Abstract
Some individuals who are infected with HIV rapidly deteriorate shortly after starting antiretroviral therapy, despite effective viral suppression. This reaction, referred to as immune reconstitution inflammatory syndrome (IRIS), is characterized by tissue-destructive inflammation and arises as CD4+ T cells re-emerge. It has been proposed that IRIS is caused by a dysregulation of the expanding population of CD4+ T cells specific for a co-infecting opportunistic pathogen. Here, we argue that IRIS instead results from hyper-responsiveness of the innate immune system to T cell help, a mechanism that may be shared by the many manifestations of IRIS that occur following the reversal of other types of immunosuppression in pathogen-infected hosts.
Depletion of CD4+ T cells during HIV infection eventually results in the loss of normal resistance to a wide range of pathogens and opportunistic infections. This susceptibility of patients with AIDS to microbial infections reflects the fundamental role of CD4+ T cells in host protective immunity. The main goal of antiretroviral therapy (ART) is to correct this immunodeficiency and re-establish protective immunity against opportunistic infections by bringing CD4+ T cell numbers back towards normal levels.
Paradoxically, however, some patients experience a rapid deterioration in response to ART, despite efficient control of HIV viraemia and no apparent drug toxicity. This adverse reaction to treatment is referred to as immune reconstitution inflammatory syndrome (IRIS), as it is thought to result from a pathological host response that occurs when the immune system is restored following ART. Although antiretroviral treatments have had a tremendous impact on prolonging the survival of patients infected with HIV, IRIS has emerged as a major problem in the clinical management of the HIV pandemic1,2–7, affecting up to 30% of individuals infected with HIV and receiving ART8,9. Thus, new approaches to treat and prevent IRIS are greatly needed to allow the safe restoration of immunity during the treatment of AIDS.
Although little is known about the mechanisms underlying IRIS, new insights into the immunopathogenesis of the syndrome have been obtained owing to an increased interest in the clinical study of the disease and a recently developed animal model. In this Opinion article, we suggest that the uncoupling of innate and adaptive immune responses during microbial infection in the absence of CD4+ T cells sets the stage for hyperactivation of innate immune cells when antigen-specific CD4+ T cell numbers are later restored following ART. Indeed, HIV-related IRIS seems to be just one manifestation of a more general phenomenon of acute immune-mediated pathology associated with the rapid reversal of immunosuppression, and a similar process may also be involved in other examples of IRIS in individuals who are HIV negative.
Risk factors in HIV-related IRIS
Several risk factors that clearly predispose individuals infected with HIV to the development of IRIS have been established. The occurrence of microbial infections near the time of ART initiation greatly increases the risk of IRIS10,11. In fact, IRIS has been associated with co-infections by a diverse array of pathogens, in particular Mycobacterium tuberculosis and Cryptococcus neoformans, and has also been associated with tumours10,12–14, and the clinical manifestations of IRIS vary according to the class of microorganism and infection site of the offending opportunistic pathogen.
There is also a strong correlation between the extent of CD4+ T cell depletion and likelihood of developing IRIS, with the most lymphopenic patients (those with the lowest CD4+ T cell counts) being at the highest risk10,11,15,16 (see below). In fact, microbial infection during severe CD4+ T cell lymphopenia seems to be the key underlying immunological scenario that sets the stage for the development of IRIS.
Following suppression of HIV replication by ART administration, depletion of CD4+ T cells ceases, and these cells begin to recover. An important hypothesis proposes that the recovering CD4+ T cells are responsible for the symptoms of IRIS. Furthermore, it is believed that CD4+ T cells specific for the opportunistic infection mount dysregulated immune responses that become pathogenic. For example, within a few days to weeks after starting ART, some individuals who are infected with HIV and co-infected with M. tuberculosis rapidly develop extremely enlarged and necrotic lymph nodes (BOX 1), and others develop pulmonary lesions or experience worsening of existing lesions17. Some studies have found that patients who are infected with HIV and develop tuberculosis-associated IRIS have a marked expansion of circulating M. tuberculosis-specific CD4+ T cells during the IRIS event compared with individuals who do not experience IRIS18,19. By contrast, CD4+ T cells specific for HIV or irrelevant pathogens not associated with IRIS in these patients (for example, cytomegalovirus) do not undergo acute expansion. However, other studies of tuberculosis-associated IRIS have failed to find a clear association between IRIS and a burst of M. tuberculosis-specific CD4+ T cells20. Therefore, the available clinical data are inconclusive about whether CD4+ T cells specific for the opportunistic pathogen become dysregulated in patients with IRIS or even whether they are involved in the pathogenesis at all.
Insights from animal models
One of the first examples of IRIS was observed during the study of immunity to Pneumocystis carinii in mice. In the course of examining the cell types involved in host control of P. carinii, it was found that severe combined immunodeficient (scid) mice harbouring a chronic P. carinii infection develop a lethal hyperinflammatory response in their lungs ~1 month following infusion of wild-type bone marrow21. Soon after, it was found that CD4+ T cells are the key mediators of this response22,23. Although it was not appreciated at the time, the disease in these mice can now be viewed as a form of IRIS, similar to that observed in individuals infected with HIV24,25. Indeed, the P. carinii-infected scid mice mimic a T cell-depleted patient who is infected with HIV and harbours an opportunistic infection, and the transfer of CD4+ T cells recapitulates the restoration of CD4+ T cells that occurs after ART. Therefore, in the case of P. carinii infection, CD4+ T cells themselves are capable of inducing IRIS. Interestingly, at the same time as these early mouse studies, it was found that patients with AIDS who are infected with mycobacterium and develop a paradoxical worsening of disease following ART with zidovudine display evidence of strong cellular immunity (measured as delayed-type hypersensitivity responses) against a purified mycobacterial protein derivative26. This first suggested that the deterioration of these patients despite effective antiviral therapy was, in fact, due to the restoration of cellular immunity26.
A model designed to study mycobacterium-associated IRIS has been developed recently using Mycobacterium avium-infected mice27 (FIG. 1). To mimic CD4+ T cell reconstitution of patients co-infected with mycobacteria and HIV, T cell-deficient (TCRαKO) mice harbouring a chronic M. avium infection are injected with a small number of purified CD4+ T cells (FIG. 1). When M. avium-infected wild-type mice containing a normal number of T cells are injected with CD4+ T cells, the mice show no symptoms, as expected. However, when CD4+ T cells are transferred into infected TCRαKO mice, there is a rapid failure of lung function, wasting and the eventual death of most animals less than 3 weeks after T cell transfer. By contrast, if TCRαKO mice are injected with CD4+ T cells before M. avium infection, rather than after, there is no reconstitution disease. If the same T cells are injected into TCRαKO mice that remain uninfected, again there is no disease.
Figure 1. Experimental IRIS requires chronic mycobacterial infection of lymphopenic hosts.
Immune reconstitution inflammatory syndrome (IRIS) can be experimentally induced in T cell-deficient mice harbouring a chronic Mycobacterium avium infection by injecting purified CD4+ T cells into the mice. Importantly, IRIS does not occur in wild-type mice infected before or after CD4+ T cell transfer. Furthermore, no IRIS-related symptoms are observed in T cell-deficient mice when T cells are transferred before or soon after the time of infection. IRIS occurs only after transfer of CD4+ T cells into lymphopenic mice that were infected with M. avium several months previously.
This mouse model has provided important insights into the mechanism of IRIS development. For example, the mouse model has allowed us to test whether interferon-γ (IFNγ) secreted by CD4+ T cells plays a part in IRIS pathology. Correlative clinical studies show a link between IRIS development and increased levels of IFNγ, so it has been hypothesized that this cytokine, which is required to control mycobacterial infections, has a major pathogenic role during IRIS18,19,28,29. However, some individuals who do not develop IRIS have similarly increased levels of IFNγ following ART20,30, indicating that overproduction of IFNγ is not sufficient to drive IRIS. Therefore, it is not possible to definitively demonstrate a mechanistic link between IFNγ-producing CD4+ T cells and IRIS on the basis of these correlative clinical studies. However, in the mouse model of M. avium-associated IRIS, Ifng–/– CD4+ T cells show a greatly reduced ability to induce wasting and mortality27, indicating that IFNγ is a major mediator of illness in this system. Importantly, a detailed analysis suggests that the level of IFNγ produced by antigen-specific CD4+ T cells does not correlate with disease severity, highlighting the importance of using complimentary experimental models to investigate the mechanisms of human IRIS.
In summary, the mouse P. carinii and M. avium models of IRIS have yielded several important insights about the underlying mechanisms of this disease. First, CD4+ T cell reconstitution itself is sufficient to drive IRIS in susceptible hosts. Second, during mycobacterium-related IRIS, IFNγ is an important mediator of immunopathology. Third, HIV infection is not required to trigger immune reconstitution disease. Last, and most important, susceptibility to IRIS develops in the context of chronic microbial infection and CD4+ T cell deficiency (FIG. 1).
Role of lymphopenia in HIV-related IRIS
The role of lymphopenia in promoting the development IRIS is not well understood. One possibility is that the lymphopenic environment alters the function of CD4+ T cells, making them more pathogenic31. When CD4+ T cells are introduced into a T cell-deficient host in the absence of cognate antigen, the cells become spontaneously activated to divide and also gain increased cytokine-producing potential32. Furthermore, antigen-driven CD4+ T cell responses are also dysregulated in lymphopenic environments. Tumour-specific tolerance33 and peripheral self-tolerance34, for example, can be overcome in lymphopenic environments.
Increased interleukin-7 (IL-7) signalling in T cells owing to the lack of competition for this cytokine has an important role in driving homeostatic proliferation of T cells in lymphopenia35,36, and it has recently been shown that patients who are infected with HIV and develop IRIS have elevated levels of IL-7 in their serum compared with patients who are infected with HIV but do not experience IRIS29. On the basis of this association between high IL-7 levels and susceptibility to IRIS, it can be hypothesized that T cells receive increased homeostatic signals in the form of IL-7 in patients with IRIS, and that this in turn promotes exaggerated T cell responses. However, increased IL-7 signalling in lymphopenic hosts drives only slow T cell proliferation rates and is not required for rapid, antigen-driven T cell expansion37. In M. avium-infected TCRαKO mice, IRIS development requires antigen recognition by T cells27, and by extension it is likely that the T cell-driven pathology in patients with IRIS also requires antigen-driven CD4+ T cell responses. Therefore, the role of increased IL-7 signalling in the pathogenesis of IRIS remains unclear.
Although patient studies cannot directly address the hypothesis that lymphopenia directly promotes IRIS by exaggerating CD4+ T cell responses though homeostatic mechanisms, this hypothesis can be tested using the M. avium mouse model of experimentally induced IRIS27. Using this system, it was found that M. avium-infected T cell receptor (TCR)-transgenic mice with a monoclonal T cell repertoire specific for an irrelevant antigen (OT-II TCR trangenics recognizing chicken ovalbumin) display severe reconstitution disease when injected with CD4+ T cells specific for M. avium. As OT-II mice contain CD4+ T cells but cannot mount normal bacterium-specific T cell responses, the lack of antigen-specific CD4+ T cells preceding T cell transfer, and not the lymphopenic environment itself, must be responsible for IRIS susceptibility in this system. By contrast, TCR-transgenic mice that contain only T cells specific for M. avium do not develop IRIS, which indicates that the presence of bacterium-specific CD4+ T cell responses during infection is sufficient to prevent susceptibility to IRIS. Therefore, it seems that lymphopenic mice become susceptible to IRIS after M. avium infection because they cannot mount bacterium-specific CD4+ T cell responses during the infection. Given that CD4+ T cells are central orchestrators of the inflammatory response to mycobacterial infection, this has led to the more specific hypothesis that infection-associated IRIS is a consequence of the recovery from immunosuppression, not simply the repopulation of the lymphopenic environment.
Immunosuppression in other forms of IRIS
Paradoxical exacerbations of disease following withdrawal of immunosuppression have also been reported in patients who are negative for HIV. These clinical examples of IRIS that occur following withdrawal of various anti-inflammatory treatments may provide insights into the mechanisms underlying the development of HIV-associated IRIS.
IRIS following withdrawal of TNF blockade
Similarly to individuals infected with HIV, patients with rheumatoid arthritis and Crohn's disease who receive tumour necrosis factor (TNF)-blocking therapies become highly susceptible to M. tuberculosis38. When a patient receiving TNF blockade develops an M. tuberculosis infection, the clinical response is to begin antibiotic treatment and immediately stop TNF blockade. In some individuals, this halting of the blockade and the subsequent rapid restoration of TNF signalling results in acute exacerbation of tuberculosis (for example, the development of new lung cavities and lymph adenopathies) rather than resolution39–45. The development of this form of tuberculosis-associated IRIS parallels the development of that which occurs in individuals infected with HIV, except that in this setting the immunosuppression is TNF blockade rather than CD4+ T cell depletion. Nonetheless, following either HIV infection or TNF blockade, the resulting immunodeficiency leads to the outgrowth of M. tuberculosis in a poorly inflamed environment, and the reversal of immunosuppression results in exaggerated responses.
The susceptibility of patients to IRIS following withdrawal of TNF blockade may provide insights into the general phenomenon of reconstitution disease. First, the fact that these patients being treated for auto-immunity are HIV negative highlights the fact that HIV infection itself is probably not required for IRIS development. Second, the existence of IRIS in T cell-replete hosts further argues against the hypothesis discussed above — that IRIS stems from the development of dysregulated CD4+ T cell responses in lymphopenic environments. Last, this clinical scenario directly demonstrates that TNF can be an important mediator of IRIS. It is interesting that susceptibility to IRIS in this setting depends on the absence of TNF signalling in the first place (that is, in the context of M. tuberculosis infection, sensitivity to TNF-induced pathology arises when TNF is initially absent).
IRIS following withdrawal of natalizumab
Another form of reconstitution disease in the absence of HIV infection occurs when patients with multiple sclerosis are treated with natalizumab, an α4 integrin-blocking therapy that prevents lymphocyte migration into the central nervous system (CNS) without depleting T cells. In some patients with multiple sclerosis, natalizumab administration leads to progressive multifocal leuko encephalopathy (PML) caused by reactivation of JC polyoma virus within the brain. When this occurs, natalizumab is discontinued, and multiple rounds of plasma exchange are often used to hasten the removal of the drug46. The goal is to quickly reverse the defect in lymphocyte migration that has led to the inability to control the JC polyoma virus infection. In some patients, however, the rapid restoration of normal lymphocyte homing to the virus-infected CNS leads to a life-threatening exacerbation of symptoms47–52. These clinical observations reveal that even a localized defect in tissue inflammation can lead to IRIS-like disease when normal cell trafficking is restored.
IRIS in transplant recipients
Although it is rare, IRIS has also been reported in kidney and liver transplant recipients53. In these patients, the pharmacological regimen that is administered to prevent graft rejection leads to susceptibility to C. neoformans infection, and withdrawal of the immunosuppressive drugs leads to a sudden exacerbation of symptoms associated with immune responses against the fungus54–58. Thus, despite the tremendous diversity in clinical histories leading to IRIS, there seems to be an underlying immunological scenario shared by these different forms of reconstitution disease.
Common factors in different forms of IRIS
One aspect common to all of the different forms of IRIS is that immune responses which are normally involved in host protection become pathogenic. For example, CD4+ T cells are required to control mycobacterial and cryptococcal infections but are likely to be central mediators of IRIS in patients co-infected with HIV. In mice, CD4+ T cells are required for optimal control of both M. avium and P. carinii, but in each case CD4+ T cell transfer into infected T cell-deficient mice is sufficient to induce IRIS. The reconstitution syndromes associated with antibody blockade are similar in this respect: TNF is required to control M. tuberculosis, but the restoration of TNF signalling leads to IRIS; and lymphocyte migration into the site of infection is required to control JC polyoma virus, but restoring T cell migration by removing natalizumab leads to PML-associated IRIS. In each case, the loss of some form of immune response leads to the inability to tolerate that response later. An understanding of why this intolerance occurs may explain why immune responses that are beneficial for the host under normal circumstances are pathogenic during IRIS.
There are informative similarities between the series of events leading to the development of HIV-associated IRIS, the mouse model of IRIS and the reconstitution syndromes described above (TABLE 1). In all of these settings, there is some initial form of infection-associated or iatrogenic immunosuppression: CD4+ T cell depletion during HIV infection, loss of TNF during blockade treatment, inhibition of lymphocyte homing during natalizumab therapy, or a genetic deficiency in T cells in TCRαKO mice. The immune deficiency leads to persistent infection by an opportunistic pathogen and high antigen loads, with the offending micro organism and infection site varying according to the particular immune defect. This results in a unique immunological environment in which there is extensive microbial infection but an incomplete pro-inflammatory response. From the clinical perspective, it is important to restore normal immunity, so ART is administered to suppress HIV replication or the relevant immunosuppressive therapy is stopped; in experimentally induced IRIS, this is recapitulated by injecting CD4+ T cells into the infected TCRαKO mice. The ensuing pathology then manifests when immunological competence is restored.
Table 1.
The diverse forms of IRIS share common features leading to the manifestation of disease
| Form of IRIS | Progression of events leading to IRIS |
|||||
|---|---|---|---|---|---|---|
| Infection or iatrogenic immunosuppression | Immune defect | High microbial load without normal inflammation | Clinical intervention | Full inflammation restored | Pathology | |
| HIV-associated | HIV | ↓ CD4+ T cells | Many, including mycobacteria and cryptococci | ART | ↑ CD4+ T cells | Many manifestations |
| Exacerbation of TB after withdrawal of TNF blockade | TNF blockade | ↓ TNF | M. tuberculosis | Stop TNF blockade | ↑ TNF | Lung pathology, lymphadenitis |
| PML-associated | α4 integrin blockade | ↓ Immune cell migration into CNS | JC polyoma virus | Stop α4 integrin blockade | ↑ Immune cell migration into CNS | Leukoencephalopathy |
| Organ transplant-associated | Anti-graft rejection treatment | Broad immunosuppression | C. neoformans | Decrease immunosuppressive treatment | Many factors? | Meningitis |
| Mouse model | TCRαKO or scid mice | T cell deficiency | M. avium or P. carinii | Inject CD4+ T cells | ↑ CD4+ T cells | Wasting, lung pathology |
ART, antiretroviral therapy; C. neoformans, Cryptococcus neoformans; CNS, central nervous system; IRIS, immune reconstitution inflammatory syndrome; M. avium, Mycobacterium avium; M. tuberculosis, Mycobacterium tuberculosis; P. carinii, Pneumocystis carinii; PML, progressive multifocal leukoencephalopathy; scid, severe combined immunodeficient; TB, tuberculosis; TCRαKO, T cell receptor-α knockout; TNF, tumour necrosis factor.
This parallel series of events that occurs before many forms of infection-associated IRIS may be central to the development of dysregulated inflammatory responses. We suggest that high microbial load in the absence of normal inflammation sets the stage for a pathological overshoot when the full inflammatory response is allowed to proceed (TABLE 1). The fact that IRIS is preceded by high infectious loads and low levels of inflammation is directly supported by the observation that individuals who are infected with HIV and develop crypto coccal meningitis as a result of IRIS have high crypto coccal antigenic loads before they begin ART treatment and also very low levels of many inflammatory biomarkers in their cerebrospinal fluid59,60.
A role for innate immune responses
Studies in the mouse model of IRIS have provided valuable insights into how chronic infection in the absence of a T cell response can predispose to IRIS.
Proposed mechanism of IRIS based on experimentally induced IRIS
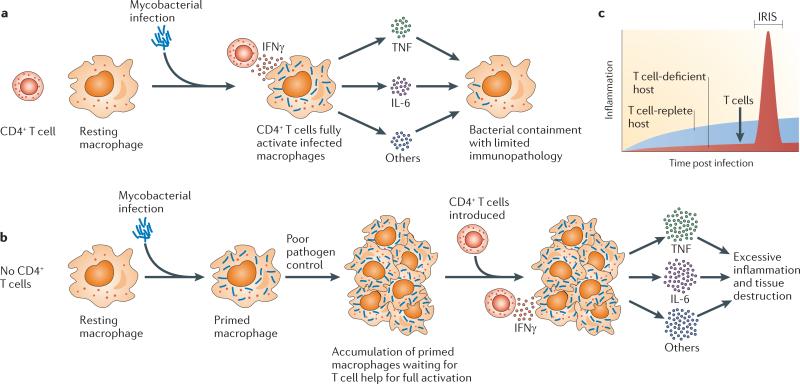
Macrophages require two signals to become fully activated: the first comes from the recognition of microorganisms through pattern recognition receptors, and the second comes from CD4+ T cell help. It has been shown that during influenza infection61 and M. tuberculosis infection62, for example, CD4+ T cell depletion results in a marked decrease in the activation of the innate immune system. Here, we suggest a model of IRIS development, based on the mouse studies of M. avium-associated IRIS, that focuses on the role of CD4+ T cells in driving the activation of myeloid cells. Importantly, this hypothesis attempts to explain how immune responses that occur during recovery from a period of lymphopenia lead to pathology, whereas immune responses to the same infection in a fully immunocompetent host do not (FIG. 2).
Figure 2. Model of IRIS.
During microbial infection of a T cell-deficient host, the uncoupling of innate and adaptive immune responses sets the stage for excessive inflammation on T cell reconstitution. a | Myeloid cells such as macrophages require two signals to become fully activated. The first involves recognition of microbial products by pattern recognition receptors, which primes the cells for further activation. The second involves interaction with interferon-γ (IFNγ)-producing CD4+ T cells, after which macro phages become fully activated and produce high levels of pro-inflammatory mediators such as tumour necrosis factor (TNF) and interleukin-6 (IL-6). Following bacterial infection of a host with a normal immune system, macro phages take up bacteria and quickly encounter effector CD4+ T cells, resulting in containment of the pathogen. b | On infection of a T cell-deficient host, infected myeloid cells become primed by microbial products but never become fully activated to exert their pro-inflammatory effector functions. As the uncontrolled infection progresses, this can result in disease owing to high levels of pathogen replication. However, over time, as increasing numbers of primed macrophages accumulate in the tissues of the host, this also creates a state of immunological hyper-responsiveness to CD4+ T cell help. When the immunosuppression is removed and antigen-specific CD4+ T cells rapidly reconstitute the infected lymphopenic host, they create a burst of IFNγ-mediated T cell help, and large numbers of primed macro phages now become fully activated en masse. This results in an acute spike in the production of pro-inflammatory mediators, which drives immunopathology during immune reconstitution inflammatory syndrome (IRIS). c | The acute inflammatory response that causes IRIS would normally not be encountered in a T cell-replete host, and thus the rapid kinetics of this activation process may be an important factor driving disease.
In a T cell-replete host, microbial recognition by cells of the innate immune system is rapidly followed by the induction of an adaptive CD4+ T cell response that drives full activation of the infected myeloid cells (FIG. 2a). In the setting of T cell deficiency, however, the innate and adaptive immune responses are temporally uncoupled from each other. In a TCRαKO mouse, the M. avium infection progresses in the absence of an adaptive immune response, and after several months there are large numbers of primed macrophages that carry a high bacterial load but have not received CD4+ T cell help to become fully activated (FIG. 2b).
We hypothesize that M. avium-associated IRIS in this mouse model results when antigen-specific CD4+ T cells are reintroduced and suddenly drive fulminant activation of mycobacterium-primed macrophages in the lungs and many other tissues of the host. This leads to a sudden spike in inflammation and tissue damage that outpaces host repair mechanisms and is responsible for the pathology (FIG. 2c). Importantly, this model provides a general mechanistic link between HIV-associated IRIS and other forms of IRIS. In each setting of reconstitution disease, some form of immunosuppression may result in the accumulation of microorganism-primed myeloid cells that then undergo full activation when immune function is restored.
Clinical evidence of dysregulated innate immune responses in HIV-related IRIS.
Recent findings have shown that patients with IRIS may have increased innate immune activation, providing support for the proposed model. One study looking at patients co-infected with HIV and M. tuberculosis demonstrated that, 2 weeks after starting ART, peripheral blood mononuclear cells from those patients who did go on to develop IRIS produced increased levels of the pro-inflammatory cytokines IL-1, IL-6, IL-8 and TNF in vitro after stimulation with heat-killed M. tuberculosis63. Moreover, several recent reports showed that IL-6 and TNF levels are increased in the serum of patients with IRIS63,64. Another study of tuberculosis-associated IRIS in individuals infected with HIV found that whole-blood cultures from patients with IRIS spontaneously (that is, without any sort of in vitro stimulation) produced increased levels of innate immune cell-derived cytokines and chemokines65.
Perhaps the most striking observations supporting a role for dysregulated myeloid responses in the development of IRIS come from a post-mortem immunohistological examination of lung tissue obtained from a patient who succumbed to lung failure during tuberculosis-associated IRIS. It was found that the lung tissue contained very few T cells and natural killer cells but had an overwhelming number of CD68+ myeloid cells within the parenchymal and alveolar spaces66. Taken together, the evidence described above supports the notion that susceptibility to IRIS is associated with heightened activation of the myeloid compartment.
Lessons for treatment of HIV-related IRIS
The proposed mechanism of IRIS described above has important implications for the management of this inflammatory disorder in individuals infected with HIV. In the case of mycobacterial and other co-infections, a common approach has been to initiate treatment of the opportunistic infection before administering ART. This strategy reduces the microbial burden present at the time of ART-driven T cell reconstitution, thereby decreasing the risk of IRIS67. However, it was found that delaying ART may result in increased overall mortality from AIDS1,2,4,6,7, and for this reason it is currently recommended that ART is administered shortly after the diagnosis has been confirmed and antimicrobial chemotherapy has been initiated in patients with severe lymphopenia68.
Although it is expected that the implementation of these guidelines will decrease overall AIDS mortality, their widespread deployment can also be expected to result in increased incidence of IRIS. This may be particularly true for severely lymphopenic individuals6 or patients with disseminated or CNS diseases69. Currently, corticosteroids are the main therapeutic option for managing IRIS events in individuals infected with HIV70. Because corticosteroids are non specific and present a risk of severe side effects, and so their use in immunocompromised individuals is counter-intuitive, alternative therapeutic approaches that more specifically target the pathways underlying IRIS are needed.
The new data implicating innate inflammatory pathways in IRIS pathology may lead to novel strategies for treating IRIS. However, they also introduce additional therapeutic challenges. The host factors upstream of IRIS pathology that have been identified so far are themselves essential mediators of host immunity, and their suppression is probably responsible for susceptibility to the opportunistic infection in the first place. Therefore, the immune interventions chosen to treat IRIS should specifically target downstream pro-inflammatory mediators (for example, cytokines and chemokines) that are not crucial for host control of the associated opportunistic infection but that do directly contribute to the pathology of IRIS.
Innate and adaptive immune responses have evolved to act in concert with each other. The uncoupling of these responses that occurs during microbial co-infection of individuals who are already infected with HIV and are depleted for CD4+ T cells predisposes these individuals for a pathological overshoot of myeloid cell function when T cell help rapidly reappears after initiation of ART. The use of experimental mouse systems to study the myeloid responses to infections in the presence or absence of CD4+ T cells will provide further insights into the basic immunological mechanisms underlying IRIS. Although non-human primate models of IRIS (macaques co-infected with M. tuberculosis and simian immunodeficiency virus) are currently unavailable, such models will also be useful, as they may more closely resemble the human syndrome. A detailed understanding of the inflammatory mechanisms responsible for IRIS will provide a rationale for safely restoring immunity in individuals infected with HIV and will facilitate clinical management of the HIV pandemic.
Box 1 Clinical manifestation of IRIS.
Immune reconstitution inflammatory syndrome (IRIS) can present as a range of manifestations, including fever, respiratory distress and cavitating lung lesions, or meningitis71. The figure shows another typical presentation of IRIS, in an individual who was infected with both HIV and Mycobacterium avium complex and who developed severe necrotizing cervical lymphadenopathy 4 weeks after the initiation of antiretroviral therapy (the necrotic areas are indicated by arrows). The enlarged lymph nodes compressed the jugular veins and were displacing the trachea, requiring pericutaneous drainage and corticosteroid therapy for relief of the obstructive symptoms.
Acknowledgements
The authors thank J. Keane for stimulating discussions during the preparation of this article. Work in the authors’ laboratories is supported by the intramural research programme of the US National Institute for Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
Competing interests statement
The authors declare no competing financial interests
Contributor Information
Daniel L. Barber, Immunobiology Section, Laboratory of Parasitic Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rm 6146, 50 South Drive, Bethesda, Maryland, 20892, USA.
Bruno B. Andrade, Immunobiology Section, Laboratory of Parasitic Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rm 6146, 50 South Drive, Bethesda, Maryland, 20892, USA.
Irini Sereti, Laboratory of Immunoregulation, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rm 11B07, 10 Center Drive, Bethesda, Maryland 20892, USA..
Alan Sher, Immunobiology Section, Laboratory of Parasitic Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rm 6146, 50 South Drive, Bethesda, Maryland, 20892, USA..
References
- 1.Zolopa A, et al. Early antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: a multicenter randomized strategy trial. PLoS ONE. 2009;4:e5575. doi: 10.1371/journal.pone.0005575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdool Karim SS, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N. Engl. J. Med. 2010;362:697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castelnuovo B, et al. Cause-specific mortality and the contribution of immune reconstitution inflammatory syndrome in the first 3 years after antiretroviral therapy initiation in an urban african cohort. Clin. Infect. Dis. 2009;49:965–972. doi: 10.1086/605500. [DOI] [PubMed] [Google Scholar]
- 4.Abdool Karim SS, et al. Integration of antiretroviral therapy with tuberculosis treatment. N. Engl. J. Med. 2011;365:1492–1501. doi: 10.1056/NEJMoa1014181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoyo-Ulloa I, et al. Impact of the immune reconstitution inflammatory syndrome (IRIS) on mortality and morbidity in HIV-infected patients in Mexico. Int. J. Infect. Dis. 2011;15:e408–e414. doi: 10.1016/j.ijid.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanc F-X, et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N. Engl. J. Med. 2011;365:1471–1481. doi: 10.1056/NEJMoa1013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Havlir DV, et al. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N. Engl. J. Med. 2011;365:1482–1491. doi: 10.1056/NEJMoa1013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.French MA, et al. Immune restoration disease after the treatment of immunodeficient HIV-infected patients with highly active antiretroviral therapy. HIV Med. 2000;1:107–115. doi: 10.1046/j.1468-1293.2000.00012.x. [DOI] [PubMed] [Google Scholar]
- 9.Ratnam I, Chiu C, Kandala N-B, Easterbrook PJ. Incidence and risk factors for immune reconstitution inflammatory syndrome in an ethnically diverse HIV type 1-infected cohort. Clin. Infect. Dis. 2006;42:418–427. doi: 10.1086/499356. [DOI] [PubMed] [Google Scholar]
- 10.Murdoch DM, Venter WDF, Feldman C, Van Rie A. Incidence and risk factors for the immune reconstitution inflammatory syndrome in HIV patients in South Africa: a prospective study. AIDS. 2008;22:601–610. doi: 10.1097/QAD.0b013e3282f4a607. [DOI] [PubMed] [Google Scholar]
- 11.Grant PM, et al. Risk factor analyses for immune reconstitution inflammatory syndrome in a randomized study of early vs. deferred ART during an opportunistic infection. PLoS ONE. 2010;5:e11416. doi: 10.1371/journal.pone.0011416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Breton G, et al. Immune reconstitution inflammatory syndrome in HIV-infected patients with disseminated histoplasmosis. AIDS. 2006;20:119–121. doi: 10.1097/01.aids.0000199014.66139.39. [DOI] [PubMed] [Google Scholar]
- 13.Nolan RC, Chidlow G, French MA. Parvovirus B19 encephalitis presenting as immune restoration disease after highly active antiretroviral therapy for human immunodeficiency virus infection. Clin. Infect. Dis. 2003;36:1191–1194. doi: 10.1086/374603. [DOI] [PubMed] [Google Scholar]
- 14.Connick E, Kane MA, White IE, Ryder J, Campbell TB. Immune reconstitution inflammatory syndrome associated with Kaposi sarcoma during potent antiretroviral therapy. Clin. Infect. Dis. 2004;39:1852–1855. doi: 10.1086/426078. [DOI] [PubMed] [Google Scholar]
- 15.Mueller M, et al. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect. Dis. 2010;10:251–261. doi: 10.1016/S1473-3099(10)70026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manabe YC, Campbell JD, Sydnor E, Moore RD. Immune reconstitution inflammatory syndrome: risk factors and treatment implications. J. Acquir. Immune Defic. Syndr. 2007;46:456–462. doi: 10.1097/qai.0b013e3181594c8c. [DOI] [PubMed] [Google Scholar]
- 17.Meintjes G, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect. Dis. 2008;8:516–523. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bourgarit A, et al. Explosion of tuberculin-specific Th1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patients. AIDS. 2006;20:F1–F7. doi: 10.1097/01.aids.0000202648.18526.bf. [DOI] [PubMed] [Google Scholar]
- 19.Bourgarit A, et al. Tuberculosis-associated immune restoration syndrome in HIV-1-infected patients involves tuberculin-specific CD4 Th1 cells and KIR-negative γδ T cells. J. Immunol. 2009;183:3915–3923. doi: 10.4049/jimmunol.0804020. [DOI] [PubMed] [Google Scholar]
- 20.Meintjes G, et al. Type 1 helper T cells and FoxP3-positive T cells in HIV-tuberculosis-associated immune reconstitution inflammatory syndrome. Am. J. Respir. Crit. Care Med. 2008;178:1083–1089. doi: 10.1164/rccm.200806-858OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roths JB, Marshall JD, Allen RD, Carlson GA, Sidman CL. Spontaneous Pneumocystis carinii pneumonia in immunodeficient mutant scid mice. Natural history and pathobiology. Am. J. Pathol. 1990;136:1173–1186. [PMC free article] [PubMed] [Google Scholar]
- 22.Roths JB, Sidman CL. Both immunity and hyperresponsiveness to Pneumocystis carinii result from transfer of CD4 but not CD8 T cells into severe combined immunodeficiency mice. J. Clin. Invest. 1992;90:673–678. doi: 10.1172/JCI115910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roths JB, Sidman CL. Single and combined humoral and cell-mediated immunotherapy of Pneumocystis carinii pneumonia in immunodeficient scid mice. Infect. Immun. 1993;61:1641–1649. doi: 10.1128/iai.61.5.1641-1649.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gigliotti F, Xu H, Wright T. Contribution of T cell subsets to the pathophysiology of Pneumocystis-related immunorestitution disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;291:L1256–L1266. doi: 10.1152/ajplung.00079.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atochina-Vasserman EN, et al. Immune reconstitution during pneumocystis lung infection: disruption of surfactant component expression and function by S-nitrosylation. J. Immunol. 2009;182:2277–2287. doi: 10.4049/jimmunol.0802775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.French MA, Mallal SA, Dawkins RL. Zidovudine-induced restoration of cell-mediated immunity to mycobacteria in immunodeficient HIV-infected patients. AIDS. 1992;6:1293–1297. doi: 10.1097/00002030-199211000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Barber DL, et al. Th1-driven immune reconstitution disease in Mycobacterium avium-infected mice. Blood. 2010;116:3485–3493. doi: 10.1182/blood-2010-05-286336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elliott JH, et al. Immunopathogenesis and diagnosis of tuberculosis and tuberculosis-associated immune reconstitution inflammatory syndrome during early antiretroviral therapy. J. Infect. Dis. 2009;200:1736–1745. doi: 10.1086/644784. [DOI] [PubMed] [Google Scholar]
- 29.Antonelli LRV, et al. Elevated frequencies of highly activated CD4+ T cells in HIV+ patients developing immune reconstitution inflammatory syndrome. Blood. 2010;116:3818–3827. doi: 10.1182/blood-2010-05-285080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan DBA, et al. TLR2-induced cytokine responses may characterize HIV-infected patients experiencing mycobacterial immune restoration disease. AIDS. 2011;25:1455–1460. doi: 10.1097/QAD.0b013e328348fb18. [DOI] [PubMed] [Google Scholar]
- 31.Krupica T, Fry TJ, Mackall CL. Autoimmunity during lymphopenia: a two-hit model. Clin. Immunol. 2006;120:121–128. doi: 10.1016/j.clim.2006.04.569. [DOI] [PubMed] [Google Scholar]
- 32.Sprent J, Surh CD. Normal T cell homeostasis: the conversion of naive cells into memory-phenotype cells. Nature Immunol. 2011;131:478–484. doi: 10.1038/ni.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown IE, Blank C, Kline J, Kacha AK, Gajewski TF. Homeostatic proliferation as an isolated variable reverses CD8+ T cell anergy and promotes tumor rejection. J. Immunol. 2006;177:4521–4529. doi: 10.4049/jimmunol.177.7.4521. [DOI] [PubMed] [Google Scholar]
- 34.King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117:265–277. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- 35.Tan JT, et al. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc. Natl Acad. Sci. USA. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schluns KS, Kieper WC, Jameson SC, Lefrançois L. Interleukin-7 mediates the homeostasis of naïve and memory CD8 T cells in vivo. Nature Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 37.Kieper WC, et al. Recent immune status determines the source of antigens that drive homeostatic T cell expansion. J. Immunol. 2005;174:3158–3163. doi: 10.4049/jimmunol.174.6.3158. [DOI] [PubMed] [Google Scholar]
- 38.Keane J, et al. Tuberculosis associated with infliximab, a tumor necrosis factor α-neutralizing agent. N. Engl. J. Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 39.Wallis RS, van Vuuren C, Potgieter S. Adalimumab treatment of life-threatening tuberculosis. Clin. Infect. Dis. 2009;48:1429–1432. doi: 10.1086/598504. [DOI] [PubMed] [Google Scholar]
- 40.Yoon YK, et al. Paradoxical response during antituberculous therapy in a patient discontinuing infliximab: a case report. J. Med. Case Rep. 2009;3:6673. doi: 10.1186/1752-1947-3-6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Belknap R, Reves R, Burman W. Immune reconstitution to Mycobacterium tuberculosis after discontinuing infliximab. Int. J. Tuberc. Lung Dis. 2005;9:1057–1058. [PubMed] [Google Scholar]
- 42.Arend SM, Leyten EMS, Franken WPJ, Huisman EM, van Dissel JT. A patient with de novo tuberculosis during anti-tumor necrosis factor-α therapy illustrating diagnostic pitfalls and paradoxical response to treatment. Clin. Infect. Dis. 2007;45:1470–1475. doi: 10.1086/522993. [DOI] [PubMed] [Google Scholar]
- 43.Szerszen A, Gupta S, Seminara D, Jarrett M, Goldstein M. Peritoneal tuberculosis complicated by immune reconstitution inflammatory syndrome in a patient treated with infliximab?: a case for adjuvant immunosuppressive therapy. J. Clin. Rheumatol. 2009;15:417–418. doi: 10.1097/RHU.0b013e3181bc9407. [DOI] [PubMed] [Google Scholar]
- 44.Garcia Vidal C, et al. Paradoxical response to antituberculous therapy in infliximab-treated patients with disseminated tuberculosis. Clin. Infect. Dis. 2005;40:756–759. doi: 10.1086/427941. [DOI] [PubMed] [Google Scholar]
- 45.Rivoisy C, Amrouche L, Carcelain G, Sereni D, Bourgarit A. Paradoxical exacerbation of tuberculosis after TNFα antagonist discontinuation: beware of immune reconstitution inflammatory syndrome. Joint Bone Spine. 2011;78:312–315. doi: 10.1016/j.jbspin.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 46.Khatri BO, et al. Effect of plasma exchange in accelerating natalizumab clearance and restoring leukocyte function. Neurology. 2009;72:402–409. doi: 10.1212/01.wnl.0000341766.59028.9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clifford DB, et al. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol. 2010;9:438–446. doi: 10.1016/S1474-4422(10)70028-4. [DOI] [PubMed] [Google Scholar]
- 48.Johnson T, Nath A. Immune reconstitution inflammatory syndrome and the central nervous system. Curr. Opin. Neurol. 2011;24:284–290. doi: 10.1097/WCO.0b013e328346be57. [DOI] [PubMed] [Google Scholar]
- 49.Van Assche G, et al. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn's disease. N. Engl. J. Med. 2005;353:362–368. doi: 10.1056/NEJMoa051586. [DOI] [PubMed] [Google Scholar]
- 50.Lindå H, et al. Progressive multifocal leukoencephalopathy after natalizumab monotherapy. N. Engl. J. Med. 2009;361:1081–1087. doi: 10.1056/NEJMoa0810316. [DOI] [PubMed] [Google Scholar]
- 51.Ryschkewitsch CF, et al. JC virus persistence following progressive multifocal leukoencephalopathy in multiple sclerosis patients treated with natalizumab. Ann. Neurol. 2010;68:384–391. doi: 10.1002/ana.22137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miravalle A, Jensen R, Kinkel RP. Immune reconstitution inflammatory syndrome in patients with multiple sclerosis following cessation of natalizumab therapy. Arch. Neurol. 2011;68:186–191. doi: 10.1001/archneurol.2010.257. [DOI] [PubMed] [Google Scholar]
- 53.Sun HY, Singh N. Opportunistic infection-associated immune reconstitution syndrome in transplant recipients. Clin. Infect. Dis. 2011;53:168–176. doi: 10.1093/cid/cir276. [DOI] [PubMed] [Google Scholar]
- 54.Legris T, et al. Immune reconstitution inflammatory syndrome mimicking relapsing cryptococcal meningitis in a renal transplant recipient. Transpl. Infect. Dis. 2011;13:303–308. doi: 10.1111/j.1399-3062.2010.00592.x. [DOI] [PubMed] [Google Scholar]
- 55.Singh N, et al. An immune reconstitution syndrome- like illness associated with Cryptococcus neoformans infection in organ transplant recipients. Clin. Infect. Dis. 2005;40:1756–1761. doi: 10.1086/430606. [DOI] [PubMed] [Google Scholar]
- 56.Crespo G, et al. Immune reconstitution syndrome after voriconazole treatment for cryptococcal meningitis in a liver transplant recipient. Liver Transpl. 2008;14:1671–1674. doi: 10.1002/lt.21601. [DOI] [PubMed] [Google Scholar]
- 57.Lanternier F, et al. Cellulitis revealing a cryptococcosis-related immune reconstitution inflammatory syndrome in a renal allograft recipient. Am. J. Transplant. 2007;7:2826–2828. doi: 10.1111/j.1600-6143.2007.01994.x. [DOI] [PubMed] [Google Scholar]
- 58.Singh N. Novel immune regulatory pathways and their role in immune reconstitution syndrome in organ transplant recipients with invasive mycoses. Eur. J. Clin. Microbiol. Infect. Dis. 2008;27:403–408. doi: 10.1007/s10096-008-0461-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boulware DR, et al. Clinical features and serum biomarkers in HIV immune reconstitution inflammatory syndrome after cryptococcal meningitis: a prospective cohort study. PLoS Med. 2010;7:e1000384. doi: 10.1371/journal.pmed.1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boulware DR, et al. Paucity of initial cerebrospinal fluid inflammation in cryptococcal meningitis is associated with subsequent immune reconstitution inflammatory syndrome. J. Infect. Dis. 2010;202:962–970. doi: 10.1086/655785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Strutt TM, et al. Memory CD4+ T cells induce innate responses independently of pathogen. Nature Med. 2010;16:558–564. doi: 10.1038/nm.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barber DL, Mayer-Barber KD, Feng CG, Sharpe AH, Sher A. CD4 T cells promote rather than control tuberculosis in the absence of PD-1-mediated inhibition. J. Immunol. 2011;186:1598–1607. doi: 10.4049/jimmunol.1003304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tadokera R, et al. Hypercytokinaemia accompanies HIV-tuberculosis immune reconstitution inflammatory syndrome. Eur. Respir. J. 2011;37:1248–1259. doi: 10.1183/09031936.00091010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boulware DR, et al. Higher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J. Infect. Dis. 2011;203:1637–1646. doi: 10.1093/infdis/jir134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oliver BG, et al. Mediators of innate and adaptive immune responses differentially affect immune restoration disease associated with Mycobacterium tuberculosis in HIV patients beginning antiretroviral therapy. J. Infect. Dis. 2010;202:1728–1737. doi: 10.1086/657082. [DOI] [PubMed] [Google Scholar]
- 66.Lawn SD, Wainwright H, Orrell C. Fatal unmasking tuberculosis immune reconstitution disease with bronchiolitis obliterans organizing pneumonia: the role of macrophages. AIDS. 2009;23:143–145. doi: 10.1097/QAD.0b013e32831d2a98. [DOI] [PubMed] [Google Scholar]
- 67.Lawn SD, Myer L, Bekker L-G, Wood R. Tuberculosis-associated immune reconstitution disease: incidence, risk factors and impact in an antiretroviral treatment service in South Africa. AIDS. 2007;21:335–341. doi: 10.1097/QAD.0b013e328011efac. [DOI] [PubMed] [Google Scholar]
- 68.Panel on Antiretroviral Guidelines for Adults and Adolescents. US Department of Health and Human Services Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescencents, 10 Jan 2011. AIDSinfo. 2011 [online] http://www.aidsinfo.nih.gov/Contentfiles/AdultandAdolescentGL.pdf.
- 69.Török ME, et al. Timing of initiation of antiretroviral therapy in human immunodeficiency virus (HIV)-associated tuberculous meningitis. Clin. Infect. Dis. 2011;52:1374–1383. doi: 10.1093/cid/cir230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meintjes G, et al. Randomized placebo-controlled trial of prednisone for paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS. 2010;24:2381–2390. doi: 10.1097/QAD.0b013e32833dfc68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.French MA. Immune reconstitution inflammatory syndrome: a reappraisal. Clin. Infect. Dis. 2009;48:101–107. doi: 10.1086/595006. [DOI] [PubMed] [Google Scholar]