Abstract
Hemorrhagic transformation (HT) associated with recombinant tissue plasminogen activator (rt-PA) complicates and limits its use in stroke. Here, we provide a focused review on the involvement of matrix metalloproteinase 9 (MMP-9) in rt-PA–associated HT in cerebral ischemia, and we review emerging evidence that the selective inhibitor of the sulfonylurea receptor 1 (Sur1), glibenclamide (U.S. adopted name, glyburide), may provide protection against rt-PA–associated HT in cerebral ischemia. Glyburide inhibits activation of MMP-9, ameliorates edema formation, swelling, and symptomatic hemorrhagic transformation, and improves preclinical outcomes in several clinically relevant models of stroke, both without and with rt-PA treatment. A retrospective clinical study comparing outcomes in diabetic patients with stroke treated with rt-PA showed that those who were previously on and were maintained on a sulfonylurea fared significantly better than those whose diabetes was managed without sulfonylureas. Inhibition of Sur1 with injectable glyburide holds promise for ameliorating rt-PA–associated HT in stroke.
Keywords: rt-PA, Sur1, glyburide, MMP-9, cerebral ischemia, stroke
Introduction
Hemorrhagic transformation (HT) is a serious complication of ischemic stroke, particularly in patients treated with recombinant tissue plasminogen activator (rt-PA). Disability and death occur more frequently in patients with HT after an ischemic stroke, especially in patients with parenchymal hematomas.1 In a review of prospective studies since 1996, the overall frequency of any HT, asymptomatic or symptomatic, in patients without antithrombotic drugs was 8.5%, and ranged from 8% to 22% when patients were treated with aspirin or heparin.2 Several factors predispose to HT, but the severity of the ischemic insult and the administration of rt-PA are two of the most important predictors.3–5 Patients with large strokes, even when not treated with rt-PA, are at high risk, with an odds ratio of 10.2 (95% CI, 3.2–32.1).6 Considering only the worst form of HT, parenchymal hemorrhage, patients treated with rt-PA are at significantly elevated risk, with an odds ratio of 3.6 (95% CI, 1.8–7.3).5
The increase in risk imposed by rt-PA is particularly frustrating to clinicians because it is entirely iatrogenic, and is encountered in the context of administering the only treatment for stroke currently approved by national medical regulatory agencies such as the US Food and Drug Administration (FDA). Thus, it is not surprising that great efforts have been made to elucidate the molecular mechanisms involved and to identify potential therapies to reduce the occurrence of symptomatic HT.
Sur1-regulated NCCa-ATP channel
The Sur1-regulated NCCa-ATP channel is transcriptionally upregulated following cerebral ischemia.7 Blockade of the channel by the specific, highly selective sulfonylurea inhibitor, glibenclamide (adopted U.S. name, glyburide), confers robust protection in rat models of stroke, including nonlethal models of thromboembolic, permanent and temporary occlusion, as well as models of malignant cerebral edema.8–10 In these models, protection is manifested as significant improvements in edema, brain swelling, lesion size, white matter preservation, neurological function, and mortality.
Following cerebral ischemia, all members of the neurovascular unit upregulate the Sur1-regulated NCCa-ATP channel, including neurons, oligodendrocytes, astrocytes, and endothelial cells.8–10 However, the role of this channel in endothelial cell swelling,8,11 which contributes to ischemia, and its role in dysfunction of interendothelial tight junctions,12 which contributes to edema formation and brain swelling, are particularly important for many of the beneficial effects observed from blocking the channel.
We previously speculated that seemingly distinct pathophysiological events, such as the formation of vasogenic edema and HT, may actually form part of a continuum of blood-brain barrier (BBB) dysfunction that is induced by cerebral ischemia.13 Given the important role of the Sur1-regulated channel in edema formation, we hypothesized that the channel also would be involved in HT. Support for this hypothesis is provided in this focused review.
MMPs, ischemia, and rt-PA
Several mechanisms have been proposed to account for the increased risk of HT associated with rt-PA. Besides clot lysis, rt-PA exerts direct effects on various brain cells,14 resulting in direct vasoactivity, cleavage of the N -methyl-D-aspartate (NMDA) NR1 subunit, which leads to excitotoxicity and amplification of damaging calcium overload,15,16 microglial activation,17 and upregulation of extracellular proteases from the matrix metalloproteinase (MMP) family.18–20 In the clinical setting, it is likely that the last of these, the upregulation of MMP, plays the leading role in HT.
Involvement of inducible MMPs in microvascular dysfunction after ischemic stroke is well accepted. MMPs are upregulated early after onset of cerebral ischemia.21–24 During the hours following ischemia, MMPs disrupt the BBB by degrading interendothelial tight junction proteins (e.g., occludin and claudin-5) and basal lamina proteins (e.g., fibronectin, laminin, collagen, proteoglycans, and others) and thereby promote edema formation, leukocyte infiltration, brain swelling, and HT.18,20,22,25–32 Two of the principal predictors of HT—large infarct volume and use of rt-PA—are associated with increased levels of MMPs, both in plasma and in brain tissue.33–36
MMP-9
Given the import role of MMPs in ischemic stroke, it is not surprising that numerous preclinical studies have investigated MMP upregulation in animal models of stroke. Here, we focus on MMP-9, recognizing that others, including MMP-2 and MMP-3, also may play prominent roles.
MMP-9 in cerebral ischemia
MMP-9 activity is increased by cerebral ischemia alone, without administration of thrombolytics. An increase in MMP-9 activity has been reported in various rat models of stroke, with ischemia induced by thromboembolism,37–39 by temporary mechanical middle cerebral artery occlusion (MCAo),21,40–42 by permanent mechanical MCAo,23 or by coagulation of the MCA.43 An increase in MMP-9 activity following ischemia also has been reported in mouse models, with ischemia induced by temporary mechanical MCAo.22 In rat models with 2-hour mechanical MCAo, increases in MMP-9 protein as well as MMP-9 activity (pro-MMP-9 and cleaved MMP-9 forms (80–92 kDa)) are observed first in the core then later in the penumbra, with levels peaking at 24 h and returning to near-baseline at 5 days. Spontaneously hypertensive rats show greater increases in MMP-9 activity than Wistar–Kyoto rats.23 The increase in MMP-9 protein associated with ischemia is due to transcriptional upregulation, with mRNA for MMP-9 increasing several-fold after ischemia/reperfusion.40,44,45 In humans, MMP-9 protein is upregulated 10-fold in the infarct core compared to contralateral tissues.46
MMP-9 with rt-PA
Work with animal models suggests that MMP-9 activity in cerebral ischemia may be further increased by rt-PA. However, it is important to note that, with rare exceptions,47,48 virtually all studies involving rodents have used a dose of rt-PA (10 mg/kg) that is ~11 times greater than the dose used clinically in humans (0.9 mg/kg). The high dose used in model systems potentially could distort what may be expected following the use of rt-PA in the clinical setting.
An increase in MMP-9 activity following ischemia plus administration of rt-PA has been reported in rat models with ischemia induced by thromboembolism37–39,49 or by temporary mechanical MCAo.40,41,50 With 2- to 3-hour mechanical MCAo, administration of rt-PA (10 mg/kg i.v.) at the time of recanalization results in a 2- to 2.5-fold increase in MMP-9 activity above levels observed with ischemia alone.40,41,50 An increase in MMP-9 activity following ischemia plus administration of rt-PA also has been reported in mouse models, with ischemia induced by temporary mechanical MCAo.40,43,44,51–53 One report studying a mouse model with mechanical MCAo indicated no increase in MMP-9 concentration by rt-PA above that observed with ischemia alone.54 Optimal induction of MMP-9 requires an interaction between thrombus and the thrombolytic agent,55 which may be of particular importance in experiments utilizing mechanical MCAo of a duration too short to result in thrombosis.
Gene suppression of MMP-9 and t-PA
MMP-9–null mice are partially protected from cerebral ischemia, purportedly due to reduced proteolytic degradation of the BBB.20,45,54 Similar results have been reported with gene suppression obtained by administering MMP-9 shRNA or siRNA.56,57
t-PA induces MMP-9 activity in the ischemic brain through a plasmin/plasminogen-independent mechanism40,44 that, paradoxically, requires endogenous t-PA. Studies with transgenic mice show that the expected increase in MMP-9 activity with ischemia alone is blunted in t-PA knockout mice, and that the administration of exogenous t-PA restores MMP-9 levels.40,44,58,59 Signaling for this t-PA–mediated mechanism requires low-density lipoprotein receptor-related protein 1.44
Inhibitors of MMP activity
Broad-spectrum MMP inhibitors
Broad-spectrum MMP inhibitors not only inhibit MMP-9 but also other MMPs upregulated during cerebral ischemia, including MMP-2, MMP-3, MMP-13, as well as MMPs required for cellular functions associated with tissue recovery. Broad spectrum MMP inhibitors that have been used in experimental studies include BB-94 (batimastat),37,42,45,60,61 BB-1101,62–65 FN-439,66 SB-3CT,67 p-aminobenzoyl-gly-pro-D-leu-D-ala-hydroxamate.31,54 Administration of broad-spectrum MMP inhibitors reduces rt-PA–induced edema and HT, improves preclinical outcomes, and may reduce infarct size in a variety of animal models. However, it is now recognized that MMPs play a crucial role in recovery from stroke, and that important functions related to recovery should not be impeded.35,66,68–70 Indeed, the beneficial effects of MMP inhibition on BBB permeability during the acute phase after injury are reportedly countered by impaired long-term recovery in rat models of stroke.64
The dual-action tetracyclines
One of the important effects of the pleitropic tetracyclines is the inhibition of MMPs.71 The mechanism by which tetracyclines inhibit MMPs is believed to involve both direct inhibition, mediated by an interaction between the tetracycline molecule and metal ions within MMP, and indirect inhibition, due to reduced MMP expression or scavenging of reactive oxygen species.71 Two agents, doxycycline72,73 and minocycline,39,74–77 have been studied in experimental stroke models. Both reduce MMP-9 expression or activity and improve preclinical outcomes. In humans with ischemic stroke who are treated either without or with rt-PA, administration of minocycline within 6 hours of ischemia significantly reduces serum MMP-9 levels at 24 h.78
Indirect inhibition with antioxidants
Reactive oxygen species are important inducers of MMP-9 expression,79 and thus are reasonable targets for “indirect” MMP inhibition. The antioxidants/free radical scavengers edaravone,41,80 quercetin,81 diphenyleneiodonium combined with dimethylsuloxide,82 and resveratrol83 have been shown to inhibit MMP upregulation induced in ischemic stroke models, in some cases after administration of rt-PA. These agents reduce MMP-9 activity and improve preclinical outcomes.84,85 Edaravone is widely used in Japan to treat acute ischemic stroke within 24 h after onset, albeit with equivocal results.86 In patients with acute ischemic stroke, edaravone has been found to suppress serum MMP-9 levels.87
Other agents with indirect effects on MMP-9
MMP-9 expression or activity after cerebral ischemia is reduced by the administration of anti-VEGF neutralizing antibody38 (but see Ref. 88), angiopoietin-1,89 the plasma serine protease, activated protein C,51 the 5-lipoxygenase inhibitor, zileuton,90 statins,91–93 estrogen,50,94,95 melatonin,96,97 and the antihypertensive, candesartan.98
Glyburide
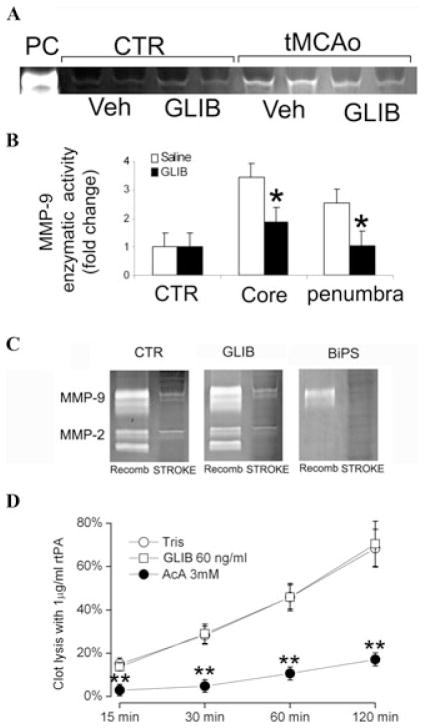
Emerging data indicate that the selective sulfonylurea inhibitor, glyburide, which inhibits Sur1-regulated NCCa-ATP channels,8,99 may inhibit MMP activity indirectly and thus complement rt-PA in rat models of stroke.48 In a rat model with 2-hour mechanical MCAo, administration of glyburide reduced MMP-9 activity in the core and penumbra by half, compared with saline controls (Figs. 1A and B).
Figure 1.
Glyburide reduces MMP-9 activity in a model of stroke, but does not inhibit MMP enzymatic activity or rt-PA–induced clot lysis. (A) Zymography of contralateral (CTR) and ipsilateral (tMCAo) tissues from vehicle (Veh) and glyburide (GLIB) treated rats 24 h after 2-hour mechanical MCAo; data from four rats are shown; methods as in Sumii and Lo.37 (B) Quantitative analysis of MMP-9 proteolytic activity in core and in penumbral tissues (n = 3 per group); *P < 0.05. (C) Zymography of recombinant MMP-9 and MMP-2 (Recomb; left in each panel) and of infarcted brain tissues (STROKE; right in each panel) in the presence of vehicle (CTR), glyburide (GLIB), and a broad-spectrum MMP inhibitor, 2R-[(4-biphenylylsulfonyl)amino]-N -hydroxy-3-phenylpropionamide (BiPS), as indicated, showing that proteolytic activity of the recombinant enzymes or of native tissues is unaffected by glyburide, but is inhibited by BiPS; from Simard et al.100 (D) Quantitative analysis of clot lysis induced by rt-PA in the presence of glyburide (GLIB), Tris buffer (used as negative control), and aminocaproic acid (AcA; used as positive control); methods as in Colucci et al.101
Unlike the broad-spectrum MMP inhibitors and the tetracyclines, glyburide does not directly inhibit MMP proteolysis (Fig. 1C), consistent with the interpretation that the inhibitory effect of glyburide on MMP activity (Figs. 1A and B) is due to reduced MMP expression or secretion or activation. In addition, glyburide does not inhibit rt-PA–induced clot lysis (Fig. 1D), a crucial property if the two are to be coadministered.
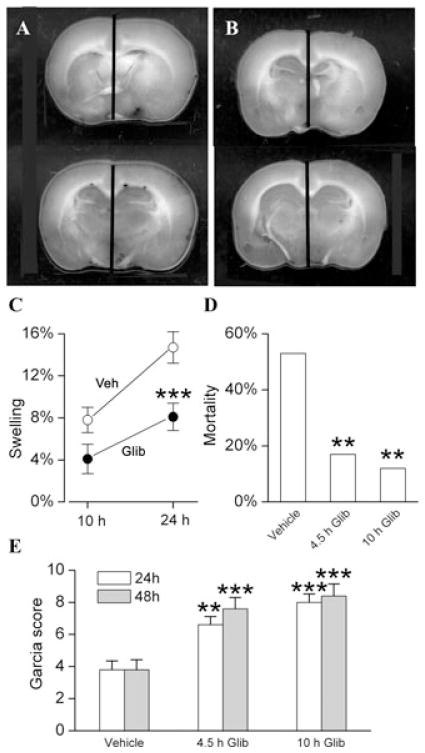
The effects of glyburide were studied in a clinically relevant model that included 4.5-h mechanical MCAo (75–90% reduction in laser Doppler flowmetry signals) plus administration at 4.5 h of rt-PA at the dose used in humans (0.9 mg/kg i.v.).48 This model of severe ischemic insult is associated with significant brain swelling and high mortality from malignant cerebral edema (Fig. 2). Administration of glyburide at the time of recanalization significantly reduced brain swelling (Figs. 2A–C). Administration of glyburide at the time of recanalization or 5.5 h later (10 h after onset of ischemia) significantly reduced mortality and improved neurological scores (Figs. 2D and E). The dose of rt-PA used in this experiment is expected to be minimally thrombolytic in rats.102 However, this dose was chosen because of its clinical relevance, and because a subthrombolytic dose mimics the situation frequently encountered in humans wherein administration of rt-PA does not lead to timely recanalization or reperfusion in patients with MCA occlusion.103,104
Figure 2.
Glyburide improves outcomes in a clinically relevant model of stroke. (A–C) Hemispheric swelling imaged at 24 h in rats administered vehicle (A) or glyburide (B) 4.5 h after onset of ischemia; the vertical bars denote the location of midline structures; the scatter plot (C) shows the percent hemispheric swelling at 10 and 24 h in rats administered vehicle (empty circles) or glyburide (filled circles) 4.5 h after onset of ischemia; 8–10 rats per group; * * *P < 0.001. (D) Mortality, assessed at 48 hours, in rats administered vehicle, glyburide at 4.5 h after onset of ischemia (4.5 h Glib) or glyburide at 10 h after onset of ischemia (10 hours Glib); 51, 41, and 25 rats in the three groups, respectively; * *P < 0.01; data at 4.5 and 10 h are not statistically different from each other (P = 0.7). (E) Garcia scores, assessed at 24 h (empty bars) and 48 h (gray bars), in rats administered vehicle, glyburide at 4.5 h after onset of ischemia (4.5 h Glib) or glyburide at 10 h after onset of ischemia (10 h Glib); same rats as in (D); * *P < 0.01; * * *P < 0.001; data at 4.5 and 10 h are not statistically different from each other (P > 0.05); from Simard et al.48
The effects of glyburide also were studied in a model with a more severe ischemic insult that included 6-hour mechanical MCAo plus administration at 6 hours of a dose of rt-PA (10 mg/kg i.v.) that is thrombolytic in rats. This model is also associated with significant brain swelling and high mortality from malignant cerebral edema, but in addition, is associated with an increased incidence of symptomatic HT (Fig. 3). Administration of glyburide at the time of recanalization significantly reduced brain swelling and symptomatic HT (Figs. 3A–D), and improved neurological scores both acutely (Fig. 3E) and over the course of 2 weeks of observation (Fig. 3F). These data suggest that glyburide may be useful for extending the treatment window for rt-PA beyond the current 4.5 h.105
Figure 3.
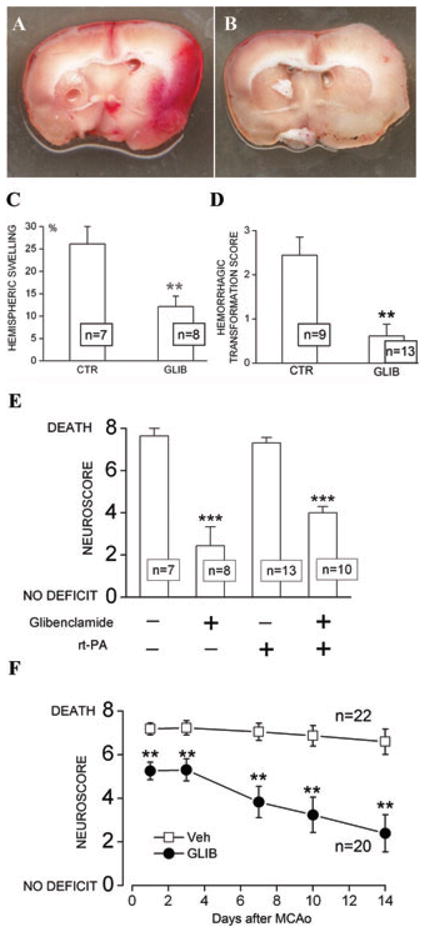
Glyburide improves outcomes in a model of stroke with delayed recanalization and administration of rt-PA. (A, B) Coronal sections from rats administered vehicle (A, CTR) or glyburide (B, GLIB); tissues obtained after death (A) or 48 h after insult (B); note that swelling and hemorrhagic transformation, which were prominent in the control, were less evident with glyburide. (C and D) Quantification of hemispheric swelling (C) and scores for hemorrhagic transformation (D) in rats administered vehicle or glyburide; the number of rats in each group is denoted in the boxes; * * P < 0.01. (E) Neuroscores 48 h after the ischemic insult in four groups of rats, with and without rt-PA, and with and without glyburide administration, as indicated. (F) Neuroscores at 1, 3, 7, 10, and 14 days after the ischemic insult in rats administered rt-PA plus vehicle (Veh) or glyburide (GLIB), as indicated; rats different from those in panel E.
For all experiments, 275–325 gm male Wistar rats (panels A, B, C, E, and F) or SHR (panel D) underwent 6-hour mechanical MCAo; after filament withdrawal, rt-PA (10 mg/kg) was infused i.v., except as indicated in panel E; vehicle or glyburide infusion was started 6 hours after onset of ischemia; all methods including surgery for MCAo, dosing of glyburide, blinded evaluations, and good laboratory practice are the same as in Simard et al.48
MMP-9 in endothelium
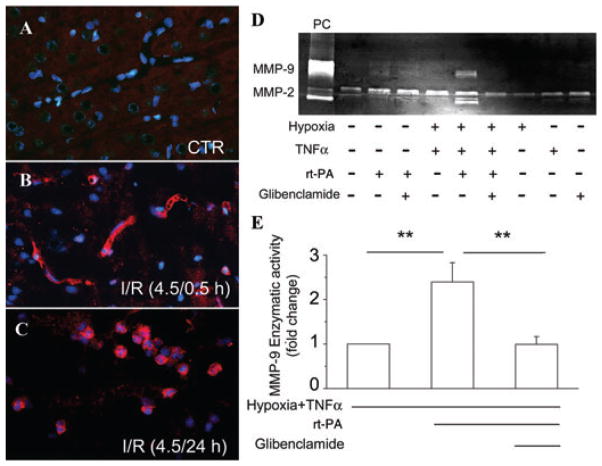
Ongoing work indicates that, following cerebral ischemia, MMP may originate from leukocytes, microglia, as well as endothelial cells.17,32,44,46,79,106,107 Immunohistochemical studies demonstrate that, early after ischemia/reperfusion in the rat (no rt-PA administered), MMP-9 upregulation is prominent in microvessels in the region of the ischemic insult, whereas later, microglia and invading neutrophils also show prominent labeling (Figs. 4A–C).108 In rat and mouse models with rt-PA administration (again, 10 mg/mL), most of the early MMP-9 upregulation colocalizes with the endothelial marker, platelet endothelial cell adhesion molecule (PECAM-1; a.k.a., CD31).40,41 Notably, these experiments show that rt-PA administration causes upregulation of MMP-9 only in ischemic endothelium, not in uninjured tissues or uninjured microvessels.40,41 The molecular explanation for this observation has not been elucidated, but it has several important implications, including for cell culture experiments (see later).
Figure 4.
MMP-9 is upregulated first in microvessels and glyburide blocks rt-PA–induced MMP-9 activity in endothelial cells. (A–C) Sections of cortex obtained from a control (uninjured) rat (A), or from a rat following ipsilateral 4.5-h ischemia plus 0.5-h (B) or 24-h (C) reperfusion, immunolabeled for MMP-9 (red), and stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue) to identify nuclei; note that early on, MMP-9 is upregulated only in microvessels, whereas later, infiltrating neutrophils are also visualized. (D) Zymography of the culture medium of brain microvascular endothelial (bEnd.3) cells exposed to the conditions indicated; note that, in cells exposed to hypoxia (1% O2) plus NF-κB activation (TNF-α), rt-PA induces robust activation of both MMP-9 and MMP-2, and that this effect was blocked by glyburide (glibenclamide), which was added at the onset of hypoxia/TNF-α exposure; positive control (PC): recombinant MMP-9/MMP-2. (E) Densitometric analysis of MMP-9 activity obtained as previously for the three conditions indicated; P < 0.01; n = 6; methods as in Woo et al.7 and Simard et al.12
In humans, immunohistochemistry of the infarcted core shows MMP-9 mainly located in blood vessels (endothelial cells/periendothelial layer), together with the presence of perivascular neutrophils.46,109 In peri-infarct areas the major source of MMP-9 is microglial cells.109 It is important to note that studies in humans are carried out on tissues long after the insult (37–108 h;109 10–87 h46), during which time patterns of protein expression and enzymatic activity are likely to change.
MMP-9 upregulation in endothelial cell cultures
The in vivo studies identifying microglia and endothelial cells as important sources of MMP have spawned experiments to examine MMP expression in cultured microglia17,44,106,107 and cultured endothelial cells. Here, we limit our discussion to work with endothelial cell cultures.
Work on endothelial cells has identified a t-PA–induced, NF-κB–dependent, MMP-9 pathway in ischemic brain endothelium in vitro. In cultured brain microvascular endothelial cells, hypoxia alone has little51 or no (Fig. 4D)79 effect on MMP-9 expression or enzymatic activity. However, the addition of stimuli that mimic the added stress associated with reperfusion (e.g., H2O2, TNF-α, thrombin, leukocyte elastase) has an important conditioning effect.51,79 When rt-PA is added to endothelial cells exposed to hypoxia plus a conditioning stimulus, MMP-9 expression and enzymatic activity are markedly upregulated (Figs. 4D and E). The MMP-9 gene promoter has binding sites for the redox sensitive transcription factor NF-κB,53 and effects of oxidative stress and of rt-PA are mediated by NF-κB: rt-PA–induced increases in MMP-9 are inhibited by the free radical scavenger, endaravone,41 and by inhibiting NF-κB signaling.51,79 The rt-PA–mediated increase in pro-MMP-9 and activated MMP-9 also is inhibited by activated protein C.51 In addition, the rt-PA–mediated increase in pro-MMP-9 and activated MMP-9 is inhibited by glyburide (Figs. 4D and E).
Sulfonylurea drugs improve outcome in diabetics with stroke
A retrospective study showed that, in patients with type 2 diabetes mellitus presenting with acute ischemic stroke, prior and continued use of sulfonylurea drugs as part of the treatment regimen for diabetes was associated with significantly better neurological and functional outcomes at discharge compared with patients whose diabetes was managed without the use of sulfonylurea drugs.110 Notably, the stroke subtype was very important for observing a salutary effect of sulfonylureas: no patient with a lacunar stroke benefited; all of the benefit was observed in patients with nonlacunar strokes.
Maintaining the use of the sulfonylurea drug after presenting with stroke was also crucial. The cohort of patients studied by Kunte et al.110 was actually a subgroup of patients previously reported by Weih et al.,111 which had included all diabetic patients presenting with acute ischemic stroke who had used a sulfonylurea drug before stroke, regardless of whether the drug was continued after hospitalization. In the combined cohort (patients still taking a sulfonylurea drug plus patients who stopped sulfonylurea drug after hospitalization), overall outcomes were less favorable, with only a nonsignificant trend toward improvement in neurological deficit by the time of discharge.
Another retrospective study suggesting possible protective effects of sulfonylurea drugs in diabetic patients with acute ischemic stroke was presented at an International Stroke Conference; however, this study also examined separately a subgroup of patients who received rt-PA.112 The data were from the Registry of the Canadian Stroke Network and concerned patients presenting to 11 stroke centers in Ontario between July 1, 2003, and March 31, 2008. Of the 15,814 patients screened, 2,448 with diabetes were admitted to hospital within 24 h of symptom onset. Information on medication use during the 48 h or more after admission was available for these patients.
Of the 2,448 patients, 1,469 had not been on and were not started on a sulfonylurea (group A); 729 had been on and were kept on a sulfonylurea (group D). Age (72.6 versus 72.6), gender (45% versus 39% female), admission serum glucose (9.7 ± 4.3 versus 11.1 ± 4.6 mmol/L), and thrombolysis (15% versus 12%) were reasonably balanced for groups A versus D, respectively. The likelihood of in-hospital mortality was less for patients in group D compared with group A (odds ratio, 0.51; 95% CI, 0.37–0.71; P < 0.05). The likelihood of neurological worsening was less for patients in group D compared with group A (odds ratio, 0.70; 95% CI, 0.55–0.89; P < 0.05).
Of the 2,448 patients, 358 were managed with rt-PA. These patients were dichotomized as previously discussed into group A (had not been on and were not started on a sulfonylurea) and group D (had been on and were kept on a sulfonylurea). The baseline characteristics of these patients were not analyzed separately. The likelihood of in-hospital mortality was less for patients in group D compared with group A (odds ratio, 0.30; 95% CI, 0.12–0.73; P < 0.05). The likelihood of neurological worsening was less for patients in group D compared with group A (odds ratio, 0.52; 95% CI, 0.28–0.98; P < 0.05). Although it is not known how well groups A and D were balanced regarding baseline characteristics, these findings are noteworthy, suggesting that the use of sulfonylureas may be beneficial in the context of rt-PA–aided recanalization/reperfusion following acute ischemic stroke.
The findings from the retrospective studies reviewed earlier, both without and with rt-PA, are limited to patients with diabetes mellitus. However, diabetic patients comprise one-third of stroke patients, diabetics are among those who suffer the worst outcomes from stroke, and stroke in diabetics has a high rate of recurrence,113,114 making it difficult to overestimate the importance of improving outcomes in this subgroup of stroke patients. A convergence of data suggests that an increased risk of symptomatic hemorrhagic transformation is a major factor in the poorer outcomes observed in diabetic patients,115,116 making the findings of these retrospective studies highly pertinent.
Glyburide may improve outcome in nondiabetics with stroke
A multicenter, prospective, open label, Phase IIa trial (the Glyburide Advantage in Malignant Edema and Stroke [GAMES]-pilot) of RP-1127 (glyburide for injection, Remedy Pharmaceuticals, Inc., New York, NY) is underway to test the effect of RP-1127 in patients with a severe anterior circulation ischemic stroke who are likely to experience clinically significant brain swelling and who are at high risk for malignant cerebral edema requiring decompressive craniectomy or predisposing to death (Clinical-Trials.gov Identifier: NCT01268683). The inclusion criteria include a baseline diffusion weighted image (DWI) lesion volume 82–210 mL, administration of i.v. rt-PA per institutional guidelines, and start of a 72-h infusion of RP-1127 no later than 10 h after the time last known at baseline. Outcome measures include safety endpoints, MRI assessments of edema, swelling, and mass effect during the first 3 days, and modified Rankin scores (mRS) at 1 and 3 months.
One of the patients enrolled in GAMES-pilot was a 68-year-old male who presented to a community hospital with an acute right middle cerebral artery stroke syndrome. He received i.v. rt-PA within 3 hours of the time last known at baseline. He was transported to the University of Maryland Medical Center, where neurological assessment showed a baseline National Institutes of Health Stroke Scale score of 21. MR imaging showed signal attenuation in the right M1 trunk, consistent with prior M1 occlusion followed by recanalization, and a baseline DWI lesion volume of 146 mL (Fig. 5). He was enrolled in GAMES-pilot and had a 72-h infusion of RP-1127 beginning ~9 h after the last known baseline infusion. His hospital course was notable for the lack of development of parenchymal hematoma, and the lack of need for intubation, osmotherapy, and decompressive craniectomy. MRI on day 3 showed modest expansion of the DWI lesion volume, but minimal mass effect (Fig. 5). Fluid attenuated inversion recovery (FLAIR) MRI on days 2 and 4 showed the highly unusual pattern of cytotoxic edema confined largely to the cortical gray matter, apparent preservation of subcortical white matter, and maintenance of gyral anatomy and of sulcal cerebrospinal fluid (Fig. 5). The one-month mRS was 4. The clinical course and the radiological findings in this patient are typical of several other patients enrolled in GAMES-pilot. A more complete report on this cohort is anticipated soon.
Figure 5.
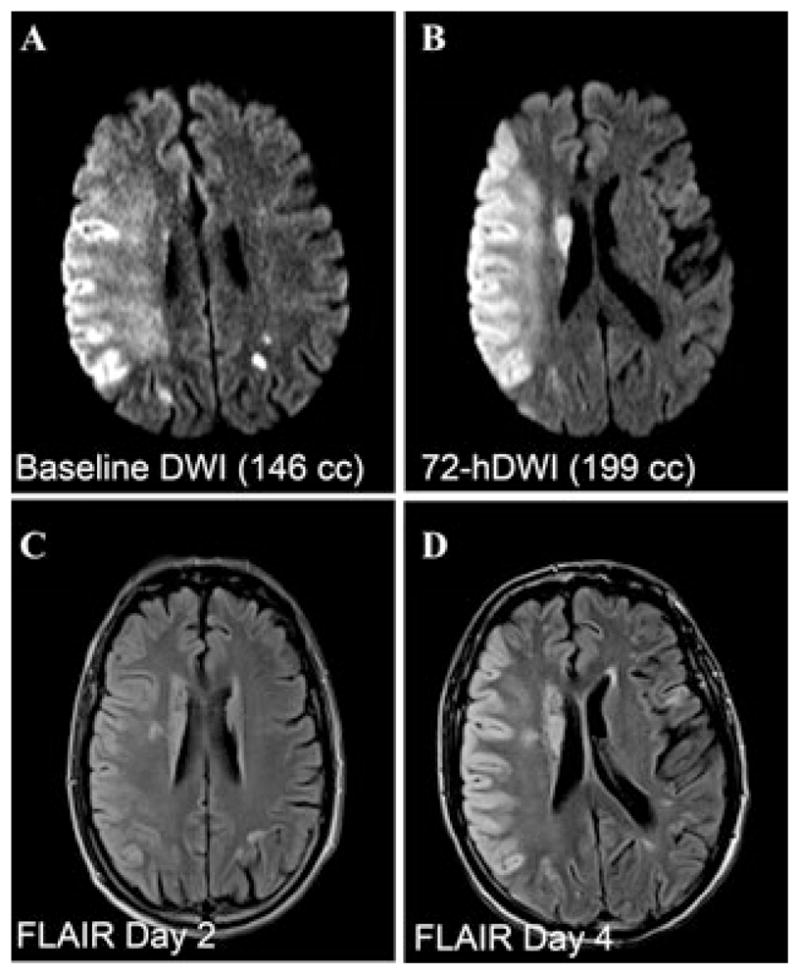
MRI of patient enrolled in GAMES-pilot treated with rt-PA followed by RP-1127 (glyburide for injection). (A–D) Diffusion weighted (DWI; A, B) and fluid attenuated inversion recovery (FLAIR; C, D) images obtained at the times indicated after onset of ischemia, in a patient who presented with an acute right MCA stroke syndrome likely from a right M1 occlusion, received conventional rt-PA treatment, and had a subsequent NIHSS score of 21. He was administered a 72-h infusion of RP-1127 beginning ~9 h after onset of ischemia.
The number of patients enrolled in GAMES-pilot is small, therefore necessitating cautious interpretation. However, early findings with these large strokes suggest that glyburide may have a role in ameliorating swelling and the development of parenchymal hematoma or of symptomatic HT that are often associated with rt-PA–aided recanalization/reperfusion following acute ischemic stroke. A phase IIb trial of PR-1127 for patients with severe anterior circulation strokes is planned.
Conclusions
Recombinant tissue plasminogen activator–associated HT mediated by MMP-9 complicates and limits the use of rt-PA in stroke. Great strides have been made in characterizing the molecular mechanisms responsible for the effect of rt-PA on MMP-9 upregulation, with the goal of rendering rt-PA safer for use in human stroke. Important progress has been made in identifying pharmaceutical agents to accomplish this goal, with edaravone and minocycline currently in use or in clinical trials in Japan or in the United States. Exciting new preclinical data suggest that inhibition of Sur1 may ameliorate rt-PA–associated HT mediated by MMP-9. The potent Sur1 inhibitor, glyburide, inhibits activation of MMP-9, ameliorates edema formation, swelling and symptomatic HT, and improves preclinical outcomes in several clinically relevant models of stroke, both with and without rt-PA treatment. A retrospective clinical study involving diabetic patients, and preliminary results from an open-label clinical trial, suggest that glyburide may complement rt-PA in patients with acute ischemic stroke.
Footnotes
Conflicts of interest
J.M.S. holds a U.S. patent (# 7,285,574), “A novel nonselective cation channel in neural cells and methods for treating brain swelling,” and is a member of the Scientific Advisory Board and holds shares in Remedy Pharmaceuticals. No support was provided by Remedy Pharmaceuticals to J.M.S. for this project.
References
- 1.Paciaroni M, Agnelli G, Corea F, et al. Early hemorrhagic transformation of brain infarction: rate, predictive factors, and influence on clinical outcome: results of a prospective multicenter study. Stroke. 2008;39:2249–2256. doi: 10.1161/STROKEAHA.107.510321. [DOI] [PubMed] [Google Scholar]
- 2.Lindley RI, Wardlaw JM, Sandercock PA, et al. Frequency and risk factors for spontaneous hemorrhagic transformation of cerebral infarction. J Stroke Cerebrovasc Dis. 2004;13:235–246. doi: 10.1016/j.jstrokecerebrovasdis.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 3.The Ninds t-PA Stroke Study Group. Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke. 1997;28:2109–2118. doi: 10.1161/01.str.28.11.2109. [DOI] [PubMed] [Google Scholar]
- 4.Khatri P, Wechsler LR, Broderick JP. Intracranial hemorrhage associated with revascularization therapies. Stroke. 2007;38:431–440. doi: 10.1161/01.STR.0000254524.23708.c9. [DOI] [PubMed] [Google Scholar]
- 5.Larrue V, Von Kummer R, Del Zoppo GJ, Bluhmki E. Hemorrhagic transformation in acute ischemic stroke. Potential contributing factors in the European Cooperative Acute Stroke Study. Stroke. 1997;28:957–960. doi: 10.1161/01.str.28.5.957. [DOI] [PubMed] [Google Scholar]
- 6.Terruso V, D’Amelio M, Di BN, et al. Frequency and determinants for hemorrhagic transformation of cerebral infarction. Neuroepidemiology. 2009;33:261–265. doi: 10.1159/000229781. [DOI] [PubMed] [Google Scholar]
- 7.Woo SK, Kwon MS, Geng Z, et al. Sequential activation of hypoxia-inducible factor 1 and specificity protein 1 is required for hypoxia-induced transcriptional stimulation of Abcc8. J Cereb Blood Flow Metab. 2012;32:525–536. doi: 10.1038/jcbfm.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simard JM, Chen M, Tarasov KV, et al. Newly expressed SUR1-regulated NC(Ca-ATP) channel mediates cerebral edema after ischemic stroke. Nat Med. 2006;12:433–440. doi: 10.1038/nm1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simard JM, Yurovsky V, Tsymbalyuk N, et al. Protective effect of delayed treatment with low-dose glibenclamide in three models of ischemic stroke. Stroke. 2009;40:604–609. doi: 10.1161/STROKEAHA.108.522409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simard JM, Tsymbalyuk N, Tsymbalyuk O, et al. Glibenclamide is superior to decompressive craniectomy in a rat model of malignant stroke. Stroke. 2010;41:531–537. doi: 10.1161/STROKEAHA.109.572644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen M, Simard JM. Cell swelling and a nonselective cation channel regulated by internal Ca2+ and ATP in native reactive astrocytes from adult rat brain. J Neurosci. 2001;21:6512–6521. doi: 10.1523/JNEUROSCI.21-17-06512.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simard JM, Geng Z, Woo SK, et al. Glibenclamide reduces inflammation, vasogenic edema, and caspase-3 activation after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2009;29:317–330. doi: 10.1038/jcbfm.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simard JM, Kent TA, Chen M, et al. Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurol. 2007;6:258–268. doi: 10.1016/S1474-4422(07)70055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaur J, Zhao Z, Klein GM, et al. The neurotoxicity of tissue plasminogen activator? J Cereb Blood Flow Metab. 2004;24:945–963. doi: 10.1097/01.WCB.0000137868.50767.E8. [DOI] [PubMed] [Google Scholar]
- 15.Nicole O, Docagne F, Ali C, et al. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat Med. 2001;7:59–64. doi: 10.1038/83358. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Monreal M, Lopez-Atalaya JP, Benchenane K, et al. Arginine 260 of the aminoterminal domain of NR1 subunit is critical for tissue-type plasminogen activator-mediated enhancement of N-methyl-D-aspartate receptor signaling. J Biol Chem. 2004;279:50850–50856. doi: 10.1074/jbc.M407069200. [DOI] [PubMed] [Google Scholar]
- 17.Siao CJ, Tsirka SE. Tissue plasminogen activator mediates microglial activation via its finger domain through annexin II. J Neurosci. 2002;22:3352–3358. doi: 10.1523/JNEUROSCI.22-09-03352.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Tsuji K, Lee SR, et al. Mechanisms of hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke. Stroke. 2004;35:2726–2730. doi: 10.1161/01.STR.0000143219.16695.af. [DOI] [PubMed] [Google Scholar]
- 19.Lo EH, Broderick JP, Moskowitz MA. tPA and proteolysis in the neurovascular unit. Stroke. 2004;35:354–356. doi: 10.1161/01.STR.0000115164.80010.8A. [DOI] [PubMed] [Google Scholar]
- 20.Asahi M, Wang X, Mori T, et al. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–7732. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujimura M, Gasche Y, Morita-Fujimura Y, et al. Early appearance of activated matrix metalloproteinase-9 and blood-brain barrier disruption in mice after focal cerebral ischemia and reperfusion. Brain Res. 1999;842:92–100. doi: 10.1016/s0006-8993(99)01843-0. [DOI] [PubMed] [Google Scholar]
- 22.Gasche Y, Fujimura M, Morita-Fujimura Y, et al. Early appearance of activated matrix metalloproteinase-9 after focal cerebral ischemia in mice: a possible role in blood-brain barrier dysfunction. J Cereb Blood Flow Metab. 1999;19:1020–1028. doi: 10.1097/00004647-199909000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg GA, Navratil M, Barone F, Feuerstein G. Proteolytic cascade enzymes increase in focal cerebral ischemia in rat. J Cereb Blood Flow Metab. 1996;16:360–366. doi: 10.1097/00004647-199605000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Heo JH, Lucero J, Abumiya T, et al. Matrix metalloproteinases increase very early during experimental focal cerebral ischemia. J Cereb Blood Flow Metab. 1999;19:624–633. doi: 10.1097/00004647-199906000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Cunningham LA, Wetzel M, Rosenberg GA. Multiple roles for MMPs and TIMPs in cerebral ischemia. Glia. 2005;50:329–339. doi: 10.1002/glia.20169. [DOI] [PubMed] [Google Scholar]
- 26.Adibhatla RM, Hatcher JF. Tissue plasminogen activator (tPA) and matrix metalloproteinases in the pathogenesis of stroke: therapeutic strategies. CNS Neurol Disord Drug Targets. 2008;7:243–253. doi: 10.2174/187152708784936608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenberg GA. Matrix metalloproteinases in neuroinflammation. Glia. 2002;39:279–291. doi: 10.1002/glia.10108. [DOI] [PubMed] [Google Scholar]
- 28.Del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab. 2003;23:879–894. doi: 10.1097/01.WCB.0000078322.96027.78. [DOI] [PubMed] [Google Scholar]
- 29.Yong VW, Power C, Forsyth P, Edwards DR. Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci. 2001;2:502–511. doi: 10.1038/35081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lo EH, Wang X, Cuzner ML. Extracellular proteolysis in brain injury and inflammation: role for plasminogen activators and matrix metalloproteinases. J Neurosci Res. 2002;69:1–9. doi: 10.1002/jnr.10270. [DOI] [PubMed] [Google Scholar]
- 31.Copin JC, Merlani P, Sugawara T, et al. Delayed matrix metalloproteinase inhibition reduces intracerebral hemorrhage after embolic stroke in rats. Exp Neurol. 2008;213:196–201. doi: 10.1016/j.expneurol.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gidday JM, Gasche YG, Copin JC, et al. Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am J Physiol Heart Circ Physiol. 2005;289:H558–H568. doi: 10.1152/ajpheart.01275.2004. [DOI] [PubMed] [Google Scholar]
- 33.Castellanos M, Leira R, Serena J, et al. Plasma metalloproteinase-9 concentration predicts hemorrhagic transformation in acute ischemic stroke. Stroke. 2003;34:40–46. [PubMed] [Google Scholar]
- 34.Ramos-Fernandez M, Bellolio MF, Stead LG. Matrix metalloproteinase-9 as a marker for acute ischemic stroke: a systematic review. J Stroke Cerebrovasc Dis. 2011;20:47–54. doi: 10.1016/j.jstrokecerebrovasdis.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Morancho A, Rosell A, Garcia-Bonilla L, Montaner J. Metalloproteinase and stroke infarct size: role for anti-inflammatory treatment? Ann NY Acad Sci. 2010;1207:123–133. doi: 10.1111/j.1749-6632.2010.05734.x. [DOI] [PubMed] [Google Scholar]
- 36.Ning M, Furie KL, Koroshetz WJ, et al. Association between tPA therapy and raised early matrix metalloproteinase-9 in acute stroke. Neurology. 2006;66:1550–1555. doi: 10.1212/01.wnl.0000216133.98416.b4. [DOI] [PubMed] [Google Scholar]
- 37.Sumii T, Lo EH. Involvement of matrix metalloproteinase in thrombolysis-associated hemorrhagic transformation after embolic focal ischemia in rats. Stroke. 2002;33:831–836. doi: 10.1161/hs0302.104542. [DOI] [PubMed] [Google Scholar]
- 38.Kanazawa M, Igarashi H, Kawamura K, et al. Inhibition of VEGF signaling pathway attenuates hemorrhage after tPA treatment. J Cereb Blood Flow Metab. 2011;31:1461–1474. doi: 10.1038/jcbfm.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murata Y, Rosell A, Scannevin RH, et al. Extension of the thrombolytic time window with minocycline in experimental stroke. Stroke. 2008;39:3372–3377. doi: 10.1161/STROKEAHA.108.514026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsuji K, Aoki T, Tejima E, et al. Tissue plasminogen activator promotes matrix metalloproteinase-9 up-regulation after focal cerebral ischemia. Stroke. 2005;36:1954–1959. doi: 10.1161/01.STR.0000177517.01203.eb. [DOI] [PubMed] [Google Scholar]
- 41.Yagi K, Kitazato KT, Uno M, et al. Edaravone, a free radical scavenger, inhibits MMP-9-related brain hemorrhage in rats treated with tissue plasminogen activator. Stroke. 2009;40:626–631. doi: 10.1161/STROKEAHA.108.520262. [DOI] [PubMed] [Google Scholar]
- 42.Pfefferkorn T, Rosenberg GA. Closure of the blood-brain barrier by matrix metalloproteinase inhibition reduces rt-PA-mediated mortality in cerebral ischemia with delayed reperfusion. Stroke. 2003;34:2025–2030. doi: 10.1161/01.STR.0000083051.93319.28. [DOI] [PubMed] [Google Scholar]
- 43.Yepes M, Sandkvist M, Moore EG, et al. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J Clin Invest. 2003;112:1533–1540. doi: 10.1172/JCI19212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang C, An J, Haile WB, et al. Microglial low-density lipoprotein receptor-related protein 1 mediates the effect of tissue-type plasminogen activator on matrix metalloproteinase-9 activity in the ischemic brain. J Cereb Blood Flow Metab. 2009;29:1946–1954. doi: 10.1038/jcbfm.2009.174. [DOI] [PubMed] [Google Scholar]
- 45.Asahi M, Asahi K, Jung JC, et al. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab. 2000;20:1681–1689. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 46.Cuadrado E, Rosell A, Penalba A, et al. Vascular MMP-9/TIMP-2 and neuronal MMP-10 up-regulation in human brain after stroke: a combined laser microdissection and protein array study. J Proteome Res. 2009;8:3191–3197. doi: 10.1021/pr801012x. [DOI] [PubMed] [Google Scholar]
- 47.Haelewyn B, Risso JJ, Abraini JH. Human recombinant tissue-plasminogen activator (alteplase): why not use the ‘human’ dose for stroke studies in rats? J Cereb Blood Flow Metab. 2010;30:900–903. doi: 10.1038/jcbfm.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simard JM, Woo SK, Tsymbalyuk N, et al. Glibenclamide – 10 hour treatment window in a clinically-relevant model of stroke. Transl Stroke Res. 2012;3:286–295. doi: 10.1007/s12975-012-0149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kelly MA, Shuaib A, Todd KG. Matrix metalloproteinase activation and blood-brain barrier breakdown following thrombolysis. Exp Neurol. 2006;200:38–49. doi: 10.1016/j.expneurol.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 50.Li M, Zhang Z, Sun W, et al. 17beta-estradiol attenuates breakdown of blood-brain barrier and hemorrhagic transformation induced by tissue plasminogen activator in cerebral ischemia. Neurobiol Dis. 2011;44:277–283. doi: 10.1016/j.nbd.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng T, Petraglia AL, Li Z, et al. Activated protein C inhibits tissue plasminogen activator-induced brain hemorrhage. Nat Med. 2006;12:1278–1285. doi: 10.1038/nm1498. [DOI] [PubMed] [Google Scholar]
- 52.Ishiguro M, Mishiro K, Fujiwara Y, et al. Phosphodiesterase-III inhibitor prevents hemorrhagic transformation induced by focal cerebral ischemia in mice treated with tPA. PLoS One. 2010;5:e15178. doi: 10.1371/journal.pone.0015178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang X, Lee SR, Arai K, et al. Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat Med. 2003;9:1313–1317. doi: 10.1038/nm926. [DOI] [PubMed] [Google Scholar]
- 54.Copin JC, Bengualid DJ, Da Silva RF, et al. Recombinant tissue plasminogen activator induces blood-brain barrier breakdown by a matrix metalloproteinase-9-independent pathway after transient focal cerebral ischemia in mouse. Eur J Neurosci. 2011;34:1085–1092. doi: 10.1111/j.1460-9568.2011.07843.x. [DOI] [PubMed] [Google Scholar]
- 55.Kahles T, Foerch C, Sitzer M, et al. Tissue plasminogen activator mediated blood-brain barrier damage in transient focal cerebral ischemia in rats: relevance of interactions between thrombotic material and thrombolytic agent. Vascul Pharmacol. 2005;43:254–259. doi: 10.1016/j.vph.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 56.Hu Q, Chen C, Khatibi NH, et al. Lentivirus-mediated transfer of MMP-9 shRNA provides neuroprotection following focal ischemic brain injury in rats. Brain Res. 2011;1367:347–359. doi: 10.1016/j.brainres.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 57.Hu Q, Chen C, Yan J, et al. Therapeutic application of gene silencing MMP-9 in a middle cerebral artery occlusion-induced focal ischemia rat model. Exp Neurol. 2009;216:35–46. doi: 10.1016/j.expneurol.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 58.Zhao BQ, Ikeda Y, Ihara H, et al. Essential role of endogenous tissue plasminogen activator through matrix metalloproteinase 9 induction and expression on heparin-produced cerebral hemorrhage after cerebral ischemia in mice. Blood. 2004;103:2610–2616. doi: 10.1182/blood-2003-03-0835. [DOI] [PubMed] [Google Scholar]
- 59.Wang YF, Tsirka SE, Strickland S, et al. Tissue plasminogen activator (tPA) increases neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice. Nat Med. 1998;4:228–231. doi: 10.1038/nm0298-228. [DOI] [PubMed] [Google Scholar]
- 60.Park CH, Shin TK, Lee HY, et al. Matrix metalloproteinase inhibitors attenuate neuroinflammation following focal cerebral ischemia in mice Korean. J Physiol Pharmacol. 2011;15:115–122. doi: 10.4196/kjpp.2011.15.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lapchak PA, Chapman DF, Zivin JA. Metalloproteinase inhibition reduces thrombolytic (tissue plasminogen activator)-induced hemorrhage after thromboembolic stroke. Stroke. 2000;31:3034–3040. doi: 10.1161/01.str.31.12.3034. [DOI] [PubMed] [Google Scholar]
- 62.Rosenberg GA, Estrada EY, Dencoff JE. Matrix metalloproteinases and TIMPs are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke. 1998;29:2189–2195. doi: 10.1161/01.str.29.10.2189. [DOI] [PubMed] [Google Scholar]
- 63.Rosenberg GA, Cunningham LA, Wallace J, et al. Immunohistochemistry of matrix metalloproteinases in reperfusion injury to rat brain: activation of MMP-9 linked to stromelysin-1 and microglia in cell cultures. Brain Res. 2001;893:104–112. doi: 10.1016/s0006-8993(00)03294-7. [DOI] [PubMed] [Google Scholar]
- 64.Sood RR, Taheri S, Candelario-Jalil E, et al. Early beneficial effect of matrix metalloproteinase inhibition on blood-brain barrier permeability as measured by magnetic resonance imaging countered by impaired long-term recovery after stroke in rat brain. J Cereb Blood Flow Metab. 2008;28:431–438. doi: 10.1038/sj.jcbfm.9600534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang Y, Estrada EY, Thompson JF, et al. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- 66.Kang SS, Kook JH, Hwang S, et al. Inhibition of matrix metalloproteinase-9 attenuated neural progenitor cell migration after photothrombotic ischemia. Brain Res. 2008;1228:20–26. doi: 10.1016/j.brainres.2008.06.056. [DOI] [PubMed] [Google Scholar]
- 67.Gu Z, Cui J, Brown S, et al. A highly specific inhibitor of matrix metalloproteinase-9 rescues laminin from proteolysis and neurons from apoptosis in transient focal cerebral ischemia. J Neurosci. 2005;25:6401–6408. doi: 10.1523/JNEUROSCI.1563-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee SR, Kim HY, Rogowska J, et al. Involvement of matrix metalloproteinase in neuroblast cell migration from the subventricular zone after stroke. J Neurosci. 2006;26:3491–3495. doi: 10.1523/JNEUROSCI.4085-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barkho BZ, Munoz AE, Li X, et al. Endogenous matrix metalloproteinase (MMP)-3 and MMP-9 promote the differentiation and migration of adult neural progenitor cells in response to chemokines. Stem Cells. 2008;26:3139–3149. doi: 10.1634/stemcells.2008-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao BQ, Tejima E, Lo EH. Neurovascular proteases in brain injury, hemorrhage and remodeling after stroke. Stroke. 2007;38:748–752. doi: 10.1161/01.STR.0000253500.32979.d1. [DOI] [PubMed] [Google Scholar]
- 71.Griffin MO, Fricovsky E, Ceballos G, Villarreal F. Tetracyclines: a pleitropic family of compounds with promising therapeutic properties. Review of the literature. Am J Physiol Cell Physiol. 2010;299:C539–C548. doi: 10.1152/ajpcell.00047.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Z, Xue Y, Jiao H, et al. Doxycycline-mediated protective effect against focal cerebral ischemia-reperfusion injury through the modulation of tight junctions and PKCdelta signaling in rats. J Mol Neurosci. 2012;47:89–100. doi: 10.1007/s12031-011-9689-x. [DOI] [PubMed] [Google Scholar]
- 73.Lee H, Park JW, Kim SP, et al. Doxycycline inhibits matrix metalloproteinase-9 and laminin degradation after transient global cerebral ischemia. Neurobiol Dis. 2009;34:189–198. doi: 10.1016/j.nbd.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 74.Koistinaho M, Malm TM, Kettunen MI, et al. Minocycline protects against permanent cerebral ischemia in wild type but not in matrix metalloprotease-9-deficient mice. J Cereb Blood Flow Metab. 2005;25:460–467. doi: 10.1038/sj.jcbfm.9600040. [DOI] [PubMed] [Google Scholar]
- 75.Machado LS, I, Sazonova Y, Kozak A, et al. Minocycline and tissue-type plasminogen activator for stroke: assessment of interaction potential. Stroke. 2009;40:3028–3033. doi: 10.1161/STROKEAHA.109.556852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nagel S, Su Y, Horstmann S, et al. Minocycline and hypothermia for reperfusion injury after focal cerebral ischemia in the rat: effects on BBB breakdown and MMP expression in the acute and subacute phase. Brain Res. 2008;1188:198–206. doi: 10.1016/j.brainres.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 77.Machado LS, Kozak A, Ergul A, et al. Delayed minocycline inhibits ischemia-activated matrix metalloproteinases 2 and 9 after experimental stroke. BMC Neurosci. 2006;7:56. doi: 10.1186/1471-2202-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Switzer JA, Hess DC, Ergul A, et al. Matrix metalloproteinase-9 in an exploratory trial of intravenous minocycline for acute ischemic stroke. Stroke. 2011;42:2633–2635. doi: 10.1161/STROKEAHA.111.618215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kolev K, Skopal J, Simon L, et al. Matrix metalloproteinase-9 expression in post-hypoxic human brain capillary endothelial cells: H2O2 as a trigger and NF-kappaB as a signal transducer. Thromb Haemost. 2003;90:528–537. doi: 10.1160/TH03-02-0070. [DOI] [PubMed] [Google Scholar]
- 80.Liu N, Shang J, Tian F, et al. In vivo optical imaging for evaluating the efficacy of edaravone after transient cerebral ischemia in mice. Brain Res. 2011;1397:66–75. doi: 10.1016/j.brainres.2011.04.038. [DOI] [PubMed] [Google Scholar]
- 81.Lee JK, Kwak HJ, Piao MS, et al. Quercetin reduces the elevated matrix metalloproteinases-9 level and improves functional outcome after cerebral focal ischemia in rats. Acta Neurochir (Wien) 2011;153:1321–1329. doi: 10.1007/s00701-010-0889-x. [DOI] [PubMed] [Google Scholar]
- 82.Nagel S, Genius J, Heiland S, et al. Diphenyleneiodonium and dimethylsulfoxide for treatment of reperfusion injury in cerebral ischemia of the rat. Brain Res. 2007;1132:210–217. doi: 10.1016/j.brainres.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 83.Gao D, Zhang X, Jiang X, et al. Resveratrol reduces the elevated level of MMP-9 induced by cerebral ischemia-reperfusion in mice. Life Sci. 2006;78:2564–2570. doi: 10.1016/j.lfs.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 84.Yamashita T, Kamiya T, Deguchi K, et al. Dissociation and protection of the neurovascular unit after thrombolysis and reperfusion in ischemic rat brain. J Cereb Blood Flow Metab. 2009;29:715–725. doi: 10.1038/jcbfm.2008.164. [DOI] [PubMed] [Google Scholar]
- 85.Zhang W, Sato K, Hayashi T, et al. Extension of ischemic therapeutic time window by a free radical scavenger, Edaravone, reperfused with tPA in rat brain. Neurol Res. 2004;26:342–348. doi: 10.1179/016164104225014058. [DOI] [PubMed] [Google Scholar]
- 86.Lapchak PA. A critical assessment of edaravone acute ischemic stroke efficacy trials: is edaravone an effective neuroprotective therapy? Expert Opin Pharmacother. 2010;11:1753–1763. doi: 10.1517/14656566.2010.493558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Isahaya K, Yamada K, Yamatoku M, et al. Effects of Edaravone, a free radical scavenger, on serum levels of inflammatory biomarkers in acute brain infarction. J Stroke Cerebrovasc Dis. 2012;21:102–107. doi: 10.1016/j.jstrokecerebrovasdis.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 88.Abumiya T, Yokota C, Kuge Y, Minematsu K. Aggravation of hemorrhagic transformation by early intraarterial infusion of low-dose vascular endothelial growth factor after transient focal cerebral ischemia in rats. Brain Res. 2005;1049:95–103. doi: 10.1016/j.brainres.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 89.Valable S, Montaner J, Bellail A, et al. VEGF-induced BBB permeability is associated with an MMP-9 activity increase in cerebral ischemia: both effects decreased by Ang-1. J Cereb Blood Flow Metab. 2005;25:1491–1504. doi: 10.1038/sj.jcbfm.9600148. [DOI] [PubMed] [Google Scholar]
- 90.Tu XK, Yang WZ, Shi SS, et al. 5-lipoxygenase inhibitor zileuton attenuates ischemic brain damage: involvement of matrix metalloproteinase 9. Neurol Res. 2009;31:848–852. doi: 10.1179/174313209X403913. [DOI] [PubMed] [Google Scholar]
- 91.Liu XS, Zhang ZG, Zhang L, et al. Atorvastatin downregulates tissue plasminogen activator-aggravated genes mediating coagulation and vascular permeability in single cerebral endothelial cells captured by laser microdissection. J Cereb Blood Flow Metab. 2006;26:787–796. doi: 10.1038/sj.jcbfm.9600227. [DOI] [PubMed] [Google Scholar]
- 92.Zhang L, Zhang ZG, Ding GL, et al. Multitargeted effects of statin-enhanced thrombolytic therapy for stroke with recombinant human tissue-type plasminogen activator in the rat. Circulation. 2005;112:3486–3494. doi: 10.1161/CIRCULATIONAHA.104.516757. [DOI] [PubMed] [Google Scholar]
- 93.Kawai H, Deguchi S, Deguchi K, et al. Protection against ischemic stroke damage by synergistic treatment with amlodipine plus atorvastatin in Zucker metabolic rat. Brain Res. 2011;1382:308–314. doi: 10.1016/j.brainres.2011.01.062. [DOI] [PubMed] [Google Scholar]
- 94.Liu R, Wen Y, Perez E, et al. 17beta-Estradiol attenuates blood-brain barrier disruption induced by cerebral ischemia-reperfusion injury in female rats. Brain Res. 2005;1060:55–61. doi: 10.1016/j.brainres.2005.08.048. [DOI] [PubMed] [Google Scholar]
- 95.Liu R, Liu Q, He S, et al. Combination therapy of 17beta-estradiol and recombinant tissue plasminogen activator for experimental ischemic stroke. J Pharmacol Exp Ther. 2010;332:1006–1012. doi: 10.1124/jpet.109.160937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hung YC, Chen TY, Lee EJ, et al. Melatonin decreases matrix metalloproteinase-9 activation and expression and attenuates reperfusion-induced hemorrhage following transient focal cerebral ischemia in rats. J Pineal Res. 2008;45:459–467. doi: 10.1111/j.1600-079X.2008.00617.x. [DOI] [PubMed] [Google Scholar]
- 97.Tai SH, Chen HY, Lee EJ, et al. Melatonin inhibits postischemic matrix metalloproteinase-9 (MMP-9) activation via dual modulation of plasminogen/plasmin system and endogenous MMP inhibitor in mice subjected to transient focal cerebral ischemia. J Pineal Res. 2010;49:332–341. doi: 10.1111/j.1600-079X.2010.00797.x. [DOI] [PubMed] [Google Scholar]
- 98.Guan W, Kozak A, El-Remessy AB, et al. Acute treatment with Candesartan reduces early injury after permanent middle cerebral artery occlusion. Transl Stroke Res. 2011;2:179–185. doi: 10.1007/s12975-010-0061-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Simard JM, Woo SK, Bhatta S, Gerzanich V. Drugs acting on SUR1 to treat CNS ischemia and trauma. Curr Opin Pharmacol. 2008;8:42–49. doi: 10.1016/j.coph.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Simard JM, Tsymbalyuk O, Ivanov A, et al. Endothelial sulfonylurea receptor 1-regulated NC Ca-ATP channels mediate progressive hemorrhagic necrosis following spinal cord injury. J Clin Invest. 2007;117:2105–2113. doi: 10.1172/JCI32041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Colucci M, Scopece S, Gelato AV, et al. In vitro clot lysis as a potential indicator of thrombus resistance to fibrinolysis–study in healthy subjects and correlation with blood fibrinolytic parameters. Thromb Haemost. 1997;77:725–729. [PubMed] [Google Scholar]
- 102.Korninger C, Collen D. Studies on the specific fibrinolytic effect of human extrinsic (tissue-type) plasminogen activator in human blood and in various animal species in vitro. Thromb Haemost. 1981;46:561–565. [PubMed] [Google Scholar]
- 103.Del Zoppo GJ, Poeck K, Pessin MS, et al. Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann Neurol. 1992;32:78–86. doi: 10.1002/ana.410320113. [DOI] [PubMed] [Google Scholar]
- 104.Von KR, Holle R, Rosin L, et al. Does arterial recanalization improve outcome in carotid territory stroke? Stroke. 1995;26:581–587. doi: 10.1161/01.str.26.4.581. [DOI] [PubMed] [Google Scholar]
- 105.Del Zoppo GJ, Saver JL, Jauch EC, Adams HP., Jr Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke. 2009;40:2945–2948. doi: 10.1161/STROKEAHA.109.192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Del Zoppo GJ, Milner R, Mabuchi T, et al. Microglial activation and matrix protease generation during focal cerebral ischemia. Stroke. 2007;38:646–651. doi: 10.1161/01.STR.0000254477.34231.cb. [DOI] [PubMed] [Google Scholar]
- 107.Del Zoppo GJ, Frankowski H, Gu YH, et al. Microglial cell activation is a source of metalloproteinase generation during hemorrhagic transformation. J Cereb Blood Flow Metab. 2012;32:919–932. doi: 10.1038/jcbfm.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Aoki T, Sumii T, Mori T, et al. Blood-brain barrier disruption and matrix metalloproteinase-9 expression during reperfusion injury: mechanical versus embolic focal ischemia in spontaneously hypertensive rats. Stroke. 2002;33:2711–2717. doi: 10.1161/01.str.0000033932.34467.97. [DOI] [PubMed] [Google Scholar]
- 109.Rosell A, Ortega-Aznar A, Alvarez-Sabin J, et al. Increased brain expression of matrix metalloproteinase-9 after ischemic and hemorrhagic human stroke. Stroke. 2006;37:1399–1406. doi: 10.1161/01.STR.0000223001.06264.af. [DOI] [PubMed] [Google Scholar]
- 110.Kunte H, Schmidt S, Eliasziw M, et al. Sulfonylureas improve outcome in patients with type 2 diabetes and acute ischemic stroke. Stroke. 2007;38:2526–2530. doi: 10.1161/STROKEAHA.107.482216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Weih M, Amberger N, Wegener S, et al. Sulfonylurea drugs do not influence initial stroke severity and inhospital outcome in stroke patients with diabetes. Stroke. 2001;32:2029–2032. [PubMed] [Google Scholar]
- 112.Silver FL, Fang J, Robertson AC, et al. Possible neuroprotective effects of sufonylureas in diabetic patients with acute ischemic stroke. Abstracts from the 2009 International Stroke Conference. Stroke. 2009;40:e156. [P19 http://stroke.ahajournals.org/cgi/reprint/40/4/e105] [Google Scholar]
- 113.Callahan A, Amarenco P, Goldstein LB, et al. Risk of stroke and cardiovascular events after ischemic stroke or transient ischemic attack in patients with type 2 diabetes or metabolic syndrome: secondary analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Arch Neurol. 2011;68:1245–1251. doi: 10.1001/archneurol.2011.146. [DOI] [PubMed] [Google Scholar]
- 114.Ergul A, Kelly-Cobbs A, Abdalla M, Fagan SC. Cerebrovascular complications of diabetes: focus on stroke. Transl Stroke Res. 2011;2:391–398. doi: 10.2174/187153012800493477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Martini SR, Kent TA. Hyperglycemia in acute ischemic stroke: a vascular perspective. J Cereb Blood Flow Metab. 2007;27:435–451. doi: 10.1038/sj.jcbfm.9600355. [DOI] [PubMed] [Google Scholar]
- 116.Elgebaly MM, Ogbi S, Li W, et al. Neurovascular injury in acute hyperglycemia and diabetes: a comparative analysis in experimental stroke. Transl Stroke Res. 2011;2:391–398. doi: 10.1007/s12975-011-0083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]