Abstract
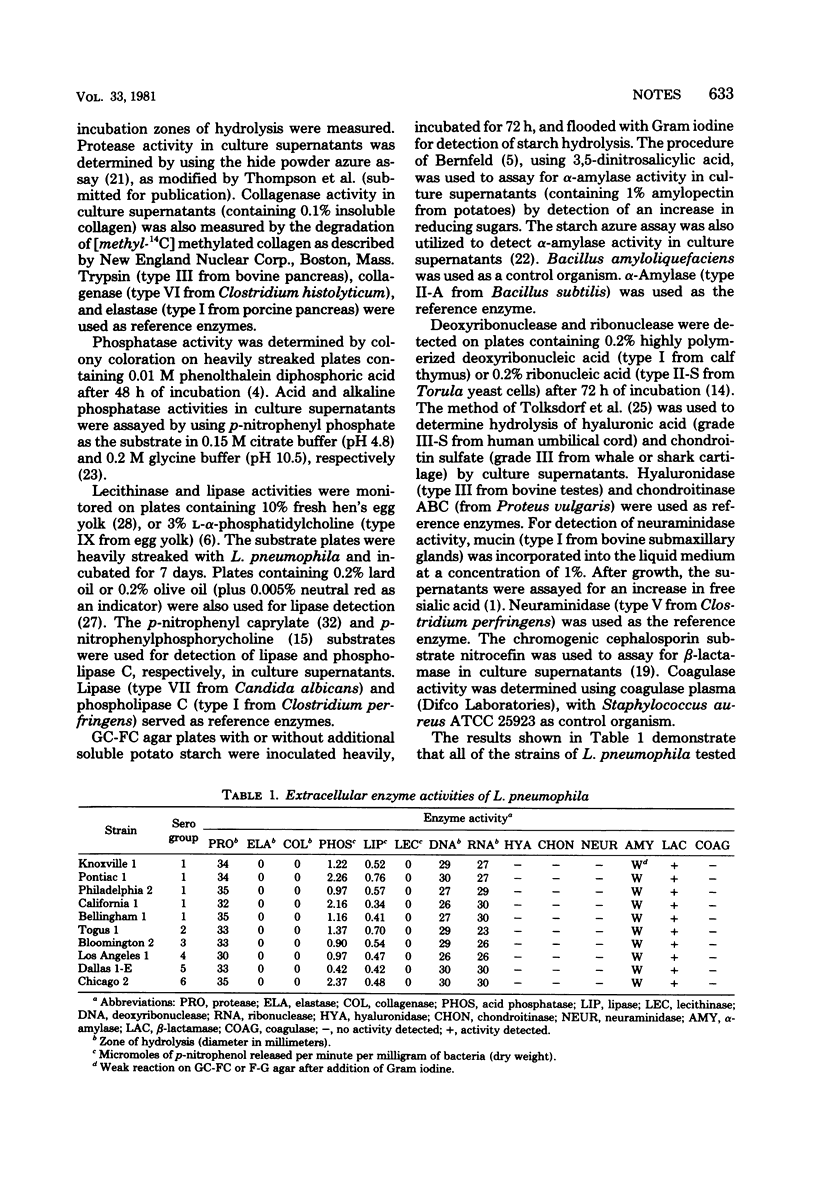
All strains of Legionella pneumophila tested produced detectable levels of extracellular protease, phosphatase, lipase, deoxyribonuclease, ribonuclease, and beta-lactamase activity. Weak starch hydrolysis was also demonstrated for all strains. Elastase, collagenase, phospholipase C, hyaluronidase, chondroitinase, neuraminidase, or coagulase were not detected in any of these laboratory-maintained strains.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARBER M., KUPER S. W. A. Identification of Staphylococcus pyogenes by the phosphatase reaction. J Pathol Bacteriol. 1951 Jan;63(1):65–68. doi: 10.1002/path.1700630108. [DOI] [PubMed] [Google Scholar]
- BERNFELD P. Enzymes of starch degradation and synthesis. Adv Enzymol Relat Subj Biochem. 1951;12:379–428. doi: 10.1002/9780470122570.ch7. [DOI] [PubMed] [Google Scholar]
- Baine W. B., Rasheed J. K., Maca H. W., Kaufmann A. F. Hemolytic activity of plasma and urine from rabbits experimentally infected with Legionella pneumophila. Rev Infect Dis. 1979 Nov-Dec;1(6):912–917. doi: 10.1093/clinids/1.6.912. [DOI] [PubMed] [Google Scholar]
- Baine W. B., Rasheed J. K., Mackel D. C., Bopp C. A., Wells J. G., Kaufmann A. F. Exotoxin activity associated with the Legionnaires disease bacterium. J Clin Microbiol. 1979 Mar;9(3):453–456. doi: 10.1128/jcm.9.3.453-456.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrisope G. L., Fox C. W., Marshall R. T. Lecithin agar for detection of microbial phospholipases. Appl Environ Microbiol. 1976 May;31(5):784–786. doi: 10.1128/aem.31.5.784-786.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser D. W., Tsai T. R., Orenstein W., Parkin W. E., Beecham H. J., Sharrar R. G., Harris J., Mallison G. F., Martin S. M., McDade J. E. Legionnaires' disease: description of an epidemic of pneumonia. N Engl J Med. 1977 Dec 1;297(22):1189–1197. doi: 10.1056/NEJM197712012972201. [DOI] [PubMed] [Google Scholar]
- Friedman R. L., Iglewski B. H., Miller R. D. Identification of a cytotoxin produced by Legionella pneumophila. Infect Immun. 1980 Jul;29(1):271–274. doi: 10.1128/iai.29.1.271-274.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu K. P., Neu H. C. Inactivation of beta-lactam antibiotics by Legionella pneumophila. Antimicrob Agents Chemother. 1979 Nov;16(5):561–564. doi: 10.1128/aac.16.5.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hébert G. A., Moss C. W., McDougal L. K., Bozeman F. M., McKinney R. M., Brenner D. J. The rickettsia-like organisms TATLOCK (1943) and HEBA (1959): bacteria phenotypically similar to but genetically distinct from Legionella pneumophila and the WIGA bacterium. Ann Intern Med. 1980 Jan;92(1):45–52. doi: 10.7326/0003-4819-92-1-45. [DOI] [PubMed] [Google Scholar]
- JEFFRIES C. D., HOLTMAN D. F., GUSE D. G. Rapid method for determining the activity of microorganisms on nucleic acids. J Bacteriol. 1957 Apr;73(4):590–591. doi: 10.1128/jb.73.4.590-591.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurioka S., Matsuda M. Phospholipase C assay using p-nitrophenylphosphoryl-choline together with sorbitol and its application to studying the metal and detergent requirement of the enzyme. Anal Biochem. 1976 Sep;75(1):281–289. doi: 10.1016/0003-2697(76)90078-6. [DOI] [PubMed] [Google Scholar]
- Legakis N. J., Papavassiliou J. Thin-layer chromatographic technique for rapid detection of bacterial phospholipases. J Clin Microbiol. 1975 Nov;2(5):373–376. doi: 10.1128/jcm.2.5.373-376.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G. K., Steigerwalt A., Feeley J. C., Wong E. S., Martin W. T., Patton C. M., Brenner D. J. Legionella gormanii sp. nov. J Clin Microbiol. 1980 Nov;12(5):718–721. doi: 10.1128/jcm.12.5.718-721.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H. E. Proteolytic action of Legionella pneumophila on human serum proteins. Infect Immun. 1980 Jan;27(1):51–53. doi: 10.1128/iai.27.1.51-53.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens J. J. The egg yolk reaction produced by several species of bacteria. J Appl Bacteriol. 1974 Mar;37(1):137–148. doi: 10.1111/j.1365-2672.1974.tb00424.x. [DOI] [PubMed] [Google Scholar]
- Rinderknecht H., Geokas M. C., Silverman P., Haverback B. J. A new ultrasensitive method for the determination of proteolytic activity. Clin Chim Acta. 1968 Aug;21(2):197–203. doi: 10.1016/0009-8981(68)90127-7. [DOI] [PubMed] [Google Scholar]
- Rinderknecht H., Wilding P., Haverback B. J. A new method for the determination of alpha-amylase. Experientia. 1967 Oct 15;23(10):805–805. doi: 10.1007/BF02146851. [DOI] [PubMed] [Google Scholar]
- SOMMER A. J. The determination of acid and alkaline phosphatase using p-nitrophenyl phosphate as substrate. Am J Med Technol. 1954 Jul-Aug;20(4):244–253. [PubMed] [Google Scholar]
- Sykes R. B., Matthew M. The beta-lactamases of gram-negative bacteria and their role in resistance to beta-lactam antibiotics. J Antimicrob Chemother. 1976 Jun;2(2):115–157. doi: 10.1093/jac/2.2.115. [DOI] [PubMed] [Google Scholar]
- TORRIANI A. Influence of inorganic phosphate in the formation of phosphatases by Escherichia coli. Biochim Biophys Acta. 1960 Mar 11;38:460–469. doi: 10.1016/0006-3002(60)91281-6. [DOI] [PubMed] [Google Scholar]
- Tsubokura M., Otsuki K., Shimohira I., Yamamoto H. Production of indirect hemolysin by Yersinia enterocolitica and its properties. Infect Immun. 1979 Sep;25(3):939–942. doi: 10.1128/iai.25.3.939-942.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren W. J., Miller R. D. Growth of Legionnaires disease bacterium (Legionella pneumophila) in chemically defined medium. J Clin Microbiol. 1979 Jul;10(1):50–55. doi: 10.1128/jcm.10.1.50-55.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K. H., Moss C. W., Hochstein D. H., Arko R. J., Schalla W. O. "Endotoxicity" of the Legionnaires' disease bacterium. Ann Intern Med. 1979 Apr;90(4):624–627. doi: 10.7326/0003-4819-90-4-624. [DOI] [PubMed] [Google Scholar]
- Wretlind B., Wadström T. Purification and properties of a protease with elastase activity from Pseudomonas aeruginosa. J Gen Microbiol. 1977 Dec;103(2):319–327. doi: 10.1099/00221287-103-2-319. [DOI] [PubMed] [Google Scholar]


