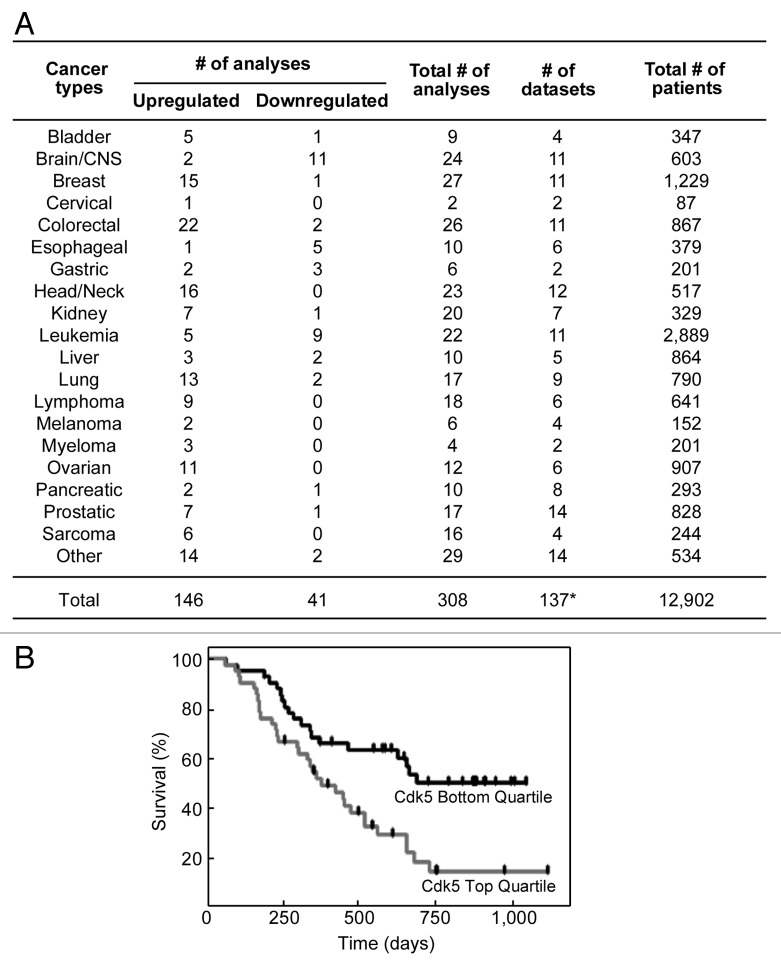
Cyclin-dependent kinase 5 (cdk5) is a small serine/threonine kinase that was identified based on its structural similarity to cdk1 and cdk2, key regulators of mammalian cell cycle progression.1 However, cdk5 is unique in that it is activated by the non-conventional cyclin, p35 or p39, which are abundant in postmitotic neurons.1 Indeed, cdk5 activity has been associated with neuronal functions, such as differentiation, migration and synaptic plasticity.1 However, there is increasing evidence suggesting that cdk5 activity is also present in non-neuronal cells and is involved in diverse functions, including DNA repair and cell cycle progression.2 Cdk5 mediates DNA repair through phosphorylation and activation of ATM,3 which is critical for double strand break repair by homologous recombination.4 In addition, silencing cdk5 sensitizes breast cancer cells to PARP inhibitors, which are highly effective against cells that are deficient in DNA double-strand break repair.2 Since no difference in PARP inhibitor sensitivity was observed when either ATM alone or both ATM and cdk5 are silenced, it appears that cdk5 and ATM operate in the same pathway.2 Interestingly, cdk5 has been shown to be required for intra-S and G2/M cell cycle checkpoints.2 Furthermore, evidence for a link between cdk5 and cancer is increasing. Cdk5 has been shown to positively regulate migration and metastasis in prostate cancer cells.5 Additionally, single nucleotide polymorphisms (SNPs) in the promoter region of the cdk5 gene have been linked to increased risk for lung cancer.6 Decreased methylation of the cdk5 promoter region, which resulted in increased cdk5 expression, was also observed in mantle cell lymphoma.7 To further investigate the link between the level of cdk5 expression and human cancers, we initially examined multiple microarray data sets (Oncomine) that measured levels of cdk5 in cancer vs corresponding normal tissues. Out of a total of 308 independent analyses of 137 microarray data sets, consisting of a total of 12,902 patients, we found that 146 analyses revealed significant upregulation of cdk5, and 41 analyses showed significant downregulation of cdk5 (Fig. 1A). Among those that showed significant upregulation of cdk5 expression are colorectal, head/neck, breast, lung, ovarian, lymphoma, prostatic, sarcoma, myeloma and bladder cancers. Conversely, brain and esophageal cancers and leukemia have significantly lower cdk5 expression. It is important to note that although 11 of the 24 analyses of brain cancer showed reduction of cdk5 levels compared with normal brain, analyses may have been compromised by comparing different cell populations, i.e., neurons that highly express cdk5 with dedifferentiated astrocytic tumor cells that express considerably lower levels of cdk5. Recently, silencing cdk5 has also been demonstrated to sensitize multiple myeloma cells to treatment with bortezomib, a proteasome inhibitor that generates considerable clinical response in newly diagnosed as well as advanced multiple myeloma patients.8 However, only 40% of patients respond to bortezomib, and most of these patients develop resistance over time. As cdk5 expression is upregulated in multiple myeloma, and cdk5 mediates bortezomib sensitivity, we investigated the possibility that cdk5 could serve as a predictive marker for patient survival following bortezomib treatment. Using the Kaplan-Meier survival analysis, we determined that, following bortezomib treatment, patients with high cdk5 expression (top quartile) have significantly lower survival compared with patients with low cdk5 expression (bottom quartile) (Fig. 1B). After 750 to 1,000 d of bortezomib treatment, survival of patients with low cdk5 expression is about 50%, while that of patients with high cdk5 expression is only about 15%. No significant difference in survival was observed between high and low cdk5-expressing cancer patients treated with dexamethasone, indicating specificity to bortezomib (data not shown). In vitro, the cdk5 inhibitors roscovitine and SCH727965 have been shown to sensitize primary myeloma cells to bortezomib treatment.8 Whether this will translate to a clinical setting remains to be determined. While the precise mechanism of bortezomib action in multiple myeloma is still unknown, it has been shown that bortezomib reduces NFκB activity and induces unfolded protein response, ER stress and immune sensitization.9 Bortezomib also inhibits the transcription of many DNA repair enzymes, including those involved in non-homologous end joining, mismatch repair, base excision repair and nucleotide excision repair.10 As cdk5 acts on the G2/M checkpoint to prevent passage into mitosis upon DNA damage,2 lack of cdk5 allows unregulated mitotic entry that increases genomic instability and, thus, apoptosis. In multiple myeloma, it is conceivable that the combination of DNA repair deficiencies related to bortezomib treatment and lack of cdk5 causes significant strain on DNA repair pathways leading to synthetic lethality. Regardless of the mechanism involved in the synergism between bortezomib treatment and lack of cdk5, it is clear from our analyses that cdk5 is generally upregulated in human cancers, and at least in multiple myeloma, patients with low cdk5 levels have significantly higher overall survival following bortezomib treatment. Thus, cdk5 could serve as a valuable prognostic marker and may allow the identification of multiple myeloma patients that will likely respond to bortezomib treatment.
Figure 1. (A) Up- and downregulation of cdk5 expression in human cancers. Analysis of cdk5 expression in cancer vs corresponding normal tissues was performed using the Oncomine microarray online data mining software with threshold p value < 0.05; fold change = all; gene rank = all; data type = mRNA.11,12 Out of 308 independent data analyses of 137 microarray data sets, consisting of a total of 12,902 patients, 146 analyses showed upregulated and 41 analyses showed downregulated expression of cdk5 compared with normal tissues. In the “other” category, cancers with upregulated cdk5 expression include uterine and parathyroid cancers, while cancers with downregulated cdk5 expression include testicular and skin cancers. *Note that several multicancer data sets were separately analyzed for different cancer types and, thus, the total number of data sets analyzed (137) does not match with the actual cumulative number of data sets (149). (B) Survival of relapsed multiple myeloma patients following bortezomib treatment is linked to cdk5 expression. Kaplan-Meier survival analyses show that patients with cdk5 expression in the highest quartile have significantly reduced survival relative to patients with cdk5 expression in the lowest quartile (p = 0.0021; n = 42).12 Bortezomib treatment (1.3 mg/m2) was performed on days 1, 2, 8 and 11 for eight 3-week cycles followed by three 5-week cycles with bortezomib administered on days 1, 8, 15 and 22. No significant difference was observed in the dexamethasone-treated group (p = 0.4891, n = 18; data not shown).
Acknowledgments
This work was supported in part by grants from the Canadian Institutes of Health Research and NSERC to K.Y.L. and J.L.R. are supported by a grant from NSERC.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/21886
References
- 1.Rosales JL, et al. Bioessays. 2006;28:1023–34. doi: 10.1002/bies.20473. [DOI] [PubMed] [Google Scholar]
- 2.Turner NC, et al. EMBO J. 2008;27:1368–77. doi: 10.1038/emboj.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tian B, et al. Nat Cell Biol. 2009;11:211–8. doi: 10.1038/ncb1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrison C, et al. EMBO J. 2000;19:463–71. doi: 10.1093/emboj/19.3.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strock CJ, et al. Cancer Res. 2006;66:7509–15. doi: 10.1158/0008-5472.CAN-05-3048. [DOI] [PubMed] [Google Scholar]
- 6.Choi HS, et al. J Hum Genet. 2009;54:298–303. doi: 10.1038/jhg.2009.29. [DOI] [PubMed] [Google Scholar]
- 7.Leshchenko VV, et al. Blood. 2010;116:1025–34. doi: 10.1182/blood-2009-12-257485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu YX, et al. Blood. 2011;117:3847–57. doi: 10.1182/blood-2010-08-304022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cvek B, et al. Curr Pharm Des. 2011;17:1483–99. doi: 10.2174/138161211796197124. [DOI] [PubMed] [Google Scholar]
- 10.Mitsiades N, et al. Blood. 2003;101:2377–80. doi: 10.1182/blood-2002-06-1768. [DOI] [PubMed] [Google Scholar]
- 11.Rhodes DR, et al. Neoplasia. 2007;9:166–80. doi: 10.1593/neo.07112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mulligan G, et al. Blood. 2007;109:3177–88. doi: 10.1182/blood-2006-09-044974. [DOI] [PubMed] [Google Scholar]