Abstract
Background
Albuminuria is an important marker for chronic kidney disease and progression to end-stage renal disease in the general population; understanding racial and ethnic differences can help inform efforts to reduce health disparities. We sought to estimate independent associations of race/ethnicity with albuminuria to determine whether observed differences were attributable to known kidney disease risk factors.
Methods
This cross-sectional study included 64,161 Kidney Early Evaluation Program (KEEP) participants, 2000–2008, with estimated glomerular filtration rate ≥ 60 mL/min/1.73 m2, not on regular dialysis, and without previous kidney transplant. Albuminuria (urine albumin-creatinine ratio [ACR] ≥ 30 mg/g) was examined by self-reported race and ethnicity. Covariates were age, sex, educational level, body mass index, diabetes status or glucose level, hypertension status or blood pressure measurement, smoking status, health insurance status, and geographic region.
Results
Albuminuria prevalence was 8% (n = 2303) in whites, 11% (n = 2310) in African Americans, 9% (n = 730) in Hispanics, 10% (n = 381) in Asians, and 15% (n = 344) in American Indians/Alaska Natives. Compared with whites, odds of albuminuria were higher for all groups after multivariate adjustment. Odds were highest for American Indians/Alaska Natives (adjusted odds ratio 1.93, 95% confidence interval 1.70–2.20), then Asians (1.42, 1.26–1.61), African Americans (1.38, 1.29–1.47), and Hispanics (1.19, 1.08–1.31).
Conclusions
In the KEEP study population, albuminuria prevalence was higher among African Americans, Hispanics, Asians, and American Indians/Alaska Natives than among non-Hispanic whites, suggesting a need for screening for early detection of kidney damage, especially among people at increased risk, in the community primary care setting.
Introduction
Prevalence of chronic kidney disease (CKD) and end-stage renal disease (ESRD) is increasing in the United States.1 Incidence and prevalence of ESRD is higher among racial and ethnic minority groups than among whites, yet rates of CKD, defined by estimated glomerular filtration rate (eGFR) below 60 mL/min/1.73 m2, are similar or lower.2 These findings highlight the complexity of racial/ethnic differences in kidney disease. Some of the confusion may be due to limitations of the serum-creatinine-based equations used to estimate kidney function.3;4 Albuminuria is an important marker for the onset and progression of CKD, and for cardiovascular disease. Because albuminuria is a direct manifestation of kidney injury, it may be a more objective measure of risk for ESRD than GFR estimated from serum creatinine.5–7 Albuminuria consistently predicts cardiovascular events and mortality in the general population, and long-term risk of ESRD.8–12 Even low levels of albuminuria, in what would currently be considered the normal range, have been shown to predict cardiovascular events and death.13;14
Some studies have shown racial/ethnic differences in microalbuminuria among diabetic patients.15;16 In the National Health and Nutrition Examination Survey (NHANES), among participants aged 20 years and older, albuminuria prevalence was higher among African Americans than among non-Hispanic whites.2 However, no study has examined differences in abnormal albuminuria, equivalent to CKD stages 1 or 2 as defined by the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines,17 among multiple racial/ethnic groups with and without diabetes and hypertension from across the United States. Different risk factor patterns for albuminuria with eGFR ≥ 60 mL/min/1.73 m2 and CKD defined by eGFR < 60 mL/min/1.73 m2 have been described.18 Understanding racial and ethnic differences regarding prevalence of albuminuria in the general population is important to help inform efforts to reduce health disparities in ESRD.
Using data from the National Kidney Foundation’s Kidney Early Evaluation Program (KEEP), we evaluated the association of race/ethnicity with albuminuria in a screened US population. We sought to estimate the independent associations of race/ethnicity with albuminuria among participants with eGFR ≥ 60 mL/min/1.73 m2 to determine whether observed racial differences were attributable to known kidney disease risk factors.
Methods
KEEP Study Population
As has been previously described, KEEP is a free, community-based, voluntary screening program designed to identify individuals at increased risk for kidney disease and to encourage follow-up care.19 KEEP screenings are conducted in urban and rural locations throughout the United States through each state’s local National Kidney Foundation affiliate19;20 Officially launched nationwide in August 2000, and now in its ninth year, KEEP has screened more than 115,000 participants from 49 states and the District of Columbia. In this study, we included eligible KEEP participants from August 2000 through December 31, 2008, aged at least 18 years, with a personal diagnosis of diabetes mellitus or hypertension, or with a family history of diabetes, hypertension, or CKD.
Screening Protocol
Screening data were collected on participant demographic characteristics, personal and family medical history, and health behaviors.19 One-time systolic and diastolic blood pressure measurements and height and weight measurements were obtained.19 Blood and urine specimens were collected and processed to determine serum creatinine, fasting blood glucose, and urine albumin levels.19 Lipid and hemoglobin A1C data became available starting May 1, 2005. Serum creatinine values for KEEP participants were calibrated against values measured at the Cleveland Clinic Research Laboratory using the Roche enzymatic assay. Subsequently, eGFR using the original (raw) serum creatinine value was recalculated using the 4-variable Modification of Diet in Renal Disease (MDRD) Study equation with the newly calibrated serum creatinine values.21;22 Spot urine specimens were collected and tested for microalbuminuria with the Micral assay (Roche Pharmaceuticals, www.roche.com) until September 2001, then with the Clinitek assay (Bayer Diagnostics, www.bayer.com).19
Study Population
Participants who had undergone kidney transplant or were on regular dialysis treatment were excluded from this analysis. From the eligible KEEP study population (n = 107,114), we included participants with a serum creatinine measurement for whom eGFR could be determined (n = 101,770). From these, we excluded 1862 participants with missing race/ethnicity data or who self-identified as non-Hispanic other race. As we were interested in participants at risk for reduced eGFR, we excluded those with eGFR < 60 mL/min/1.73 m2 (n = 24,150). Of the 75,758 participants with eGFR ≥ 60 mL/min/1.73 m2 , data for all variables of interest were present for 85% (n = 64,161), who constituted our study population.
Predictor Variables and Covariates
Race and ethnicity information, obtained by self-report at the time of screening using the KEEP questionnaire, was categorized into five racial/ethnic groups: non-Hispanic white, African American, Asian, American Indian/Alaska Native, and Hispanic. Persons of Hispanic origin may be of any race; however, these race groups included only persons of non-Hispanic origin.
Potential covariates were determined a priori as characteristics believed to influence CKD risk: age; sex; obesity; diabetes; hypertension; smoking; family history of diabetes, hypertension, or CKD; educational level; presence of health insurance; and geographic region. Age was determined by self-reported date of birth at the time of screening and categorized into four groups: 18–45 years, 46–60 years, 61–75 years, and > 75 years. Obesity was defined as body mass index ≥ 30 kg/m2. Diabetes was defined by participant self-report, or by fasting glucose values ≥ 126 mg/dL or nonfasting glucose values ≥ 140 mg/dL. Hypertension was defined by participant self-report, or by systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg in the absence of diabetes or CKD, and systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 80 mmHg with diabetes or CKD present.
Smoking status was self-reported and categorized as current or former/never. Family history of diabetes, hypertension, or kidney disease was determined by participant self-report of a first-degree relative with the condition. Educational level was self-reported and categorized as less than high school or high school equivalent and higher. Health insurance status was dichotomized according to participant self-report of health insurance coverage or not at the time of screening. To account for potential regional differences in CKD by race/ethnicity, we categorized the United States into the four census geographic regions: Northeast (Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, Vermont, New Jersey, New York, Pennsylvania); Midwest (Illinois, Indiana, Michigan, Ohio, Wisconsin, Iowa, Kansas, Minnesota, Missouri, Nebraska, North Dakota, South Dakota); South (Delaware, District of Columbia, Florida, Georgia, Maryland, North Carolina, South Carolina, Virginia, West Virginia, Alabama, Kentucky, Mississippi, Tennessee, Arkansas, Louisiana, Oklahoma, Texas); and West (Arizona, Colorado, Idaho, Montana, Nevada, New Mexico, Utah, Wyoming, Alaska, California, Hawaii, Oregon, Washington).
Outcome Variables
Our main outcome variable was abnormal albuminuria, or urine albumin-creatinine ratio (ACR) ≥ 30 mg/g. Because we limited our study population to participants with eGFR ≥ 60 mL/min/1.73 m2 , presence of albuminuria was equivalent to CKD stages 1 or 2 according to Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines.17 We further categorized albuminuria as microalbuminuria (ACR 30–299 mg/g) and macroalbuminuria (ACR ≥ 300 mg/g). KEEP does not record urine ACR as a specific number, but as < 30, 30–299, or ≥ 300 mg/g.
Statistical Analysis
We used a complete-case analysis approach, in which we analyzed participants with available data for all covariates of interest. Demographics were similar for included and excluded participants, except that participants excluded due to missing values (n = 11,595) were slightly older (mean age 55.7 vs. 52.1 years) with slightly higher prevalence of diabetes (35% vs. 31%) and hypertension (71% vs. 66%). We compared demographic characteristics and CKD risk factor prevalence by race/ethnicity using chi-squared analyses.
We compared mean age and clinical and laboratory characteristics across racial/ethnic groups using ANOVA. We tabulated the proportions and respective 95% confidence intervals of participants with albuminuria (ACR ≥ 30 mg/g) by race/ethnicity, and the proportions with microalbuminuria (ACR 30–299 mg/g) and macroalbuminuria (ACR ≥ 300 mg/g).
We conducted univariate and multivariate logistic regression analyses to determine the association of race/ethnicity with presence of albuminuria among participants with eGFR ≥ 60 mL/min/1.73 m2. Albuminuria was the dependant variable. Age as a categorical variable, along with the other a priori covariates, was included in the multivariate analyses. We repeated the same analysis to determine the association of race/ethnicity with presence of macroalbuminuria.
Lastly, we conducted a subgroup analysis among participants without diabetes or hypertension. We tabulated the proportions with albuminuria (ACR ≥ 30 mg/g) by race/ethnicity, and conducted univariate and multivariate logistic regression analyses using the same covariates, other than diabetes and hypertension.
Statistical analyses were performed with the SAS statistical package (release 9.1; SAS Institute Inc., www.sas.com). The Institutional Review Board (IRB) at Hennepin County Medical Center approved the KEEP study, including research protocol, process of obtaining informed consent, and data management procedures. Cleveland Clinic IRB approved this study.
Results
Of the 64,161 KEEP participants in our study, 45% (n = 28,579) were white, 33% (n = 21,435) were African American, 12% (n = 7954) were Hispanic, 6% (n = 3912) were Asian, and 4% (n = 2281) were American Indian/Alaska Native. Most participants were women, and the overall mean age was 52 years (Table 1). More than half of American Indian/Alaska Native and African American participants were obese; less than a quarter of Asian participants were obese. Hispanics and American Indians/Alaska Natives were least likely to self-report having health insurance at the time of screening. African American participants had higher mean systolic blood pressure compared with the other groups (Table 2).
Table 1.
Baseline Demographic Characteristics of KEEP Participants With eGFR ≥ 60 mL/min/1.73m2, Stratified by Race/Ethnicity
| Characteristics | White, % | African American, % |
Hispanic, % | Asian, % | American Indian/Alaska Native, % |
P* |
|---|---|---|---|---|---|---|
| n | (n = 28,579) | (n = 21,435) | (n = 7954) | (n = 3912) | (n = 2281) | |
| Age, mean (SD) | 55 (15) | 51 (14) | 46 (14) | 52 (14) | 48 (15) | < 0.001 |
| Women | 65 | 72 | 67 | 62 | 74 | < 0.001 |
| Obesity | 41 | 53 | 42 | 18 | 57 | < 0.001 |
| Diabetes | 32 | 30 | 30 | 29 | 39 | < 0.001 |
| Hypertension | 67 | 70 | 55 | 55 | 61 | < 0.001 |
| Current smoker | 12 | 13 | 11 | 6 | 26 | < 0.001 |
| Family history | ||||||
| Diabetes | 55 | 63 | 65 | 52 | 78 | < 0.001 |
| Hypertension | 78 | 85 | 74 | 78 | 73 | < 0.001 |
| Kidney disease | 17 | 20 | 22 | 17 | 24 | < 0.001 |
| High school | ||||||
| education or higher | 92 | 88 | 66 | 88 | 82 | < 0.001 |
| Health insurance, yes | 88 | 81 | 53 | 77 | 68 | < 0.001 |
| Region | < 0.001 | |||||
| Northeast | 26 | 18 | 21 | 36 | 9 | |
| Midwest | 16 | 13 | 14 | 5 | 20 | |
| South | 45 | 66 | 44 | 18 | 31 | |
| West | 13 | 3 | 21 | 41 | 40 | |
Note: N = 64,161
SD, standard deviation.
Chi-square analyses, except for mean age, for which ANOVA was used.
Table 2.
Baseline Clinical and Laboratory Characteristics of KEEP Participants With eGFR ≥ 60 mL/min/1.73m2, Stratified by Race/Ethnicity
| Characteristics | White | African American |
Hispanic | Asian | American Indian/Alaska Native |
P |
|---|---|---|---|---|---|---|
| No. | (n = 28,579) | (n = 21,435) | (n = 7954) | (n = 3912) | (n = 2281) | |
| BMI (kg/m2) | 29.7 (6.7) | 31.6 (7.1) | 29.8 (6.3) | 26.1 (5.6) | 31.9 (7.0) | < 0.001 |
| Waist circumference (inches) | 39.5 (6.6) | 39.9 (6.5) | 38.4 (5.7) | 36.3 (6.5) | 42.3 (8.3) | < 0.001 |
| SBP (mmHg) | 132.8 (18.9) | 134.0 (19.8) | 128.5 (19.1) | 127.2 (18.8) | 128.5 (17.5) | < 0.001 |
| DBP (mmHg) | 79.1 (11.0) | 81.5 (11.8) | 78.2 (11.1) | 78.0 (11.2) | 77.3 (11.2) | < 0.001 |
| Total cholesterol (mg/dL)* | 198.7 (41.9) | 197.6 (40.6) | 200.0 (41.1) | 197.3 (38.1) | 188.8 (38.5) | 0.2 |
| HDL cholesterol (mg/dL)* | 53.9 (16.8) | 58.7 (17.4) | 50.2 (14.5) | 53.0 (15.7) | 55.7 (14.9 | < 0.001 |
| LDL cholesterol (mg/dL)* | 106.5 (35.3) | 107.6 (34.9) | 108.3 (31.6) | 102.5 (30.8) | 100.8 (34.9) | 0.6 |
| Triglycerides (mg/dL)* | 169.0 (121.0) | 119.3 (82.3) | 178.5 (135.0) | 161.6 (114.8) | 192.5 (147.9) | < 0.001 |
| Fasting blood glucose (mg/dL) | 105.7 (37.7) | 106.8 (43.1) | 108.0 (41.6) | 106.5 (30.1) | 114.8 (51.5) | 0.02 |
| Hemoglobin A1C (%)*† | 7.0 (1.6) | 7.2 (1.9) | 7.6 (1.9) | 7.8 (2.1) | 7.6 (2.1) | < 0.001 |
Note: N = 64,161
BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SD, standard deviation.
Data available only after May 1, 2005.
Only participants with diabetes.
Values shown are mean (SD).
Note: conversion factors for units: total, LDL and HDL cholesterol in mg/dL to mmol/L; ×0.02586; triglycerides in mg/dL to mmol/L, × 0.01129; Glucose in mg/dL to mmol/L, ×.05551.
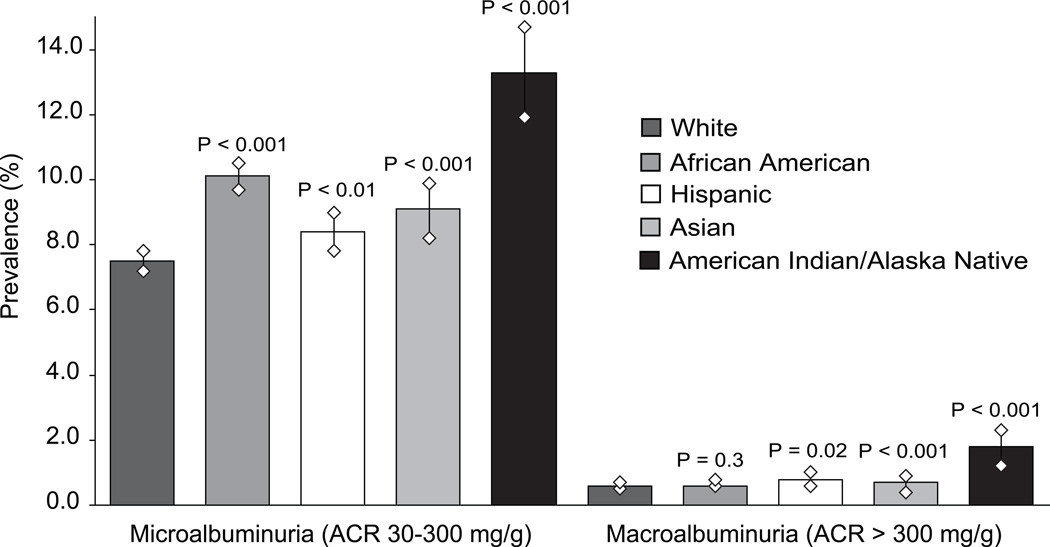
Abnormal albuminuria (urinary ACR ≥ 30 mg/g) was present in 8% (n = 2303) of whites, 11% (n = 2310) of African Americans, 9% (n = 730) of Hispanics, 10% (n = 381) of Asians, and 15% (n = 344) of American Indians/Alaska Natives. Stratifying the outcome by microalbuminuria (ACR 30–299 mg/g) and macroalbuminuria (ACR ≥ 300 mg/g) showed significant racial/ethnic differences (Figure 1), with higher prevalence of macroalbuminuria among Hispanics and American Indians/Alaska Natives than among whites.
Figure 1.
Racial/ethnic differences in prevalence of microalbuminuria and macroalbuminuria, Kidney Early Evaluation Program (KEEP) participants, 2000–2008. Diamond shapes represent upper and lower limits of 95% confidence intervals for prevalence estimates. P-values shown are chi-square comparisons for each racial/ethnic group to non-Hispanic whites; global chi-square P-values for microalbuminuria and macroalbuminuria were < 0.001. ACR, albumin-creatinine ratio.
Odds of albuminuria were higher for all groups compared with whites in both unadjusted and multivariate adjusted analyses, about 20% higher for Hispanics, 40% higher for African Americans and Asians, and 90% higher for American Indians/Alaska Natives (Table 3). Our subgroup analyses of participants without diabetes or hypertension showed no association between race/ethnicity and albuminuria in either unadjusted or adjusted analyses except for American Indians/Alaska Natives, whose odds of albuminuria were twice as high as odds for whites (Table 3).
Table 3.
Odds Ratios of Albuminuria by Race/Ethnicity Among KEEP Participants, 2000–2008
| eGFR ≥ 60 (n = 64,161) |
eGFR ≥ 60 w/o DM or HTN (n = 19,036) |
|||
|---|---|---|---|---|
| Race/Ethnicity | Unadjusted OR (95% CI) |
Adjusted* OR (95% CI) |
Unadjusted OR (95% CI) |
Adjusted† OR (95% CI) |
| White | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| African American | 1.38 (1.30–1.46)‡ | 1.38 (1.29–1.47)‡ | 1.06 (0.88–1.29) | 1.06 (0.87–1.30) |
| Hispanic | 1.15 (1.06–1.26)§ | 1.19 (1.08–1.31)§ | 1.05 (0.83–1.33) | 1.00 (0.77–1.31) |
| Asian | 1.23 (1.10–1.38)§ | 1.42 (1.26–1.61)‡ | 1.03 (0.75–1.41) | 1.03 (0.74–1.43) |
| American Indian/Alaska Native | 2.03 (1.79–2.29)‡ | 1.93 (1.70–2.20)‡ | 2.04 (1.46–2.84)‡ | 2.09 (1.47–2.98)‡ |
eGFR given in mL/min/1.73 m2 ; conversion factor for mL/s/1.73m^2, ×0.01667. CI, confidence interval; eGFR, estimated glomerular filtration rate; OR, odds ratio; DM, diabetes mellitus; HTN, hypertension; ref, reference.
Adjusted for age; sex; obesity; diabetes; hypertension; smoking status; family history of diabetes, hypertension, or kidney disease; education level, presence of health insurance, and geographic region.
Adjusted for all above except diabetes and hypertension.
P < 0.001
P < 0.05
In our examination of the association of race/ethnicity with macroalbuminuria, even after multivariate adjustment, American Indians/Alaska Natives retained nearly 3-fold odds of macroalbuminuria compared with whites (Table 4). Odds of macroalbuminuria were also about 40% higher for Hispanics than for whites. However, odds ratios for the other groups were only modestly elevated and did not reach statistical significance.
Table 4.
Odds Ratios for Macroalbuminuria by Race/Ethnicity Among KEEP Participants With eGFR ≥ 60 mL/min/1.73m2
| Race/Ethnicity | Unadjusted OR (95% CI) | Adjusted* OR (95% CI) |
|---|---|---|
| White | 1.00 (ref) | 1.00 (ref) |
| African American | 1.14 (0.91–1.43) | 1.14 (0.90–1.45) |
| Hispanic | 1.40 (1.05–1.88)† | 1.38 (1.01–1.90)† |
| Asian | 1.22 (0.81–1.84) | 1.28 (0.84–1.97) |
| American Indian/Alaska Native | 3.13 (2.21–4.44)‡ | 2.71 (1.87–3.93)‡ |
N = 64,161. CI, confidence interval; OR, odds ratio; ref, reference.
Adjusted for age; sex; obesity; diabetes; hypertension; smoking status; family history of diabetes, hypertension, or kidney disease; education level; presence of health insurance; and geographic region.
P < 0.05
P < 0.001
Discussion
We found higher prevalence of albuminuria among African Americans, Hispanics, Asians, and American Indians/Alaska Natives compared with non-Hispanic whites in the KEEP population, and the differences persisted even after adjustment for demographics, diabetes, hypertension, obesity, and socioeconomic factors. Furthermore, the racial/ethnic differences were not uniform. Risk for prevalent macroalbuminuria was highest for Hispanics and American Indians/Alaska Natives. Among participants without known diabetes or hypertension, only American Indians/Alaska Natives were at higher risk for prevalent albuminuria. While diabetes and hypertension seem to play major roles in higher prevalence of albuminuria among all racial/ethnic groups, further independent risk appears to be present among American Indians/Alaska Natives in isolation of these established risk factors.
Reasons for the higher albuminuria prevalence among American Indians/Alaska Natives are likely multi-factorial and may include genetics, environmental factors, or further residual confounding. The population comprises more than 500 unique tribes that are culturally diverse and geographically dispersed in rural and urban settings.23 Diabetes, an important risk factor for albuminuria, is increasing in this population.18;24;25 Hypertension is also a strong and independent risk factor for albuminuria in this population.18 American Indians/Alaska Natives may have some shared genetic predisposition to albuminuria,26 or the risk may be related to environmental factors that are unaccounted for, or to exposure to toxins such as heavy metals like lead, cadmium, and uranium.27;28 Some of the increased risk observed among American Indians/Alaska Natives without diabetes or hypertension could be related to higher prevalence of earlier stages of these conditions, such as prediabetes and prehypertension, metabolic syndrome, or cardiovascular disease that we were unable to account for in this study.29
Prior studies of albuminuria have focused on diabetic patients, on a specific health maintenance organization group, or on population samples, such as NHANES, with racial/ethnic representation limited to African Americans and Mexican Americans. Our study examined differences in prevalent albuminuria among multiple racial/ethnic groups from throughout the United States and included participants with and without known diabetes and hypertension. Among African Americans, Hispanics, and Asians, we found higher prevalence of albuminuria consistent with prior studies.2;15;16 These results were not surprising, given the strong association of albuminuria with development of ESRD11;12;30;31 and the known differences in ESRD incidence and prevalence among racial/ethnic minorities.2 The risk for macroalbuminuria was also particularly strong among Hispanics, and could be related to their burden of diabetes and hypertension.32 Prevention, early detection, and aggressive treatment of diabetes and hypertension might help reduce racial differences in albuminuria.
The National Kidney Foundation released a position statement on testing for CKD, which can be done with two simple tests: a urine test to detect proteinuria and a blood test for information to estimate GFR.33 According to the National Kidney Disease Education Program, health care professionals should screen persons with diabetes, persons with hypertension at the time it is diagnosed and then every three years, and persons with family history of ESRD at least every three years; most of these recommendations are opinion based.34 Providers should also consider that the risk of kidney failure is higher for African Americans, American Indians/Alaska Natives, and Asians.34 Our findings strongly suggest an indication to consider screening all American Indians and Alaska Natives aged older than 18 years for albuminuria, in addition to those with diabetes as recommended by the Indian Health Service.35 In other groups, screening for albuminuria in people with diabetes or hypertension and family history of CKD may be adequate, particularly among African Americans, Hispanics, and Asians. Screening for albuminuria identifies individuals at increased renal risk.36 Such screening may be cost-effective among people with diabetes and hypertension,37 and it is an important part of a public health approach to CKD.38;39
As this is a cross-sectional study, we cannot determine causality or account for changes in outcomes or risk factors over time. Our cohort is derived from a group of voluntary, screened participants, of which more than 60% were women, in KEEP, a program that targets individuals at elevated risk for kidney disease. Therefore, our results may overestimate prevalence of albuminuria among the racial/ethnic groups represented. However, CKD in the KEEP cohort has been shown to be similar to CKD in the subgroup of participants with CKD in NHANES.40 Some of the data are based on self-report from questionnaires and therefore subject to potential recall and ascertainment bias, but ascertainment of disease status by self-report has been shown to be valid.41 In general, participants with and without missing data in our study were similar, except that those with missing data had a slightly higher prevalence of diabetes and hypertension; thus we may have underestimated the effect of these two conditions in our results. As single measurements of urine albumin and creatinine were used rather than repeated measurements over time as recommended for clinical practice,5 prevalence of albuminuria might be overestimated. However, single urine samples have been accepted as adequate for the detection of albuminuria.42 Lastly, we did not have information regarding treatment with specific medications indicated for albuminuria, such as angiotension-converting enzyme inhibitors, which can cause regression of albuminuria. If these agents were prescribed differently among the racial/ethnic groups, this could potentially be a missed confounder in this study.
Effective screening of specific racial/ethnic groups at increased risk may lead to improved detection of early CKD, allowing early intervention that may help those at highest risk for complications such as cardiovascular disease and progression to ESRD. Future studies are needed to assess the effectiveness of earlier screening for albuminuria in these at-risk populations for prevention of renal outcomes, cardiovascular outcomes, and mortality.
Acknowledgements
The authors wish to thank Shane Nygaard, BA, and Nan Booth, MSW, MPH, of the Chronic Disease Research Group, for manuscript preparation and editing, respectively.
Support: The Kidney Early Evaluation Program (KEEP)™ is a program of the National Kidney Foundation, Inc., and is supported by Amgen, Abbott, Novartis, Siemens, Genentech, Genzyme, Nephroceuticals, Pfizer, LifeScan, and Suplena. Dr. Shlipak’s work was supported by the American Heart Association Established Investigator Award, and R01 DK 066488 from NIDDK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors report that they have no relevant financial interests.
REFERENCES
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Renal Data System: USRDS 2008 Annual Data Report: Atlas of Chronic Kidney Disease & End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2008. [Google Scholar]
- 3.Rule AD, Jacobsen SJ, Schwartz GL, et al. A comparison of serum creatinine-based methods for identifying chronic kidney disease in hypertensive individuals and their siblings. Am J Hypertens. 2006;19:608–614. doi: 10.1016/j.amjhyper.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 4.Rule AD, Rodeheffer RJ, Larson TS, et al. Limitations of estimating glomerular filtration rate from serum creatinine in the general population. Mayo Clin Proc. 2006;81:1427–1434. doi: 10.4065/81.11.1427. [DOI] [PubMed] [Google Scholar]
- 5.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Kidney Disease Outcome Quality Initiative. Am J Kidney Dis. 2002;39(suppl 1):S1–S266. [PubMed] [Google Scholar]
- 6.Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, et al. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia. 1989;32:219–226. doi: 10.1007/BF00285287. [DOI] [PubMed] [Google Scholar]
- 7.Keane WF, Eknoyan G. Proteinuria, albuminuria, risk, assessment, detection, elimination (PARADE): a position paper of the National Kidney Foundation. Am J Kidney Dis. 1999;33:1004–1010. doi: 10.1016/s0272-6386(99)70442-7. [DOI] [PubMed] [Google Scholar]
- 8.Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 9.Hillege HL, Fidler V, Diercks GF, et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106:1777–1782. doi: 10.1161/01.cir.0000031732.78052.81. [DOI] [PubMed] [Google Scholar]
- 10.Verhave JC, Gansevoort RT, Hillege HL, et al. An elevated urinary albumin excretion predicts de novo development of renal function impairment in the general population. Kidney Int. 2004;66(suppl 92):S18–S21. doi: 10.1111/j.1523-1755.2004.09205.x. [DOI] [PubMed] [Google Scholar]
- 11.Iseki K, Kinjo K, Iseki C, et al. Relationship between predicted creatinine clearance and proteinuria and the risk of developing ESRD in Okinawa, Japan. Am J Kidney Dis. 2004;44:806–814. [PubMed] [Google Scholar]
- 12.Ishani A, Grandits GA, Grimm RH, et al. Association of single measurements of dipstick proteinuria, estimated glomerular filtration rate, and hematocrit with 25-year incidence of end-stage renal disease in the multiple risk factor intervention trial. J Am Soc Nephrol. 2006;17:1444–1452. doi: 10.1681/ASN.2005091012. [DOI] [PubMed] [Google Scholar]
- 13.Arnlov J, Evans JC, Meigs JB, et al. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation. 2005;112:969–975. doi: 10.1161/CIRCULATIONAHA.105.538132. [DOI] [PubMed] [Google Scholar]
- 14.Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, et al. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation. 2004;110:32–35. doi: 10.1161/01.CIR.0000133312.96477.48. [DOI] [PubMed] [Google Scholar]
- 15.Bryson CL, Ross HJ, Boyko EJ, et al. Racial and ethnic variations in albuminuria in the US Third National Health and Nutrition Examination Survey (NHANES III) population: associations with diabetes and level of CKD. Am J Kidney Dis. 2006;48:720–726. doi: 10.1053/j.ajkd.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 16.Young BA, Katon WJ, Von KM, et al. Racial and ethnic differences in microalbuminuria prevalence in a diabetes population: the pathways study. J Am Soc Nephrol. 2005;16:219–228. doi: 10.1681/ASN.2004030162. [DOI] [PubMed] [Google Scholar]
- 17.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(suppl 1):S32–S36. [PubMed] [Google Scholar]
- 18.Jolly SE, Li S, Chen SC, et al. Risk factors for chronic kidney disease among American Indians and Alaska Natives--findings from the Kidney Early Evaluation Program. Am J Nephrol. 2009;29:440–446. doi: 10.1159/000174857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown WW, Peters RM, Ohmit SE, et al. Early detection of kidney disease in community settings: the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2003;42:22–35. doi: 10.1016/s0272-6386(03)00405-0. [DOI] [PubMed] [Google Scholar]
- 20.National Kidney Foundation. KEEP Kidney Early Evaluation Program Annual Data Report 2006. Am J Kidney Dis. 2007;49(suppl 1):S1–S160. [Google Scholar]
- 21.Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 23.U.S. Department of Commerce Economics and Statistics Administration. [Accessed August 17, 2009];United States Census Bureau, The American Indian and Alaska Native Populations: Census 2000 Brief. Available at http://www.census.gov/prod/2002pubs/c2kbr01-15.pdf.
- 24.Burrows NR, Geiss LS, Thompson T, Acton KJ. [Accessed August 17, 2009];Diabetes Prevalence Among American Indians and Alaska Natives and the Overall Population --- United States, 1994--2002. 2003 Aug 2;52(30):702–704. Available at http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5230a3.htm. [Google Scholar]
- 25.Kasiske BL, Rith-Najarian S, Casper ML, et al. American Indian heritage and risk factors for renal injury. Kidney Int. 1998;54:1305–1310. doi: 10.1046/j.1523-1755.1998.00106.x. [DOI] [PubMed] [Google Scholar]
- 26.Mottl AK, Vupputuri S, Cole SA, et al. Linkage analysis of albuminuria. J Am Soc Nephrol. 2009;20:1597–1606. doi: 10.1681/ASN.2008080895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonick HC. Nephrotoxicity of cadmium & lead. Indian J Med Res. 2008;128:335–352. [PubMed] [Google Scholar]
- 28.Mao Y, Desmeules M, Schaubel D, et al. Inorganic components of drinking water and microalbuminuria. Environ Res. 1995;71:135–140. doi: 10.1006/enrs.1995.1075. [DOI] [PubMed] [Google Scholar]
- 29.Lucove J, Vupputuri S, Heiss G, et al. Metabolic syndrome and the development of CKD in American Indians: the Strong Heart Study. Am J Kidney Dis. 2008;51:21–28. doi: 10.1053/j.ajkd.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Mogensen CE, Christensen CK. Predicting diabetic nephropathy in insulin-dependent patients. N Engl J Med. 1984;311:89–93. doi: 10.1056/NEJM198407123110204. [DOI] [PubMed] [Google Scholar]
- 31.Bigazzi R, Bianchi S, Baldari D, et al. Microalbuminuria predicts cardiovascular events and renal insufficiency in patients with essential hypertension. J Hypertens. 1998;16:1325–1333. doi: 10.1097/00004872-199816090-00014. [DOI] [PubMed] [Google Scholar]
- 32.Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006. Diabetes Care. 2009;32:287–294. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vassalotti JA, Stevens LA, Levey AS. Testing for chronic kidney disease: a position statement from the National Kidney Foundation. Am J Kidney Dis. 2007;50:169–180. doi: 10.1053/j.ajkd.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 34.National Kidney Disease Education Program (NKDEP) [Accessed August 17, 2009];An initiative of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (NIH), U.S. Department of Health and Human Services (DHHS) Available at http://www.nkdep.nih.gov/index.htm.
- 35.Indian Health Service (IHS) Division of Diabetes Treatment and Prevention. [Accessed August 17, 2009];IHS Standards of Care for Adults with Type 2 Diabetes. Available at http://www.ihs.gov/medicalprograms/diabetes/.
- 36.van der Velde, Halbesma N, de Charro FT, et al. Screening for albuminuria identifies individuals at increased renal risk. J Am Soc Nephrol. 2009;20:852–862. doi: 10.1681/ASN.2008060655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palmer AJ, Valentine WJ, Chen R, et al. A health economic analysis of screening and optimal treatment of nephropathy in patients with type 2 diabetes and hypertension in the USA. Nephrol Dial Transplant. 2008;23:1216–1223. doi: 10.1093/ndt/gfn082. [DOI] [PubMed] [Google Scholar]
- 38.Jaar BG, Khatib R, Plantinga L, et al. Principles of screening for chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:601–609. doi: 10.2215/CJN.02540607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Norris K, Nissenson AR. Race, gender, and socioeconomic disparities in CKD in the United States. J Am Soc Nephrol. 2008;19:1261–1270. doi: 10.1681/ASN.2008030276. [DOI] [PubMed] [Google Scholar]
- 40.Whaley-Connell AT, Sowers JR, McFarlane SI, et al. Diabetes mellitus in CKD: Kidney Early Evaluation Program (KEEP) and National Health and Nutrition and Examination Survey (NHANES) 1999–2004. Am J Kidney Dis. 2008;51(suppl 2):S21–S29. doi: 10.1053/j.ajkd.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 41.Okura Y, Urban LH, Mahoney DW, et al. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol. 2004;57:1096–1103. doi: 10.1016/j.jclinepi.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 42.Nathan DM, Rosenbaum C, Protasowicki VD. Single-void urine samples can be used to estimate quantitative microalbuminuria. Diabetes Care. 1987;10:414–418. doi: 10.2337/diacare.10.4.414. [DOI] [PubMed] [Google Scholar]