ABSTRACT
The pituitary gland is composed of hormone-producing cells essential for homeostasis and reproduction. Pituitary cells are sensitive to endocrine feedback in the adult and can have altered hormonal secretion from exposure to the endocrine disruptor bisphenol A (BPA). BPA is a prevalent plasticizer used in food and beverage containers, leading to widespread human exposure. Although prenatal exposure to BPA can impact reproductive function in the adult, the effects of BPA on the developing pituitary are unknown. We hypothesized that prenatal exposure to low doses of BPA impacts gonadotroph cell number or parameters of hormone synthesis. To test this, pregnant mice were administered 0.5 μg/kg/day of BPA, 50 μg/kg/day of BPA, or vehicle beginning on Embryonic Day 10.5. At parturition, pituitaries from female offspring exposed in utero to either dose of BPA had increased proliferation, as assessed by mKi67 mRNA levels and immunohistochemistry. Coincidently, gonadotroph number also increased in treated females. However, we observed a dichotomy between mRNA levels of Lhb and Fshb. Female mice exposed to 0.5 μg/kg/day BPA had increased mRNA levels of gonadotropins and the gonadotropin-receptor hormone (GNRH) receptor (Gnrhr), which mediates GNRH regulation of gonadotropin production and release. In contrast, mice treated with 50 μg/kg/day of BPA had decreased gonadotropin mRNA levels, Gnrhr and Nr5a1, a transcription factor required for gonadotroph differentiation. No other pituitary hormones were altered on the day of birth in response to in utero BPA exposure, and male pituitaries showed no change in the parameters tested. Collectively, these results show that prenatal exposure to BPA affects pituitary gonadotroph development in females.
Keywords: anterior pituitary, bisphenol A (BPA), development, developmental biology, endocrine disruptors, environmental contaminants and toxicants, follicle-stimulating hormone (FSH), gonadotrophs, gonadotropins, luteinizing hormone (LH), pituitary
Developmental exposure to low doses of bisphenol A increases proliferation and alters gonadotroph differentiation in the pituitary.
INTRODUCTION
The pituitary gland develops from an invagination of the oral ectoderm during embryonic development to form the structure known as the Rathke pouch. This structure contains highly proliferative progenitor cells that receive signals from the neighboring neural and oral ectoderm to initiate transcription factor expression in a spatial and temporal manner [1–3]. This process is necessary for differentiation of the distinct hormone-producing cell types [4]. The extent to which cell specification is hardwired, or the ability of these signals to be modified by the in utero environment, is currently unknown.
Gestational exposure to chemicals that mimic or inhibit hormone action, commonly known as endocrine-disrupting chemicals (EDCs), can interfere with the hypothalamic-pituitary-gonadal (HPG) axis and lead to infertility or disease in adults [5]. One of the most abundant EDCs is bisphenol A (BPA), which has been found in 95% of adult human urine samples tested and can accumulate in reproductive organs [6]. The acceptable human intake of BPA is referred to as the oral reference dose (ORfD) and is calculated by the U.S. Environmental Protection Agency to be 50 μg/kg/day [7]. Additionally, BPA has the ability to cross the placental barrier, but the fetus has limited abilities to metabolize it, leading to a high accumulation of BPA in fetal and placental tissue to levels that are equal to or greater than the ORfD [8].
Exposure to BPA has adverse effects on reproductive processes in both male and female rodents. Both embryonic and neonatal exposure to BPA at low doses can result in permanent reductions in fertility and fecundity, accompanied by advanced pubertal onset and abnormal estrous cyclicity [9–11]. Disruption has been documented at all levels of the HPG axis. The sexually dimorphic differences seen in the hypothalamic anteroventral periventricular nucleus (AVPV), critical for the luteinizing hormone (LH) surge, are diminished by BPA exposure [12]. Additionally, low dose in utero exposure can alter the morphology of the uterus, decrease the number of ovarian follicles in females, and decrease testis size and reduce sperm count in males [13–15]. At the level of the pituitary, embryonic or postnatal administration of BPA leads to decreased gonadotropin-receptor hormone (GNRH)-induced LH secretion in adults [10, 16]. Similarly, prepubertal BPA exposure also causes a reduction of serum LH and Lhb mRNA in males and females [17–19]. Although these data suggest that BPA exposure might impact pituitary physiology, nothing is known about the effect of BPA exposure on development of gonadotrophs, the cell type that synthesizes and secretes LH and follicle-stimulating hormone (FSH).
The process of gonadotroph specification requires a sequential activation of transcription factors within the developing pituitary. A key initiating event is the expression of Pitx2 and Gata2 and the absence of Pit1 [20, 21]. For example, in mice with pituitary-specific Gata2 loss of function, gonadotroph number is severely decreased, and the circulating levels of LH and FSH are reduced [22]. Additionally, mice with decreased pituitary expression of Pitx2 have reduced expansion of the Rathke pouch early in development and fail to activate several transcription factors critical for gonadotroph differentiation. This results in diminished or complete absence of Lhb and Fshb depending on the severity of the mutation [20, 23]. Subsequently, the transcription factors Egr1 and Nr5a1 (Sf1) are key components of initiating either Lhb or both Lhb and Fshb transcription, respectively, during development [24–26]. It is currently unknown if in utero exposure to EDCs can impact these initial stages of gonadotroph formation or gonadotroph cell number.
Based on the ability of embryonic exposure to BPA to disrupt HPG axis function and the ability of BPA to accumulate in fetal tissue [8], we hypothesized that prenatal exposure to BPA at or below the ORfD results in changes in pituitary development, especially in gonadotrophs. The present study was designed to examine the impact of maternal BPA exposure on proliferation and cell specification in the pituitary of male and female offspring at birth.
MATERIALS AND METHODS
Animals
Timed pregnancies of mice on a mixed FVB, C57BL/6 background were generated. The morning after a vaginal plug was observed was designated as e0.5 and the day of parturition was designated as P1. Pregnant females were dosed orally once a day with either 0.5 or 50 μg/kg/day of 2,2-bis(4-hydroxyphenyl)propane-4,4′-isopropylidenediphenol (BPA) dissolved in ethanol and diluted in tocopherol-stripped corn oil or with tocopherol-stripped corn oil alone as a control. Mice were dosed from e10.5 through e18.5. Mice born to treated mothers were euthanized on P1 for experiments. For each treatment group, from six to eight individual pituitaries were examined. Pups were collected from five to seven different litters per treatment group. Mice used for characterization of the onset of prolactin (PRL) expression were from a CD-1 background taken at P1, P5, P10, and 4–6 mo of age, with six biological replicates for each age. All animals were housed in a facility with a 12L:12D photoperiod and fed a standard mouse diet. The University of Illinois at Urbana-Champaign Institutional Animal Care and Use Committee approved all procedures.
Immunohistochemistry
Heads from P1 mice were fixed overnight in 3.7% formaldehyde in PBS (pH 7.2) at 4°C, dehydrated in a graded series of ethanol at room temperature, and embedded in paraffin for immunohistochemistry. Paraffin blocks were sectioned (thickness, 6 μm) and prepared for immunohistochemistry as previously described [27].
Pituitary cell populations were examined with antibodies against LHβ (1:1000, AFP22238790GPOLHB; National Hormone and Pituitary Program [NHPP], National Institute of Diabetes and Digestive and Kidney Diseases), FSHβ (1:1000, 85GP9691bFSHB; NHPP), PRL (1:1000, lot AFP425_10_91; NHPP), and mKi67 (1:100; Dako). Biotin-conjugated secondary antibodies were used (1:250) and amplified with Streptavidin-Cy3 (1:250; Jackson ImmunoResearch Laboratories, Inc.). All experiments contained a slide processed without primary antibody as a control.
For colocalization experiments, blocking and incubation of the primary antibody occurred as above, with mKi67 (1:100; BD Pharmingen), FSHβ (1:1000, 85GP9691bFSHB; NHPP), SOX2 (1:250; Millipore), and PIT1 (1:500; a gift from Dr. Simon Rhodes, Indiana University, Purdue University, Indianapolis). An anti-mouse secondary antibody conjugated to biotin was used with mKi67, whereas anti-rabbit secondary antibody conjugated to Cy3 fluorophore was used with FSHβ, SOX2, and PIT1. Streptavidin-conjugated DyLight 488 fluorophore was used as a tertiary antibody to detect the anti-mouse biotin. All secondary and streptavidin-conjugated antibodies were purchased from Jackson ImmunoResearch and were used at a concentration of 1:200. Controls for these experiments included a slide processed without mKi67 and a slide processed without FSH, PIT1, or SOX2 antibodies.
All slides were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; 1:1000; Sigma) and visualized at 100× and 400× magnification using a Leica DM2560 microscope. Photographs were taken using a Retiga 2000R camera (Q-Imaging) and acquired using Q-Capture Pro software (Q-Imaging). Images were processed using Adobe Photoshop CS2.
Quantitative RT-PCR
Pituitaries isolated from individual P1 mice were stored in RNAlater (Ambion) at −20°C. RNAlater was removed, and RNA was isolated from individual pituitaries using an RNAqueous-Micro Kit (Ambion) as per the manufacturer's protocol. All cDNA was synthesized from 6 μl of RNA using the ProtoScript M-MuLV First Strand cDNA Synthesis Kit (New England Biolabs). A no-enzyme control was also prepared and used as a negative control. Pituitary cDNA was amplified using gene-specific primers (Table 1) and SYBR green mix (Bio-Rad Laboratories) on a Bio-Rad iQ5 real-time PCR machine. Data were analyzed with the standard comparative (ΔΔCt) method. For each sample, the mean Ct for the gene of interest and for the control gene, Gapdh, was calculated as an average of the duplicates of that sample. The ΔCt was calculated by subtracting the mean Gapdh Ct value from the mean gene of interest Ct value. The ΔΔCt was calculated as the difference between the ΔCt between the treatment groups and the vehicle control groups, or the vehicle control groups and the vehicle control groups to give the normalized level of target gene. The relative fold-change of expression was then equaled to 2(-ΔΔCt) for each sample. The error bars in the figures represent the SEM of the relative fold-change for each group. A sample size of from six to eight individual pituitaries, run in duplicate, was used for all groups. Pups were collected from five to seven different litters per treatment group.
TABLE 1.
List of primer sequences.
Cell Quantification
To quantify the number of gonadotrophs present in the pituitary, four animals per treatment group were used. For each animal, a total of five slides was chosen approximately 70 μm apart and spanning the entirety of the pituitary gland (360 μm in total). Each slide contained two sections (thickness, 6 μm). The number of LHβ- or FSHβ-immunopositive cells were counted and divided by the total number of DAPI-positive cells. Cell quantification data are shown as the average percentage of LHβ- or FSHβ-immunopositive cells over the average number of total cells throughout the pituitary for each group.
Statistical Analysis
Statistical significance was determined using ANOVA and subsequent comparisons using a two-tailed t-test in StatPlus (AnalystSoft) and Microsoft Excel, respectively. All P values of less than 0.05 were considered to be statistically significant.
RESULTS
Proliferation in the Pituitary of Females Is Increased Due to In Utero BPA Exposure
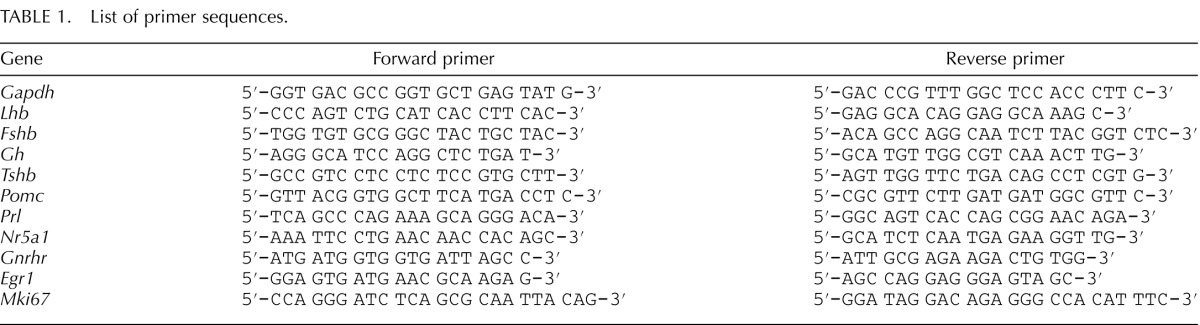
Previous studies analyzing BPA exposure have shown increases in proliferation of a lactotrope pituitary cell line in vitro [28]. Embryonic exposure to BPA has also been shown to cause increased proliferation in endometrial glandular epithelial cells and prostate ductal epithelial cells in vivo [29, 30]. To identify if BPA exposure during embryonic development resulted in altered proliferation in the pituitary, pituitaries were collected from pups exposed to two low doses of BPA, 0.5 and 50 μg/kg/day, in utero. To examine proliferation, we used immunohistochemistry analysis and quantitative RT-PCR (qRT-PCR) to assess the expression of mKi67, which is present in cells that are actively progressing through the cell cycle. mKi67-immunoreactive cell number appeared to be greater in both the 0.5 μg/kg/day dose (Fig. 1, B and E) and the 50 μ/kg/day dose of BPA (Fig. 1, C and F) in females compared to the vehicle control (Fig. 1, A and D). In parallel, mKi67 mRNA levels showed significant increases in females of both treatment groups (Fig. 1J). Surprisingly, mKi67 immunoreactivity did not appear to be altered in males between the vehicle control (Fig. 1G), the 0.5 μg/kg/day dose (Fig. 1H), and the 50 μg/kg/day dose of BPA (Fig. 1I), which is reflected in the comparable mKi67 mRNA levels in all treatment groups (Fig. 1K).
FIG. 1.
BPA exposure increased proliferation in the pituitary of females. The number of mKi67-immunoreactive cells appeared to be greater in the female pituitaries of both the 0.5 μg/kg/day dose (B and E) and the 50 μg/kg/day dose (C and F) of BPA treatment groups compared to the vehicle control (A and D). In males, there appeared to be no difference in mKi67-immunoreactive cells for any condition (G–I). mKi67 mRNA levels were significantly increased in females of both treatment groups (J; P = 0.0005, 0.5 μg/kg/day; P = 0.0375, 50 μg/kg/day. mKi67 mRNA levels in males showed no change compared to the vehicle control group (K). All qRT-PCR values are normalized to the housekeeping gene Gapdh, and values are represented relative to the vehicle control group. Significance in J and K is noted by an asterisk (*P ≤ 0.05, n = 4 [immunohistochemistry], n = 6–8 [qRT-PCR]). AL, anterior lobe; IL, intermediate lobe; PL, posterior lobe. Original magnification ×100 (A–C) and ×400 (D–I); bar = 50 μm (A–I).
Gonadotroph Cell Number Is Increased by In Utero BPA Exposure
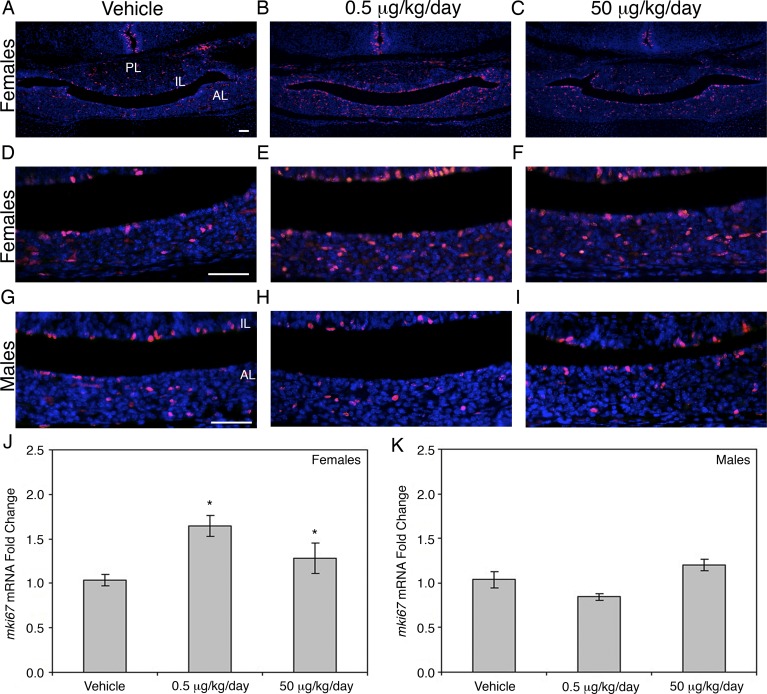
Exposure to BPA during critical developmental periods has been shown to impact gonadotroph function. Therefore, we used immunohistochemistry for LHβ to visualize the effects of in utero exposure to BPA on gonadotroph cell number throughout the pituitary of females. LHβ immunostaining revealed an increase in gonadotroph number in both the 0.5 μg/kg/day (Fig. 2, B and E) and the 50 μg/kg/day BPA treatment groups (Fig. 2, C and F) compared to the vehicle control (Fig. 2, A and D). Quantification of the number of LHβ-immunopositive (Fig. 2G) and FSHβ-immunopositive (Fig. 2H) cells confirmed a significant increase in gonadotroph cell number.
FIG. 2.
LHβ and FSHβ positive cells were increased by in utero BPA exposure. Immunohistochemical detection of LHβ showed an increase in the pituitaries of both the 0.5 μg/kg/day (B and E) and the 50 μg/kg/day (C and F) BPA-treated mice compared to the vehicle control (A and D). LHβ-immunoreactive (G; P = 0.029, 0.5 μg/kg/day; P = 0.049, 50 μg/kg/day and FSHβ-immunoreactive (H; P = 0.020, 0.5 μg/kg/day; P = 0.002, 50 μg/kg/day cells are shown as the average percentage of positive cells compared to total cells in the anterior pituitary. Significance in G and H is noted by an asterisk (*P ≤ 0.05, n = 4). AL, anterior lobe; IL, intermediate lobe; PL, posterior lobe. Original magnification ×100 (A–C) and ×400 (D–F); bar = 50 μm (A–F).
Progenitor Proliferation Appears to Increase in Females Exposed to BPA
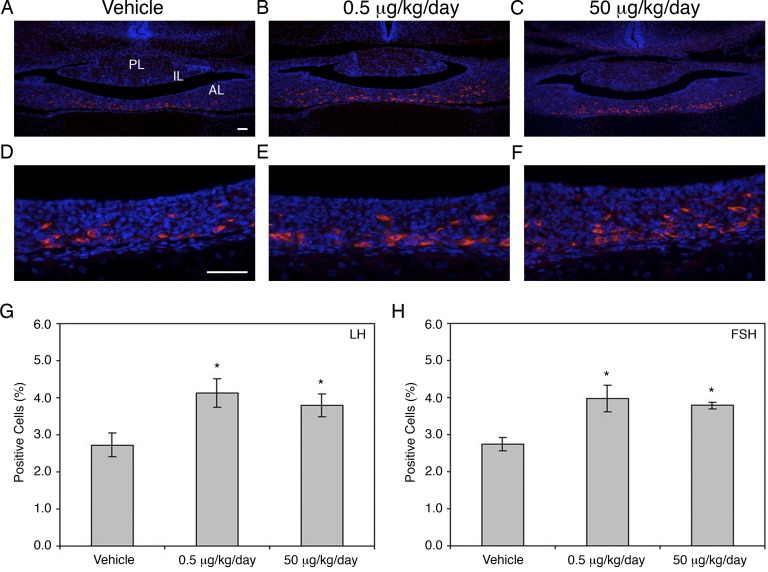
To identify if the increase noted in gonadotrophs was a direct result of an increase in their proliferation, immunohistochemistry was used to double-label mKi67 (nuclear, green) and FSHβ (cytoplasmic, red). Despite an increase of gonadotrophs in both the 0.5 μg/kg/day (Fig. 3B) and the 50 μg/kg/day (Fig. 3C) treatment groups compared to the vehicle control (Fig. 3A, solid arrow; for quantification, see Fig. 2), the majority of proliferating cells were not gonadotropin positive (arrowhead), although a few colabeled cells were detected in all groups (outlined arrow).
FIG. 3.
BPA appeared to increase proliferation in pituitary progenitor cells. Few mKi67 (green) and FSHβ (red) colabeled cells were found in the female vehicle control pituitary (A), which does not appear to be changed by either the 0.5 μg/kg/day dose (B) or the 50 μg/kg/day dose (C) of BPA. Similarly, there appeared to be no difference in mKi67-immunoreactive (green) and PIT1-immunoreactive (red) cells, with a few double-labeled cells detected in all conditions (D–F). Many SOX2-positive progenitor cells in the cleft region (red) were double labeled with mKi67 (green) in both treatment groups (H and I, bracket), compared to only a few in the vehicle control (G, bracket). A representative cell immunoreactive with mKi67 only is denoted with an arrowhead in each panel. Cells immunoreactive only for FSH, PIT1, or SOX2 are shown with solid arrows, and outlined arrows indicate double-labeled cells. AL, anterior lobe; IL, intermediate lobe. n = 4–5. Original magnification ×400; bar = 50 μm (A–I).
The pituitary-specific transcription factor PIT1 is necessary to produce somatotropes, lactotropes, and thyrotropes [21]. To identify if the proliferation of these PIT1-positive cell types was altered by BPA exposure, immunohistochemistry was used to double-label mKi67 (nuclear, green) and PIT1 (nuclear, red). Several double-labeled cells were detected (outlined arrow) in the control (Fig. 3D), 0.5 μg/kg/day (Fig. 3E), and the 50 μg/kg/day (Fig. 3F) treatment groups; however, the majority of the proliferating cells (arrowhead) were not PIT1 positive (solid arrow).
The well-characterized transcription factor SOX2 is essential for stem cell self-renewal and progenitor maintenance. In the pituitary, progenitor cells containing SOX2 are located in abundance near the cleft, lining the intermediate lobe of the pituitary and scattered throughout the anterior lobe [31, 32]. Because the localization of the proliferating cells in mice treated with BPA appeared similar to SOX2-containing cells, immunohistochemistry was used to determine if the amount of proliferating SOX2 cells increased. Cells were double labeled using mKi67 (nuclear, green) and SOX2 (nuclear, red). In the vehicle control group (Fig. 3G), although some SOX2-positive cells were colocalized with mKi67 (outlined arrow), many SOX2 cells were not proliferating (solid arrow). In both the 0.5 μg/kg/day (Fig. 3H) and the 50 μg/kg/day (Fig. 3I) treatment groups, the number of mKi67-positive cells appeared to be increased (arrowhead), and many of these cells colocalized with SOX2 (outlined arrow).
In Utero BPA Exposure Alters Gonadotropin mRNA Levels and Factors Important for Gonadotroph Differentiation in Females
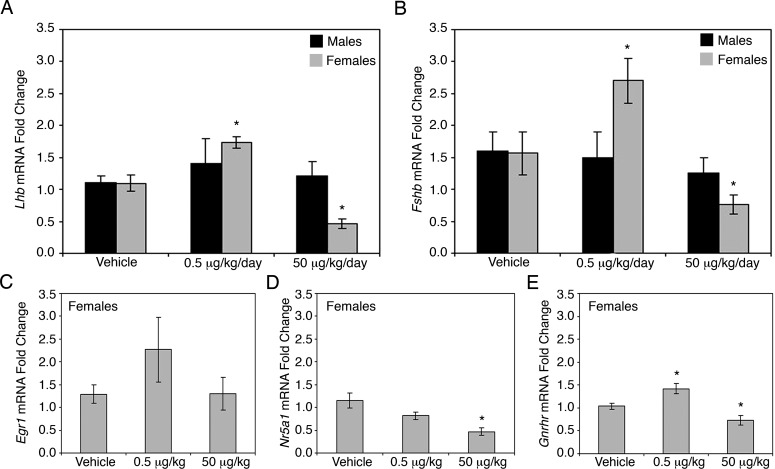
Because an increase in gonadotroph cell number was detected in the pituitaries of BPA-treated female offspring at birth, we examined gonadotropin mRNA levels using qRT-PCR. Females exposed to 0.5 μg/kg/day of BPA during embryonic development showed a significant increase in both Lhb (Fig. 4A) and Fshb mRNA (Fig. 4B), whereas males exposed to the same conditions showed no changes in expression (Fig. 4, A and B). However, females exposed to the higher BPA dose of 50 μg/kg/day showed a significant decrease in mRNA levels of both Lhb and Fshb, whereas males exposed to the same conditions again showed no effects (Fig. 4, A and B). Because gonadotroph number was increased in both treatment groups but Lhb and Fshb mRNA levels were differentially altered, we examined mRNA levels of markers known to be important in gonadotropin synthesis. Egr1 and Nr5a1 (steroidogenic factor-1) are key components of gonadotropin synthesis [25, 26, 33]. GNRH receptor (GNRHR) is essential for communication between the pituitary and the hypothalamus, as well as for initiating and maintaining gonadotroph hormone expression [34–36]. Although not significantly altered, Egr1 showed a trend towards an increase in the lowest treatment group, but no changes were observed for the 50 μg/kg/day treatment group as compared to vehicle (Fig. 4C). Nr5a1 had significantly decreased mRNA levels in the 50 μg/kg/day treatment group and no change in the 0.5 μg/kg/day treatment group (Fig. 4D). Gnrhr mRNA levels paralleled Lhb and Fshb mRNA levels, with a significant increase in the 0.5 μg/kg/day treatment group and a significant decrease in the 50 μg/kg/day treatment group as compared to vehicle treated controls (Fig. 4E).
FIG. 4.
Female gonadotropin mRNA levels and expression of factors controlling gonadotroph differentiation were altered by in utero BPA exposure. Levels of Lhb (A; P = 0.004) and Fshb (B; P = 0.018) mRNA were significantly increased in females exposed to BPA at a dose of 0.5 μg/kg/day compared to the vehicle control. Females exposed to 50 μg/kg/day of BPA showed a significant decrease in mRNA levels of both Lhb (A; P = 0.002) and Fshb (B; P = 0.032). No difference was found in the mRNA levels of Lhb (A) or Fshb (B) in males exposed to the same doses. In females, the mRNA levels for Egr1 showed a trend towards an increase in the lowest treatment group (P = 0.079), but no change was observed for the 50 μg/kg/day treatment group (C). Nr5a1 mRNA levels were significantly decreased in the 50 μg/kg/day treatment group (D; P = 0.002). Gnrhr mRNA levels showed a significant increase in the 0.5 μg/kg/day treatment group (E; P = 0.011)and a significant decrease in the 50 μg/kg/day treatment group (E; P = 0.033). All qRT-PCR values are normalized to the housekeeping gene Gapdh and are represented relative to the vehicle control group. Significance is noted by an asterisk (*P ≤ 0.05, n = 7–8).
Levels of PRL Protein and mRNA Are Undetectable at P1, and In Utero BPA Exposure Does Not Induce Expression
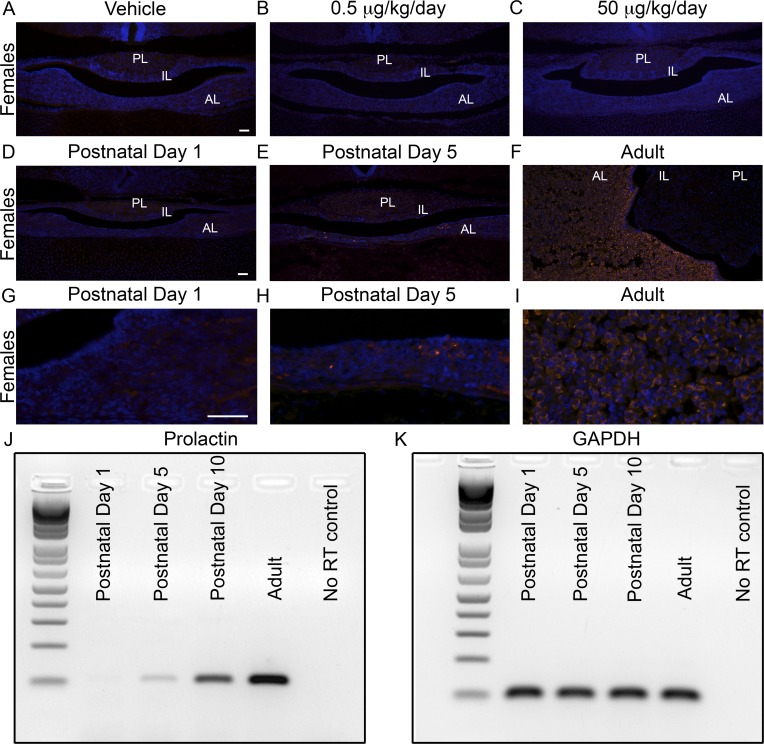
It is known that BPA can stimulate PRL production from lactotropes both in vivo and in vitro in the adult [28, 37–39] and can induce lactotrope proliferation [40, 41]. To determine if lactotropes were increased in response to BPA at P1, immunohistochemistry was used to examine PRL expression. No detection of PRL was found in any treatment group (Fig. 5, A-C). Additionally, in both male and female offspring at P1, Prl mRNA levels for all treatment groups were below the level of detection for qRT-PCR (data not shown). To determine when in the course of development PRL was detectable, a time course using pituitaries of CD-1 mice collected at P1, P5, P10, and adulthood was immunostained for PRL. No PRL-positive cells were observed at P1 (Fig. 5, D and G), a small number of cells were visible at P5 (Fig. 5, E and H), many more cells were positive at P10 (data not shown), and a large amount of cells were positive by adulthood (Fig. 5, F and I). Because mRNA must turn on before protein is detectable, we used RT-PCR to characterize when Prl mRNA reaches detectable levels. Similar to the immunohistochemistry data, Prl mRNA levels were not detectable at P1, only present in low levels at P5, and easily detectable at P10 and adulthood (Fig. 5, J and K).
FIG. 5.
PRL expression was not detectable at P1, and BPA exposure did not induce its expression. PRL-immunoreactive cells were not present at P1 in vehicle-treated female pituitaries (A) or those exposed to a 0.5 μg/kg/day (B) or 50 μg/kg/day (C) dose of BPA. A time course of PRL expression in CD-1 mice revealed no immunoreactive cells at P1 (D and G), a few cells at P5 (E and H), and many cells by adulthood (F and I). Prl mRNA was similarly not detectable in P1 pituitaries, and expression increased from P5 to adulthood (J). Gapdh is shown as a loading control (K). n = 4 (Immunohistochemistry), n = 6 (RT-PCR). AL, anterior lobe; IL, intermediate lobe; PL, posterior lobe. Original magnification ×100 (A–F) and ×400 (G–I); bar = 50 μm (A–I).
BPA Exposure Does Not Alter mRNA Levels of Major Hormones Produced by Somatotropes, Corticotropes, or Thyrotropes at P1
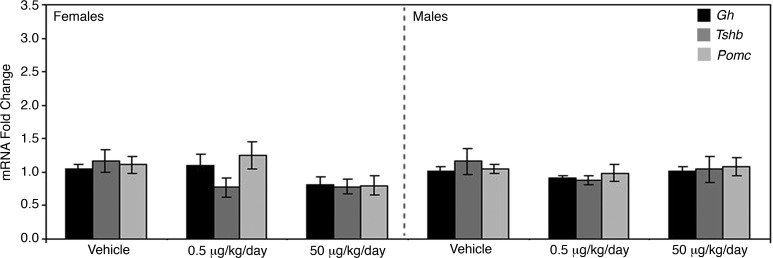
To elucidate if any other pituitary cell type was affected by in utero BPA exposure, the mRNA levels of hormones produced by corticotropes, somatotropes, and thyrotropes were analyzed. No changes were detected in the mRNA levels of pro-opiomelanocortin (Pomc), growth hormone (Gh), or thyroid-stimulating hormone (Tshb) for either male or female mice treated with BPA as compared to their vehicle-treated counterparts (Fig. 6).
FIG. 6.
The mRNA levels for the major hormones secreted by somatotropes, thyrotropes, and corticotropes showed no change in response to BPA exposure. The mRNA levels of hormones produced by somatotropes (Gh), thyrotropes (Tshb), and corticotropes (Pomc) of both males and females appeared unaffected by either dose of BPA tested. All qRT-PCR values are normalized to the housekeeping gene Gapdh and are represented relative to the vehicle control group.
DISCUSSION
Pituitary development relies on coordination between progenitor proliferation and differentiation of the distinct hormone-producing cell lineages (for review, see [4]). Failure to establish a pituitary with the proper cohort of cells at birth can result in hormonal imbalances and infertility. The extent to which the in utero environment can impact development of the pituitary is unknown. Using an in vivo model, we have shown that BPA exposure at environmentally relevant levels disrupts normal pituitary development. Female offspring are sensitive to BPA exposure, and the gonadotrophs appear to be the main hormone-secreting cell type affected in the pituitary at birth. Furthermore, we have shown that the level of exposure is critical when examining the effects of BPA: Pituitary proliferation and gonadotroph number are affected similarly at both doses, but gonadotropin mRNA levels have a unique response in each dose.
Normally, pituitary progenitor cells will actively proliferate in response to signaling from the surrounding hypothalamic tissue during development [42, 43]. This helps to ensure appropriate number and specification of cells during organogenesis as well as to maintain stem cell populations in the adolescent and adult pituitary [44, 45]. The present results show that in utero exposure to BPA can increase pituitary proliferation. The mechanism by which BPA promotes pituitary proliferation is unknown; however, one possibility is that the action of BPA on the pituitary may be estrogenic. Developmental BPA exposure can increase proliferation in other estrogen-sensitive organs, such as the uterus and mammary gland [30, 46–48]. Furthermore, maternal exposure to estrogens or other estrogenic compounds, such as methoxychlor, has been shown to increase gonadotroph cell numbers [49, 50]. It is well established that the adult pituitary is highly sensitive to estrogen exposure, because estradiol treatment after a gonadectomy results in increased pituitary proliferation and exposure to estrogenic compounds, such as diethylstilbestrol, increases proliferation and promotes pituitary tumor formation [51, 52]. Besides estrogen receptors (ERs), BPA can act antagonistically with other hormone receptors, including thyroid hormone receptor (THR) and androgen receptor (AR) [53, 54], which are present in the embryonic pituitary [55, 56]. However, no evidence has been reported to date to indicate that activation or inhibition of THR or AR can influence pituitary cell number and specification during development. Additional studies are needed to address the mechanism of action of BPA at the level of the pituitary, but the results of the present study are consistent with a potential effect at ERs.
The present results show that exposure to BPA during embryonic development causes a significant increase in gonadotroph cell number in the pituitary. Interestingly, we did not observe any changes in mRNA levels of other pituitary hormones. These results are similar to those of other studies that have shown CD-1 mice exposed neonatally and through adolescence to BPA showed no changes in Tshb, Gh, or Prl mRNA [57]. However, it is somewhat surprising that no effect on lactotrope cell numbers was found, because in the adult pituitary, BPA exposure increases numbers of PRL cells in estrogen-sensitive rats (F344) in vivo and proliferation of somatolactotrope (GH3) cell lines in vitro [28]. In the present study, PRL protein and mRNA were too low to be detected in all of the treatment conditions, indicating that at the time point we examined, the initiation of PRL expression is not affected by BPA exposure. Overall, these data suggest that BPA can augment the proliferation normally occurring in the embryonic pituitary and potentially direct uncommitted cells to the gonadotroph fate on the day of birth. This idea would be consistent with the studies showing that estradiol or methoxychlor can cause general proliferation increases and subsequent gonadotroph specification [49, 50]. Although the mechanism for this is unknown, it may be that intermediate cells are produced from the stem/progenitor cell that have many different receptors to sense the environment and differentiate depending on what signals are present.
Gonadotroph specification occurs through expression of developmentally regulated transcription factors and is reinforced by GNRH signaling late in gestation. Interestingly, despite seeing an increased number of gonadotrophs in response to both doses of BPA tested, the higher dose of 50 μg/kg/day was found to suppress Lhb and Fshb mRNA levels. We hypothesize that the increase in gonadotroph number may be established early in development and that the overall suppression of gonadotropin mRNA may be a result of a secondary action of BPA at the higher dose. The decrease in gonadotropin mRNA may be a result of a decrease in sensitivity of the gonadotrophs to GNRH, which is supported by the reduction seen in Gnrhr mRNA levels. GNRHR is expressed on the surface of pituitary gonadotroph cells and mediates the release of LH and FSH. It is also necessary for gonadotropin specification, because mice lacking Gnrhr have severely decreased LHβ-immunopositive cells and a decreased number of FSHβ-immunopositive cells [58]. In addition, we find Nr5a1 (Sf1) mRNA levels are also decreased in the higher dose of BPA tested. Nr5a1 is necessary for proper LH levels [26, 33]. These results indicate that although BPA can alter gonadotroph number, it may also impact Lhb and Fshb transcription through an unexplored mechanism. It is important to note that time of exposure and dose may be critical factors in determining the outcome of BPA administration. For example, adult exposure to BPA at higher levels than those used in the present study resulted in increases in Lhb and Fshb mRNA [57, 59], however, gonadotroph number was not examined.
One of the most significant findings of the present study is that embryonic BPA exposure only affects the female pituitary. One possible explanation is that sexually dimorphic development of the anteroventral periventricular nucleus (AVPV) of the hypothalamus is disrupted by BPA, leading to altered pituitary function. Males have higher circulating testosterone concentrations than females in the late prenatal and early postnatal periods. Testosterone is aromatized to estradiol locally in the brain, which is critical in the masculinization of the AVPV [60, 61]. BPA administration after birth specifically affects female rats, leading to a reduction of Kiss1 expression in the AVPV [62]. KISS1 neurons are intimately involved in gonadotropin release from the pituitary at puberty, providing a potential link to the female-specific pituitary changes we see in response to BPA. However, the effect of exposure to BPA on the hypothalamus before birth has not been described, and it is unclear if the development of sexually dimorphic nuclei in the hypothalamus has any effect on prenatal pituitary development. Another possibility, suggested by human studies, is that the pituitary is sexually dimorphic as well, because gonadotroph numbers are greater in females than in males during early embryogenesis [63]. It is unknown, though, if the rodent pituitary is sexually dimorphic before birth or differentially exposed to estradiol during development. Evidence exists that postnatally, male pituitaries have higher levels of aromatase than female pituitaries [64], similar to the hypothalamus, but again, if this is also true during embryogenesis is unknown. Although BPA exposure has been shown to have a sex-specific effect in the brain, the nature of its mode of action in developing female pituitaries will clearly warrant further investigation.
Understanding the consequences of BPA exposure on the developing pituitary is critical because of its widespread use and evidence for long-term reproductive effects of prenatal exposure (for review, see [65–68]). The present study defines the effect of BPA exposure during the critical period of pituitary organogenesis. We demonstrate the potential for low, environmentally relevant doses of BPA to have an effect on gonadotroph cell number and specification in female pituitaries. This knowledge is a critical step in furthering our understanding of how the pituitary responds to exogenous chemical exposure during development and suggests that the ORfD set by the government may be too high for sensitive subgroups, such as pregnant women.
ACKNOWLEDGMENT
The authors thank Dr. Ann Nardulli at the University of Illinois Urbana-Champaign for use of equipment and all members of the Raetzman Lab for technical support and reading of the manuscript.
Footnotes
Supported by National Institutes of Health grant R01DK076647 (L.T.R.), P20ES018163 (J.A.F), University of Illinois at Urbana-Champaign Research Board Grant 12174 (L.T.R.), and the Billie A. Field Fellowship (W.W. and Z.R.C).
REFERENCES
- Watkins-Chow DE, Camper SA. How many homeobox genes does it take to make a pituitary gland? Trends Genet 1998;14:284 290 [DOI] [PubMed] [Google Scholar]
- Rosenfeld MG, Briata P, Dasen J, Gleiberman AS, Kioussi C, Lin C, O'Connell SM, Ryan A, Szeto DP, Treier M. Multistep signaling and transcriptional requirements for pituitary organogenesis in vivo. Recent Prog Horm Res 2000;55:1 13 [PubMed] [Google Scholar]
- Wagner J, Lepore D, Thomas P. Differentiation of mouse embryonic stem cells into growth hormone and prolactin expressing cells in vitro. Mol Cell Endocrinol 2007;273:68 74 [DOI] [PubMed] [Google Scholar]
- Zhu X, Zhang J, Tollkuhn J, Ohsawa R, Bresnick EH, Guillemot F, Kageyama R, Rosenfeld MG. Sustained Notch signaling in progenitors is required for sequential emergence of distinct cell lineages during organogenesis. Genes Dev 2006;20:2739 2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Park MS, Lee HS. Endocrine disrupting chemicals: human exposure and health risks. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 2006;24:183 224 [DOI] [PubMed] [Google Scholar]
- Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect 2005;113:391 395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Integrated Risk Information System (IRIS). Bisphenol A (CASRN 80-05-7). In: U.S. EPA IRIS Substance File [Internet]. http://www.epa.gov/iris/subst/0356.htm. Accessed 24 Februrary 2012.
- Takahashi O, Oishi S. Disposition of orally administered 2,2-bis(4-hydroxyphenyl)propane (bisphenol A) in pregnant rats and the placental transfer to fetuses. Environ Health Perspect 2000;108:931 935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adewale HB, Jefferson WN, Newbold RR, Patisaul HB. Neonatal bisphenol-A exposure alters rat reproductive development and ovarian morphology without impairing activation of gonadotropin-releasing hormone neurons. Biol Reprod 2009;81:690 699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BS, Murray MK, Damassa DA, King JC, Soto AM. Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ Health Perspect 2001;109:675 680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabaton NJ, Wadia PR, Rubin BS, Zalko D, Schaeberle CM, Askenase MH, Gadbois JL, Tharp AP, Whitt GS, Sonnenschein C, Soto AM. Perinatal exposure to environmentally relevant levels of bisphenol A decreases fertility and fecundity in CD-1 mice. Environ Health Perspect 2011;119:547 552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul HB, Fortino AE, Polston EK. Neonatal genistein or bisphenol-A exposure alters sexual differentiation of the AVPV. Neurotoxicol Teratol 2006;28:111 118 [DOI] [PubMed] [Google Scholar]
- Markey CM, Wadia PR, Rubin BS, Sonnenschein C, Soto AM. Long-term effects of fetal exposure to low doses of the xenoestrogen bisphenol-A in the female mouse genital tract. Biol Reprod 2005;72:1344 1351 [DOI] [PubMed] [Google Scholar]
- Rodriguez HA, Santambrosio N, Santamaria CG, Munoz-de-Toro M, Luque EH. Neonatal exposure to bisphenol A reduces the pool of primordial follicles in the rat ovary. Reprod Toxicol 2010;30:550 557 [DOI] [PubMed] [Google Scholar]
- Goyal HO, Robateau A, Braden TD, Williams CS, Srivastava KK, Ali K. Neonatal estrogen exposure of male rats alters reproductive functions at adulthood. Biol Reprod 2003;68:2081 2091 [DOI] [PubMed] [Google Scholar]
- Fernandez M, Bianchi M, Lux-Lantos V, Libertun C. Neonatal exposure to bisphenol A alters reproductive parameters and gonadotropin releasing hormone signaling in female rats. Environ Health Perspect 2009;117:757 762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura D, Yanagiba Y, Duan Z, Ito Y, Okamura A, Asaeda N, Tagawa Y, Li C, Taya K, Zhang SY, Naito H, Ramdhan DHet al. Bisphenol A may cause testosterone reduction by adversely affecting both testis and pituitary systems similar to estradiol. Toxicol Lett 2010;194:16 25 [DOI] [PubMed] [Google Scholar]
- Collet SH, Picard-Hagen N, Viguie C, Lacroix MZ, Toutain PL, Gayrard V. Estrogenicity of bisphenol A: a concentration-effect relationship on luteinizing hormone secretion in a sensitive model of prepubertal lamb. Toxicol Sci 2010;117:54 62 [DOI] [PubMed] [Google Scholar]
- Akingbemi BT, Sottas CM, Koulova AI, Klinefelter GR, Hardy MP. Inhibition of testicular steroidogenesis by the xenoestrogen bisphenol A is associated with reduced pituitary luteinizing hormone secretion and decreased steroidogenic enzyme gene expression in rat Leydig cells. Endocrinology 2004;145:592 603 [DOI] [PubMed] [Google Scholar]
- Suh H, Gage PJ, Drouin J, Camper SA. Pitx2 is required at multiple stages of pituitary organogenesis: pituitary primordium formation and cell specification. Development 2002;129:329 337 [DOI] [PubMed] [Google Scholar]
- Dasen JS, O'Connell SM, Flynn SE, Treier M, Gleiberman AS, Szeto DP, Hooshmand F, Aggarwal AK, Rosenfeld MG. Reciprocal interactions of Pit1 and GATA2 mediate signaling gradient-induced determination of pituitary cell types. Cell 1999;97:587 598 [DOI] [PubMed] [Google Scholar]
- Charles MA, Saunders TL, Wood WM, Owens K, Parlow AF, Camper SA, Ridgway EC, Gordon DF. Pituitary-specific Gata2 knockout: effects on gonadotrope and thyrotrope function. Mol Endocrinol 2006;20:1366 1377 [DOI] [PubMed] [Google Scholar]
- Gage PJ, Suh H, Camper SA. Dosage requirement of Pitx2 for development of multiple organs. Development 1999;126:4643 4651 [DOI] [PubMed] [Google Scholar]
- Lee SL, Sadovsky Y, Swirnoff AH, Polish JA, Goda P, Gavrilina G, Milbrandt J. Luteinizing hormone deficiency and female infertility in mice lacking the transcription factor NGFI-A (Egr-1). Science 1996;273:1219 1221 [DOI] [PubMed] [Google Scholar]
- Topilko P, Schneider-Maunoury S, Levi G, Trembleau A, Gourdji D, Driancourt MA, Rao CV, Charnay P. Multiple pituitary and ovarian defects in Krox-24 (NGFI-A, Egr-1)-targeted mice. Mol Endocrinol 1998;12:107 122 [DOI] [PubMed] [Google Scholar]
- Zhao L, Bakke M, Parker KL. Pituitary-specific knockout of steroidogenic factor 1. Mol Cell Endocrinol 2001;185:27 32 [DOI] [PubMed] [Google Scholar]
- Goldberg LB, Aujla PK, Raetzman LT. Persistent expression of activated Notch inhibits corticotrope and melanotrope differentiation and results in dysfunction of the HPA axis. Dev Biol 2011;358:23 32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz R, Brown NG, Allen DL, Bigsby RM, Ben-Jonathan N. The environmental estrogen bisphenol A stimulates prolactin release in vitro and in vivo. Endocrinology 1997;138:1780 1786 [DOI] [PubMed] [Google Scholar]
- Timms BG, Howdeshell KL, Barton L, Bradley S, Richter CA, vom Saal FS. Estrogenic chemicals in plastic and oral contraceptives disrupt development of the fetal mouse prostate and urethra. Proc Natl Acad Sci U S A 2005;102:7014 7019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-de-Toro M, Markey CM, Wadia PR, Luque EH, Rubin BS, Sonnenschein C, Soto AM. Perinatal exposure to bisphenol-A alters peripubertal mammary gland development in mice. Endocrinology 2005;146:4138 4147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Gremeaux L, Fu Q, Liekens D, Van Laere S, Vankelecom H. Pituitary progenitor cells tracked down by side population dissection. Stem Cells 2009;27:1182 1195 [DOI] [PubMed] [Google Scholar]
- Fauquier T, Rizzoti K, Dattani M, Lovell-Badge R, Robinson IC. SOX2-expressing progenitor cells generate all of the major cell types in the adult mouse pituitary gland. Proc Natl Acad Sci U S A 2008;105:2907 2912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Bakke M, Krimkevich Y, Cushman LJ, Parlow AF, Camper SA, Parker KL. Steroidogenic factor 1 (SF1) is essential for pituitary gonadotrope function. Development 2001;128:147 154 [DOI] [PubMed] [Google Scholar]
- Kaiser UB, Sabbagh E, Katzenellenbogen RA, Conn PM, Chin WW. A mechanism for the differential regulation of gonadotropin subunit gene expression by gonadotropin-releasing hormone. Proc Natl Acad Sci U S A 1995;92:12280 12284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger LL, Haisenleder DJ, Dalkin AC, Marshall JC. Regulation of gonadotropin subunit gene transcription. J Mol Endocrinol 2004;33:559 584 [DOI] [PubMed] [Google Scholar]
- Wu S, Wilson MD, Busby ER, Isaac ER, Sherwood NM. Disruption of the single copy gonadotropin-releasing hormone receptor in mice by gene trap: severe reduction of reproductive organs and functions in developing and adult mice. Endocrinology 2010;151:1142 1152 [DOI] [PubMed] [Google Scholar]
- Stoker TE, Robinette CL, Britt BH, Laws SC, Cooper RL. Prepubertal exposure to compounds that increase prolactin secretion in the male rat: effects on the adult prostate. Biol Reprod 1999;61:1636 1643 [DOI] [PubMed] [Google Scholar]
- Velasco-Marinero E, Herrero-Payo JJ, Carretero-Gonzalez J. Changes in pituitary and prolactin cells of Wistar rats after two dental fillings with bisphenolic resins. Arch Oral Biol 2011;56:592 598 [DOI] [PubMed] [Google Scholar]
- Ramos JG, Varayoud J, Kass L, Rodriguez H, Costabel L, Munoz-De-Toro M, Luque EH. Bisphenol A induces both transient and permanent histofunctional alterations of the hypothalamic-pituitary-gonadal axis in prenatally exposed male rats. Endocrinology 2003;144:3206 3215 [DOI] [PubMed] [Google Scholar]
- Goloubkova T, Ribeiro MF, Rodrigues LP, Cecconello AL, Spritzer PM. Effects of xenoestrogen bisphenol A on uterine and pituitary weight, serum prolactin levels and immunoreactive prolactin cells in ovariectomized Wistar rats. Arch Toxicol 2000;74:92 98 [DOI] [PubMed] [Google Scholar]
- Chun TY, Gorski J. High concentrations of bisphenol A induce cell growth and prolactin secretion in an estrogen-responsive pituitary tumor cell line. Toxicol Appl Pharmacol 2000;162:161 165 [DOI] [PubMed] [Google Scholar]
- Ericson J, Norlin S, Jessell TM, Edlund T., Integrated FGF. and BMP signaling controls the progression of progenitor cell differentiation and the emergence of pattern in the embryonic anterior pituitary. Development 1998;125:1005 1015 [DOI] [PubMed] [Google Scholar]
- Potok MA, Cha KB, Hunt A, Brinkmeier ML, Leitges M, Kispert A, Camper SA. WNT signaling affects gene expression in the ventral diencephalon and pituitary gland growth. Dev Dyn 2008;237:1006 1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouin J, Bilodeau S, Roussel-Gervais A. Stem cells, differentiation and cell cycle control in pituitary. Front Horm Res 2010;38:15 24 [DOI] [PubMed] [Google Scholar]
- Ikeda H, Yoshimoto T. Developmental changes in proliferative activity of cells of the murine Rathke's pouch. Cell Tissue Res 1991;263:41 47 [DOI] [PubMed] [Google Scholar]
- Zhu X, Rosenfeld MG. Transcriptional control of precursor proliferation in the early phases of pituitary development. Curr Opin Genet Dev 2004;14:567 574 [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Maffini MV, Wadia PR, Sonnenschein C, Rubin BS, Soto AM. Exposure to environmentally relevant doses of the xenoestrogen bisphenol-A alters development of the fetal mouse mammary gland. Endocrinology 2007;148:116 127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Sugihara A, Uchida K, Sato T, Ohta Y, Katsu Y, Watanabe H, Iguchi T. Developmental effects of perinatal exposure to bisphenol-A and diethylstilbestrol on reproductive organs in female mice. Reprod Toxicol 2002;16:107 116 [DOI] [PubMed] [Google Scholar]
- Masutomi N, Shibutani M, Takagi H, Uneyama C, Lee KY, Hirose M. Alteration of pituitary hormone-immunoreactive cell populations in rat offspring after maternal dietary exposure to endocrine-active chemicals. Arch Toxicol 2004;78:232 240 [DOI] [PubMed] [Google Scholar]
- Wu YJ. Chen da W, Liu JL, Zhang JH, Luo HS, Cui S. Estradiol promotes pituitary cell proliferation and gonadotroph differentiation at different doses and with different mechanisms in chick embryo. Steroids 2009;74:441 448 [DOI] [PubMed] [Google Scholar]
- Nolan LA, Levy A. The effects of testosterone and oestrogen on gonadectomized and intact male rat anterior pituitary mitotic and apoptotic activity. J Endocrinol 2006;188:387 396 [DOI] [PubMed] [Google Scholar]
- Zhao W, Shen H, Yuan F, Li G, Sun Y, Shi Z, Zhang Y, Wang Z. Induction stage-dependent expression of vascular endothelial growth factor and aquaporin-1 in diethylstilbestrol-treated rat pituitary. Eur J Histochem 2009;53:53 60 [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Bansal R, Parris C. Bisphenol-A, an environmental contaminant that acts as a thyroid hormone receptor antagonist in vitro, increases serum thyroxine, and alters RC3/neurogranin expression in the developing rat brain. Endocrinology 2005;146:607 612 [DOI] [PubMed] [Google Scholar]
- Lee HJ, Chattopadhyay S, Gong EY, Ahn RS, Lee K. Antiandrogenic effects of bisphenol A and nonylphenol on the function of androgen receptor. Toxicol Sci 2003;75:40 46 [DOI] [PubMed] [Google Scholar]
- Crocoll A, Zhu CC, Cato AC, Blum M. Expression of androgen receptor mRNA during mouse embryogenesis. Mech Dev 1998;72:175 178 [DOI] [PubMed] [Google Scholar]
- Bradley DJ, Towle HC, Young WS., III. Spatial and temporal expression of alpha- and beta-thyroid hormone receptor mRNAs, including the beta 2-subtype, in the developing mammalian nervous system. J Neurosci 1992;12:2288 2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi W, Lee CK, Yeung WS, Giesy JP, Wong MH, Zhang X, Hecker M, Wong CK. Effect of perinatal and postnatal bisphenol A exposure to the regulatory circuits at the hypothalamus-pituitary-gonadal axis of CD-1 mice. Reprod Toxicol 2011;31:409 417 [DOI] [PubMed] [Google Scholar]
- Wen S, Ai W, Alim Z, Boehm U. Embryonic gonadotropin-releasing hormone signaling is necessary for maturation of the male reproductive axis. Proc Natl Acad Sci U S A 2010;107:16372 16377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee JS, Kim RO, Seo JS, Kang HS, Park CB, Soyano K, Lee J, Lee YM, Lee JS. Bisphenol A modulates expression of gonadotropin subunit genes in the hermaphroditic fish, Kryptolebias marmoratus. Comp Biochem Physiol C Toxicol Pharmacol 2010;152:456 466 [DOI] [PubMed] [Google Scholar]
- Weisz J, Ward IL. Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses, and neonatal offspring. Endocrinology 1980;106:306 316 [DOI] [PubMed] [Google Scholar]
- Poling MC, Kauffman AS. Sexually dimorphic testosterone secretion in prenatal and neonatal mice is independent of kisspeptin-Kiss1r and GnRH signaling. Endocrinology 2012;153:782 793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Mickens JA, McCaffrey KA, Leyrer SM, Patisaul HB. Neonatal bisphenol A exposure alters sexually dimorphic gene expression in the postnatal rat hypothalamus. Neurotoxicology 2012;33:23 36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan SL, Grumbach MM. The ontogenesis of human foetal hormones. II. Luteinizing hormone (LH) and follicle stimulating hormone (FSH).Acta Endocrinol (Copenh) 1976;81:808 829 [DOI] [PubMed] [Google Scholar]
- Carretero J, Vazquez G, Rubio M, Blanco E, Juanes JA, Perez E, Burks D, Vazquez R. Postnatal differentiation of the immunohistochemical expression of aromatase P450 in the rat pituitary gland. Histol Histopathol 2003;18:419 423 [DOI] [PubMed] [Google Scholar]
- Welshons WV, Nagel SC, vom Saal FS. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure.Endocrinology 2006;147:S56 S69 [DOI] [PubMed] [Google Scholar]
- Golub MS, Wu KL, Kaufman FL, Li LH, Moran-Messen F, Zeise L, Alexeeff GV, Donald JM. Bisphenol A: developmental toxicity from early prenatal exposure. Birth Defects Res B Dev Reprod Toxicol 2010;89:441 466 [DOI] [PubMed] [Google Scholar]
- Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, Vandenbergh JG, Walser-Kuntz DR, vom Saal FS. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol 2007;24:199 224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BS. Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. J Steroid Biochem Mol Biol 2011;127:27 34 [DOI] [PubMed] [Google Scholar]