Abstract
OBJECTIVE
Obesity leads to severe long-term complications and reduced life expectancy. Roux-en-Y gastric bypass (RYGB) surgery induces excessive and continuous weight loss in (morbid) obesity, although it causes several abnormal anatomical and physiological conditions.
RESEARCH DESIGN AND METHODS
To distinctively unveil effects of RYGB surgery on β-cell function and glucose turnover in skeletal muscle, liver, and gut, nondiabetic, morbidly obese patients were studied before (pre-OP, five female/one male, BMI: 49 ± 3 kg/m2, 43 ± 2 years of age) and 7 ± 1 months after (post-OP, BMI: 37 ± 3 kg/m2) RYGB surgery, compared with matching obese (CONob, five female/one male, BMI: 34 ± 1 kg/m2, 48 ± 3 years of age) and lean controls (CONlean, five female/one male, BMI: 22 ± 0 kg/m2, 42 ± 2 years of age). Oral glucose tolerance tests (OGTTs), hyperinsulinemic-isoglycemic clamp tests, and mechanistic mathematical modeling allowed determination of whole-body insulin sensitivity (M/I), OGTT and clamp test β-cell function, and gastrointestinal glucose absorption.
RESULTS
Post-OP lost (P < 0.0001) 35 ± 3 kg body weight. M/I increased after RYGB, becoming comparable to CONob, but remaining markedly lower than CONlean (P < 0.05). M/I tightly correlated (τ = −0.611, P < 0.0001) with fat mass. During OGTT, post-OP showed ≥15% reduced plasma glucose from 120 to 180 min (≤4.5 mmol/L), and 29-fold elevated active glucagon-like peptide-1 (GLP-1) dynamic areas under the curve, which tightly correlated (r = 0.837, P < 0.001) with 84% increased β-cell secretion. Insulinogenic index (0–30 min) in post-OP was ≥29% greater (P < 0.04). At fasting, post-OP showed approximately halved insulin secretion (P < 0.05 vs. pre-OP). Insulin-stimulated insulin secretion in post-OP was 52% higher than before surgery, but 1–2 pmol/min2 lower than in CONob/CONlean (P < 0.05). Gastrointestinal glucose absorption was comparable in pre-OP and post-OP, but 9–26% lower from 40 to 90 min in post-OP than in CONob/CONlean (P < 0.04).
CONCLUSIONS
RYGB surgery leads to decreased plasma glucose concentrations in the third OGTT hour and exaggerated β-cell function, for which increased GLP-1 release seems responsible, whereas gastrointestinal glucose absorption remains unchanged but lower than in matching controls.
The increasing prevalence of obesity has affected between ∼10 and 30% of the adult population in Western countries (1). Since obesity represents a major cluster of the cardiometabolic syndrome, it favors development of type 2 diabetes mellitus (T2DM), arterial hypertension, dyslipidemia, and heart and vessel diseases, all of which may reduce the individual’s quality of life and lead to premature death (1–3).
Despite this rather desperate prognosis for the obese, there is currently a lack of effective medication. So far, the most effective treatment for obese people is bariatric surgery (4). Out of several operation procedures, Roux-en-Y gastric bypass (RYGB) surgery has been well established for inducing long-term weight loss (5). Patients who have undergone this surgery not only lose a substantial amount of weight but also show clear improvements with regard to all clusters of the cardiometabolic syndrome, including glycemia (5,6).
Several publications have dealt with obese subjects with and without T2DM before and after bariatric surgery. The majority of T2DM patients benefit from the resolution of T2DM (7,8). Whole-body insulin sensitivity improves in both T2DM and nondiabetic subjects but clear amelioration seems to occur only after substantial weight loss (7–10).
However, despite all of these beneficial effects, RYGB surgery also carries risks for the individuals, not only from the operation itself but also from long-term unwanted effects that may diminish the patient’s quality of life, in particular malabsorption because of the altered anatomy of the gastrointestinal tract. In addition, episodes of pronounced postprandial hypoglycemia were reported in patients after bariatric surgery (11,12). Using oral d-xylose loading tests in diabetic rats and humans, it was reported that bariatric surgery did not affect carbohydrate absorption (13,14). Nevertheless, the two monosaccharides d-xylose and d-glucose differ in metabolism and absorption, which is passive for d-xylose (14), whereas glucose absorption is primarily an active process.
Postprandial hypoglycemia in humans may result from glucose malabsorption and/or from more pronounced insulin release due to increased glucagon-like peptide-1 (GLP-1) release (15). In order to refrain from these possible short- and long-term complications, it certainly appears desirable to mimic the proven beneficial effects of RYGB surgery by the prescription of currently available remedies. To this end, however, the comprehensive knowledge of all RYBP effects seems essential.
Thus, we aimed to precisely unveil the distinctive gastrointestinal, endocrine, and metabolic alterations by comparing patients before (pre-OP) and after (post-OP) RYGB surgery. In order to exclude the well-known, pronounced effects of glucose toxicity on whole-body insulin sensitivity and β-cell function (16), we only recruited nondiabetic subjects with morbid obesity. For a more precise comparison, we recruited not only a healthy age- and sex-matched control group with normal BMI (CONlean), but also another obese control group (CONob) that was precisely matched for the major anthropometric measures of the patients after surgery (3). In all study participants, an oral glucose tolerance test (OGTT) and a hyperinsulinemic-isoglycemic clamp test were performed, twice (before and about half a year after RYGB) in the patients but only once in the control subjects. The present report extends previously published data (3) by a more in-depth analysis of the effects of RYGB surgery on 1) insulin sensitivity, 2) insulin secretion from β-cells during OGTT and clamp test, 3) gastrointestinal glucose absorption using recently developed modeling analyses, 4) hepatic glucose production (HGP) with hepatic insulin sensitivity, and 5) GLP-1 release. We hypothesized that after RYGB surgery, the decreased glycemia after a glucose load would result, to an extent, from alterations in insulin and GLP-1 release, gut glucose absorption, and/or HGP.
RESEARCH DESIGN AND METHODS
Study participants
Morbidly obese (BMI ≥40 kg/m2) subjects planning to undergo RYGB surgery were referred to our department for precise preoperative examination, as described in detail elsewhere (3,6). Our diagnostics also included an OGTT to avoid underdiagnosing diabetes, unless already known or diagnosable from fasting glucose concentrations according to the American Diabetes Association criteria (6,17,18). In addition, patients were tested for Cushing syndrome and thyroid dysfunction (6). Additional exclusion criteria for this study were current or former medication known to influence glucose homeostasis, severe (chronic) disease, pregnancy, and/or breastfeeding (3,6).
Finally, six nondiabetic, morbidly obese patients agreed to participate in the entire study program, which included both an OGTT and a hyperinsulinemic-isoglycemic clamp test both before (pre-OP) and ∼6 months after (post-OP) RYGB surgery. Two control groups, who only once underwent OGTTs and clamp tests, were enrolled: a lean control group (CONlean; n = 6), matched for age and sex to pre-OP and post-OP, and another obese control group (CONob; n = 6), with age, sex, and BMI comparable to post-OP (Table 1). The study was performed according to the Declaration of Helsinki, and the protocol was approved by the local ethical board. All study participants gave written informed consent.
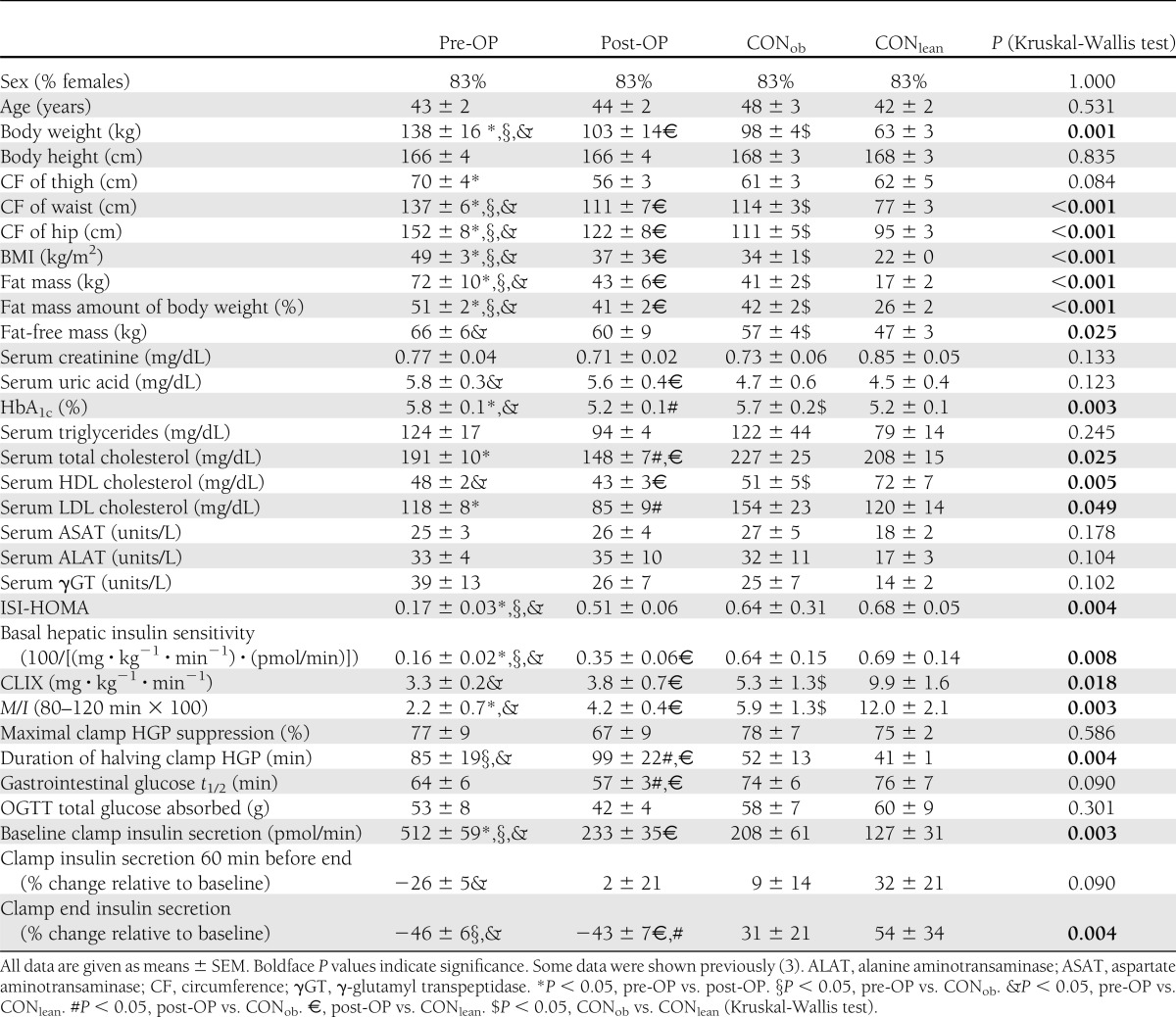
Table 1.
Anthropometric and baseline characteristics, as well as results of hepatic (ISI-HOMA and basal hepatic insulin sensitivity) and whole-body (CLIX and M) insulin sensitivity, HGP, gastrointestinal glucose half-life, total absorption of OGTT glucose, and insulin secretion before and during the clamp test in patients before (pre-OP; n = 6) and after (post-OP; n = 6) RYGB, and obese (CONob; n = 6) and lean (CONlean; n = 6) controls
OGTT
After an overnight fast for at least 10 h, a 75-g OGTT (Gluco-Drink75; Roche Diagnostics, Vienna, Austria) was performed for 3 h with frequent blood sampling (0, 10, 20, 30, 40, 60, 90, 120, 150, and 180 min) for instant determination of plasma glucose and subsequent analysis of plasma hormones (3,6,19–22).
Clamp test
After another overnight fast for at least 10 h, two catheters (Vasofix; Braun, Melsungen, Germany) were inserted into one antecubital vein of the left and right arm for blood sampling and infusions, respectively. A primed-continuous infusion (0–5 min, 4 mg · kg lean body weight; thereafter, 0.04 mg · min−1 lean body weight) of d-[6,6-2H2]glucose (98% enriched; Cambridge Isotope Laboratories, Andover, MA) was started 120 min before the start of the clamp to determine HGP, as described in detail previously (3,22–24). The isoglycemic clamp glucose target was determined from the mean value of three fasting plasma glucose measurements. However, in the case of a value <80 mg/dL, the clamp target was set to 80 mg/dL, and in the case of a value >100 mg/dL, the clamp goal was 100 mg/dL (3,22–24). Hyperinsulinemic-isoglycemic clamp tests were performed for 140 min in patients and 120 min in control subjects with primed-continuous regular insulin (Actrapid; NovoNordisk, Bagsvaerd, Denmark) infusion (40 mU · min−1 · m−2 body surface) (3,6,19–22,25). Plasma glucose was measured every 5 min and maintained at the clamp goal comparably among all four groups (P > 0.24) by infusing variable amounts of a 20% d-glucose solution, enriched with d-[6,6-2H2]glucose to ∼2% mole percent excess (3,22–24).
Bariatric surgery
The patients underwent laparoscopic long-limb RYGB in a standardized antegastric, antecolic manner. The alimentary limb measured 150 cm and the biliopancreatic limb 75 cm, as previously described (3).
Measurements
Body weight and fat (-free) mass were measured by the Tanita Bioimpedance Balance body composition analyzer (Tanita International Division, Yiewsley, U.K.). Plasma insulin and C-peptide were analyzed by commercially available radioimmunoassays from Linco Research (St. Charles, MO) and plasma free fatty acid (FFA) concentrations with a microfluorimetric assay (Wako, Richmond, VA) (3,22–24). For GLP-1 measurement at 0 and 60 min in the patients, a dipeptidylpeptidase-IV inhibitor of Linco Research was added to the plasma samples to assess active GLP-1 concentrations by using an ELISA (Millipore, Billerica, MA). Routine laboratory parameters were determined from serum/plasma at our local Department of Medical and Chemical Laboratory Diagnostics (www.kimcl.at). Mole percent excess of d-[6,6-2H2]glucose was measured as previously described (3,22–24).
Calculations
HGP and measures/surrogates of insulin sensitivity, such as glucose infusion rates, M value (M), whole-body insulin sensitivity (M/I), the Clamp-Like Index (CLIX), and insulin sensitivity index/homeostatic model assessment (ISI-HOMA; best suited for assessing hepatic insulin sensitivity) (26,27), were calculated as previously described (3,6,18–25,28) (see also Supplementary Methods and Supplementary Fig. 1A and B). Dynamic area under the curve (ΔAUC) was calculated as previously described (3,20) (Supplementary Methods). We exploited mechanistic mathematical modeling to apply our recently developed methods of gastrointestinal glucose absorption during the OGTT (20,28), given in 10–30-min intervals (as indicated), and insulin secretion during insulin infusion (17,25) (Supplementary Methods). The latter can be seen as in vivo β-cell function assessable during the clamp test (17), whereas for that during the OGTT, the insulinogenic index (IGI) for the first 30 min (18,28), the slope of C-peptide for the first 60 min, and ΔAUC of C-peptide during the entire OGTT were calculated (Supplementary Methods). Basal hepatic insulin sensitivity was calculated as 100/(HGP × basal insulin secretion). Hepatic insulin sensitivity during clamp test (i.e., duration of halving clamp HGP by insulin), OGTT total glucose absorbed, and glucose half-life (t1/2) in the gastrointestinal tract were determined as described in the Supplementary Methods and previously (20,28).
Statistical analyses
All data are given as means ± SEM. Comparisons of groups were performed by using the nonparametric Kruskal-Wallis test. In addition, repeated-measures ANOVA with post hoc least significant difference test was used to compare single values during the OGTT (for glucose every hour) and the clamp test. Linear and nonlinear, nonparametric methods were used for correlation analyses using Pearson linear correlation coefficient r and Kendall rank correlation coefficientτ, respectively (24,28). Differences were considered statistically significant at P values ≤0.05. Statistical analyses were performed using SPSS (SPSS, Inc., Chicago, IL) computer software.
RESULTS
Anthropometric and basal (laboratory) characteristics
The four groups were similar in sex and age (Table 1). Post-OP lost 35 ± 3 kg, 6.7 ± 0.8 months after RYGB (P < 0.0001). The BMI of pre-OP was markedly higher than that of the other three groups (each P < 0.001), whereas post-OP and CONob had similar BMI, which was much greater than that of CONlean (each P < 0.001). HbA1c was similar between pre-OP and CONob, but higher by 0.6–0.7% than that in post-OP and CONlean (each P < 0.005). After surgery, total and LDL cholesterol concentrations were reduced by 23 and 28%, respectively, in post-OP (P < 0.009 vs. pre-OP). HDL cholesterol was markedly lower by >30% in pre-OP and post-OP (P < 0.004) than that in CONlean, and did not change after surgery.
OGTT
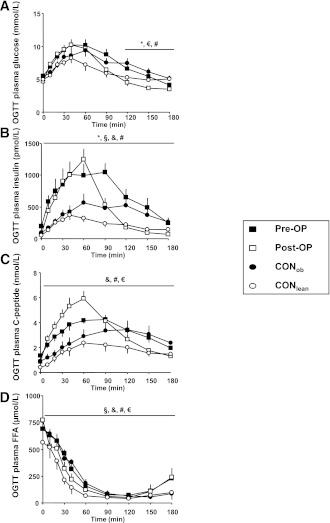
From 120 to 180 min, post-OP had a glucose level ≤4.5 mmol/L (≤80 mg/dL), which was lower than that in pre-OP, CONob, and CONlean (each P < 0.05). In post-OP, plasma insulin and plasma C-peptide concentrations showed a markedly different time course (P < 0.05 vs. pre-OP, CONob, and/or CONlean) (Fig. 1B and C). OGTT plasma FFAs were higher in pre-OP and post-OP (P < 0.05 vs. CONob and CONlean) and did not change from before to after surgery conditions (Fig. 1D).
Figure 1.
Plasma concentrations of glucose (A), insulin (B), C-peptide (C), and FFAs (D) during OGTT in obese patients before (pre-OP; n = 6; ■) and after RYGB surgery (post-OP; n = 6; □), as well as matching obese (CONob; n = 6; ●) and lean controls (CONlean; n = 6; ○), which were previously presented (3). Kruskal-Wallis test: *P < 0.05, pre-OP vs. post-OP; §P < 0.05, pre-OP vs. CONob; &P < 0.05, pre-OP vs. CONlean; #P < 0.05, post-OP vs. CONob; €, post-OP vs. CONlean.
Hyperinsulinemic-isoglycemic clamp test
Plasma glucose and insulin concentrations during the clamp test were similar among the four groups. Plasma C-peptide concentrations before and during the clamp were higher in post-OP, when compared with at least one of the other groups (each P < 0.05). Plasma FFAs were comparable among the four groups (Supplementary Fig. 2A–D).
Whole-body insulin sensitivity
Clamp M/I (Table 1) was highest (each P < 0.05 vs. the other groups) in CONlean; post-OP had higher clamp M/I by 2.0 (mg · kg−1 · min−1)/(µU/mL) than before surgery (each P < 0.05). M/I did not differ between post-OP and CONob. The OGTT insulin sensitivity marker CLIX also displayed very similar values but did not detect a significant difference between pre-OP and post-OP (Table 1). However, each group CLIX result was comparable to M/I (P ≥ 0.1). For glucose infusion rates and M see Supplementary Fig. 1A and B.
HGP and hepatic insulin sensitivity (surrogates)
At baseline, HGP was 23% lower in CONob compared with CONlean (P < 0.04). HGP remained less suppressible in post-OP after 90 and 120 min by 53 and 96% compared with CONob (P < 0.05). Basal hepatic insulin sensitivity was very low in pre-OP (P < 0.005 vs. both controls) and improved in post-OP, but was still lower than that in CONlean (P < 0.05) (Table 1). ISI-HOMA, which is a good surrogate for basal hepatic insulin sensitivity (26), virtually displayed the same results for pre-OP, CONob, and CONlean, but not for post-OP (Table 1). However, each group result in basal hepatic insulin sensitivity was comparable to that of ISI-HOMA (P ≥ 0.2).
During the clamp test, post-OP had elevated HGP from 90 to 120 min (each P < 0.05 vs. CONob). Although the maximum clamp HGP suppression by insulin was not different among the four groups (Table 1), the duration by insulin for halving clamp HGP was approximately doubled in pre-OP and post-OP, when compared with one or both control groups (each P < 0.05).
β-Cell function and active GLP-1 secretion
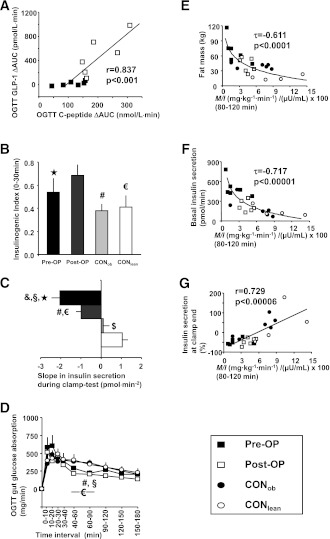
During OGTT, insulin secretion within the first hour, as reflected by C-peptide slope, was ∼75% higher in post-OP, when compared with the other three groups (each P < 0.05) (Supplementary Fig. 1C). Despite similar fasting values, the AUC of active GLP-1 during OGTT was more than twofold higher (P < 0.03) in post-OP (990 ± 147 pmol/L · min) than pre-OP (407 ± 194 pmol/L · min). The OGTT ΔAUC of C-peptide was 93% higher (P < 0.04 vs. pre-OP) in post-OP and also very closely associated with active GLP-1 ΔAUC (r = 0.837, P < 0.001) (Fig. 2A). The IGI was also greater by at least 30% in post-OP (each P < 0.01) (Fig. 2B).
Figure 2.
Correlation of OGTT GLP-1 and C-peptide ΔAUCs (A), OGTT IGI (0–30 min) (B), slope of clamp test insulin release (C), and OGTT gut glucose absorption (D), as well as correlations of M/I with body fat mass (E), basal insulin secretion (F), and insulin secretion at clamp end (in percent change relative to baseline) (G), in obese patients before (pre-OP; n = 6; ■ or black columns) and after RYGB surgery (post-OP; n = 6; □ or dark-gray columns), as well as matching obese (CONob; n = 6; ● or light-gray columns) and lean controls (CONlean; n = 6; ○ or white columns). Kruskal-Wallis test: ★P < 0.05, pre-OP vs. post-OP; §P < 0.05, pre-OP vs. CONob; &P < 0.05, pre-OP vs. CONlean; #P < 0.05, post-OP vs. CONob; €, post-OP vs. CONlean; $P < 0.05, CONob vs. CONlean.
Basal insulin secretion (Table 1) was between two- and fourfold higher in pre-OP (each P < 0.002 vs. all other groups), and fell after surgery in post-OP (P = 0.01 vs. pre-OP), becoming comparable to that in CONob, but remained nearly doubled when compared with CONlean (P < 0.05). When examining insulin secretion alteration during hyperinsulinemia (i.e., the clamp test) by calculating its slope, insulin secretion declined the most in pre-OP, and significantly less in post-OP, but rose in CONob, and still more in CONlean (each P < 0.05, each group vs. the other) (Fig. 2C). On the other hand, when expressing insulin secretion alteration during hyperinsulinemia as percent relative to baseline, insulin release at 60 min before clamp end was lower in pre-OP, but not in post-OP, CONob, and CONlean (Table 1 and Fig. 2G). At clamp end, both pre-OP and post-OP showed a decline in clamp insulin secretion, whereas CONob and CONlean displayed an increase (Table 1 and Fig. 2G), when compared with baseline.
Gut glucose absorption
Gastrointestinal glucose absorption was similar in the patients before and after surgery, and also between both control groups. However, from 40 to 90 min, and 60 to 90 min, after the start of OGTT, post-OP and pre-OP, respectively, showed lower glucose absorption rates by 120–192 mg/min (each P < 0.05) (Fig. 2D). The gastrointestinal t1/2 of glucose and total glucose absorbed were comparable among all four groups (Table 1).
Correlation analyses
M/I (final two 20-min intervals pooled) was negatively related to body weight (τ = −0.611, P < 0.0001), BMI (τ = −0.607, P < 0.00006), fat mass (τ = −0.611, P < 0.0001) (Fig. 2E), and basal insulin secretion (τ = −0.717, P < 0.00001) (Fig. 2F), but positively related to CLIX (r = 0.745, P < 0.00003), basal hepatic insulin sensitivity (r = 0.743, P < 0.00007), and the relative change in clamp end insulin secretion, when compared with baseline (r = 0.729, P < 0.00006) (Fig. 2G). Basal hepatic insulin sensitivity was positively associated with ISI-HOMA (r = 0.594, P < 0.003). At the start of the clamp test, fasting FFA concentrations correlated positively with basal HGP (r = 0.495, P < 0.02). Gastrointestinal glucose t1/2 was negatively related to body weight (τ = −0.348, P < 0.02) and fat-free mass (τ = −0.399, P < 0.01), and tended to negatively correlate with BMI (τ = −0.268, P = 0.066) and fat mass (τ = −0.269, P = 0.066).
CONCLUSIONS
The major and novel findings of this study are 1) the increased first-phase β-cell secretion in post-OP is accompanied by a markedly increased secretion of GLP-1 that closely correlates to dynamic C-peptide AUCs, 2) insulin secretion during sustained hyperinsulinemia improves after weight loss after RYGB surgery, but is still lower than in matching obese controls, 3) basal hepatic insulin sensitivity improves after surgery, whereas insulin-mediated HGP suppressibility remains impaired, and 4) gastrointestinal glucose absorption is similar after surgery, when compared with conditions before, but lower within the second OGTT hour, when compared with both control groups.
Surgical procedure
The patients underwent laparoscopic long-limb RYGB at 150 cm from the gastrojejunostomy, which is performed rather rarely, but not uncommonly, as ∼9% of all bariatric operations are performed this way (29). However, even longer-limb RYGB methods are in use, all of which seem to induce pronounced and comparable weight loss in the long run (30).
OGTT
Plasma glucose concentrations were not different between pre-OP and post-OP within the first two OGTT hours. The IGI was greater in post-OP than all other groups. A very probable explanation for this pronounced insulin release in response to hyperglycemia is the exaggerated secretion of GLP-1 in post-OP, as previously reported (12,31). We found an elevation of OGTT GLP-1 AUC by more than twofold, which is in line with the results of Laferrère et al. (31) who reported an approximately fourfold GLP-1 AUC increase after RYGB. This finding seems to be well supported by the very close association between the ΔAUCs of GLP-1 and C-peptide (Fig. 2A).
Insulin secretion and β-cell function
Most recently, Nannipieri et al. (8) reported an ∼40% improvement of β-cell function in nondiabetic patients ∼1 year after RYGB, in accordance with our findings of a 34% IGI amelioration (Fig. 2B). Assuming that the improved insulin secretion during OGTT in post-OP was predominantly because of higher GLP-1 release, we used mechanistic mathematical modeling to calculate insulin secretion during sustained hyperinsulinemia. We found that basal insulin secretion, which was tremendously elevated before surgery in the morbidly obese, decreased after surgery by ∼55%, but was not normalized, when compared with CONlean. We have previously shown that insulin itself is able to modulate its own secretion (17,25). Thus, we considered that if β-cell function was actually improved/exaggerated after RYGB surgery and was even more pronounced than in healthy controls, which can be concluded from the higher IGI, this must hold true also for insulin release during hyperinsulinemia. However, our mathematical modeling (based on C-peptide analyses) showed that insulin-induced insulin secretion in post-OP was higher than before surgery, but even worse than in CONob, matching for major anthropometric characteristics, and much worse than in CONlean as well. From this, it follows that increased β-cell function during OGTT appears to mostly result from higher GLP-1 concentrations, as supported by studies using the GLP-1 antagonist exendin-(9-39)-amide (32).
Of note, insulin secretion is induced by GLP-1 under hyperglycemic conditions only. However, despite a rather short half-life in the circulation, insulin has a relatively prolonged bioactivity, which lasts up to an hour (21). This may maintain pronounced glucose utilization even when glucose concentrations are already low.
Whole-body insulin sensitivity
After surgery, post-OP displayed higher sensitivity to insulin, as displayed by M/I (Table 1), when compared with conditions before surgery. However, this improved insulin sensitivity was comparable between post-OP and CONob. In addition, the measures of insulin sensitivity very tightly correlated with the prevailing fat mass, as visible in Fig. 2E. This relates also to post-OP. Thus, our findings are completely in line with that of Campos et al. (9), who showed an improvement of insulin sensitivity after substantial weight loss in post-RYGB patients after 6 months, but not 2 weeks. However, the changes observed may not only be attributed to the sole loss of body fat mass but could also be related to a negative energy balance.
Guidone et al. (10) showed that whole-body insulin sensitivity had been increased by 50% in formerly T2DM patients, who recovered from diabetes 1 week after RYGB surgery. Our present investigations in nondiabetic patients before and after RYGB with smaller differences in insulin sensitivity may therefore also lead to the interpretation that the impressive improvement in insulin resistance seen in formerly diabetic patients (10) is mostly due to the lack of glucose toxicity, which reduces insulin sensitivity by ∼50% (16).
HGP and hepatic insulin sensitivity
In this study, we have developed a novel formula to calculate basal hepatic insulin sensitivity (Table 1) by putting fasting HGP and insulin secretion into the nominator. Astonishingly, this new formula turned out to well reflect ISI-HOMA (Table 1), which was described as a reliable hepatic insulin sensitivity surrogate (26,27). From this novel formula, we found an amelioration of basal hepatic insulin sensitivity in post-OP, when compared with pre-OP conditions. This finding goes along with our previous report, in which we showed hepatic insulin resistance to be improved after RYGB (3). This study also investigated insulin-mediated HGP suppressibility (which is also regarded as the liver’s sensitivity to insulin during hyperinsulinemia) (1,23) by assessing the duration during the clamp test for basal HGP reduction by 50%. Thereby, we found that this duration was comparable between pre-OP and post-OP, but was twofold elevated in post-OP, when compared with controls (Table 1). This seemed surprising, because amelioration of fasting HGP would have been expected to go along with improved insulin-mediated HGP suppression, as precisely studied in T2DM patients (23). Maximal suppression of HGP by insulin, however, was similar among the four groups, indicating that the HGP reduction by insulin in the four groups is time, but not dose, dependent.
Gastrointestinal glucose absorption
The glucose absorption during OGTT was not different when comparing patients before and after surgery, as well as both control groups to each other. These results seem surprising because they rather point away from a pronounced malabsorption after RYGB, but are in line with previous studies applying oral d-xylose loading tests in diabetic rats and humans (13,14). However, pre-OP and post-OP had slightly lower gut glucose absorption rates from 60 to 90 min, and from 40 to 90 min, respectively, than the controls. Again, gastrointestinal glucose t1/2 in all participants was negatively associated with body dimensions, as described previously (20,28).
Clinical relevance
This study provided evidence that a major part of the observed effects after RYGB surgery in morbidly obese patients appears to be predominantly mediated via GLP-1 release during oral absorption, but also by an improvement in basal hepatic insulin sensitivity, as shown herein and previously (3), going along with reduced fasting insulin secretion. Interestingly, reduced glucose absorption is detectable, but does not seem to play an important role, because glucose absorption profiles were comparable before and after RYGB.
Limitations
A weakness of this study might be the rather low number of participants per group. However, our exclusion criteria were quite strict because the absence of diabetes was required. This, however, could warrant the absence of glucose toxicity effects that would have blurred measurements of whole-body insulin sensitivity, which is known to be deteriorated by 40–50% in the presence of diabetes (16). In addition, this study’s setting was also rather extensive for the patients because they had to undergo many more visits than needed for both preoperative and follow-up examinations.
Final conclusions
Gastric bypass surgery leads to pronounced weight loss of ∼35 kg within about half a year, which was accompanied by slightly improved whole-body insulin sensitivity, pronouncedly lower glucose concentrations between 2 and 3 h after a glucose load, and greater β-cell function during an OGTT, for which increased GLP-1 release may be responsible, but without any changes in glucose absorption. Basal hepatic insulin sensitivity improved at fasting, whereas insulin-mediated HGP suppression remained unalterably prolonged. Insulin secretion during hyperinsulinemia was higher after than before surgery, but still worse than in matching controls. This suggests that in RYGB patients, augmented GLP-1 secretion and slightly lower gut glucose absorption in general (which was also present before surgery), as well as, to some (minor) extent, improved basal hepatic insulin sensitivity with reduced fasting insulin secretion are the main contributors to the observed gastrointestinal, endocrine, and metabolic alterations, which are mostly beneficial, but also include unwanted, and thus threatening, effects, such as pronounced postprandial hypoglycemia.
Supplementary Material
Acknowledgments
M.P.-S. was employed as a post-doctorate on a grant from the Austrian Society of Internal Medicine (“Josef Skoda-Award”), which was competitively awarded to M.K. H.E. and M.K. are supported by the WWTF (Vienna Science and Technology Fund). The Relationship between Insulin Sensitivity and Cardiovascular Disease (RISC) project (www.egir.org) directed by the European Group for the Study of Insulin Resistance (EGIR) since 2001 (current Vienna subcontractors: C.-H.A. and A.L.) is supported by European Union Grant QLG1-CT-2001-01252 and by AstraZeneca. No other potential conflicts of interest relevant to this article were reported.
C.-H.A. researched data, performed mathematic calculations, analyzed data, and wrote and revised the manuscript. A.T. and G.Pa. performed data analysis, contributed to discussion, and reviewed and edited the manuscript. M.P.-S. researched and analyzed data. G.Pr., M.S., B.L., M.G.B., A.L., and M.K. reviewed and edited the manuscript. H.E. researched data and performed data analysis. C.-H.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors are grateful to all volunteers for their participation and to H. Lentner and A. Hofer (Medical University of Vienna) for skillful care of the study participants. The authors also thank the staff of the laboratory of the Division of Endocrinology and Metabolism and of the Department of Medical and Chemical Laboratory Diagnostics (Medical University of Vienna) for precise hormone and metabolite analyses, including gas chromatography–mass spectrometry measurements.
The authors thank Dr. D. Borrow, Specialized Hospital Complex Agathenhof, for re-editing the manuscript.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-0197/-/DC1.
References
- 1.Anderwald CH. Adipotoxicity revisited: shifting from metabolic to cardiometabolic risk profile. Journal of Clinical Metabolism & Diabetes 2010;1:61–75 [Google Scholar]
- 2.Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab 2004;89:2583–2589 [DOI] [PubMed] [Google Scholar]
- 3.Promintzer-Schifferl M, Prager G, Anderwald C, et al. Effects of gastric bypass surgery on insulin resistance and insulin secretion in nondiabetic obese patients. Obesity (Silver Spring) 2011;19:1420–1426 [DOI] [PubMed] [Google Scholar]
- 4.Sjöström L, Lindroos AK, Peltonen M, et al. Swedish Obese Subjects Study Scientific Group Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683–2693 [DOI] [PubMed] [Google Scholar]
- 5.Lima MM, Pareja JC, Alegre SM, et al. Acute effect of Roux-en-Y gastric bypass on whole-body insulin sensitivity: a study with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab 2010;95:3871–3875 [DOI] [PubMed] [Google Scholar]
- 6.Jankovic D, Wolf P, Anderwald CH, et al. Prevalence of endocrine disorders in morbidly obese patients and the effects of bariatric surgery on endocrine and metabolic parameters. Obes Surg 2012;22:62–69 [DOI] [PubMed] [Google Scholar]
- 7.Camastra S, Gastaldelli A, Mari A, et al. Early and longer term effects of gastric bypass surgery on tissue-specific insulin sensitivity and beta cell function in morbidly obese patients with and without type 2 diabetes. Diabetologia 2011;54:2093–2102 [DOI] [PubMed] [Google Scholar]
- 8.Nannipieri M, Mari A, Anselmino M, et al. The role of beta-cell function and insulin sensitivity in the remission of type 2 diabetes after gastric bypass surgery. J Clin Endocrinol Metab 2011;96:E1372–E1379 [DOI] [PubMed] [Google Scholar]
- 9.Campos GM, Rabl C, Peeva S, et al. Improvement in peripheral glucose uptake after gastric bypass surgery is observed only after substantial weight loss has occurred and correlates with the magnitude of weight lost. J Gastrointest Surg 2010;14:15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guidone C, Manco M, Valera-Mora E, et al. Mechanisms of recovery from type 2 diabetes after malabsorptive bariatric surgery. Diabetes 2006;55:2025–2031 [DOI] [PubMed] [Google Scholar]
- 11.Patti ME, McMahon G, Mun EC, et al. Severe hypoglycaemia post-gastric bypass requiring partial pancreatectomy: evidence for inappropriate insulin secretion and pancreatic islet hyperplasia. Diabetologia 2005;48:2236–2240 [DOI] [PubMed] [Google Scholar]
- 12.Meier JJ, Butler AE, Galasso R, Butler PC. Hyperinsulinemic hypoglycemia after gastric bypass surgery is not accompanied by islet hyperplasia or increased beta-cell turnover. Diabetes Care 2006;29:1554–1559 [DOI] [PubMed] [Google Scholar]
- 13.Rubino F, Forgione A, Cummings DE, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg 2006;244:741–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang G, Agenor K, Pizot J, et al. Accelerated gastric emptying but no carbohydrate malabsorption 1 year after gastric bypass surgery (GBP). Obes Surg. 15 April 2012. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodieux F, Giusti V, D’Alessio DA, Suter M, Tappy L. Effects of gastric bypass and gastric banding on glucose kinetics and gut hormone release. Obesity (Silver Spring) 2008;16:298–305 [DOI] [PubMed] [Google Scholar]
- 16.Cline GW, Magnusson I, Rothman DL, Petersen KF, Laurent D, Shulman GI. Mechanism of impaired insulin-stimulated muscle glucose metabolism in subjects with insulin-dependent diabetes mellitus. J Clin Invest 1997;99:2219–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mari A, Tura A, Natali A, et al. RISC Investigators Influence of hyperinsulinemia and insulin resistance on in vivo β-cell function: their role in human β-cell dysfunction. Diabetes 2011;60:3141–3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stadler M, Pacini G, Petrie J, Luger A, Anderwald C, RISC Investigators Beta cell (dys)function in non-diabetic offspring of diabetic patients. Diabetologia 2009;52:2435–2444 [DOI] [PubMed] [Google Scholar]
- 19.Anderwald-Stadler M, Krebs M, Promintzer M, et al. Plasma obestatin is lower at fasting and not suppressed by insulin in insulin-resistant humans. Am J Physiol Endocrinol Metab 2007;293:E1393–E1398 [DOI] [PubMed] [Google Scholar]
- 20.Anderwald C, Gastaldelli A, Tura A, et al. Mechanism and effects of glucose absorption during an oral glucose tolerance test among females and males. J Clin Endocrinol Metab 2011;96:515–524 [DOI] [PubMed] [Google Scholar]
- 21.Anderwald C, Anderwald-Stadler M, Promintzer M, et al. The Clamp-Like Index: a novel and highly sensitive insulin sensitivity index to calculate hyperinsulinemic clamp glucose infusion rates from oral glucose tolerance tests in nondiabetic subjects. Diabetes Care 2007;30:2374–2380 [DOI] [PubMed] [Google Scholar]
- 22.Promintzer M, Krebs M, Todoric J, et al. Insulin resistance is unrelated to circulating retinol binding protein and protein C inhibitor. J Clin Endocrinol Metab 2007;92:4306–4312 [DOI] [PubMed] [Google Scholar]
- 23.Anderwald C, Bernroider E, Krššák M, et al. Effects of insulin treatment in type 2 diabetic patients on intracellular lipid content in liver and skeletal muscle. Diabetes 2002;51:3025–3032 [DOI] [PubMed] [Google Scholar]
- 24.Anderwald C, Pfeiler G, Nowotny P, et al. Glucose turnover and intima media thickness of internal carotid artery in type 2 diabetes offspring. Eur J Clin Invest 2008;38:227–237 [DOI] [PubMed] [Google Scholar]
- 25.Anderwald C, Tura A, Grassi A, et al. Insulin infusion during normoglycemia modulates insulin secretion according to whole-body insulin sensitivity. Diabetes Care 2011;34:437–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes 2006;55:1430–1435 [DOI] [PubMed] [Google Scholar]
- 27.Brestoff JR, Clippinger B, Spinella T, von Duvillard SP, Nindl BC, Arciero PJ. An acute bout of endurance exercise but not sprint interval exercise enhances insulin sensitivity. Appl Physiol Nutr Metab 2009;34:25–32 [DOI] [PubMed] [Google Scholar]
- 28.Anderwald C, Tura A, Winhofer Y, et al. Glucose absorption in gestational diabetes mellitus during an oral glucose tolerance test. Diabetes Care 2011;34:1475–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buchwald H, Williams SE. Bariatric surgery worldwide 2003. Obes Surg 2004;14:1157–1164 [DOI] [PubMed] [Google Scholar]
- 30.Murr MM, Balsiger BM, Kennedy FP, Mai JL, Sarr MG. Malabsorptive procedures for severe obesity: comparison of pancreaticobiliary bypass and very very long limb Roux-en-Y gastric bypass. J Gastrointest Surg 1999;3:607–612 [DOI] [PubMed] [Google Scholar]
- 31.Laferrère B, Teixeira J, McGinty J, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab 2008;93:2479–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salehi M, Prigeon RL, D’Alessio DA. Gastric bypass surgery enhances glucagon-like peptide 1-stimulated postprandial insulin secretion in humans. Diabetes 2011;60:2308–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.