Abstract
Protein Tyrosine Kinase 6 (PTK6) is an intracellular tyrosine kinase that has distinct functions in normal epithelia and cancer. It is expressed primarily in nondividing epithelial cells in the normal intestine where it promotes differentiation. However after DNA damage, PTK6 is induced in proliferating progenitor cells where it contributes to apoptosis. We examined links between PTK6 and the tumor suppressor p53 in the isogenic p53+/+ and p53−/− HCT116 colon tumor cell lines. We found that p53 promotes expression of PTK6 in HCT116 cells, and shRNA-mediated knockdown of PTK6 leads to reduced induction of the CDK inhibitor p21. Knockdown of PTK6 enhances apoptosis in HCT116 cells with wild type p53, following treatment of cells with γ-radiation, doxorubicin, or 5-fluorouracil. No differences in activation of AKT, ERK1/2, or ERK5, known PTK6 regulated prosurvival signaling proteins, were detected. However, activity of STAT3, a PTK6 substrate, was impaired in cells with knockdown of PTK6 following DNA damage. In contrast to its role in the normal epithelium following DNA damage, PTK6 promotes survival of cancer cells with wild type p53 by promoting p21 expression and STAT3 activation. Targeting PTK6 in combination with use of chemotherapeutic drugs or radiation may enhance death of colon tumor cells with wild type p53.
INTRODUCTION
Protein tyrosine kinase 6 (PTK6; also called BRK or Sik) is an intracellular tyrosine kinase distantly related to the Src-family of tyrosine kinases. While PTK6 is not expressed in the normal mammary gland or ovarian epithelium, it is frequently overexpressed in breast and ovarian tumors [reviewed in (1, 2)]. In normal tissues, PTK6 is most highly expressed in non-dividing, differentiated epithelial cells of the gastrointestinal tract (3–5). PTK6 is also expressed in the normal prostate where it is localized to the epithelial nuclei, but its nuclear localization is lost in prostate disease and prostate tumors (6).
Characterization of the Ptk6-null mouse revealed increased intestinal epithelial cell proliferation and impaired enterocyte differentiation that correlated with enhanced activation of AKT, when compared with wild type control mice. In addition, nuclear β-catenin was more readily detected in Ptk6-null mice (7). PTK6 can directly associate with β-catenin and inhibit β-catenin/TCF regulated transcription. Ptk6-null BAT-GAL mice, containing a β-catenin-activated LacZ reporter transgene, displayed increased levels of β-galactosidase expression in the colon (8).
Even though PTK6 promotes epithelial cell differentiation and cell cycle exit in the normal intestine, alterations in PTK6 localization and/or expression can impact its function. While PTK6 is primarily restricted to differentiated nondividing cells in the small and large intestine, PTK6 is induced in proliferating progenitor crypt epithelial cells following γ-irradiation (9), or treatment with the carcinogen azoxymethane (AOM) (10). Disruption of the Ptk6 gene impairs DNA damage induced apoptosis in the mouse. Induction of PTK6 in colonic crypts following AOM injection correlated with increased apoptosis, compensatory proliferation and tumorigenesis. Reduced tumor development was correlated with impaired STAT3 activation in the colons of Ptk6 null mice (10),
The induction of PTK6 expression following DNA damage in vivo led us to explore potential links between this tyrosine kinase and the tumor suppressor protein p53, which is frequently mutated in colon cancer (11). p53 is a transcription factor that is stabilized following DNA damage. In intestinal tissues, p53-dependent (12, 13) and independent apoptosis (14) may occur following irradiation. Induction of expression of the CDK inhibitor p21 may prevent cells from undergoing apoptosis (15), and the ability of p53 to promote expression of p21 has been shown to play a protective role in the intestine (16). Mice lacking either p53 or its downstream target p21 are more sensitive to developing GI toxicity syndrome in response to radiation injury (17).
The aim of our study was to determine if p53 regulates induction of PTK6 expression following DNA damage, and if PTK6 modulates colon cancer cell sensitivity to chemotherapeutic agents. We utilized HCT116 cells, which were derived from human colorectal carcinoma epithelial cells, and contain a wild type p53 gene. These cells respond normally to DNA-damaging agents through induction of p53 followed by cell cycle arrest (18). HCT116 p53−/− cells were produced by deletion of both alleles of p53 through homologous recombination (19). Utilizing isogenic HCT116 p53+/+ and p53 −/− cells, we found that knockdown of PTK6 expression enhances apoptosis in p53+/+ HCT116 colon cancer cells following DNA damage induced by γ-irradiation, doxorubicin and 5-fluorouracil. Along with increased apoptosis, PTK6 knockdown cells also displayed decreased survival with impaired STAT3 activation and decreased p21 levels. These data suggest that kinase inhibitors targeting PTK6 may enhance sensitivity of colon cancer cells to chemotherapeutic agents.
MATERIALS AND METHODS
Cell Lines
The p53+/+ and p53−/− HCT116 human colorectal carcinoma cell lines were a gift from Dr. Bert Vogelstein (John Hopkins, Baltimore, MD). Cells were cultured in Dulbecco modified Eagle medium (DMEM) containing 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. Immortalized young adult mouse colon (YAMC) control cells were provided by Robert Whitehead (Vanderbilt University Medical Center, Nashville, TN). Control YAMC and YAMC cells derived from Ptk6 −/− mice (20, 21) were cultured in RPMI 1640 media containing 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin and INF-γ (5 U/ml) and grown at 33°C. The Ptk6+/+ and Ptk6−/− YAMC cell lines were genotyped and characterized in the authors’ laboratory (21). No additional authentication of cell lines was done by the authors.
Cell Treatments
For γ-irradiation experiments, cells were plated at 4 × 105 cells/10 cm dish 16 hours before treatment. Fresh growth medium was added to the cells and they were exposed to 20 Gy of gamma irradiation and harvested at 0, 3, 6, 12, 24, 48 or 72 hours post treatment. Both floating and attached cells were harvested for protein lysates.
For doxorubicin and 5-fluorouracil (5FU) experiments, cells were plated at 1 × 106 cells/10 cm dish 16 hours before treatment. Fresh medium containing either DMSO(control cells) or 5 μM or 10 μM of doxorubicin (Fisher Scientific, Waltham, MA) per dish or 300 μM of 5-Fluorouracil (Sigma-Aldrich, St. Louis, MO) was added per dish, and cells were incubated for 24 hours at 37°C. Both floating and attached cells were harvested for protein lysates.
PTK6 Knockdown
The Mission™ TCR shRNA Target Set directed against PTK6 in the pLKO.1 lentiviral expression vector was purchased from Sigma-Aldrich. Lentiviruses expressing TCRN0000021549 (shRNA 49), TCRN0000021552 (shRNA 52) and empty vector were produced in the HEK293FT packaging cell line by co-transfection with compatible packaging plasmids HIVtrans and VSVG as described previously (8). Cells were infected with retrovirus (50% viral supernatant and 50% growth medium containing 5 μg/ml polybrene) and placed in selection medium containing 2 μg/ml puromycin for 2 weeks for selection of stable pools.
PTK6 Overexpression
HCT116 pLKO.1 vector (control) and shRNA49 PTK6 knockdown cells were transfected using the Lipofectamine 2000 (Invitrogen, Carlsbad, CA) as per the manufacturer’s instructions. For ectopic PTK6 overexpression experiments, cells were transfected with DNA encoding full-length wild-type PTK6 (pcDNA3.Myc.PTK6) or empty vector as a control (pcDNA3). Cells were plated in 10 cm dishes, transfected with 8 μg DNA and incubated at 37°C for 12 hours prior to treatment with doxorubicin or 5FU.
Immunoblotting and Antibodies
Immunoblotting was performed as previously described (7). Antibodies were obtained from the following sources: human PTK6, Millipore (Temecula, CA); AKT (9272), Phospho-AKT (Thr308), cleaved-Caspase 3, cleaved PARP, ERK 1/2 (9102), Phospho-ERK 1/2 (Thr202/Tyr204), ERK5 (3372), Phospho-ERK5 (Thr218/Tyr220), STAT3 (9132) and phospho-STAT3 (Tyr705), Cell Signaling Technology (Beverly, MA); p53 (DO-1) HRP, Santa Cruz Biotechnology, Inc (Santa Cruz, CA); β-actin, Sigma- Aldrich (St. Louis, MO); and p21, BD Biosciences (Franklin Lakes, NJ). Secondary antibodies (donkey anti-rabbit or sheep anti-mouse conjugated to horseradish peroxidase) (Amersham Biosciences, Piscataway, NJ) were detected by chemiluminescence with SuperSignal West Dura Extended Duration Substrate (Thermo Scientific, Rockford, IL).
Flow Cytometry
The annexin V-PE staining kit (Enzo Life Sciences, Farmingdale, NY) was used for the detection of apoptotic cells, according to manufacturer’s instructions. This kit uses a dual-staining protocol in which the cells show fluorescence of both annexin V (apoptotic cells) 7AAD (necrotic cells or late apoptotic cells). HCT116 cells were exposed to 20 Gy of γ-irradiation and harvested 48 hours post treatment. Cells were trypsinized and 1 × 105 cells were washed with PBS, processed for labeling with annexin V-7AAD, and analyzed by flow cytometry.
Colony Formation Assay
Cells were seeded at a density of 1 × 105 cells/plate on 10 cm dishes. Control (non-irradiated) or irradiated cells exposed to 20 Gy of γ-irradiation were grown for 7 days. Cells were stained with crystal violet solution (0.1% crystal violet and 25% methanol in water) for 30 minutes at room temperature.
RNA Extraction and Quantitative Real-Time (qRT)-PCR
Total RNA was isolated from cells using TRIzol (Invitrogen) according to manufacture’s instructions. To generate cDNA for real-time PCR, total RNA was reverse transcribed using the SuperScript III First-Strand kit (Invitrogen). Quantitative real-time PCR (RT-PCR) was performed using the MyiQ single color real time PCR detection system (Bio-Rad, Hercules, CA). RT-PCR amplification was always done in triplicate and a melting curve was performed for each analysis to ensure the amplification of one product. The levels of each gene of interest were normalized against the levels of 18S mRNA, which was used as an internal control. The following primers were used: human p21 (Sense, 5′-AAG ACC ATG TGG ACC TGT -3′; Antisense, 5′-GGT AGA AAT CTG TCA TGC TG -3′), human PTK6 (Sense, 5′-TGT TCC TGC TCT TCC CAG TT-3′; Antisense, 5′-TGG GAG GAA AGA ACC CTT GA-3′) (22) and human 18S (Sense, 5′-TTG ACT CAA CAC GGG AAA CC-3′; Antisense, 5′-ACC CAC GGA ATC GAG AAA GA-3′).
Data Analysis
Results are shown as the mean ± SD. P-values were determined using the 2-tailed Student’s T test (Microsoft Excel, 2010). A difference was considered statistically significant if the P-value was equal to or less than 0.05. Immunoblot band densities were quantified using NIH ImageJ (23).
RESULTS
PTK6 expression is induced following γ-irradiation in HCT116 cells
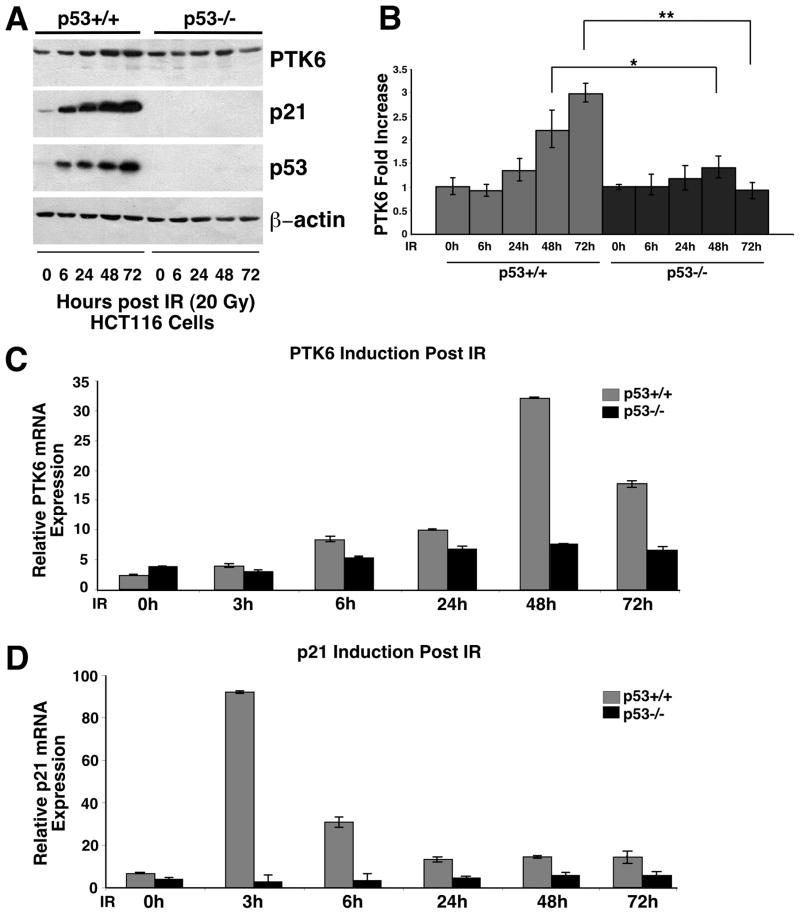
Following DNA damage, PTK6 is induced in the proliferating crypt epithelial cells in the small intestine (9) and colon (10), where it promotes apoptosis following damage. Apoptosis in the intestine following γ-irradiation is regulated by p53-dependent (12, 24) and p53-independent (14) mechanisms. To determine if p53 regulates induction of PTK6 expression following DNA damage, we turned to the isogenic HCT116 p53+/+ and p53−/− human colon cancer cell lines (25) that express moderate levels of endogenous PTK6. Both cell lines were subjected to 20 Gy of γ-irradiation and harvested at 0, 6, 24, 48 and 72 hours post treatment. Protein lysates were prepared and analyzed by immunoblotting. Induction of PTK6 was observed by 24 hours after irradiation, with peak levels at 48 hours (Figure 1A, B). While PTK6 was induced in both HCT116 p53+/+ and p53−/− cell lines following irradiation, significantly higher levels of PTK6 protein were detected in p53+/+ HCT116 cells (Figure 1A, B) suggesting a p53-dependent component in PTK6 induction. Rapid accumulation of p53 and activation of the p53 target gene p21 were detected in wild type HCT116 cells following irradiation (Figure 1A).
Figure 1. PTK6 expression increases following γ-irradiation in HCT116 cells.
(A) HCT116 p53+/+ and p53−/− cells were exposed to 20 Gy of γ-irradiation and harvested after 0,, 6, 24, 48 and 72 hours. Immunoblotting was performed using antibodies against PTK6, p21 and p53. β-actin was examined as a loading control. (B) The ratio of PTK6 toβ-actin was quantified using NIH ImageJ to determine the fold increase of PTK6 (* P< 0.05; ** P< 0.01, Bars +/− SD). (C) Quantitative real-time PCR analysis of PTK6 and p21 transcripts were normalized to levels of 18S rRNA, which was used an internal control (Bars +/− SD).
To further explore links between p53 and PTK6 induction, expression of PTK6 and the p53 target gene p21 were examined by quantitative real-time (RT)-PCR. HCT116 p53+/+ and p53−/− cell lines were subjected to 20 Gy of γ-irradiation and harvested at 0, 3, 6, 24, 48 and 72 hours post treatment. Induction of PTK6 mRNA expression was observed, with an approximately 30-fold increase in expression at 48 hours post irradiation in the HCT116 p53+/+ cells (Figure 1C). However, the p21 gene, which is a direct target of p53 (26), showed a 90-fold induction by 3 hours post irradiation in HCT116 p53+/+ cells (Figure 1D). Induction of PTK6 mRNA is consistent with the induction of PTK6 protein expression at 48 hours post irradiation, but unlike the p21 gene that is induced by 3 hours post irradiation, the PTK6 gene does not appear to be a direct transcriptional target of p53.
Knockdown of PTK6 expression sensitizes HCT116 cells to γ-irradiation-induced DNA damage
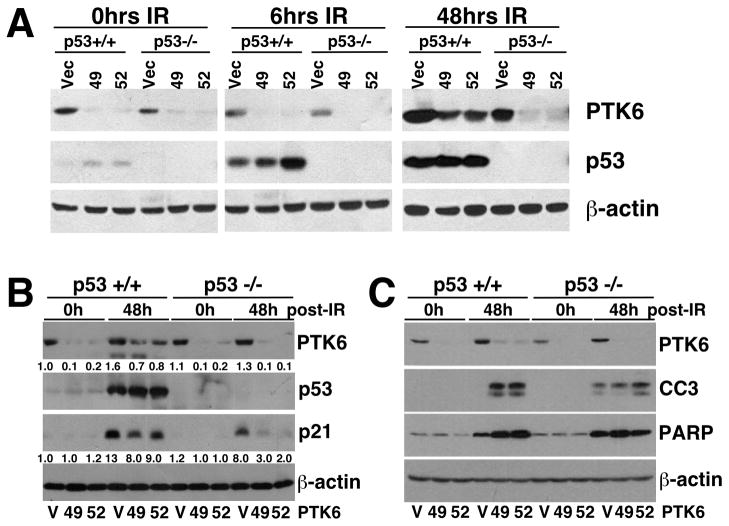
DNA-damaging agents including γ-irradiation and chemotherapeutic drugs, such as doxorubicin and 5FU, are the basis of most current cancer treatment regimens. Given the important role of p53 in DNA damage-induced apoptosis, we wanted to assess the potential crosstalk between p53 and PTK6 in regulating DNA damage-induced apoptosis following γ-irradiation. HCT116 p53+/+ and p53−/− cell lines containing empty vector (V) or one of two different shRNAs (49, 52) that target PTK6 were subjected to 20 Gy of γ-irradiation and harvested at 0, 6 and 48 hours post treatment. DNA damage-induced expression of p53 was detected in HCT116 p53+/+ vector control and stable PTK6 knockdown cell lines by 6 hours (Figure 2A). Additionally, p21 expression was induced following γ-irradiation in both HCT116 p53+/+ and p53−/− cells, but reduced p21 expression was observed in PTK6 knockdown cells (49, 52) versus control cells (V) (Figure 2B). These results support the findings that PTK6 expression enhances p21 expression, possibly leading to survival of colon cancer cells following DNA damage. Immunoblotting with antibodies specific for cleaved Caspase 3 and cleaved PARP further demonstrates that knockdown of PTK6 leads to enhanced apoptosis in the p53+/+ HCT116 cells (Figure 2C).
Figure 2. Knockdown of PTK6 in HCT116 cells enhances apoptosis following γ-irradiation.
(A) Stable HCT116 p53+/+ and p53 −/− cells containing empty vector shRNA (V) or one of two different shRNAs (49 or 52) that target PTK6 were exposed to 20Gy of γ-irradiation and harvested at 0, 6 and/or 48 hours post-treatment. Immunoblotting was performed using antibodies against PTK6 and p53; (B) PTK6, p53 and p21; and (C) PTK6 and the cleaved forms of Caspase 3 and PARP. β-actin was examined as a loading control.
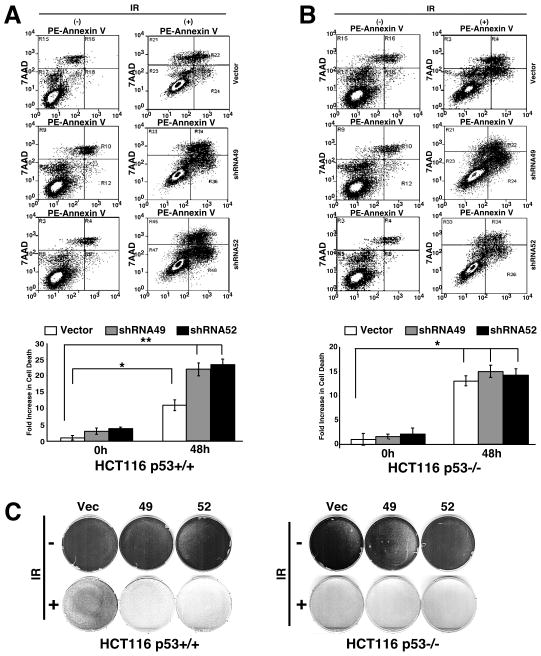
The PTK6 knockdown cells were subjected to γ-irradiation and incubated with PE Annexin V and 7AAD, and cell death was analyzed by flow cytometry. PE Annexin V staining can identify early stages of apoptosis, while staining cells with 7AAD detects the late stages of cell death resulting from either apoptosis or necrosis. Both HCT116 p53+/+ and p53−/− cells displayed increased apoptosis following irradiation (Figure 3A, B). Stable knockdown of PTK6 in HCT116 p53+/+ cells led to increased apoptosis compared with vector control cells (Figure 3A), while PTK6 expression had little to no effect on apoptosis in HCT 116 p53−/− cells following irradiation (Figure 3B). To further examine the impact that knockdown of PTK6 had on cell growth and survival, we performed colony assays with HCT116 p53+/+ and p53−/− PTK6 knockdown cells following 20 Gy of γ-irradiation. Colony formation was assessed at 7 days post irradiation. Although 20 Gy of γ-irradiation leads to dramatic induction of cell death in both HCT116 p53+/+ and p53−/− cells, a population of cells survived and gave rise to colonies (Figure 3C). Both HCT116 p53+/+ and p53−/− cells displayed decreased survival following γ-irradiation with a more dramatic decrease observed in HCT116 p53−/− cells (Figure 3C). Stable knockdown of PTK6 led to a more striking decrease in survival in HCT116 p53+/+ cells compared with vector control cells,
Figure 3. Knockdown of PTK6 in HCT116 cells leads to increased apoptosis and decreased survival following γ-irradiation.
HCT116 p53+/+ (A) and p53−/− (B) cells containing empty vector shRNA (Vector) or one of two different shRNAs (shRNA49, shRNA52) were stained with PE Annexin V and 7AAD and assayed by flow cytometry 0 (−) and 48 (+) hours post-20 Gy γ-irradiation. (A) Quantification of fold-increase in cell death for HCT116 p53+/+ (*P< 0.006, **P< 0.002; Bars +/− SD) and (B) HCT116 p53−/−(*P< 0.004; Bars +/− SD) shown in plots. (C) Colony formation assays were performed after seven days with HCT116 p53+/+ and p53−/− cells containing empty vector (Vector) or one of two different shRNAs (shRNA49, shRNA52) that target PTK6. Cells were untreated (− IR) or exposed to 20 Gy of γ-irradiation (+ IR).
Knockdown of PTK6 expression sensitizes HCT116 cells to DNA damage by chemotherapeutic drugs
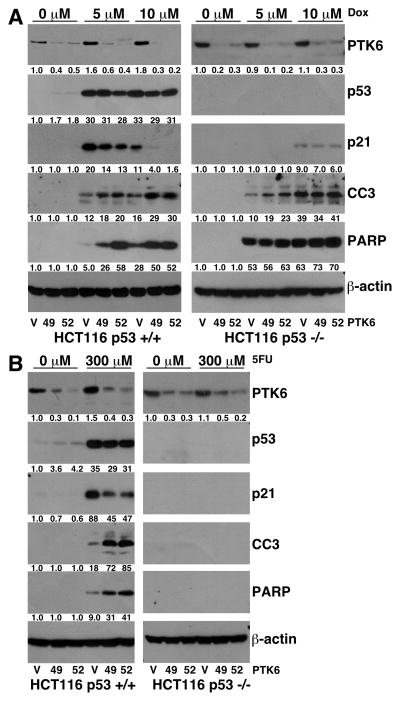
Doxorubicin is a DNA-damaging anthracycline antibiotic used in chemotherapy treatment for a wide range of malignancies (27). The chemotherapeutic agent 5FU is one of the most effective adjuvant therapies for patients with colon cancer (28). Both agents induce apoptosis in sensitive cancer cells. To determine if PTK6 modulates colon cancer cell sensitivity to chemotherapeutic agents, HCT116 p53+/+ and p53 −/− cells expressing vector control (V) or shRNAs targeting PTK6 (49 and 52) were treated with doxorubicin or 5FU for 24 hours, and cell lysates were prepared and examined by immunoblotting. Induction of PTK6 was observed following treatment with doxorubicin (Figure 4A) with a more marked increase detected in HCT116 p53+/+ cells as compared with p53−/− cells. In HCT116 p53+/+ cells treated with doxorubicin, the highest levels of apoptosis, marked by cleaved caspase-3 and cleaved PARP levels, were detected in PTK6 knockdown cells compared with vector control cells (Figure 4A). PTK6 expression had little effect on apoptosis in HCT116 p53−/− cells following drug treatment. Induction of p21 was also detected in HCT116 p53+/+ cells following treatment, with significantly higher levels observed in vector control cells compared to PTK6 knockdown cells (Figure 4A). A slight induction of p21 was observed in HCT116 p53−/− cells following 10 μM of doxorubicin indicating a p53-independent mode of regulation. However, PTK6 knockdown had no significant impact on p21 induction in HCT116 p53−/− cells following doxorubicin treatment.
Figure 4. PTK6 knockdown leads to enhanced apoptosis in HCT116 p53 +/+ cells following treatment with chemotherapeutic DNA-damaging drugs.
(A) HCT116 p53+/+ and p53−/− cells containing empty vector shRNA (V) or one of two different shRNAs (49, 52) that target PTK6 were treated with either DMSO or 5 or 10 μM of doxorubicin (Dox) and harvested 24 hours post treatment. Immunoblotting was performed using antibodies against PTK6, p53, p21 cleaved Caspase 3 and cleaved PARP. β-actin was examined as a loading control. (B) HCT116 p53+/+ and p53−/− cells containing empty vector shRNA (V) or one of two different shRNAs (49, 52) that target PTK6 were treated with either DMSO or 300 μM of 5FU and harvested 24 hours post treatment. Immunoblotting was performed using antibodies against PTK6, p53, p21, cleaved Caspase 3 and cleaved PARP. β-actin was examined as a loading control.
The effect of 5FU on apoptosis has been attributed to the ability of this drug to induce the activity of p53. Mutation or deletion of p53 has been shown to lead to resistance of cells to 5FU (19, 28). HCT116 p53+/+ cells treated with 5FU show a similar trend to that of doxorubicin with both induction of PTK6 expression and enhanced apoptosis in PTK6 knockdown cells (Figure 4B). 5FU treatment did not cause detectable apoptosis in the HCT116 p53−/− cells (Figure 4B). Induction of p21 was also detected in HCT116 p53+/+ cells following 5FU treatment, with significantly higher levels observed in vector control cells compared to PTK6 knockdown cells (Figure 3B). Little to no expression of p21 was observed in HCT116 p53−/− cells following drug treatment, as p21 induction by DNA-damaging agents is primarily regulated by p53 (29). These data suggest that knockdown of PTK6 expression promotes apoptosis of HCT116 p53+/+ cells and enhances the response of colon tumor cells to doxorubicin and 5FU.
Ectopic expression of recombinant PTK6 protects against DNA damage-induced apoptosis
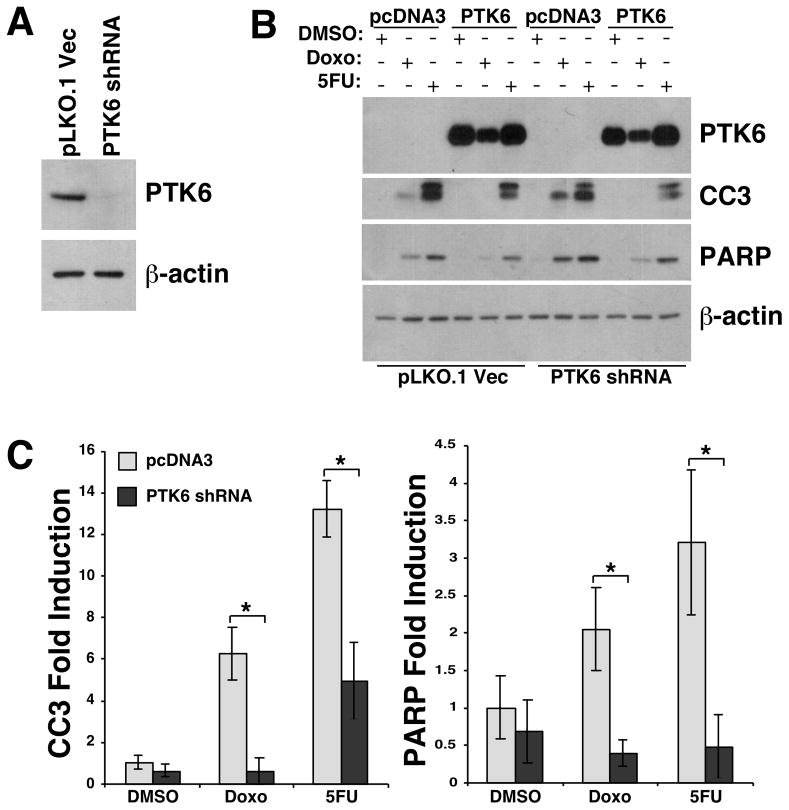
To verify that the enhanced apoptosis in PTK6 knockdown cells was not due to off-target shRNA effects, we reintroduced recombinant PTK6 into these cells. The shRNA49 targets the 3′ untranslated region of Ptk6, which is not present in the recombinant construct, so we used the shRNA49 and pLKO.1 vector control stable cell lines for ectopic PTK6 expression (PTK6 shRNA, pLKO.1) (Figure 5A). Following transient transfection of wild-type recombinant PTK6 or empty vector control (pcDNA3), cells were treated with doxorubicin or 5FU for 24 hours, and cell lysates were prepared and examined by immunoblotting (Figure 5B). In PTK6 knockdown cells (PTK6 shRNA), doxorubicin- and 5FU-induced cleavage of Caspase 3 and PARP were significantly reduced by ectopic wild type PTK6 (Figure 5C). Rescue of apoptosis by expression of ectopic PTK6 confirms that the enhanced apoptosis of PTK6 knockdown cells in response to DNA damage was due to reduced PTK6 expression in those cells.
Figure 5. Ectopic expression of recombinant PTK6 rescues DNA damage-induced apoptosis in PTK6 stable knockdown cells.
(A) Endogenous PTK6 detected by immunoblotting of lysates prepared from pLKO.1 vector and shRNA49 (PTK6 shRNA) stable HCT116 cell lines. (B) Recombinant PTK6 or pcDNA3 vector was introduced by transient transfection into HCT116 cells stably expressing PTK6 shRNA or PLKO1 vector (control). Cells were treated for 24 hours with 10 uM doxorubicin, 300 mM 5-FU, or DMSO control. Immunoblotting was performed using antibodies against PTK6, cleaved Caspase 3 and cleaved PARP; β-actin was examined as a loading control. (C) The ratios of cleaved Caspase 3 and cleaved PARP relative to β-actin in control vector (pcDNA3) and PTK6 knockdown cells (PTK6 shRNA) were quantified using NIH ImageJ (* P < 0.05, bars +/− SD).
Targeting PTK6 impairs STAT3 activation following DNA damage
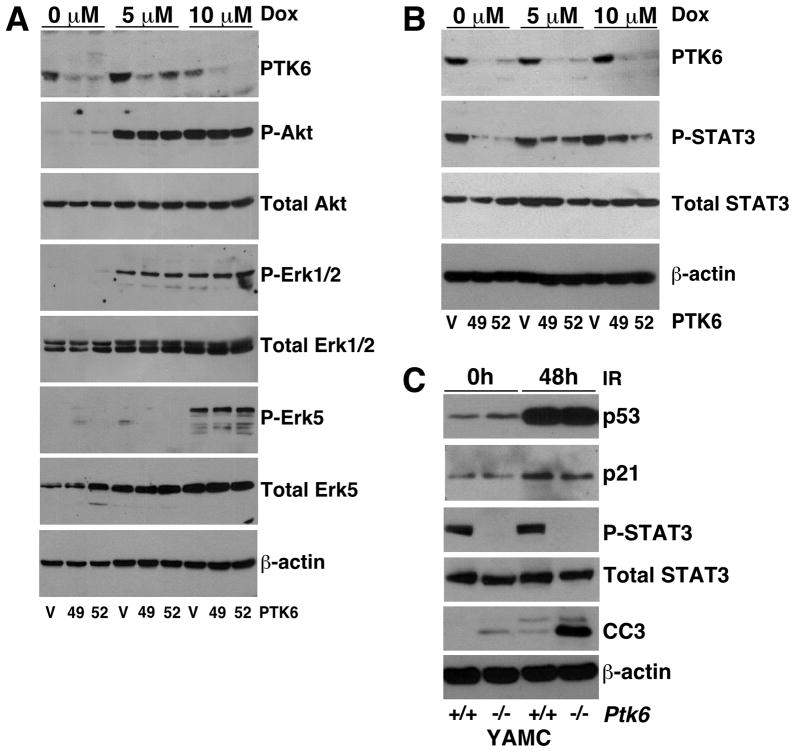
To identify signaling pathways downstream of PTK6 that might play a role in cell survival, we examined the expression and activation of, AKT, ERK1/2, ERK5 and STAT3. Previously, we showed that AKT and ERK1/2 activation was increased following disruption of PTK6 in the mouse small intestine following γ-irradiation (9). To determine if these pro-survival pathways were affected, HCT116 p53+/+ cells with stable knockdown of PTK6 and control cells were treated with 5 μM or 10 μM of doxorubicin for 24 hours and activation of AKT and ERK1/2 was examined using phospho-specific antibodies. We detected increased activation of all signaling proteins following DNA damage, but knockdown of PTK6 had no impact on activation (Figure 6A). We also examined ERK5 activation as it has been shown to play a role in cancer cell survival and proliferation (30). PTK6, in complex with ERK5, has been shown to regulate cell migration in keratinocytes and breast cancer cells following receptor activation (31, 32). Similar to AKT and ERK1/2, we observed an increase in phospho-ERK5 levels following treatment, but PTK6 expression had no effect on ERK5 activation (Figure 6A).
Figure 6. STAT3 activation is impaired in PTK6 knockdown cells following DNA damage.
(A) HCT116 p53+/+ cells containing empty vector shRNA (V) or one of two different shRNAs (49, 52) that target PTK6 were treated with either DMSO or 5 or 10 μM of doxorubicin (Dox) and harvested 24 hours post treatment. Immunoblotting was performed using antibodies against PTK6, Phospho-AKT, Total AKT, Phospho-ERK1/2, Total ERK1/2, Phospho-ERK5 and Total ERK5. β-actin was examined as a loading control. (B) HCT116 p53+/+ cells containing empty vector shRNA (V) or shRNAs (49, 52) were treated with either DMSO or 5 or 10 μM of doxorubicin (Dox) and harvested 24 hours post treatment. Immunoblotting was performed using antibodies against PTK6, Phospho-STAT3 and Total STAT3. β-actin was examined as a loading control. (C) Immunoblot analysis of lysates from wild type (+/+) and Ptk6 −/− YAMC cells that were untreated or exposed to 20 Gy of γ-irradiation and harvested 48 hours post treatment. Immunoblotting was performed using antibodies against p53, p21, phospho-STAT3, total STAT3 and cleaved Caspase 3. β-actin was examined as a loading control.
STAT3 has been shown to promote proliferation and survival leading to colon tumorigenesis (33, 34). PTK6 phosphorylates and activates STAT3 in cell lines (35) and increased STAT3 activation was observed in Ptk6 +/+ mice, compared with Ptk6 −/− mice, after AOM administration (10). We examined STAT3 activation in cell lysates prepared from vector control (V) and PTK6 stable knockdown HCT116 p53+/+ cells (49 and 52) that were treated with 5 μM or 10 μM of doxorubicin for 24. We examined STAT3 activation by examining phosphorylation of STAT3 tyrosine residue 705 (P-STAT3). Highest levels of P-STAT were detected in the vector control cells. Following doxorubicin treatment there was an increase in STAT3 activation in PTK6 knockdown cells (49, 52) but not to the levels observed in vector control cells (Figure 6B).
We also examined STAT3 activation in immortalized young adult mouse colon (YAMC) cells from wild type and Ptk6 −/− mice (21). This cell culture model system represents normal colon epithelial cells (21). Lysates were prepared from untreated or γ-irradiated Ptk6 +/+ and Ptk6 −/− YAMC cells. Interestingly, both p53 and p21 were induced in irradiated control and Ptk6 null YAMC cells, which carry a temperature-sensitive mutant of the SV40 large T gene for immortalization and have impaired p53 activity. In Ptk6−/− YAMC cells, p21 levels were slightly lower than in wild type control YAMCs post irradiation. Previously, we showed that PTK6 regulates basal STAT3 activity in YAMC cells (10). Similar results were obtained from YAMC cells following γ-irradiation treatment. Baseline levels of phospho-STAT3 are higher in Ptk6 +/+ YAMC cells compared with Ptk6 −/− YAMC cells. In cells that were exposed to 20 Gy of γ-irradiation and harvested at 48 hours post treatment, active STAT3 was detected only in Ptk6 +/+ cells (Figure 6C). Consistent with the HCT116 cells, we observed increased cleaved Caspase 3 levels in Ptk6 −/− YAMC cells compared with the wild type cells following radiation. These data suggest that the protective role of PTK6 in response to DNA damage may function through activation of the prosurvival transcription factor STAT3.
DISCUSSION
Functions of PTK6 are not well understood and a growing body of evidence indicates that PTK6 signaling outcomes are context-dependent. PTK6 has growth-suppressive or growth-promoting functions dependent on its localization in different intracellular compartments (8, 36–40). While it is not expressed in normal mammary gland, high level expression of PTK6 in a majority of breast tumors examined may promote ERBB family, MET [reviewed in (2)] and IGF-1 receptor signaling (41). Studies of PTK6 in the normal intestine indicated that PTK6 restrained growth and promoted differentiation of gut epithelial cells (7). However, PTK6 induction in progenitor cells following DNA damage led to inhibition of prosurvival signaling and apoptosis (9). Although characterization of PTK6 in the normal gastrointestinal tract suggested that it might function as a tumor suppressor, recent in vivo studies demonstrated that disruption of Ptk6 impairs STAT3 activation and subsequent tumorigenesis following carcinogen administration (10).
These studies are the first to link PTK6 with the tumor suppressor p53 in intestinal cells. Radiation and chemotherapeutic agents stabilize and activate p53, which regulates genes that encode proteins that function in cell cycle regulation, survival and apoptosis (42). Recently p53 has been implicated in preventing the epithelial-mesenchymal transition (43). We show that PTK6 expression is positively regulated by p53-dependent mechanisms following DNA damage in HCT116 cells. We found that PTK6 protein and mRNA levels were induced in HCT116 p53+/+ cells following γ-irradiation (Figure 1A-C). However, unlike the p21 gene that is induced by 3 hours post γ-irradiation, the PTK6 gene, which is induced at peak levels at 48 hours post γ-irradiation, does not appear to be a direct target of p53 (Figure 1C, D).
Our data indicate that PTK6 expression confers resistance of colon cancer cells to DNA damaging agents, such as doxorubicin and 5FU and γ-radiation. Unlike what we observed in the small intestine (9) and colon (10) in vivo, knockdown of PTK6 in HCT116 p53+/+ and p53−/− cells led to enhanced apoptosis in HCT116 p53+/+ cells following γ-radiation, doxorubicin and 5FU treatment. Increased apoptosis was also observed in YAMC Ptk6−/− cells (Figure 6C). These data suggest that knockdown of PTK6 in colon cancer cells will enhance sensitivity to DNA damage induced apoptosis.
One of the best-characterized p53 targets, the CDK inhibitor p21, is expressed in differentiated intestinal epithelial cells similar to PTK6 (44). In several systems, p21 has been shown to promote cell survival by inducing cell cycle arrest and DNA repair [reviewed in (15, 45)]. The ability of p53 to activate p21 has a protective effect in the intestinal epithelium following high doses of radiation (17, 46, 47). Expression of p21 has been correlated with the resistance of human colon cancer cell lines to chemotherapeutic agents (19, 48). Knockdown of PTK6 in HCT116 p53+/+ cells led to decreased p21 expression following treatment with γ-irradiation, doxorubicin and 5FU (Figures 2 and 3). These data suggest a novel cross talk between p53 and PTK6 that merits further exploration. The ability of PTK6 to contribute to p21 expression following DNA damage provides one mechanism by which it may promote cell survival.
Along with an increase in apoptosis, PTK6 knockdown cells displayed decreased survival following γ-irradiation in the HCT116 p53+/+ cells (Figure 3C). STAT3 activation has been shown to play a vital role in a variety of cellular processes including proliferation, migration and survival (49). In the gastrointestinal tract, STAT3 has been shown to play a critical role in the initiation and progression of colitis associated colon cancer (33, 34). STAT3 has been shown to play an important role in intestinal stem cell survival (50), and it will be interesting to determine contributions of STAT3 and PTK6 to putative colon cancer stem cells. STAT3 was identified as a substrate of PTK6 (35), and we determined that PTK6 regulates activation of STAT3 in the colon after AOM induced DNA damage, in established tumors and in the HCT116 colon cancer cell line (10). Consistent with previous results, STAT3 activation was impaired in HCT116 p53+/+ cells following doxorubicin treatment (Figure 6B) and in YAMC Ptk6 knockout cells after γ-irradiation (Figure 6C). The ability of PTK6 to regulate STAT3 activity could contribute to resistance of colon cancer cells to DNA damaging agents.
In a complex tissue such as the colon, epithelial cells located on different positions along the crypt-villus axis are subjected to different signals emanating from the environment. When PTK6 is induced in crypt epithelial cells following DNA damage, it contributes to the apoptotic response (9, 10). In contrast, PTK6 promotes resistance to DNA damage-induced apoptosis in human colon cancer cells. Development of colon cancers is accompanied by mutations and alterations in a number of genes and pathways, changing the cellular context for PTK6 signaling. Knockdown of PTK6 following DNA damage led to reduced p21 expression and STAT3 activity, leading to enhanced apoptosis and decreased survival. These data suggest that identification of kinase inhibitors that specifically target PTK6 may enhance sensitivity of colon cancer cells to chemotherapeutic agents and radiation.
Acknowledgments
Grant Support: This work was supported by NIH Grant DK44525 (A. L. Tyner). J. J. Gierut received support from a NRSA/NIH Institutional T32 training grant DK07739, and an AGA Foundation Graduate Student Research Fellowship Award.
We thank the laboratories of Dr. Bert Vogelstein (Johns Hopkins University) and Dr. Robert Whitehead (Vanderbilt University) for providing cell lines for the studies.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Brauer PM, Tyner AL. Building a better understanding of the intracellular tyrosine kinase PTK6 - BRK by BRK. Biochimica et biophysica acta. 2010;1806:66–73. doi: 10.1016/j.bbcan.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostrander JH, Daniel AR, Lange CA. Brk/PTK6 signaling in normal and cancer cell models. Current opinion in pharmacology. 2010;10:662–9. doi: 10.1016/j.coph.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siyanova EY, Serfas MS, Mazo IA, Tyner AL. Tyrosine kinase gene expression in the mouse small intestine. Oncogene. 1994;9:2053–7. [PubMed] [Google Scholar]
- 4.Vasioukhin V, Serfas MS, Siyanova EY, Polonskaia M, Costigan VJ, Liu B, et al. A novel intracellular epithelial cell tyrosine kinase is expressed in the skin and gastrointestinal tract. Oncogene. 1995;10:349–57. [PubMed] [Google Scholar]
- 5.Llor X, Serfas MS, Bie W, Vasioukhin V, Polonskaia M, Derry J, et al. BRK/Sik expression in the gastrointestinal tract and in colon tumors. Clin Cancer Res. 1999;5:1767–77. [PubMed] [Google Scholar]
- 6.Derry JJ, Prins GS, Ray V, Tyner AL. Altered localization and activity of the intracellular tyrosine kinase BRK/Sik in prostate tumor cells. Oncogene. 2003;22:4212–20. doi: 10.1038/sj.onc.1206465. [DOI] [PubMed] [Google Scholar]
- 7.Haegebarth A, Bie W, Yang R, Crawford SE, Vasioukhin V, Fuchs E, et al. Protein tyrosine kinase 6 negatively regulates growth and promotes enterocyte differentiation in the small intestine. Molecular and Cellular Biology. 2006;26:4949–57. doi: 10.1128/MCB.01901-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palka-Hamblin HL, Gierut JJ, Bie W, Brauer PM, Zheng Y, Asara JM, et al. Identification of beta-catenin as a target of the intracellular tyrosine kinase PTK6. J Cell Sci. 2010;123:236–45. doi: 10.1242/jcs.053264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haegebarth A, Perekatt AO, Bie W, Gierut JJ, Tyner AL. Induction of protein tyrosine kinase 6 in mouse intestinal crypt epithelial cells promotes DNA damage-induced apoptosis. Gastroenterology. 2009;137:945–54. doi: 10.1053/j.gastro.2009.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gierut J, Zheng Y, Bie W, Carroll RE, Ball-Kell S, Haegebarth A, et al. Disruption of the Mouse Protein Tyrosine Kinase 6 Gene Prevents STAT3 Activation and Confers Resistance to Azoxymethane. Gastroenterology. 2011;141:1371–80. e2. doi: 10.1053/j.gastro.2011.06.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol. 2011;6:479–507. doi: 10.1146/annurev-pathol-011110-130235. [DOI] [PubMed] [Google Scholar]
- 12.Clarke AR, Gledhill S, Hoper ML, Bird CC, Wyllie AH. p53 dependence of early apoptotic and proliferative responses within the mouse intestinal epithelium following gamma-irradiation. Oncogene. 1994;9:1767–73. [PubMed] [Google Scholar]
- 13.Merritt AJ, Potten CS, Kemp CJ, Hickman JA, Balmain A, Lane DP, et al. The role of p53 in spontaneous and radiation-induced apoptosis in the gastrointestinal tract of normal and p53-deficient mice. Cancer Res. 1994;54:614–7. [PubMed] [Google Scholar]
- 14.Merritt AJ, Allen TD, Potten CS, Hickman JA. Apoptosis in small intestinal epithelial from p53-null mice: evidence for a delayed, p53-independent G2/M-associated cell death after gamma-irradiation. Oncogene. 1997;14:2759–66. doi: 10.1038/sj.onc.1201126. [DOI] [PubMed] [Google Scholar]
- 15.Gartel AL, Tyner AL. The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer Ther. 2002;1:639–49. [PubMed] [Google Scholar]
- 16.Gudkov AV, Komarova EA. Pathologies associated with the p53 response. Cold Spring Harb Perspect Biol. 2010;2:a001180. doi: 10.1101/cshperspect.a001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirsch DG, Santiago PM, di Tomaso E, Sullivan JM, Hou WS, Dayton T, et al. p53 controls radiation-induced gastrointestinal syndrome in mice independent of apoptosis. Science. 2010;327:593–6. doi: 10.1126/science.1166202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waldman T, Kinzler KW, Vogelstein B. p21 is necessary for the p53-mediated G1 arrest in human cancer cells. Cancer Res. 1995;55:5187–90. [PubMed] [Google Scholar]
- 19.Bunz F, Hwang PM, Torrance C, Waldman T, Zhang Y, Dillehay L, et al. Disruption of p53 in human cancer cells alters the responses to therapeutic agents [see comments] J Clin Invest. 1999;104:263–9. doi: 10.1172/JCI6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whitehead RH, Robinson PS. Establishment of conditionally immortalized epithelial cell lines from the intestinal tissue of adult normal and transgenic mice. American journal of physiology Gastrointestinal and liver physiology. 2009;296:G455–60. doi: 10.1152/ajpgi.90381.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitehead RH, Robinson PS, Williams JA, Bie W, Tyner AL, Franklin JL. Conditionally immortalized colonic epithelial cell line from a Ptk6 null mouse that polarizes and differentiates in vitro. Journal of Gastroenterology and Hepatology. 2008;23:1119–24. doi: 10.1111/j.1440-1746.2008.05308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brauer PM, Zheng Y, Evans MD, Dominguez-Brauer C, Peehl DM, Tyner AL. The Alternative Splice Variant of Protein Tyrosine Kinase 6 Negatively Regulates Growth and Enhances PTK6-Mediated Inhibition of beta-Catenin. PLoS ONE. 2011;6:e14789. doi: 10.1371/journal.pone.0014789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasband WS. ImageJ. U S National Institutes of Health; Bethesda, Maryland, USA: 1997–2011. http://imagej.nih.gov/ij/ [Google Scholar]
- 24.Merritt AJ, Potten CS, Kemp CJ, Hickman JA, Balmain A, Lane DP, et al. The role of p53 in spontaneous and radiation induced apapotosis in the gastrointestinal tract of normal and p53 deficient mice. Cancer Res. 1994;5420:614–7. [PubMed] [Google Scholar]
- 25.Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, et al. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 26.El-Deiry WS, Harper WJ, O’Connor PM, Velculescu VE, Canman CE, Jackman J, et al. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 1994;54:1169–74. [PubMed] [Google Scholar]
- 27.Weiss RB. The anthracyclines: will we ever find a better doxorubicin? Seminars in oncology. 1992;19:670–86. [PubMed] [Google Scholar]
- 28.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nature reviews Cancer. 2003;3:330–8. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 29.Macleod K, Sherry N, Hannon G, Beach D, Tokino T, Kinzler K, et al. p53-dependent and independent expression of p21 during cell growth, differentiation, and DNA damage. Genes&Dev. 1995;9:935–44. doi: 10.1101/gad.9.8.935. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Tournier C. Regulation of cellular functions by the ERK5 signalling pathway. Cellular signalling. 2006;18:753–60. doi: 10.1016/j.cellsig.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Castro NE, Lange CA. Breast tumor kinase and extracellular-signal-regulated kinase 5 mediate Met receptor signaling to cell migration in breast cancer cells. Breast Cancer Res. 2010;12:R60. doi: 10.1186/bcr2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Locatelli A, Lofgren KA, Daniel AR, Castro NE, Lange CA. Mechanisms of HGF/Met Signaling to Brk and Sam68 in Breast Cancer Progression. Horm Cancer. 2011 doi: 10.1007/s12672-011-0097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–13. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bollrath J, Phesse TJ, von Burstin VA, Putoczki T, Bennecke M, Bateman T, et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15:91–102. doi: 10.1016/j.ccr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Liu L, Gao Y, Qiu H, Miller WT, Poli V, Reich NC. Identification of STAT3 as a specific substrate of breast tumor kinase. Oncogene. 2006;25:4904–12. doi: 10.1038/sj.onc.1209501. [DOI] [PubMed] [Google Scholar]
- 36.Haegebarth A, Heap D, Bie W, Derry JJ, Richard S, Tyner AL. The nuclear tyrosine kinase BRK/Sik phosphorylates and inhibits the RNA-binding activities of the Sam68-like mammalian proteins SLM-1 and SLM-2. J Biol Chem. 2004;279:54398–404. doi: 10.1074/jbc.M409579200. [DOI] [PubMed] [Google Scholar]
- 37.Kim HI, Lee ST. Oncogenic Functions of PTK6 Are Enhanced by Its Targeting to Plasma Membrane But Abolished by Its Targeting to Nucleus. Journal of biochemistry. 2009 doi: 10.1093/jb/mvp050. [DOI] [PubMed] [Google Scholar]
- 38.Brauer PM, Zheng Y, Wang L, Tyner AL. Cytoplasmic retention of protein tyrosine kinase 6 promotes growth of prostate tumor cells. Cell Cycle. 2010;9:4190–9. doi: 10.4161/cc.9.20.13518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng Y, Gierut J, Wang Z, Miao J, Asara JM, Tyner AL. Protein Tyrosine Kinase 6 Protects Cells from Anoikis by Directly Phosphorylating Focal Adhesion Kinase and Activating AKT. Oncogene. 2012 doi: 10.1038/onc.2012.427. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng Y, Asara JM, Tyner AL. Protein-tyrosine Kinase 6 Promotes Peripheral Adhesion Complex Formation and Cell Migration by Phosphorylating p130 CRK-associated Substrate. The Journal of Biological Chemistry. 2012;287:148–58. doi: 10.1074/jbc.M111.298117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Irie HY, Shrestha Y, Selfors LM, Frye F, Iida N, Wang Z, et al. PTK6 regulates IGF-1-induced anchorage-independent survival. PLoS ONE. 2010;5:e11729. doi: 10.1371/journal.pone.0011729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vousden KH, Prives C. Blinded by the Light: The Growing Complexity of p53. Cell. 2009;137:413–31. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 43.Muller PA, Vousden KH, Norman JC. p53 and its mutants in tumor cell migration and invasion. The Journal of Cell Biology. 2011;192:209–18. doi: 10.1083/jcb.201009059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gartel AL, Serfas MS, Gartel M, Goufman E, Wu GS, El-Deiry WS, et al. p21 (WAF1/CIP1) expression is induced in newly nondividing cells in diverse epithelial and during differentiation of the Caco-2 intestinal cell line. Exp Cell Res. 1996;227:171–81. doi: 10.1006/excr.1996.0264. [DOI] [PubMed] [Google Scholar]
- 45.Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nature reviews Cancer. 2009;9:400–14. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Komarova EA, Kondratov RV, Wang K, Christov K, Golovkina TV, Goldblum JR, et al. Dual effect of p53 on radiation sensitivity in vivo: p53 promotes hematopoietic injury, but protects from gastro-intestinal syndrome in mice. Oncogene. 2004;23:3265–71. doi: 10.1038/sj.onc.1207494. [DOI] [PubMed] [Google Scholar]
- 47.Leibowitz BJ, Qiu W, Liu H, Cheng T, Zhang L, Yu J. Uncoupling p53 functions in radiation-induced intestinal damage via PUMA and p21. Molecular cancer research : MCR. 2011;9:616–25. doi: 10.1158/1541-7786.MCR-11-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ravizza R, Gariboldi MB, Passarelli L, Monti E. Role of the p53/p21 system in the response of human colon carcinoma cells to Doxorubicin. BMC cancer. 2004;4:92. doi: 10.1186/1471-2407-4-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matthews JR, Sansom OJ, Clarke AR. Absolute requirement for STAT3 function in small-intestine crypt stem cell survival. Cell death and differentiation. 2011 doi: 10.1038/cdd.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]