During intrathymic T cell development, a huge repertoire of T cells with different antigen specificities are generated through somatic recombination at the T cell receptor (TCR) loci, which equips T cells with the capacity to recognize diverse microbial and environmental antigens. Within such a repertoire of T cells, self-reactive T cells exist and, if not properly controlled, can cause self-inflicted damage to tissues and result in autoimmune diseases. Under normal situations, such self-reactive T cells are kept in check by multiple peripheral tolerance mechanisms, including induction of anergy.1
In the peripheral lymphoid organs, most T cells reside in a naïve resting state. Naïve T cells can be readily activated to become effector T cells that perform an immune function after engagement of the TCR with foreign peptides presented by antigen-presenting cells. T cell activation is accompanied by proliferation, enlargement in size, production of effector molecules (such as cytokines) and high metabolic rate. To induce full T cell activation, the TCR signal alone is not sufficient; concurrent signals from costimulatory molecules, such as CD28 and cytokine receptors, are also required. In the absence of co-stimulation, the TCR signal alone induces T cell anergy rather than full activation. Anergic T cells are hyporesponsive to TCR restimulation, even in the presence of proper costimulation; they are metabolically inert, defective in proliferation and impaired in cytokine production. T cell anergy is important not only for self-tolerance, but also for contributing to tumor immune evasion.1 Thus, understanding the mechanisms governing T cell anergy should provide therapeutic strategies for combating autoimmune diseases and cancer.
The tumor suppressor TSC1, in association with TSC2, inhibits the activation of the mammalian target of rapamycin complex 1 (mTORC1) via the GAP activity of the TSC1/2 complex. Several recent studies have revealed the critical role of TSC1 in normal T cell homeostasis, survival and quiescence;2-5 in B cell development;6 in mast cell survival and function7 and in proper innate immune responses and endotoxin shock.8 We have recently found that TSC1 is expressed at higher levels in anergic T cells than in activated T cells.9 In T cells, mTOR is activated following TCR engagement via the PI3K/Akt and the RasGRP1-Ras-Erk1/2 pathways (Fig. 1).10 Recent studies have demonstrated that mTOR performs crucial regulatory roles in effector T cell differentiation, inducible regulatory T cell differentiation, T cell trafficking and memory T cell responses to viral pathogens. Given the role of mTOR in T cell activation, we hypothesized that TSC1 may play an important role in T cell anergy by modulating mTOR activity. This hypothesis is proved right by our most recent studies using mice with T cell-specific deletion of TSC1.9 While WT anergic T cells contain low mTORC1 activity, TSC1-deficient (TSC1KO) T cells pretreated with anergizing condition maintained mTORC1 signaling at a level similar to WT-activated T cells, supporting that TSC1 is critical for decreased mTOR activity in anergic T cells. In vitro, WT naïve CD4 T cells become anergic after TCR simulation and when CTLA4-Ig was added to block CD28-mediated costimulation. These T cells produced much less IL-2 and IFNγ and proliferated less than fully activated T cells after TCR and anti-CD28 restimulation. However, TSC1KO CD4 T cells that underwent similar anergy-inducing treatment retained the ability to produce these cytokines and proliferated vigorously. In addition, the low metabolic rate typically seen in anergic T cells was not observed in TSC1KO T cells following anergizing treatment. The resistance of TSC1-deficient T cells to anergy was further confirmed in vivo using the Staphylococcus enterotoxin B (SEB) superantigen-induced TCRVβ8+ T cell anergy model. Ultimately, aged and TSC1-deficient mice develop autoimmune diseases in the thyroid gland and liver. The autoimmune diseases in TSC1KO mice appear mild, which could be partly due to the propensity of TSC1 effector T cells to death. Additionally, it is unclear whether regulatory T cell function is altered in absence of TSC1.
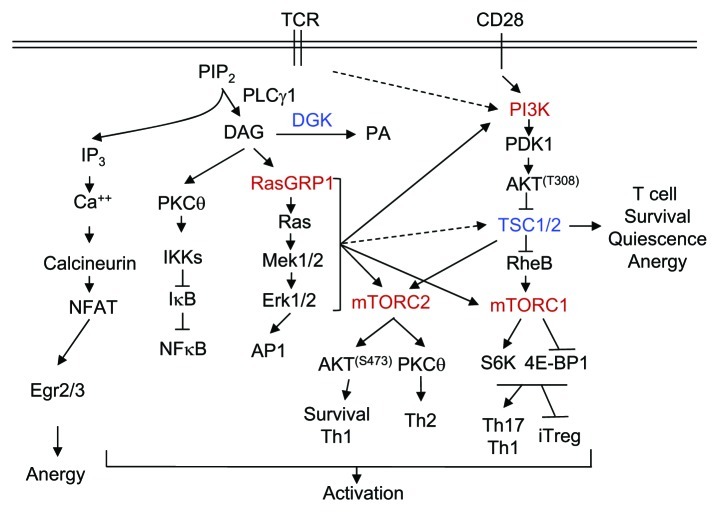
Figure 1. TSC1/2-mTOR signaling in T cell activation and tolerance. TCR engagement activates PLCγ1, which hydrolyzes PIP2 to generate DAG and IP3, two important second messengers that trigger the activation of multiple signal cascades. IP3 triggers Ca2+ influx, which, in turn, induces the activation of the calcineurin-NFAT pathway. DAG associates and activates RasGRP1 and PKCθ, resulting in the activation of the Ras-Erk1/2-AP1 and IKK-NFκB pathways, respectively. CD28 provides costimulation and enhances PI3K-Akt activation. The Ca2+-NFAT pathway alone induces T cell anergy by increasing expression of anergy-promoting molecules. This pathway, in concert with DAG-mediated pathways, induces T cell activation. DGKs convert DAG to PA and, thus, inhibit T cell activation. In T cells, the RasGRP1-Ras-Erk1/2 pathway as well as the PI3K-Akt pathway, is important for mTORC1 and mTORC2 signaling. TSC1 inhibits mTORC1, but promotes mTORC2 signaling and is important for T cell survival, quiescence and anergy.
The resistance of TSC1KO T cells to anergy is correlated with increased mTORC1 signaling and can be reverted by rapamycin treatment, indicating that TSC1 promotes T cell anergy via inhibiting mTORC1.9 Interestingly, the inducible T cell costimulator (ICOS) expression is increased in TSC1KO T cells, and blocking ICOS signaling partially renders TSC1KO T cells sensitive to anergy, suggesting that TSC1 inhibits ICOS expression to ensure the dependence on CD28 co-stimulation for T cell activation. In addition, the upregulation of anergy-promoting molecules, such as Egr2/3, Itch, Grail and DGKζ, was impaired in TSC1KO T cells following anergizing treatment, raising the possibility that TSC1 may promote T cell anergy via multiple mechanisms. Further investigation of how TSC1/2-mTOR may control the expression of ICOS and anergy-promoting molecules and how TSC1/2 themselves are regulated in T cells should provide additional insight into the mechanism’s control of T cell anergy and tolerance.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/22235
References
- 1.Chappert P, et al. Curr Opin Immunol. 2010;22:552–9. doi: 10.1016/j.coi.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Brien TF, et al. Eur J Immunol. 2011;41:3361–70. doi: 10.1002/eji.201141411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Q, et al. J Immunol. 2011;187:1106–12. doi: 10.4049/jimmunol.1003968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang K, et al. Nat Immunol. 2011;12:888–97. doi: 10.1038/ni.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang L, et al. PLoS One. 2012;7:e30592. doi: 10.1371/journal.pone.0030592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benhamron S, et al. Eur J Immunol. 2011;41:2390–6. doi: 10.1002/eji.201041336. [DOI] [PubMed] [Google Scholar]
- 7.Shin J, et al. Blood. 2012;119:3306–14. doi: 10.1182/blood-2011-05-353342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan H, et al. J Immunol. 2012;188:3658–66. doi: 10.4049/jimmunol.1102187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie DL, et al. Proc Natl Acad Sci USA. 2012;109:14152–7. doi: 10.1073/pnas.1119744109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorentla BK, et al. Blood. 2011;117:4022–31. doi: 10.1182/blood-2010-08-300731. [DOI] [PMC free article] [PubMed] [Google Scholar]