Abstract
The major capsid protein (L1) of human papillomaviruses (HPV) expressed in heterologous systems assembles into virus-like particles (VLPs). We report cloning and expression of codon optimized HPV L1 genes of the two high-risk HPV types 16 and 18 in methylotropic yeast, Pichia pastoris. The VLPs produced in P. pastoris were subjected to three step purification method involving density gradient centrifugations and size exclusion chromatography. The enriched VLPs were characterized using conformation-specific monoclonal antibodies in ELISA and by transmission electron microscopy. Mice immunized with a bivalent HPV16 and HPV18 VLPs developed high serum antibody titers to both HPV types that persisted for 190 days post vaccination. Serum of mice immunized with the HPV-VLP preparations could neutralize homologous pseudoviruses in an in vitro assays. Our results demonstrate that the L1 proteins expressed in P. pastoris fold properly as evidenced by assembly into VLPs and induction of type-specific neutralizing antibody response in mice. This work constitutes a step towards developing an alternate production platform for generating an affordable HPV vaccine to meet the needs of developing countries.
Keywords: Cervical cancer, Prophylactic vaccine, Virus like particles, Yeast, Pseudovirus neutralization assay
1. Introduction
Cancer of uterine cervix is the second most common cancer in women. Approximately 520,000 new cases and 274,000 deaths have been reported each year worldwide [1]. The incident rate of cervical cancer in India is approximately 132,000 per year; which is nearly 52% of the recorded incidence of the disease in the Asia-Pacific region [2]. Nearly 73,000 women die of cervical cancer each year in India [1]; thereby it holds the dubious distinction of accounting for nearly a quarter of all cervical cancer deaths recorded globally [2]. It is estimated that by the year 2025, the incidence rates in the developing world would account for nearly 80% of the global rates [1].
Human papillomavirus (HPV) is an epitheliotropic double stranded DNA virus of approximately 8 kb in size. The HPV genome encodes for non-structural-proteins (E1, E2, E4, E5, E6 and E7) and structural proteins (L1 and L2). There are over 120 HPV types reported [3–5]. Persistent infection by high-risk HPV types is the single most important factor for the induction of the cervical cancer [6]. Amongst high-risk HPV types, HPV16 and 18 are the genotypes most frequently associated with cervical cancer across the world. Overall HPV type prevalence in cervical cancer in India was found to be in the following order, HPV16, 18, 31, 33, 35, 39, 45, 52, 56, 58, 59 and HPV68 [7]. Together, HPV16 and HPV18 types contribute to nearly 80% of the entire uterine cervical cancer incidence in India [8].
Cervical cancer takes several years to establish after HPV infection; therefore periodic screening of uterine cervix and removal of premalignant lesions can result in a substantial reduction of cervical cancer related mortality. However, frequent monitoring of cervical exfoliates for an abnormality requires a large number of trained professionals and persistent public funding to support the requisite infrastructure [9,10]. Consequently, organized cervical screening programs are either absent or have minimal presence in poor resource countries [7]. Prophylactic vaccination has the potential to augment cervical screening programs, promoting the reduction in disease burden by prevention of HPV infection [11].
Recombinant major capsid protein L1 of papillomavirus self-assembles to form virus-like particles (VLPs) when expressed in heterologous systems. These VLPs have been proven to be highly immunogenic and are attractive candidates for developing a prophylactic vaccine [12–14]. The VLP-based bivalent HPV16/18 or quadrivalent HPV6/11/16/18 vaccines have been successfully introduced into the market. These vaccines offer near 100% protection against lesions caused by incident infection by the HPV types present in the vaccines [15–17]. Despite the introduction of these prophylactic vaccines into the market, the prohibitive cost of the vaccines is likely to affect their availability to women in developing countries [11,18]. It is likely that regional production of prophylactic HPV vaccine would not only help in bringing down the cost but also facilitate bridging the demand and supply gap. Current vaccines available in the market contain VLPs expressed either in insect cells (recombinant baculovirus infected Trichoplusia ni Hi5 cells) or in Saccharomyces cerevisiae. Use of various other expression platforms for producing HPV VLPs has been reported by several investigators [19,20,24]. Expression and purification of HPV16 L1 from Pichia pastoris was demonstrated by some investigators [21–23]. However to our knowledge, a detailed characterizations of the P. pastoris expressed VLPs and their ability to induce neutralizing antibodies have not been reported thus far. Evaluation of neutralizing activity is critical since the immunodominant epitopes recognized by L1 neutralizing antibodies are conformation dependent and even assembly into VLPs does not guarantee induction of neutralizing antibodies by an L1 vaccine [24].
In this manuscript, we describe the cloning of codon optimized genes for stable expression of the major capsid protein (L1) of HPV16 and HPV18; their expression in P. pastoris strain GS115, purification and characterization of the VLPs. This study is first to describe cloning and expression of HPV18 L1 in P. pastoris.
2. Materials and methods
2.1. Cells, expression vector and cell-culture media
Human embryonic Kidney cells (HEK 293 FT; Invitrogen, USA) were used for producing the HPV pseudoviruses. Methylotropic yeast P. pastoris strain GS115, yeast expression vector pPICZB and the media for growing human embryonic kidney cells (HEK 293 FT) were procured from Invitrogen, USA. Expression vectors required for producing HPV16 and HPV18 pseudo-viruses were previously published (http://home.ccr.cancer.gov/LCO/packaging.htm for details).
2.2. Yeast growth and expression media
P. pastoris growth and induction media components were procured from Hi-Media Labs, India. All other chemical including fine-chemicals were sourced from Sigma Chemical Company, USA and Merck, India.
2.3. HPV VLP conformation specific monoclonal antibodies
HPV16 and 18 VLP conformation monoclonal antibodies H16.V5 and H18.G10 were kindly provided by Prof. Neil Christensen, Pennsylvania State University, USA.
2.4. Animals usage
Four to six week old female BALB/c mice were used for the experiments after obtaining the requisite animal ethics approval. The animals were reared in individually ventilated cages (Tecniplast, Italy).
2.5. Cloning of the major capsid protein genes of HPV16 and HPV18
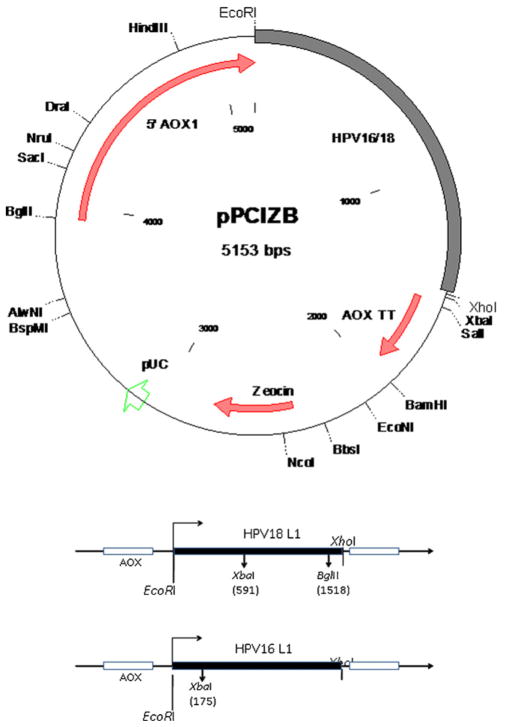
DNA sequences coding for the major capsid protein encoding gene (L1) of HPV16 (Gen Bank accession number ABV21641) and HPV18 (Gen Bank accession number AAQ92369) were codon optimized for expression in P. pastoris. Synthetic gene constructs for this purpose were procured from GeneArt (Regensburg, Germany). The codon optimized L1 genes were PCR amplified from the synthetic construct using Pfu DNA polymerase (Qiagen, Germany). Major capsid protein encoding genes of HPV16 L1 or HPV18 L1, also referred to as HPV L1 construct in this manuscript, were cloned into P. pastoris expression cassette pPICZB B using EcoRI and XhoI restriction enzymes (New England Bio-labs, USA). The HPV L1 genes cloned into pPICZB B were verified further using restriction fragment analysis and DNA sequencing.
2.6. Integration of the expression cassette into P. pastoris
The plasmid clones containing the HPV L1 genes were used to transform P. pastoris GS115 (Invitrogen, USA) using Pichia EasyComp™ Kit (Invitrogen, USA). Transformants harbouring HPV L1 genes were selected on Yeast-extract Peptone Dextrose (YPD) plates containing 200 μg/ml Zeocin (Invitrogen, USA). Integration of HPV L1 gene into P. pastoris was PCR verified using promoter specific primers (AOX primers) and the L1 gene specific primers for either HPV16 or HPV18 types. The nucleotide sequences of primers used in the present study were as following.
AOX For: 5′ GACTGGTTCCAATTGACAAGAC 3′;
AOX Rev: 5′GCAAATGGCATTCTGACATCC3′;
16 L1 For: 5′ACTAGAATTCATGTCTTTGTGGTTGCCATCT3′;
16 L1 Rev: 5′ATCACTCGAGTTATTACAATTTTCTCTTCTT3′;
18 L1 For: 5′GAATTCATGGCTTTGTGGAGACCATCT3′; and
18 L1 Rev: 5′CTCGAGTACACTTTCTAGCTCTAAC3′
2.7. Expression and characterization of HPV major capsid protein
P. pastoris transformants containing the HPV L1 genes were grown overnight in a shake-flask containing YPD medium supplemented with 100 μg/ml Zeocin and the cells were transferred into freshly prepared Buffered Minimal Glycerol (BMGH) medium containing 100 μg/ml Zeocin and 0.004% (w/v) L-histidine. After an overnight growth recombinant P. pastoris cells were induced for expression using Buffered Minimal Methanol (BMMH) medium supplemented with 0.004% L-histidine. In order to induce expression of the recombinant protein from P. pastoris, 0.5% (v/v) of methanol was added every 24 h to the culture until 72 h of growth. The cells were harvested after 96 h of growth and stored at −70 °C until further use.
2.8. SDS-PAGE and Western blotting
Either the recombinant P. pastoris cell lysate or enriched HPV L1 protein fractions were resolved on 10% SDS PAGE after heating the samples for 10 min at 75 °C in SDS-PAGE sample loading buffer containing β-Mercaptoethanol [25]. The polyacrylamide gel was stained using Coomassie Blue for visualizing the protein. HPV L1 proteins resolved on SDS-PAGE gel were also transferred to PVDF membrane (GE Healthcare, USA) using semi-dry protein transfer apparatus (Bio-Rad, USA). The membrane was probed using anti-HPV L1 specific monoclonal antibody CamVir-1 (1:500; Bio-Trend GmBH, Germany) [26].
2.9. Stability analysis of recombinant P. pastoris HPV16 and 18 clones
Recombinant P. pastoris HPV16 and 18 clones cultured in 10 ml BMMH media (containing 0.5% methanol) for 24 h at 30 °C in a shaking incubator. The OD600nm of the 24 h culture reached to 12, cells were diluted to 0.5 OD600nm in fresh 10 ml of BMMH media and grown at 30 °C in a shaking incubator for 24 h. Four subsequent subcultures were made, designated as passage number 1 through 4, and the expression of HPVL1 was analyzed on Western blotting using HPVL1 specific monoclonal antibody.
2.10. Purification of HPV16 and HPV18 VLP
Induced cells of the recombinant P. pastoris expressing HPV L1 proteins were harvested and resuspended in harvest buffer (0.2 M MOPS containing 2 mM MgCl2; pH 7.0) and Benzonase Grade-I (Merck, USA) was added to the cell suspension. The cell suspension was incubated for 3 h (at 4 °C), following which the cells were lysed over three passage through the cell disrupter (Constant Cell Disruption systems, UK) at 30,000 psi pressure. Cell-lysate was clarified by centrifuging at 2057 × g for 15 min to remove the cell debris. To the clarified cell lysates, PMSF and NaCl were added to the final concentration of 1 mM and 0.5 M respectively and dialyzed overnight (4 °C) against 0.2 M MOPS containing 0.5 M NaCl (pH 7.0). The dialyzed cell lysate was centrifuged at 130, 357 × g (Hitachi CP 70MX, Japan) for 4 h and the pellet was reconstituted in the harvest buffer. Further, the reconstituted pellets were fractionated on sucrose gradients (10–40%) by centrifuging at 130, 357 × g for 4 h at 4 °C (P28S2 Rotor, Hitachi) [27]. The pellet obtained from the sucrose gradient centrifugation was resuspended in the harvest buffer, which were loaded onto a 2% cross linked agarose bead column (Agarose Bead Technologies, Portugal) pre-equilibrated with DPBS containing 0.8 M NaCl. Ten fractions of 1 ml each were collected separately from the agarose columns loaded with the reconstituted pellet. These fractions were analyzed on SDS-PAGE and Western blotting using HPV L1 specific mouse monoclonal antibody (CamVir-1) for the presence of HPV L1 proteins. The fractions showing high concentration of HPV L1 proteins were further purified using Iodixanol density gradient centrifugation (GE Healthcare, Ireland) as described by Buck et al. [28].
2.11. Pseudovirus preparation
HPV pseudovirus used to evaluate the presence of HPV neutralizing antibody were produced in HEK 293 FT cells by transient co-transfection of plasmids encoding the papillomavirus structural genes, L1 and L2 (psheLL plasmids), together with a reporter plasmid (secretory alkaline phosphatase, a.k.a. SEAP). The size of the reporter gene construct was similar to the HPV genome; hence this vector is also referred to as a pseudogenome. Method for preparing the pseudovirus is described by Buck et al. [28]. Briefly, HEK 293 FT cells were co-transfected with either p16sheLL or p18sheLL constructs (coding for HPV16 or HPV18 capsid proteins L1 and L2 respectively) along with the reporter gene pYSEAP using lipofectamine 2000 (Invitrogen, USA), as per the manufacturer’s instructions. Cells were harvested and the pseudoviruses (PsV) were released from HEK 293 FT cells using detergent Brij-58 (Sigma, USA) and matured by overnight incubation at 37 °C. The PsV preparations were fractionated on Iodixanol density gradient. For this purpose DPBS containing 0.8 M NaCl were used to dilute 65.2% (w/v) the Iodixanol stock solution to get a final concentration of 27%, 33%, and 39%.
Density gradient was created by overlaying (39%, 33% and 27%) Iodixanol step wise in approximately 1.4 ml aliquots. Clarified cell lysate was overlaid onto the iodixanol gradient and centrifuged at 242,700 × g for 4 h at 22 °C (P50S2 Rotor, Hitachi). The samples were collected in 500 μl fractions, which were analyzed on SDS-PAGE. Fractions showing high intensity bands at ~56 kDa were pooled and analyzed for the presence of DNA. Psuedoviruses were aliquoted and frozen at −80 °C until further use.
2.12. Protein estimation and VLPs quantification
Protein concentration was estimated using Micro-BCA kit (Sigma) as per the manufacturer’s instruction. Enriched VLPs were quantified by VLP ELISA using a preparation of ‘calibrator VLPs’ having a concentration of 1 mg/ml produced in mammalian HEK 293 FT cells, as previously described [27]. Concentration of the purified VLPs was determined by comparing the slope obtained for calibrator VLPs with the slope obtained for the VLPs purified from P. pastoris.
2.13. ELISA
The HPV16 or 18 VLPs (100 ng/well) were coated separately into Maxisorp™ 96 well microtiter plates (Nunc, Denmark) in PBS and incubated overnight at 4 °C. The remaining active surface in the wells were blocked using 2% (w/v) skimmed milk solution in PBS containing 0.05% (v/v) Tween-20 (PBST), the plates were incubated for 2 h at 37 °C. The ELISA plates were washed thrice with PBST using automated plate washing machine (PW40 Bio-Rad, USA). A HPV16 L1 specific monoclonal antibody CamVir-1, which recognizes a linear epitope common to many HPVs was added (1:500) and the plates were incubated at 37 °C for 1 h. After washing thrice with PBST, sheep anti-mouse HRPO conjugate (Sigma, USA) was added at 1:5000 dilutions, following which the plates were incubated further at 37 °C for 1 h. The ELISA reaction was developed using H2O2 (0.03% v/v) as substrate and 3-3′-5-5′-Tetramethylbenzidine (TMB, Sigma–Aldrich, USA) as chromogenic indicator. Similarly, proper folding of HPV16 or 18 VLPs was verified using conformation specific monoclonal antibodies H16.V5 and H18.G10 for HPV16 and HPV18 respectively [29].
2.14. Transmission electron microscopy of HPV16 or 18 VLPs
Purified HPV16 and 18 VLPs were adsorbed on carbon coated copper grids and negatively stained with 2% uranyl acetate. Grids were air dried prior to examination under transmission electron microscope (Hitachi, H-7500). Samples were viewed either at 53,000× or 50,000× magnification.
2.15. Immunogenicity studies in mice
Enriched HPV16 VLPs were adsorbed onto aluminium hydroxide gel (pH 7.3) in three different concentrations viz. 2 μg, 10 μg and 20 μg per dose. Similarly, purified HPV18 VLPs were adsorbed onto aluminium hydroxide gel (pH 7.3) in three different concentrations viz. 1 μg, 3 μg and 5 μg. The adsorbed VLPs were administered intramuscularly into 4–6 weeks old female BALB/c mice, seven mice were used in each group. Two booster doses were administered on 14th and 28th days post primary immunization (Table 1). Serum samples were collected on the 35th day post primary immunization and the presence of HPV VLP specific antibodies were analyzed using enzyme immune assay. Similarly, 2 μg of HPV16 and 1 μg of 18 VLPs were co-adsorbed onto aluminium hydroxide gel. Aluminium hydroxide gel was used as placebo in this study. The experimental details are given in Table 2.
Table 1.
Dosage and immunization schedule of female BALB/c mice immunized with monovalent formulation of either HPV16 or HPV18 VLPs expressed in Pichia pastoris. Last column shows the geometric mean titer of serum antibody in immunized mice. Range of antibody titers in each group is mentioned in parenthesis.
| Group | Treatment (VLP) | Dose
|
No of mice | Immunization days | Bleeding days | GMT Serum antibody (range) | |
|---|---|---|---|---|---|---|---|
| Quantity (in μg) | Number of doses | ||||||
| 1 | HPV16 | 2 | 3 | 7 | 0, 14 and 28 | 0, 14, 28 and 35 | 64,000 (16,000–128,000) |
| 2 | HPV16 | 10 | 3 | 7 | 0, 14 and 28 | 0, 14, 28 and 35 | 47,551 (8000–256,000) |
| 3 | HPV16 | 20 | 3 | 7 | 0, 14 and 28 | 0, 14, 28 and 35 | 105,002 (64,000–256,000) |
| 4 | HPV18 | 1 | 3 | 7 | 0, 14 and 28 | 0, 14, 28 and 35 | 78,016 (16,000–256,000) |
| 5 | HPV18 | 3 | 2 | 7 | 0 and 28 | 0, 14, 28 and 35 | 172,275 (128,000–256,000) |
| 6 | HPV18 | 5 | 3 | 7 | 0, 14 and 28 | 0, 14, 28 and 35 | 95,103 (32,000–256,000) |
| 7 | Placebo | Al-Hydroxide | 3 | 7 | 0, 14 and 28 | 0, 14, 28 and 35 | 0 |
Table 2.
Dosage and immunization schedule of female BALB/c mice immunized with bivalent formulation of HPV16 and HPV18 VLPs expressed in Pichia pastoris. Last column shows the geometric mean titers of 35th and 190th day serum antibody in immunized mice. Range of antibody titers in each group is mentioned in parenthesis.
| Group | Treatment | Dosage (μg) | No of mice | Immunization days | Bleeding days | GMT Serum antibody (range)
|
|||
|---|---|---|---|---|---|---|---|---|---|
| Against 16 VLP
|
Against 18 VLP
|
||||||||
| 35th day | 190th day | 35th day | 190th day | ||||||
| 1 | Bivalent (HPV16 and 18 VLP) vaccine | HPV16 VLP – 2 μg and HPV18 VLP – 1 μg | 7 | 0, 14 and 28 | 0, 14, 28, 35 and 190 | 5796 (800–25,600) | 317 (100–800) | 2625 (1600–25,600) | 400 (100–1600) |
| 2 | Placebo | Aluminium Hydroxide | 7 | 0, 14 and 28 | 0, 14, 28, 35 and 190 | 0 | 0 | 0 | 0 |
The serum antibody titer was determined as the Geometric Mean of the maximum serum dilution of all immunized animals that gave OD450nm value above the cut-off; standard deviation for the group was enumerated. The cut-off value was determined by adding the Geometric mean of OD450nm value of pre-immune mice sera to 3 times the standard deviation in the OD450nm value.
2.16. Pseudovirus neutralization assay
The HPV16 and HPV18 pseudovirus were prepared by transiently expressing HPV16 or HPV18 L1–L2 constructs in HEK-293FT (as described in Section 2.11). Secretory alkaline phosphatase activity was detected by Great Escape SEAP Chemiluminescence Kit 2.0™ (Clonetech, USA) [28]. Decrease in secretory alkaline phosphatase activity indicated inhibition of Pseudovirus infecting 293FT cells.
2.17. Statistical analysis
Geometric mean titers were compared to evaluate Immune and neutralizing responses. Graphs and statistical analysis of data were performed using Origin Pro 7.5 software (Origin Lab Corporation, USA).
3. Results
3.1. Construction of recombinant P. pastoris expressing the major capsid protein of HPV16 and HPV18
Human papillomavirus 16 L1 and 18 L1 genes codon optimized for expression in P. pastoris were cloned separately into P. pastoris expression vector pPICZB. The cloning strategy is detailed in Fig. 1 Cloning of the HPV L1 genes into pPICZB was verified by restriction enzyme analysis and sequencing. Presences of HPV16 or HPV18 L1 genes in the recombinant P. pastoris were confirmed using PCR (data not shown). One P. pastoris clone each for HPV16 and HPV18 L1 was selected based on their stable expression for further studies.
Fig. 1.
Schematic diagram of HPV16 and HPV18 constructs. The filled boxes represent HPV16 or HPV18 L1 genes. Internal restriction enzyme sites are marked with nucleotide position in parenthesis. Both HPV16 and HPV18 L1 genes are cloned downstream of AOX promoter using EcoRI and XhoI enzymes.
3.2. SDS-PAGE and Western blotting
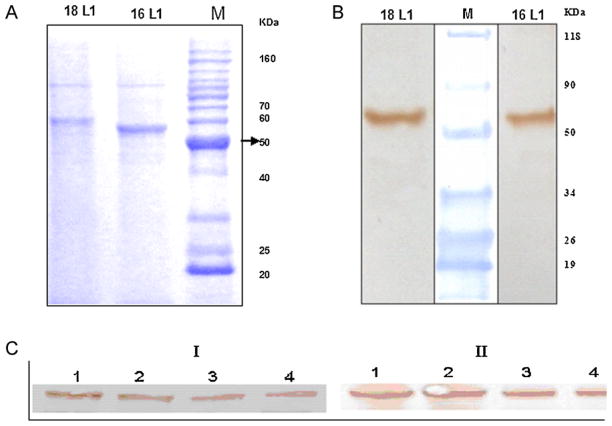
HPV16 and 18 VLPs were purified as described in Section 2. The purified VLP fractions were resolved on SDS-PAGE and stained using Coomassie brilliant blue. The gel showed protein band of approximately 56 kDa in elution fractions. The observed molecular weight of the enriched HPV18 L1 is higher than that of HPV16 L1. This discrepancy may be due to the amino acid composition and/or the theoretical mass of HPV18 L1 being higher than that of HPV16 L1 (Fig. 2A). The purified protein samples were also analyzed using Western blot, which was probed with HPV L1 specific monoclonal antibody. Protein band of approximately 56 kDa Molecular weight was observed on the blot, confirming the presence of HPV major capsid proteins L1 (Fig. 2B).
Fig. 2.
SDS-PAGE and Western Blot analysis of Pichia pastoris expressed HPV16 and HPV18 major capsid protein L1. Purified HPV16 and 18 L1 resolved on SDS PAGE (4–20% Gradient pre-cast gel; Pierce) Stained with Coomassie Brilliant Blue. The HPV16 and 18 L1 protein bands were seen at ~56 kDa. Western analysis demonstrating specific reactivity of Pichia pastoris expressed HPV16 and 18 L1 with anti-HPV L1 monoclonal antibody CamVir-1 (1:1000 Dilutions) showing protein band at ~56 kDa. Expression analysis of Pichia HPV16 and 18 clones by Western blotting. Crude cell lysate was probed using HPVL1 specific monoclonal antibody (CAMVIR1; 1:1000 dilution), the blot was developed using 3,3′-Diaminobenzidine. I. Pichia HPV16 L1 clone. Lanes 1–4 contain crude cell lysate of Pichia pastoris clones at passage I, II, III and IV respectively. L1 protein band in all passages levels show very similar density. II. Pichia HPV18 L1 clone. Lanes 1–4 contain crude cell lysate of Pichia pastoris clones at passage I, II, III and IV respectively. HPV L1 protein band in all passage levels show similar density.
3.3. Stability analysis of recombinant P. pastoris HPV16 and 18 clones
Stability of the recombinant P. pastoris HPV16 and 18 clones were assessed based on L1 expression by Western blotting of four consecutive passages of each clone. Further these blots were subjected to densitometry analysis and the results indicated that these clones were stable in all four consecutive passages (Fig. 2C and Supplementary Fig. 1).
3.4. Transmission electron microscopy of P. pastoris expressed HPV16 and 18 VLPs
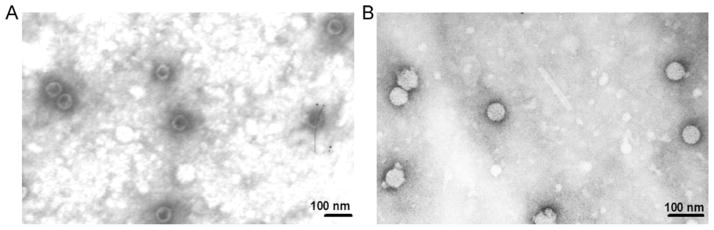
The purified VLPs of HPV16 and HPV18 were examined under transmission electron microscope at magnification of 53,000× and 50,000× respectively. Particles of diameter of ~53 nm were observed in both HPV16 and HPV18 purified protein samples (Fig. 3A and B).
Fig. 3.
Transmission electron microscopy of purified HPV VLPs. A. Transmission electron microscopy of purified HPV16 VLPs (magnification 53,000× and bar100 nm). B. Transmission electron microscopy of purified HPV18 VLPs (magnification 50,000× and bar100 nm).
3.5. Reactivity to conformation specific antibody in ELISA
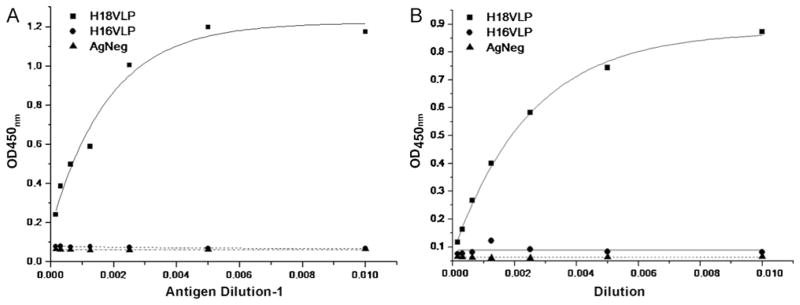
Purified HPV16 and 18 VLPs were coated separately on 96 well Maxisorp ELISA plates. The VLPs reacted to conformation specific monoclonal antibodies i.e.; H16.V5 and H18.G10 specific for HPV16 and 18 respectively (Fig. 4A and B). The purified VLPs showed high type-specific reactivity but no cross reactivity.
Fig. 4.
EIA activity of purified HPV16 and 18 VLPs. A. Reactivity of Pichia pastoris expressed HPV16 VLPs with mouse monoclonal antibody H16.V5 specific to the HPV16 VLPs. The antibody did not cross react with HPV18 VLPs. The lysate of Naïve Pichia pastoris was used as negative control B. Reactivity of Pichia pastoris expressed HPV18 VLPs with mouse monoclonal antibody H18.G10 specific to the HPV18 VLPs. The antibody did not cross react with HPV16 VLPs. The lysate of Naïve Pichia pastoris was used as negative control.
Using this ELISA format and calibrator VLPs having a concentration of 1 mg/ml, yield of the purified HPV16 and 18 VLPs were estimated. The estimated yields of HPV16 VLP and HPV18 VLPs were 9.5 mg/l and 6.4 mg/l respectively.
3.6. Immune response to the HPV16 or HPV18 VLPs
Mice immunized with three doses of VLPs adsorbed onto aluminium hydroxide showed high serum IgG response against P. pastoris expressed HPV VLPs. The geometric mean titers (GMT) of serum antibody for HPV16 VLP after 35th day post primary immunization in mice administered with 2 μg, 10 μg and 20 μg of HPV16 VLP are mentioned in Table 1. Mice immunized with 2 μg VLPs gave GMT of 64,000 (±48,000; titers range from 16,000 to 128,000), the GMT in mice immunized with 10 μg was found to be 47,551 (±55,232; titers range from 8000 to 256,000) and GMT in mice immunized with 20 μg VLPs was 105,002 (±90,509; titers range from 64,000 to 256,000). A group of mice (Group 5; Table 1) were administered only two dose of HPV18 VLPs to evaluate sero-conversion potential. We evaluated the immune efficacy between two dose and three dose in mice and further extensive studies may play the significant role in future commercial vaccine regimen. We chose to have higher antigenic payload of 3 μg/mice for this group. The GMT serum antibody titers after 35th day post primary immunization in mice administered with HPV18 VLP having concentration 1 μg, 3 μg and 5 μg and the results were given Table 1. The serum antibody GMT of mice immunized with 1 μg found to be 78,016 (±101,493; titers range from 16,000 to 256,000), the GMT in mice immunized with 3 μg found to be 172,275 (±68,418; titers range from 128,000 to 256,000) and GMT in mice immunized with 5 μg of HPV18 VLP was 95,103 (±112,163; titers range from 32,000 to 256,000).
3.7. Immune response in mice co-administered with HPV16 and HPV18 VLPs
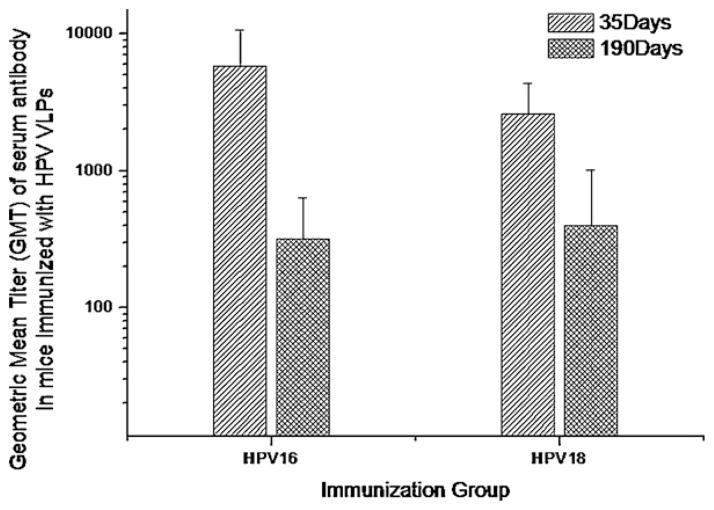
Purified HPV16 and HPV18 VLPs were co-adsorbed onto aluminium hydroxide gel and administered to mice as described in Table 2. Geometric mean titer of serum antibody on 35th day post primary immunization in mice against HPV16 VLP was 5796 (±4916), while the GMT against HPV18 VLP was 2625 (±1752). All animals were maintained for 190 days to assess the longevity of anti-HPV antibody titers in serum. Serum samples drawn on 190th day post primary immunization had serum antibody titer of 317 (±314) against HPV16 VLP, while the antibody titer against HPV18 VLP was 400 (±609) (Fig. 5). These results indicated that the VLPs expressed in P. pastoris were able to elicit long lasting serum antibody titers in mice.
Fig. 5.
Longevity of immune response in mice co-administered with HPV16 and HPV18 VLPs formulation made from HPV VLPs expressed in Pichia pastoris. Geometric mean titer (GMT) of serum antibody (IgG) titers against HPV16 and 18 VLPs in BALB/c mice co-administered with HPV16 and HPV18 VLPs formulation group. Error bar indicates ±SD.
3.8. Pseudovirus neutralization assay
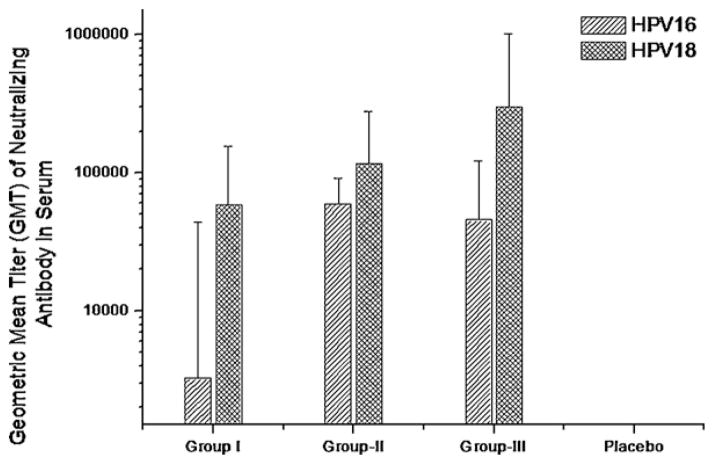
The presence of neutralizing antibodies is crucial in preventing HPV infection by VLP vaccines. The neutralizing antibody titers were measured in an in vitro pseudovirus neutralization assays. Geometric mean titers (GMT) of serum neutralizing antibody resulting in >50% inhibition of secretory alkaline phosphatase activity on 35th day post primary immunization in mice administered with 2 μg HPV16 VLP was estimated to be 3263 (±40,337; antibody titer range 59.85–63,345). Similarly, neutralizing antibody titer in mice administered with 10 μg of HPV16 VLP was 59,599 (±32,261; antibody titer range 36,603–107,812) while mice administered with 20 μg HPV16 VLP had the neutralizing antibody titer of 45,556 (±75,834; antibody titer range 3809–211,596) and HPV16 placebo group titer was 38 (±2.37; antibody titer range 35.7–40.28) (Fig. 6).
Fig. 6.
Measurement of virus specific neutralizing antibody titer in mice sera immunized with monovalent vaccine formulated using Pichia pastoris expressed HPV16 VLPs or HPV18 VLPs. Geometric mean titer (GMT) of neutralizing serum antibody of mice immunized with various antigenic payloads of HPV16 or 18 VLP produced in Pichia pastoris. Neutralization titer is reported as maximum sera dilution resulting in >50% inhibition of SEAP activity. Group I of HPV16 is 2 μg and HPV18 is 1 μg; Group II of HPV16 is 10 μg and HPV18 is 3 μg; Group III of HPV16 indicate 20 μg and HPV18 is 5 μg – 16pla: HPV16 Placebo group; 18pla: HPV18 placebo group.
The Geometric mean titers (GMT) of serum neutralizing antibody on 35th day post primary immunization of mice immunized with 1 μg of HPV18 VLP was 58,550.52 (±97,801; antibody titer range 17,876–263,430). Neutralizing antibody titer of 116,181 (±163,130; antibody titer range 41,968–475,022) was estimated for mice immunized with 3 μg of HPV18 VLP, while mice administered with 5 μg of HPV18 VLP had the neutralizing antibody titer of 302,010 (±720,245; antibody titer range 47,088–19,760,000) and HPV18 placebo group titer was 40 (antibody titer range <40) (Fig. 6).
4. Discussion
Prophylactic vaccines hold promise in cervical cancer management, but the costs of the available vaccines are beyond the reach of most people in the low resource developing countries, where the disease burden is high [30,31]. Establishment of regional manufacturing capabilities could improve vaccine availability at an affordable cost [32].
Analysis of patents in prophylactic HPV vaccine developments suggests that intellectual property rights may not be an impediment in manufacturing of the first generation HPV L1-VLP based vaccine if manufacturers choose different formulations or strains [33].
Yeasts with the heterologous gene integrated into the chromosome provide a stable alternative for recombinant gene expression. Integration of genes for expression under the Alcohol oxidase promoter is known to induce high level of expression in P. pastoris [34].
In the present study, we assessed the potential of P. pastoris as a platform to express the recombinant HPV16 and 18 VLPs. We report the purification and characterization of HPV16 and 18 VLPs, whose genes were cloned and expressed in P. pastoris strain GS115. The codon optimized expression of HPV16 L1 in P. pastoris have been reported earlier [22,23]. In this study, a stable clone of P. pastoris that express HPV16 L1 and another expressing HPV18 L1 major capsid protein were generated. Stability of these clones was assessed by L1 expression up to four consecutive passages using western blot analysis and densitometry. All passages showed similar density of L1 protein indicating that clones were stable. The size of the expressed HPV16 and 18 L1 proteins on SDS-PAGE and western blots were of ~56 kDa. We noticed in our studies that conventional method of sample preparation for electrophoresis where samples are denatured by boiling at 95 °C resulted in the L1 specific band that subscribed to a much higher size (~90 kDa) than the expected ~56 kDa (Supplementary Fig. 2). The tendency of L1 proteins to multimerize upon boiling has been suggested in previous reports therefore samples were processed for electrophoresis at 75 °C. The P. pastoris expressed HPV16 and 18 L1 proteins self assembled into VLPs, which were purified by size exclusion chromatography followed by gradient ultra-centrifugation. Our purification methods yields were 9.5 mg/l and 6.4 mg/l of HPV16 and HPV18 VLPs respectively. There is need for improving the VLP yields significantly by optimizing the production and purification procedures, which will make the production platform more viable for commercial exploitation.
P. pastoris expressed VLPs were visualized under TEM to determine correct folding and conformation specific monoclonal antibodies were analyzed using enzyme immune assay. Variability in the VLP size was previously reported [35]. The purified VLPs of HPV16 and 18 VLPs showed variable particle size having a mean of approximately ~53 nm. The HPV16 and 18 VLPs were also assessed for their immunological efficacy in eliciting neutralizing antibody response in mice. These VLPs also showed specific reactivity to the mouse monoclonal antibodies specific to the conformation of HPV16 and 18 VLPs (Fig. 4A and B); neither HPV16 nor HPV18 VLPs cross reacted with the monoclonal antibodies specific to the conformation of the other HPV types.
The purified HPV16 and 18 VLPs were adsorbed onto aluminium hydroxide gel and administered to mice either singly or in combination. Taking a clue from our observation of the high immunogenicity with HPV18 VLP in mice, where the VLPs were found more immunogenic (data not shown), we decided to use lower the dose of HPV18 VLP compared to the dose of HPV16 VLP in bivalent composition. Mice immunized with these formulations elicited high serum IgG response against each VLPs. Mice immunized with HPV16 VLP showed some dose concentration dependent response and the mice immunized with 20 μg of HPV16 VLP showed greater than a 4 log serum IgG titer. Mice immunized with HPV18 VLP did not show any dose dependency, and even the mice immunized with 1 μg VLPs were able to generate similar serum antibody titers as higher concentration VLP groups, indicating that the HPV18 VLP preparations were highly immunogenic.
A group of mice received 3 μg doses of HPV18 VLP. This group was used to evaluate whether one booster immunization was sufficient to elicit high titers. The results indicate that mice receiving just one booster generated equally strong antibody responses. Administration of bivalent vaccine containing of HPV16 and 18 VLPs in Balb/c mice elicited high serum IgG titers, which remained high even on 190 days post primary immunization. This indicates that the P. pastoris expressed VLPs induce durable antibody responses. The serum antibody titers in mice obtained for VLPs administered singly were significantly higher when compared to VLPs administered in combination. A detailed study is required to determine immunological competition between the antigens in mice.
Expression and purification of HPV16 VLP have been documented earlier [21,22]. Bazan et al. used heamagglutination inhibition (HAI) assay to demonstrate the presence of neutralizing antibody in HPV16 VLP immunized mice. The HAI assay lacks sensitivity and can measure only a subset of HPV neutralizing antibody. Hence, the use of HAI assay for quantification of HPV antibodies may not be considered as reliable method. We have employed an in vitro assay that is designated “Gold Standard” by WHO advisory group [36]. In the work detailed in the present manuscript, we have quantified the neutralizing antibody response in this well validated and sensitive assay, which is significant step forward in the validation of the P. pastoris expression platform as a proof-of-concept for producing HPV VLPs.
In order to efficiently prevent the onset of virus infection from homologous HPV type, it is desired that the vaccine elicit type specific neutralizing antibody response. These were analyzed using in vitro HPV pseudovirus neutralization assay. We noted that the pseudovirus neutralization titers elicited by HPV18 VLPs were higher than those elicited by HPV16 VLPs. This trend was similar to those obtained for VLP binding antibody titers. Interestingly, for S. cerevisiae-derived VLPs, VLP binding antibody titers induced HPV18 VLPs appear to be lower than those induced by HPV16 VLPs [37].
5. Conclusion
The immunogenicity studies in mice described in this manuscript suggest P. pastoris based HPV16 and 18 VLPs may be effective prophylactic vaccines. In order to address the region specific distribution of high-risk HPV types in women, vaccine containing additional HPV types prevalent in specific geographical region is desirable. A similar strategy, as outlined in this manuscript, for preparing recombinant HPV VLPs may be followed to create an effective multivalent vaccine to address the cervical cancer burden in specific geographical regions.
Supplementary Material
Acknowledgments
Authors wish to thank Dr. Ravindra Sisodiya and Mr. N.M. Ponnanna for their help in the animal experiments. The efforts in maintaining the cell-line by Dr. R. Ramya, Mr. V. Siva Kumar and Mr. Bala Obulapathi are also acknowledged.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.vaccine.2011.07.071.
References
- 1.Human papillomavirus and related cancers in India. WHO/ICO Information Centre on HPV and Cervical Cancer (HPV Information Centre) Summary Report. 2010:4–5.
- 2.Parkin DM, Louie KS, Clifford G. Burden and trends of type-specific human papillomavirus infections and related diseases in the Asia Pacific region. Vaccine. 2008;S26:M1–16. doi: 10.1016/j.vaccine.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Human papillomaviruses. Vol. 90. Lyon: IARC Press; 2007. IARC monographs on the evaluation of carcinogenic risks to humans; pp. 179–327. [PMC free article] [PubMed] [Google Scholar]
- 4.Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. 2003;16:1–17. doi: 10.1128/CMR.16.1.1-17.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Villier EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. 2004;324:17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 6.Bosch FX, de Sanjose S. Human papillomavirus and cervical cancer—burden and assessment of causality. J Natl Cancer Inst Monogr. 2003;31:3–13. doi: 10.1093/oxfordjournals.jncimonographs.a003479. [DOI] [PubMed] [Google Scholar]
- 7.Sankaranarayanan R, Bhatla N, Gravitt PE, Basu P, Esmy PO, Ashrafunnessa KS, et al. Human papillomavirus infection and cervical cancer prevention in India, Bangladesh, Sri Lanka and Nepal. Vaccine. 2008;26(Suppl 12):43–52. doi: 10.1016/j.vaccine.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Bharadwaja M, Hussain S, Nasare V, Das BC. HPV & HPV vaccination: issues in developing countries. Indian J Med Res. 2009;130:327–33. [PubMed] [Google Scholar]
- 9.Hakama M, Miller AB, Day NE. Screening for cancer of the uterine cervix IARC working group on cervical cancer screening. IARC Lyon: IARC Press; 1986. pp. 133–44. [Google Scholar]
- 10.Sankaranarayanan R, Esmy PO, Rajkumar R, Muwonge R, Swaminathan R, Shanthakumari S. Effect of visual screening on cervical cancer incidence and mortality in Tamil Nadu, India: a cluster-randomized trial. Lancet. 2007;370:398–406. doi: 10.1016/S0140-6736(07)61195-7. [DOI] [PubMed] [Google Scholar]
- 11.Schiller JT, Davies P. Delivering on the promise: HPV vaccines and cervical cancer. Nat Rev Microbiol. 2004;2(4):343–7. doi: 10.1038/nrmicro867. [DOI] [PubMed] [Google Scholar]
- 12.Villa LL. Prophylactic HPV vaccines: reducing the burden of HPV-related diseases. Vaccine. 2006;24(Suppl 1):S23–8. doi: 10.1016/j.vaccine.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Kirnbauer R, Booy F, Chang N, Lowy DR, Schiller JT. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci U S A. 1992;89:12180–4. doi: 10.1073/pnas.89.24.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou J, Sun XY, Stenzel DJ, Frazer IH. Expression of vaccinia recombinant HPV 16 L1 and L2 ORF proteins in epithelial cells is sufficient for assembly of HPV virion-like particles. Virology. 1991;185(1):251–7. doi: 10.1016/0042-6822(91)90772-4. [DOI] [PubMed] [Google Scholar]
- 15.Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martinus CM, et al. Sustained efficacy up to 4. 5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow up from a randomized control trial. Lancet. 2006;367:1247–55. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- 16.Schiller JT, Castellsauge X, Villa LL, Hildesheim A. An update of prophylactic human papillomavirus L1 virus-like-particles vaccine clinical trial results. Vaccine. 2008;26:53–61. doi: 10.1016/j.vaccine.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928–43. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 18.Basu P, Chowdhury D. Cervical cancer screening & HPV vaccination: a comprehensive approach to cervical cancer control. Indian J Med Res. 2009;130:241–6. [PubMed] [Google Scholar]
- 19.Bimelt S, Sonnewald U, Galmbachar P, Willmitzer L, Muller M. Production of human papillomaviruses type 16 virus-like particles in transgenic plants. J Virol. 2003;77:9211–20. doi: 10.1128/JVI.77.17.9211-9220.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varsani A, Williamson AL, Rose RC, Jaffer M, Rybicki EP. Expression of human papillomavirus type 16 major capsid protein in transgenic Nicotiana tabacum cv.Xanthi. Arch Virol. 2003;148:1771–86. doi: 10.1007/s00705-003-0119-4. [DOI] [PubMed] [Google Scholar]
- 21.Liu DW, Zhang Y, Yu XH, Jiang C, Chen Y, Wu Y, et al. Assembly and immunogenicity of human papillomavirus type 16 major capsid protein (HPV16 L1) in Pichia pastoris. Chem Res Chin Univ. 2007;23(2):200–3. [Google Scholar]
- 22.Bazan SB, de Alencar Muniz Chaves A, Aires KA, Cianciarullo AM, Garcea RL, Ho PL. Expression and characterization of HPV-16 L1 capsid protein in Pichia pastoris. Arch Virol. 2009;154(10):1609–17. doi: 10.1007/s00705-009-0484-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel MC, Patkar KK, Basu A, Mohandas KM, Mukhopadhyaya R. Production of immunogenic human papillomavirus-16 major capsid protein derived virus like particles. Indian J Med Res. 2009;130:213–8. [PubMed] [Google Scholar]
- 24.Inglis S, Shaw A, Koenig S. HPV vaccines: commercial research & development. Vaccine. 2006;24(Suppl 3):S99–105. doi: 10.1016/j.vaccine.2006.05.119. [DOI] [PubMed] [Google Scholar]
- 25.Cook JC, Joyce JG, George HA, Schultz LD, Hurni WM, Jansen KU, et al. Purification of virus-like particles of recombinant human papillomavirus type 11 major capsid protein L1 from Saccharomyces cerevisiae. Protein Expr Purif. 1999;17:477–84. doi: 10.1006/prep.1999.1155. [DOI] [PubMed] [Google Scholar]
- 26.Zhou J, Sun XY, Davies H, Crawford L, Park D, Frazer IH. Definition of linear antigenic regions of the HPV 16 L1 capsid protein using synthetic virion-like particles. Virology. 1992;189:592–9. doi: 10.1016/0042-6822(92)90582-a. [DOI] [PubMed] [Google Scholar]
- 27.Nardelli-Haefliger D, Roden RB, Benyacoub J, Sahli R, Kraehenbuhl JP, Schiller JT, et al. Human papillomavirus type 16 virus-like particles expressed in attenuated Salmonella typhimurium elicit mucosal and systemic neutralizing antibodies in mice. Infect Immun. 1997;65(8):3328–36. doi: 10.1128/iai.65.8.3328-3336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buck CB, Pastrana DV, Lowy DR, Schiller JT. Generation of HPV pseudovirions using transfection and their use in neutralization assays. Methods Mol Med. 2005;119:445–62. doi: 10.1385/1-59259-982-6:445. [DOI] [PubMed] [Google Scholar]
- 29.Christensen ND, Dillner J, Eklund C, Carter JJ, Wipf GC, Reed CA, et al. Surface conformational and linear epitopes on HPV-16 and HPV-18 L1 virus-like particles as defined by monoclonal antibodies. Virology. 1996;223:174–84. doi: 10.1006/viro.1996.0466. [DOI] [PubMed] [Google Scholar]
- 30.Ferlay J, Bray F, Pisani P, Parkin DM. Cancer incidence, mortality and prevalence worldwide. Lyon: IARC Press; 2004. IARC Cancer Base No. 5. version 2.0. [Google Scholar]
- 31.Sankaranarayanan R. Overview of cervical cancer in the developing world FIGO 6th Annual report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S205–10. doi: 10.1016/S0020-7292(06)60035-0. [DOI] [PubMed] [Google Scholar]
- 32.Sankaranarayanan R. HPV vaccination: the promise & problems. Indian J Med Res. 2009;130:322–6. [PubMed] [Google Scholar]
- 33.Padmanabhan S, Amin T, Sampat B, Cook-Deegan R, Chandrasekharan S. Intellectual property, technology transfer and manufacture of low-cost HPV vaccines in India. Nat Biotechnol. 2010;28:671–8. doi: 10.1038/nbt0710-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cregg JM, Cereghino JI, Shi J, Higgins DR. Recombinant protein expression in Pichia pastoris. Mol Biotechnol. 2000;16:23–52. doi: 10.1385/MB:16:1:23. [DOI] [PubMed] [Google Scholar]
- 35.Hu Y, Zandi R, Anavitarte A, Knobler CM, Gelbart WM. Packaging of a polymer by a viral capsid: the interplay between polymer length and capsid size. Biophys J. 2008;94:1428–36. doi: 10.1529/biophysj.107.117473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization. Expert Committee on Biological Standardization Guidelines to assure the quality, safety and efficacy of recombinant human papillomavirus virus-like particle vaccines. Geneva: WHO; 2006. http://screening.iarc.fr/doc/WHOvaccineguidelines2006.pdf. [Google Scholar]
- 37.Brown DR, Garland SM, Ferris DG, Joura E, Steben M, James M, et al. The humoral response to Gardasil® over four years as defined by total IgG and competitive Luminex immunoassay. Hum Vaccin. 2011;7(2):230–8. doi: 10.4161/hv.7.2.13948. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.