Abstract
Excessive signaling by receptor tyrosine kinases (RTKs) can cause cancer. What molecular mechanisms normally control RTK signaling? Are they defective in tumors? If so, should therapeutics be developed to restore particular regulatory pathways to cancer cells? These questions have been approached through mechanistic studies of a prototypical RTK, the epidermal growth factor receptor (EGFR). EGFR signaling is mediated and regulated by both signaling and trafficking effectors. The amplitude of receptor-proximal signals changes as EGFRs move along the degradative trafficking pathway from the cell surface, to endosomes, and into lysosomes. To optimize therapeutic suppression of receptor oncogenicity, it may be crucial to target EGFRs that are signaling from a specific site in the trafficking pathway. Research suggests that EGFRs at the plasma membrane produce the bulk of the global transcriptional response to EGF. EGFRs localized between the internalization and early endosome fusion stages of the pathway enrich the expression of transcripts associated with cancer. EGFRs at later trafficking checkpoints controlled by the endosomal sorting complex required for transport (ESCRT) complexes II and III do not contribute substantially to the EGFR-mediated transcriptional response. These results suggest that therapeutics targeting the receptors at the earliest stages of degradative trafficking might be most effective.
Tight regulation of epidermal growth factor receptor (EGFR) activity is essential for normal vertebrate development and survival. Insufficient EGFR signaling causes death in the perinatal period, whereas excessive signaling is observed in many human cancers of epithelial origin. Some rationally designed therapeutic agents have shown promise in the laboratory and in the clinic, but they are not effective against all receptor-positive tumors. Can new agents be designed for maximal disruption of EGFR oncogenicity? Perhaps, but a better understanding of where and how EGFRs signal is needed first. Two publications (1, 2) suggest that EGFRs at or near the plasma membrane are largely responsible for the acute transcriptional response to receptor activation and therefore constitute the pool to target.
EGFR signaling involves complex cascades influenced by many variables. These include (i) the nature of the extracellular stimulus (often a ligand that binds to EGFR specifically) (3–6), (ii) the amount and duration of the presence of the ligand (7), (iii) whether the receptor forms homodimers or heterodimers (8–11), and (iv) which cytosolic regulatory proteins bind to the autophosphorylated receptors (6, 12–20). Signaling cascades that are activated by direct binding of their adaptors or effectors to EGFR include the mitogen-activated protein kinase (MAPK) pathway (mediated by the adaptors Grb2 and Shc) (21, 22), the phosphatidylinositol 3-kinase (PI3K) pathway acting through the kinase Akt and the transcription factor nuclear factor κB (PI3K/Akt/NF-κB; mediated by the adaptors Grb2 and Gab1) (23), and the phospholipase C–γ (PLC-γ) pathway (mediated by direct recruitment of PLC-γ to the receptor) (24). It has been widely assumed that changes in EGFR-proximal signaling events, such as the recruitment of these proteins to EGFR or their phosphorylation, translate to changes in distal signaling responses, such as transcription or cell proliferation.
The profile of EGFR-bound proteins changes with time after receptor activation. This remodeling of signaling complexes correlates with EGFR endocytosis and movement through the degradative pathway (Fig. 1). The link between EGFR relocation and changes in receptor-proximal MAPK signaling has been investigated in various ways, including: (i) time course studies of phosphorylation and activation of EGFR, Shc, and MAPK by phosphorylation-specific immunoblotting (25); (ii) quantitative immunoprecipitation and immunoblot analysis of MAPK pathway effector and EGFR interactions over the time scale of degradative trafficking (26); and (iii) fluorescence resonance energy transfer studies demonstrating the interaction of EGFR and signaling pathway regulators during receptor trafficking (27).
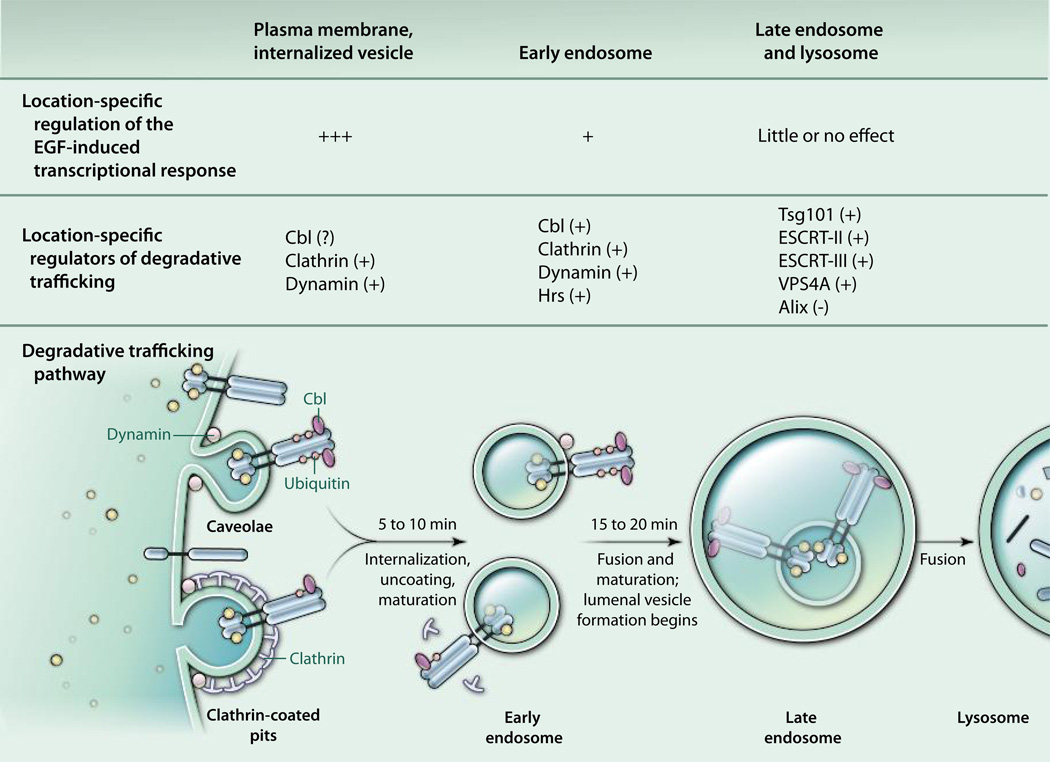
Fig. 1.
Proteins that regulate EGFR trafficking and the receptor-induced transcriptional response. A subset of the regulatory proteins that increase (+) or decrease (−) each process is listed above the site of action. The question mark next to Cbl indicates the arguable function of the protein in promoting EGFR internalization. The times shown in minutes (min) are the approximate periods required to move activated receptors from the plasma membrane to the indicated compartments. The reports by Brankatschk et al. (1) and Sousa et al. (2) have mapped EGF-induced signaling events to distinct trafficking checkpoints.
The ectopic expression of wild-type trafficking regulators and their mutant counterparts has been used to map the cellular sites where EGFRs signal. Dominant-negative mutant proteins arrest receptor transit at specific trafficking checkpoints. After comparing the signaling patterns from normally trafficking versus impeded EGFRs, investigators have assigned receptor-proximal signaling events to cellular locations upstream or downstream of each checkpoint. This approach has been applied to proteins that function early in EGFR trafficking at the plasma membrane, at multiple sites along the endosomal compartment, and at the lysosome.
Clathrin is a structural component of plasma membrane coated pits in which EGFRs can accumulate before internalization. The guanosine triphosphatase dynamin controls the internalization of clathrin-coated pits and caveolae at the plasma membrane (28). Cbl is a RING type E3 ubiquitin ligase that binds and ubiquitylates activated EGFRs at the cell surface and on endosomes (17, 29, 30). A role for Cbl in the control of EGFR internalization remains a subject for debate. However, Cbl controls the fate of internalized EGFRs by promoting the fusion of EEA1-positive endosomes that bear internalized EGFRs on their limiting membranes (31). Endosome fusion is essential for the subsequent destruction of EGFRs in lysosomes.
ESCRTs (endosomal sorting complex required for transport) are four distinct multiprotein complexes that regulate specific steps in endosomal trafficking (32). Some ESCRT-0 and ESCRT-I components bear ubiquitin-interacting motifs and retain ubiquitylated cargoes in the limiting membranes of endosomes. The timely fusion of EGFR-bearing endosomes with other endosomes requires the ESCRT-0 component Hrs, a ubiquitin-binding protein that is phosphorylated and degraded in an EGF- and Cbl-dependent manner, thus triggering an Hrs-dependent trafficking switch that drives EGFRs along the degradative pathway (31, 33). Tsg101 is a ubiquitin-binding constituent of ESCRT-I that also binds directly to ubiquitylated EGFRs (34). It promotes receptor movement from the limiting membranes of endosomes to intralumenal endosomal vesicles, but this action can be suppressed by the Tsg101-binding protein Alix (35).
ESCRTs II and III act downstream of ESCRT-I to facilitate the delivery of EGFRs into the lumenal vesicles of multivesicular bodies. Activated receptors associated with ESCRTs II and III undergo deubiquitylation before their transport into endosome lumens. The adenosine triphosphatase VPS4A disassembles the EGFR and ESCRT complexes at the limiting membrane of late endosomes just before EGFR translocation, thereby recycling ESCRT components back to the cytosol (36, 37). The functions of these various regulators of cargo trafficking have been investigated by using ectopic expression of each protein and mutants thereof.
To better define the subcellular sites of EGFR signal transduction using an alternative approach, Brankatschk et al. (1) and Sousa et al. (2) analyzed cells in which pools of clathrin, dynamin, Cbl, Hrs, Tsg101, Alix, VPS4A, or various combinations of these proteins had been depleted. Both groups concluded that the bulk of EGF-induced EGFR signaling occurs through multiple pathways initiated mostly by receptors at the plasma membrane. Normalizing their data to minimize the impact of EGF treatment on the overall size of the transcript pool, Brankatschk et al. further demonstrated that a group of internalized receptors trafficking toward the endosome fusion stage doubled the expression of specific transcripts associated with cancer, cell proliferation, and signaling through the NF-κB and cytokine signaling pathways. Beyond the endosome fusion stage, no substantive additional transcriptional response was detected.
The knock-down targets of Brankatschk et al. (1) and Sousa et al. (2) differed. Sousa and colleagues evaluated signaling outcomes in mouse fibroblasts that were conditionally depleted of dynamins 1 and 2, a situation that suppresses EGFR internalization and degradation (2). Across the range of physiological EGF concentrations and relative to their control counterparts, EGFRs of the knockout cells exhibited greater tyrosine phosphorylation and greater and prolonged ubiquitylation. Isoforms of the signal effector Shc in these cells also exhibited greater or prolonged phosphorylation, or, depending on the isoform, both. However, these increases in immediate signaling readouts did not translate into differences in MAPK or Akt activation as determined by qualitative phosphospecific immunoblotting. Because EGFRs that were retained at the cell surface due to dynamin 1 and 2 depletion produced the same receptor-proximal MAPK and Akt activation patterns as EGFRs that trafficked normally, the investigators concluded that the bulk of the MAPK response and Akt activation is controlled primarily by EGFRs at the plasma membrane.
Brankatschk and colleagues drew the same conclusion about MAPK signaling by quantifying signaling events in HeLa cells in which trafficking regulators were either present at normal amounts or were depleted by small interfering RNA knockdown (1). Individual targets for knockdown included Hrs, Tsg101, Alix, and VPS4A. Targets for combinatorial depletion included clathrin plus dynamin 2 and c-Cbl plus Cbl-b. Additional variables, such as EGF concentration and the period of cell exposure to ligand, were tested for their impact on EGFR signaling. Assays of MAPK signaling over the time scale of receptor activation and degradative trafficking included (i) immunoblot detection of phosphorylated EGFR, MEK, and MAPKs; (ii) ELK1-luciferase reporter assays; (iii) quantitative real-time reverse transcription–polymerase chain reaction (qRT-PCR) analysis of endogenous EGR1 and FOS messenger RNAs; and (iv) microarray and NanoString analyses of EGF-induced transcription. The NanoString approach provides a high-sensitivity digital transcript profile for hundreds of target sequences simultaneously, without the potential for bias arising from PCR amplification (38, 39). To extend the investigation beyond HeLa cells, transcriptional responses were also quantified in MCF10A cells.
Defined knockdowns had specific effects on some MAPK-responsive transcripts, as shown by the ELK-1 reporter assays and the microarray and NanoString analyses of endogenous transcripts. For example, the combinatorial depletion of clathrin plus dynamin 2 or c-Cbl plus Cbl-b, two conditions that increased the proportion of EGFR at the plasma membrane, produced clustered transcriptional profiles with large increases in many EGF-induced transcripts at all time points tested. Not all of these transcripts were cancer-associated. Depletion of Hrs alone, which compromised EGFR trafficking at the limiting membranes of endosomes, affected fewer transcripts in microarray assays than the double knockdown conditions, but Hrs depletion more than doubled the abundance of about 25 transcripts, several of which are associated with cancer, cell proliferation, and NF-κB and cytokine signaling. These results were confirmed in subsequent NanoString analyses using Ingenuity IPA software, which showed that Hrs depletion had a stronger impact on EGF-induced transcriptional events than did the depletion of other ESCRT components.
Specific depletion of Tsg101 had even less impact on EGF-induced transcription, whereas the loss of Alix or VPS4A had no notable effect on EGF-induced transcription. From these data, Brankatschk et al. (1) proposed that the transcriptional response to EGFR activation is elicited primarily by receptors at the cell surface and that trafficking regulators downstream of the ESCRT-0 checkpoint have little impact on the abundance of EGF-induced, cancer-associated transcripts.
An important qualification of this conclusion is that immunofluorescence imaging of EGFR and the early endosomal marker EEA1 in the double knock-down cells of Brankatschk et al. (depleted of clathrin plus dynamin 2 or c-Cbl plus Cbl-b) showed a minor population of activated EGFRs colocalizing with EEA1. It is possible that the increase in cancer-associated transcripts that occurred in both of the double knockdown conditions, as well as upon individual Hrs depletion, was elicited by internalized EGFRs that had not yet passed the endosome fusion checkpoint controlled by clathrin, dynamin 2, Cbl, and Hrs. This interpretation is consistent with previous reports that dynamin, clathrin, and Cbl ultimately promote EGFR degradation at the level of EEA1-positive endosomes, where they gate ubiquitylated receptors toward later checkpoints in the degradative pathway (31, 40, 41). In the case of Cbl, its ability to increase receptor degradation has been linked to its ability to enhance Hrs tyrosine phosphorylation and degradation to promote endosome fusion (31).
Another outcome of Brankatschk et al. (1) of particular importance for interpreting results in the signaling field is that receptor-proximal events may not be accurate indicators of the transcriptional response. The amplitude and duration of receptor-proximal signaling events, such as phosphorylation of EGFR, Akt, and MAPK, which have been viewed widely as reliable portents of distal signaling events, do not always translate to changes in the EGFR-mediated transcriptional response, at least over the short term.
On the basis of the complementary data of Sousa et al. (2) and Brankatschk et al. (1), as well as existing literature (42), it appears that the EGFRs at the cell surface and at early stages of the degradative trafficking pathway are the ones to target for the suppression of oncogenic signaling. Modulating the activity of receptors that have passed an endosome fusion checkpoint may not effectively reduce their transforming potential. This possibility is a caution to investigators who propose the development of therapeutic agents to disrupt EGFR activity or trafficking at sites beyond the endosomal checkpoint controlled by Cbl, Hrs, dynamin, and clathrin, such as those controlled by ESCRTs I, II, and III.
Acknowledgments
Funding: Research in the authors’ laboratory is supported by NIH grant CA109685 to N.L.L.
References and Notes
- 1.Brankatschk B, Wichert SP, Johnson SD, Schaad O, Rossner MJ, Gruenberg J. Regulation of the EGF transcriptional response by endocytic sorting. Sci. Signal. 2012;5:ra21. doi: 10.1126/scisignal.2002351. [DOI] [PubMed] [Google Scholar]
- 2.Sousa LP, Lax I, Shen H, Ferguson SM, De Camilli P, Schlessinger J. Suppression of EGFR endocytosis by dynamin depletion reveals that EGFR signaling occurs primarily at the plasma membrane. Proc. Natl. Acad. Sci. U.S.A. 2012;109:4419–4424. doi: 10.1073/pnas.1200164109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.French AR, Tadaki DK, Niyogi SK, Lauffenburger DA. Intracellular trafficking of epidermal growth factor family ligands is directly influenced by the pH sensitivity of the receptor/ ligand interaction. J. Biol. Chem. 1995;270:4334–4340. doi: 10.1074/jbc.270.9.4334. [DOI] [PubMed] [Google Scholar]
- 4.Reddy CC, Wells A, Lauffenburger DA. Comparative mitogenic potencies of EGF and TGF alpha and their dependence on receptor-limitation versus ligand-limitation. Med. Biol. Eng. Comput. 1998;36:499–507. doi: 10.1007/BF02523222. [DOI] [PubMed] [Google Scholar]
- 5.Ouyang X, Gulliford T, Huang G, Epstein RJ. Transforming growth factor-alpha short-circuits downregulation of the epidermal growth factor receptor. J. Cell. Physiol. 1999;179:52–57. doi: 10.1002/(SICI)1097-4652(199904)179:1<52::AID-JCP7>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 6.Stern KA, Place TL, Lill NL. EGF and amphiregulin differentially regulate Cbl recruitment to endosomes and EGF receptor fate. Biochem. J. 2008;410:585–594. doi: 10.1042/BJ20071505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sigismund S, Woelk T, Puri C, Maspero E, Tacchetti C, Transidico P, Di Fiore PP, Polo S. Clathrin-independent endocytosis of ubiquitinated cargos. Proc. Natl. Acad. Sci. U.S.A. 2005;102:2760–2765. doi: 10.1073/pnas.0409817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wada T, Qian XL, Greene MI. Intermolecular association of the p185neu protein and EGF receptor modulates EGF receptor function. Cell. 1990;61:1339–1347. doi: 10.1016/0092-8674(90)90697-d. [DOI] [PubMed] [Google Scholar]
- 9.Goldman R, Levy RB, Peles E, Yarden Y. Heterodimerization of the erbB-1 and erbB-2 receptors in human breast carcinoma cells: A mechanism for receptor transregulation. Biochemistry. 1990;29:11024–11028. doi: 10.1021/bi00502a002. [DOI] [PubMed] [Google Scholar]
- 10.Graus-Porta D, Beerli RR, Daly JM, Hynes NE. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997;16:1647–1655. doi: 10.1093/emboj/16.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waterman H, Alroy I, Strano S, Seger R, Yarden Y. The C-terminus of the kinase-defective neuregulin receptor ErbB-3 confers mitogenic superiority and dictates endocytic routing. EMBO J. 1999;18:3348–3358. doi: 10.1093/emboj/18.12.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rozakis-Adcock M, McGlade J, Mbamalu G, Pelicci G, Daly R, Li W, Batzer A, Thomas S, Brugge J, Pelicci PG, Schlessinger J, Pawson T. Association of the Shc and Grb2/Sem5 SH2-containing proteins is implicated in activation of the Ras pathway by tyrosine kinases. Nature. 1992;360:689–692. doi: 10.1038/360689a0. [DOI] [PubMed] [Google Scholar]
- 13.Fazioli F, Minichiello L, Matoska V, Castagnino P, Miki T, Wong WT, Di Fiore PP. Eps8, a substrate for the epidermal growth factor receptor kinase, enhances EGF-dependent mitogenic signals. EMBO J. 1993;12:3799–3808. doi: 10.1002/j.1460-2075.1993.tb06058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margolis B, Li N, Koch A, Mohammadi M, Hurwitz DR, Zilberstein A, Ullrich A, Pawson T, Schlessinger J. The tyrosine phosphorylated carboxyterminus of the EGF receptor is a binding site for GAP and PLC-gamma. EMBO J. 1990;9:4375–4380. doi: 10.1002/j.1460-2075.1990.tb07887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson D, Koch CA, Grey L, Ellis C, Moran MF, Pawson T. Binding of SH2 domains of phospholipase C gamma 1, GAP, and Src to activated growth factor receptors. Science. 1990;250:979–982. doi: 10.1126/science.2173144. [DOI] [PubMed] [Google Scholar]
- 16.Thien CB, Langdon WY. EGF receptor binding and transformation by v-cbl is ablated by the introduction of a loss-of-function mutation from the Caenorhabditis elegans sli-1 gene. Oncogene. 1997;14:2239–2249. doi: 10.1038/sj.onc.1201193. [DOI] [PubMed] [Google Scholar]
- 17.Levkowitz G, Waterman H, Zamir E, Kam Z, Oved S, Langdon WY, Beguinot L, Geiger B, Yarden Y. c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev. 1998;12:3663–3674. doi: 10.1101/gad.12.23.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levkowitz G, Waterman H, Ettenberg SA, Katz M, Tsygankov AY, Alroy I, Lavi S, Iwai K, Reiss Y, Ciechanover A, Lipkowitz S, Yarden Y. Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol. Cell. 1999;4:1029–1040. doi: 10.1016/s1097-2765(00)80231-2. [DOI] [PubMed] [Google Scholar]
- 19.Muthuswamy SK, Gilman M, Brugge JS. Controlled dimerization of ErbB receptors provides evidence for differential signaling by homo- and heterodimers. Mol. Cell. Biol. 1999;19:6845–6857. doi: 10.1128/mcb.19.10.6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lill NL, Douillard P, Awwad RA, Ota S, Lupher Jr. ML, Miyake S, Meissner-Lula N, Hsu VW, Band H. The evolutionarily conserved N-terminal region of Cbl is sufficient to enhance down-regulation of the epidermal growth factor receptor. J. Biol. Chem. 2000;275:367–377. doi: 10.1074/jbc.275.1.367. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura KD, Martinez R, Weber MJ. Tyrosine phosphorylation of specific proteins after mitogen stimulation of chicken embryo fibroblasts. Mol. Cell. Biol. 1983;3:380–390. doi: 10.1128/mcb.3.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooper JA, Sefton BM, Hunter T. Diverse mitogenic agents induce the phosphorylation of two related 42,000-dalton proteins on tyrosine in quiescent chick cells. Mol. Cell. Biol. 1984;4:30–37. doi: 10.1128/mcb.4.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bjorge JD, Chan TO, Antczak M, Kung HJ, Fujita DJ. Activated type I phosphatidylinositol kinase is associated with the epidermal growth factor (EGF) receptor following EGF stimulation. Proc. Natl. Acad. Sci. U.S.A. 1990;87:3816–3820. doi: 10.1073/pnas.87.10.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meisenhelder J, Suh PG, Rhee SG, Hunter T. Phospholipase C-gamma is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro. Cell. 1989;57:1109–1122. doi: 10.1016/0092-8674(89)90048-2. [DOI] [PubMed] [Google Scholar]
- 25.Haugh JM, Huang AC, Wiley HS, Wells A, Lauffenburger DA. Internalized epidermal growth factor receptors participate in the activation of p21(ras) in fibroblasts. J. Biol. Chem. 1999;274:34350–34360. doi: 10.1074/jbc.274.48.34350. [DOI] [PubMed] [Google Scholar]
- 26.Burke P, Schooler K, Wiley HS. Regulation of epidermal growth factor receptor signaling by endocytosis and intracellular trafficking. Mol. Biol. Cell. 2001;12:1897–1910. doi: 10.1091/mbc.12.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sorkin A. Internalization of the epidermal growth factor receptor: role in signalling. Biochem. Soc. Trans. 2001;29:480–484. doi: 10.1042/bst0290480. [DOI] [PubMed] [Google Scholar]
- 28.Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J. Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Melker AA, van der Horst G, Calafat J, Jansen H, Borst J. c-Cbl ubiquitinates the EGF receptor at the plasma membrane and remains receptor associated throughout the endocytic route. J. Cell Sci. 2001;114:2167–2178. doi: 10.1242/jcs.114.11.2167. [DOI] [PubMed] [Google Scholar]
- 30.Ravid T, Heidinger JM, Gee P, Khan EM, Goldkorn T. c-Cbl-mediated ubiquitinylation is required for epidermal growth factor receptor exit from the early endosomes. J. Biol. Chem. 2004;279:37153–37162. doi: 10.1074/jbc.M403210200. [DOI] [PubMed] [Google Scholar]
- 31.Visser Smit GD, Place TL, Cole SL, Clausen KA, Vemuganti S, Zhang G, Koland JG, Lill NL. Cbl controls EGFR fate by regulating early endosome fusion. Sci. Signal. 2009;2:ra86. doi: 10.1126/scisignal.2000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rusten TE, Vaccari T, Stenmark H. Shaping development with ESCRTs. Nat. Cell Biol. 2012;14:38–45. doi: 10.1038/ncb2381. [DOI] [PubMed] [Google Scholar]
- 33.Sun W, Yan Q, Vida TA, Bean AJ. Hrs regulates early endosome fusion by inhibiting formation of an endosomal SNARE complex. J. Cell Biol. 2003;162:125–137. doi: 10.1083/jcb.200302083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Babst M, Odorizzi G, Estepa EJ, Emr SD. Mammalian tumor susceptibility gene 101 (TSG101) and the yeast homologue, Vps23p, both function in late endosomal trafficking. Traffic. 2000;1:248–258. doi: 10.1034/j.1600-0854.2000.010307.x. [DOI] [PubMed] [Google Scholar]
- 35.Falguières T, Luyet PP, Bissig C, Scott CC, Velluz MC, Gruenberg J. In vitro budding of intralumenal vesicles into late endosomes is regulated by Alix and Tsg101. Mol. Biol. Cell. 2008;19:4942–4955. doi: 10.1091/mbc.E08-03-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sachse M, Strous GJ, Klumperman J. ATPase-deficient hVPS4 impairs formation of internal endosomal vesicles and stabilizes bilayered clathrin coats on endosomal vacuoles. J. Cell Sci. 2004;117:1699–1708. doi: 10.1242/jcs.00998. [DOI] [PubMed] [Google Scholar]
- 37.Sun W, Vida TA, Sirisaengtaksin N, Merrill SA, Hanson PI, Bean AJ. Cell-free reconstitution of multivesicular body formation and receptor sorting. Traffic. 2010;11:867–876. doi: 10.1111/j.1600-0854.2010.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL, Fell HP, Ferree S, George RD, Grogan T, James JJ, Maysuria M, Mitton JD, Oliveri P, Osborn JL, Peng T, Ratcliffe AL, Webster PJ, Davidson EH, Hood L, Dimitrov K. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat. Biotechnol. 2008;26:317–325. doi: 10.1038/nbt1385. [DOI] [PubMed] [Google Scholar]
- 39.Malkov VA, Serikawa KA, Balantac N, Watters J, Geiss G, Mashadi-Hossein A, Fare T. Multiplexed measurements of gene signatures in different analytes using the Nanostring nCounter Assay System. BMC Res Notes. 2009;2:80. doi: 10.1186/1756-0500-2-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raiborg C, Wesche J, Malerød L, Stenmark H. Flat clathrin coats on endosomes mediate degradative protein sorting by scaffolding Hrs in dynamic microdomains. J. Cell Sci. 2006;119:2414–2424. doi: 10.1242/jcs.02978. [DOI] [PubMed] [Google Scholar]
- 41.Schroeder B, Weller SG, Chen J, Billadeau D, McNiven MA. A Dyn2-CIN85 complex mediates degradative traffic of the EGFR by regulation of late endosomal budding. EMBO J. 2010;29:3039–3053. doi: 10.1038/emboj.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bache KG, Stuffers S, Malerød L, Slagsvold T, Raiborg C, Lechardeur D, Wälchli S, Lukacs GL, Brech A, Stenmark H. The ESCRT-III subunit hVps24 is required for degradation but not silencing of the epidermal growth factor receptor. Mol. Biol. Cell. 2006;17:2513–2523. doi: 10.1091/mbc.E05-10-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]