journal of Experimental Psychology. 1968;78:283–289. doi: 10.1037/h0026440. [DOI] [PubMed] [Google Scholar]
- Fox R, Rasche F. Binocular rivalry and reciprocal inhibition. Perception & Psychophysics. 1969;5:215–217. [Google Scholar]
- Freeman AW, Nguyen V A. Controlling binocular rivalry. Vision Research. 2001;41:2943–2950. doi: 10.1016/s0042-6989(01)00181-x. [DOI] [PubMed] [Google Scholar]
- Kleffner DA, Ramachandran V S. On the perception of shape from shading. Perception & Psychophysics. 1992;52:18–36. doi: 10.3758/bf03206757. [DOI] [PubMed] [Google Scholar]
- Kohn H. Some personality variables associated with binocular rivalry. Psychological Record. 1960;10:9–13. [Google Scholar]
- Kovács I, Papathomas TV, Yang M, Feher A. When the brain changes its mind: Interocular grouping during binocular rivalry. Proceedings of the National Academy of Sciences of the USA. 1996;93:15508–15511. doi: 10.1073/pnas.93.26.15508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmkuhle SW, Fox R. Effect of binocular rivalry suppression on the motion aftereffect. Vision Research. 1974;15:855–859. doi: 10.1016/0042-6989(75)90266-7. [DOI] [PubMed] [Google Scholar]
- Levelt WJM. On Binocular Rivalry. Soesterberg, The Netherlands: Institute for Perception RVO- TNO; 1965. [Google Scholar]
- Logothetis NK. Single units and conscious vision. Philosophical Transactions of the Royal Society of London. 1998;353:1801–1818. doi: 10.1098/rstb.1998.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenceau J, Shiffrar M. The influence of terminators on motion integration across space. Vision Research. 1992;32:263–273. doi: 10.1016/0042-6989(92)90137-8. [DOI] [PubMed] [Google Scholar]
- Lumer ED, Friston K, Rees G. Neural correlates of perceptual rivalry in the human brain. Science. 1998;280:1930–1934. doi: 10.1126/science.280.5371.1930. [DOI] [PubMed] [Google Scholar]
- Mueller TJ, Blake R. A fresh look at the temporal dynamics of binocular rivalry. Biological Cybernetics. 1989;61:223–232. doi: 10.1007/BF00198769. [DOI] [PubMed] [Google Scholar]
- Necker L A. Observations on some remarkable phenomena seen in Switzerland; and an optical phenomenon which occurs on viewing a figure of a crystal or geometrical solid. London and Edinburgh Philosophical Magazine and Journal of Science. 1832;1:329–337. [Google Scholar]
- Ngo TT, Miller SM, Liu GB, Pettigrew J D. Binocular rivalry and perceptual coherence. Current Biology. 2000;10(4):134–136. doi: 10.1016/s0960-9822(00)00399-7. [DOI] [PubMed] [Google Scholar]
- Nguyen VA, Freeman AW, Wenderoth P. The depth and selectivity of suppression in binocular rivalry. Perception & Psychophysics. 2001;63:348–360. doi: 10.3758/bf03194475. [DOI] [PubMed] [Google Scholar]
- Norman HF, Norman JF, Bilotta J. The temporal course of suppression during binocular rivalry. Perception. 2000;29:831–841. doi: 10.1068/p3085. [DOI] [PubMed] [Google Scholar]
- Ooi TL, He ZJ. Binocular rivalry and visual awareness: the role of attention. Perception. 1999;28:551–574. doi: 10.1068/p2923. [DOI] [PubMed] [Google Scholar]
- O’Shea RP, Crassini B. Interocular transfer of the motion after-effect is not reduced by binocular rivalry. Vision Research. 1981;21:801–804. doi: 10.1016/0042-6989(81)90177-2. [DOI] [PubMed] [Google Scholar]
- Polonsky A, Blake R, Braun J, Heeger DJ. Neuronal activity in human primary visual cortex correlates with perception during binocular rivalry. Nature Neuroscience. 2000;3:1153–1159. doi: 10.1038/80676. [DOI] [PubMed] [Google Scholar]
- Rubin E. Visuell wahrgenommene Figuren. Kopenhagen: Glydenaske boghandel; 1921. [Google Scholar]
- Tong F, Engel SA. Interocular rivalry revealed in the human cortical blind-spot representation. Nature. 2001;411:195–199. doi: 10.1038/35075583. [DOI] [PubMed] [Google Scholar]
- Tong F, Nakayama K, Vaughan JT, Kanwisher N. Binocular rivalry and visual awareness in human extrastriate cortex. Neuron. 1998;21:753–759. doi: 10.1016/s0896-6273(00)80592-9. [DOI] [PubMed] [Google Scholar]
- Tononi G, Srinivasan R, Russell DP, Edelman G M. Investigating neural correlates of conscious perception by frequency-tagged neuromagnetic responses. Proceedings of the National Academy of Sciences of the USA. 1998;95:3198–3203. doi: 10.1073/pnas.95.6.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade NJ, Wenderoth P. The influence of colour and contour rivalry on the magnitude of the tilt after-effect. Vision Research. 1978;18:827–835. doi: 10.1016/0042-6989(78)90123-2. [DOI] [PubMed] [Google Scholar]
- Walker P. Binocular rivalry: Central or peripheral selective processes? Psychological Bulletin. 1978;85:376–389. [Google Scholar]
- Walker P, Powell DJ. The sensitivity of binocular rivalry to changes in the nondominant stimulus. Vision Research. 1979;19:247–249. doi: 10.1016/0042-6989(79)90169-x. [DOI] [PubMed] [Google Scholar]
- Wallach H, O’Connell DN. The kinetic depth effect. Journal of Experimental Psychology. 1953;45:205–217. doi: 10.1037/h0056880. [DOI] [PubMed] [Google Scholar]
- Whittle P, Bloor DC, Pocock S. Some experiments on figural effects in binocular rivalry. Perception & Psychophysics. 1968;4:183–188. [Google Scholar]