Abstract
OBJECTIVE
The central nervous system mechanisms of defenses against falling plasma glucose concentrations, and how they go awry and result in iatrogenic hypoglycemia in diabetes, are not known. Hypoglycemic plasma glucose concentrations of 55 mg/dL (3.0 mmol/L) cause symptoms, activate glucose counterregulatory systems, and increase synaptic activity in a network of brain regions including the dorsal midline thalamus in humans. We tested the hypothesis that slightly subphysiological plasma glucose concentrations of 65 mg/dL (3.6 mmol/L), which do not cause symptoms but do activate glucose counterregulatory systems, also activate brain synaptic activities.
RESEARCH DESIGN AND METHODS
We measured relative regional cerebral blood flow (rCBF), an index of synaptic activity, in predefined brain regions with [15O]water positron emission tomography, symptoms, and plasma epinephrine and glucagon concentrations during a 2-h euglycemic (90 mg/dL) to hypoglycemic (55 mg/dL) clamp (n = 20) or a 2-h euglycemic to slight subphysiological (65 mg/dL) clamp (n = 9) in healthy humans.
RESULTS
Clamped plasma glucose concentrations of 65 mg/dL did not cause hypoglycemic symptoms, but raised plasma epinephrine and glucagon concentrations and increased rCBF (P = 0.007) only in the dorsal midline thalamus.
CONCLUSIONS
Slightly subphysiological plasma glucose concentrations increase synaptic activity in the dorsal midline thalamus in humans.
Iatrogenic hypoglycemia is the limiting factor in the glycemic management of diabetes (1). It causes recurrent morbidity in most people with type 1 diabetes and many with advanced type 2 diabetes, and is sometimes fatal (2). Indeed, as many as 1 in 10 people with type 1 diabetes may die of treatment-induced hypoglycemia (3). In addition, hypoglycemia per se causes a vicious cycle of recurrent hypoglycemia and that barrier generally precludes maintenance of euglycemia over a lifetime of diabetes.
Hypoglycemia in diabetes is generally the result of the interplay of therapeutic hyperinsulinemia and compromised physiological and behavioral defenses against falling plasma glucose concentrations (1). The compromised defenses include: 1) loss of a decrease in insulin, 2) loss of an increase in glucagon, and 3) attenuation of the central nervous system (CNS)–mediated increase in sympathoadrenal activity as plasma glucose levels fall. The attenuated adrenomedullary epinephrine response causes defective glucose counterregulation and the attenuated sympathetic neural response is largely responsible for hypoglycemia unawareness. These are the two components of hypoglycemia-associated autonomic failure in diabetes (1). The mechanisms of the normal CNS-mediated sympathoadrenal response to hypoglycemia and how it goes awry in diabetes are not known, but it is thought to involve a cerebral network that includes the thalamus (4–7). It is our premise that insight into the CNS physiology of the sympathoadrenal response in nondiabetic humans will lead to insight into its pathophysiology in people with diabetes.
There is a hierarchy among the responses to falling plasma glucose concentrations in humans (4). Among the CNS-mediated responses, the glycemic threshold for epinephrine secretion is 65–70 mg/dL and for symptoms is 50–55 mg/dL. It is not known if there is a hierarchy among the brain synaptic responses.
Measurements of regional cerebral blood flow (rCBF), an index of synaptic activity, with [15O]water positron emission tomography (PET) indicate that frank hypoglycemia (e.g., clamped plasma glucose concentrations of 50–55 mg/dL) causes increases in rCBF in a network of interconnected brain regions including the dorsal midline thalamus and the medial prefrontal cortex (anterior cingulate) among other sites in humans (5–7). Indeed, recent antecedent hypoglycemia results in a greater increase in synaptic activity in the dorsal midline thalamus during subsequent hypoglycemia (6). Because slightly subphysiological plasma glucose concentrations of 65 mg/dL also stimulate epinephrine secretion but do not cause symptoms (4), we tested the hypothesis that such slightly subphysiological plasma glucose concentrations also activate brain synaptic activities in humans.
RESEARCH DESIGN AND METHODS
Subjects
Twenty-nine healthy individuals gave their written consent to participate in this study, which was approved by the Washington University Human Research Protection Office and conducted at the Washington University Clinical Research Unit (CRU) and Neurology-Neurosurgery Intensive Care Unit PET Research Facility. Participants were 14 women and 15 men with a mean (± SE) age of 26.4 ± 1.2 years and a mean BMI of 24.0 ± 0.8 kg/m2. All subjects were in good health based on a medical history and physical examination. They were taking no medications (aside from an oral contraceptive or a stable dose of thyroxine) and had normal fasting plasma glucose and creatinine concentrations, hematocrits, and electrocardiograms. None had a personal or first-degree relative history of diabetes, or a personal history of psychiatric, neurologic, or cardiovascular conditions.
Experimental design
Subjects were admitted to the CRU in the morning after a 10-h overnight fast. They remained supine throughout the study. Two intravenous catheters were inserted into arm veins (for infusions), and one intravenous catheter was inserted into a dorsal hand vein with that hand kept in a ∼55°C Plexiglas box (for arterialized venous sampling). Twenty subjects (10 men and 10 women: mean age 27.0 ± 1.4 years; mean BMI 23.8 ± 0.8 kg/m2) underwent hyperinsulinemic (regular human insulin in a dose of 2.0 mU·kg−1·min−1; Novo Nordisk, Bagsværd, Denmark), euglycemic (90 mg/dL [5.0 mmol/L] × ∼2 h), and then hypoglycemic (55 mg/dL [3.0 mmol/L]) × ∼2 h) clamps using variable infusions of 20% dextrose based on plasma glucose determinations (YSI Glucose Analyzer 2; Yellow Springs Instruments, Yellow Springs, OH) every 5 minutes. The other nine subjects (5 men and 4 women: mean age 25.2 ± 2.8 years; mean BMI 24.8 ± 1.7 kg/m2) underwent the exact same procedure, but the lower glucose clamp was 65 mg/dL (3.6 mmol/L) × ∼2 h. [15O]Water PET measurements of rCBF were performed four times at 15-min intervals during the second hour of euglycemia and four times at 15-min intervals during the second hour of hypoglycemia and obtained in all 29 subjects. Arterialized venous samples for the analytes described below were drawn and blood pressures and heart rates were recorded every 30 min, and the electrocardiogram was monitored throughout. Hypoglycemic symptom scores were determined every 30 min during the clamps. Subjects were asked to score (from 0, none, to 6, severe) six neurogenic (autonomic) symptoms—heart pounding, shaky/tremulousness and nervous/anxious (adrenergic) and sweaty, hungry and tingling (cholinergic)—and six neuroglycopenic symptoms—(difficulty thinking/confused, tired/drowsy, weak, warm, faint, and dizzy) (8). After completion of the glucose clamps/PET studies, a brain magnetic resonance imaging (MRI) scan was obtained on the same day.
Analytical methods
Plasma glucose concentrations were measured with a glucose oxidase method (YSI Glucose Analyzer, Yellow Springs Instruments). Plasma insulin concentrations were measured with a two-site chemiluminescent assay (Immulite 1000; Siemens Corp., Los Angeles, CA), and plasma glucagon concentrations were measured with a radioimmunoassay (Millipore, Temecula, CA). Plasma epinephrine and norepinephrine concentrations were measured with a single isotope derivative (radioenzymatic) method (9). Relevant systemic variables during euglycemia and hypoglycemia were contrasted with a t test for paired data. P values less than 0.05 were considered to indicate significant differences.
Positron emission tomography (PET) and MRI
MRI scan acquisition.
Each subject also underwent one session of structural MRI of the brain with a 1.5 T system (Magnetom Sonata; Siemens, Erlangen, Germany). A three-dimensional magnetization-prepared rapid gradient echo (MPRAGE) sequence (1900/1100/3.9 repetition time/inversion time/echo time [ms]; flip angle = 15°) was acquired in a sagittally oriented plane (160 mm thick, 128 partitions, 256-mm field of view) and reconstructed into a 256 × 256 matrix (1 × 1 × 1.25 mm pixels).
PET scan acquisition.
Details of the [15O]water PET acquisition and analysis methods at our institution have been reported in detail in prior publications (6,10). Studies were acquired with a Siemens/CTI (Knoxville, TN) ECAT EXACT HR 47 tomograph using the two-dimensional mode (interslice septa extended) (10). Subjects were positioned in the scanner so that the entire brainstem was included within the 15-cm axial field of view, which restricted the view of the most superior aspect of the cortex in some subjects as a result. A transmission scan was collected at each scan session for PET data reconstruction. Four rCBF scans were collected during euglycemia and 4 were collected during hypoglycemia to measure relative rCBF with multiple 40-s emission scans after bolus intravenous injection of 50 mCi of [15O]water (1.85 GBq) (11–13).
PET scan analyses.
PET images were reconstructed using filtered back projection. Attenuation correction for individual scans was created by forward projection from coregistered transmission images. Reconstructed images were smoothed with a three-dimensional Gaussian filter to a resolution of 10 mm full width at half maximum. All individual PET images were normalized using mean whole-brain counts. Relative rCBF was calculated as the ratio of regional PET counts to mean whole-brain PET counts. Each subject’s eight rCBF PET scans and their single MPRAGE image were coregistered to each other (14), and then coregistered to a standard mean blood flow image in Talairach atlas space using a nine-parameter fit (15). The four euglycemic and the four hypoglycemic images for each subject were averaged for each condition to increase statistical precision (16). Location of regions of interest with significant change were expressed in x, y, and z stereotactic coordinates in millimeters with positive values representing right, anterior, and superior, respectively.
Whole-brain, voxel-wise PET analyses.
To explore the entire PET image space and determine if other brain regions other than our a priori defined set were differentially activated by levels of hypoglycemia, we ran a voxel-wise analysis with SPM8 (Statistical Parametric Mapping, Version 8) (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) using a random-effects model with multiple-comparisons correction for the entire volume. This approach searches all of the image space without regard for anatomical boundaries and determines if there are any regions with statistically reliable effects. SPM computes T values and P values at each voxel for the comparison of interest (e.g., hypoglycemia vs. euglycemia). Clusters of at least 90 contiguous voxels that surpassed a threshold of P < 0.005 were examined. SPM compares these clusters with the number of possible clusters expected by chance to determine if they are statistically significant and decreases the probability of identifying false-positive clusters in the analysis (17,18). The coordinates for the center of the regions from the SPM analyses (matched to the Montreal Neurological Institute [MNI] brain) were interrogated on the Talairach Daemon software and corroborated visually on the Talairach atlas.
RESULTS
Counterregulatory hormone and symptom responses
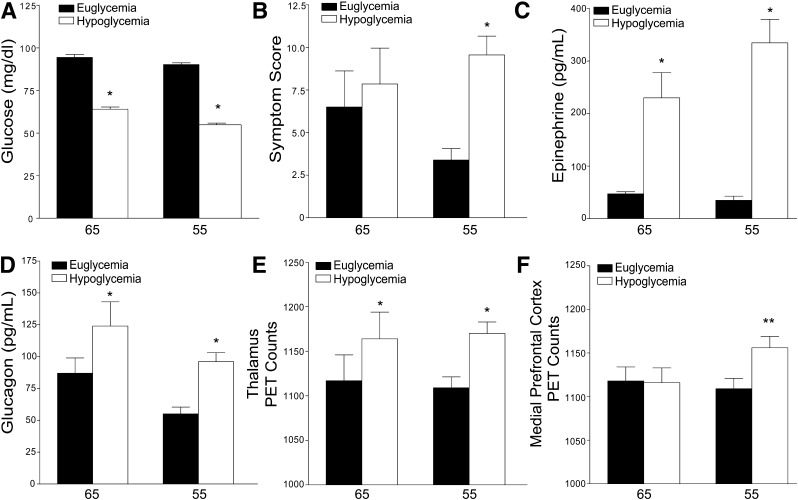
Both low glucose levels (Fig. 1A) increased sympathoadrenal responses (Fig. 1C). They also increased plasma glucagon levels (Fig. 1D). Hypoglycemic symptoms were elicited only at the 55-mg/dL glucose level (Fig. 1B).
Figure 1.
Mean (± SE) plasma glucose concentrations, symptom scores, plasma epinephrine and glucagon concentrations, and relative rCBF (as measured by PET counts) in the dorsal midline thalamus and in the medial prefrontal cortex during hyperinsulinemic-euglycemic 90 mg/dL and hypoglycemic clamps at 65 mg/dL, “65,” and 55 mg/dL, “55.” P < 0.01* or < 0.05**, euglycemia (black bar) to hypoglycemia (white bar). The glucose values (A) are means over the second hour of each of the euglycemic and hypoglycemic clamps. The symptom scores (B), plasma epinephrine concentrations (C), and plasma glucagon concentrations (D) are means over the second hour of each euglycemic and hypoglycemic clamp when the PET scans are being obtained. The rCBF data (E and F) are based on averages of the four [15O]water PET studies during the second hour of each euglycemic and hypoglycemic clamp.
rCBF
We have used the indirect technique of measuring changes in rCBF to identify regions of increased synaptic activity during hypoglycemia (11–13,19). A significant increase in rCBF was confirmed in the dorsal midline thalamus (x = 0, y = −21, z = 8) (P = 0.002) and medial prefrontal cortex (x = 0, y = 33, z = 15) (P = 0.014), but not the lateral orbital prefrontal cortex, at the 55 mg/dL glucose level when compared with euglycemia as previously identified (5). At a glucose level of 65 mg/dL, a significant increase in rCBF was found in the dorsal midline thalamus (P = 0.007) when compared with euglycemia (Fig. 1E), but not in the other regions of interest such as the medial prefrontal cortex (P = 0.85) (Fig. 1F) or lateral orbitoprefrontal cortex (x = 32, y = 38, z = 4) (P = 0.14). We also interrogated the amygdala (x = ±22, y = −6, z = −16), but failed to find a change in blood flow at a glucose level of 65 mg/dL or 55 mg/dL (P = 0.7 and P = 0.5, respectively).
SPM8 analysis identified statistically significant areas of rCBF increase in the bilateral thalamus (right: voxels: 243, T: 6.99, P < 0.001, x = 11, y = −33, z = 12; left: voxels: 92, T: 5.01, P < 0.001, x = −3, y = −19, z = 12) and brainstem region (right: voxels: 248, T: 5.73, P < 0.001, x = 9, y = −31, z = −14; left: voxels: 123, T: 4.38, P < 0.001, x = 5, y = −19, z = −2) at the 55-mg/dL glucose level, but only in the thalamus at a glucose level of 65 mg/dL (voxels: 102, T = 8.12, P = 0.007, x = −9, y = −21, z = 4). The absence of a detectable rCBF change in the medial frontal prefrontal cortex at 55 mg/dL with this methodology should not be interpreted as evidence against activation of this region, because this method of analysis is less sensitive for detecting changes on a priori identified regions because it corrects for multiple comparisons and a factor of multiple thresholding across the entire brain space.
CONCLUSIONS
These measurements of rCBF with [15O]water PET document that a slightly subphysiological plasma glucose concentration of 65 mg/dL (3.6 mmol/L) increases synaptic activity at least predominantly, perhaps selectively, in the dorsal midline thalamus in humans. This novel finding extends the evidence that the thalamus is involved in the physiology of glucose counterregulation to a slight reduction in plasma glucose that activates glucose counterregulatory systems, including epinephrine secretion, but is not low enough to cause symptoms of hypoglycemia. Thus, the dorsal midline thalamus is potentially relevant to the pathogenesis of iatrogenic hypoglycemia in diabetes (6).
The dorsal midline thalamus and the medial prefrontal cortex are part of an integrated cerebral network that mediates autonomic responses to various visceral stimuli (20–22) and may mediate hypothalamic activity and sympathoadrenal outflow from the brain during hypoglycemia. In a model of hypoglycemia-associated autonomic failure in diabetes produced in nondiabetic humans, we found that the effect of recent antecedent hypoglycemia to attenuate sympathoadrenal and symptomatic responses to subsequent hypoglycemia was associated with increased synaptic activation in the dorsal midline thalamus, and only in that brain region, during subsequent hypoglycemia (6). Based on that finding, and the phenomenon of habituation of responses to recurrent stress (23), we suggested that the thalamus may exert an inhibitory effect on hypothalamic sympathoadrenal outflow during recurrent hypoglycemia (6) and that mechanism might result in hypoglycemia-associated autonomic failure in diabetes (6).
The dorsal midline thalamus may function as a relay to modulate the hypothalamic response to falling plasma glucose levels based on previous physiological experience. It receives both internal and external sensory inputs that can provide influence over visceral and autonomic functions (20,21,24). There are both direct and indirect outputs from the dorsal midline thalamus to the hypothalamus. It can project indirectly to the hypothalamus via the medial prefrontal cortex and amygdala (20,21,25–27), or the paraventricular nucleus of the thalamus can project directly to the ventromedial hypothalamus (25). Given the neuroanatomical connections of the dorsal midline thalamus within the postulated network involved in responses to hypoglycemia, the thalamic activation could be due to excitatory or inhibitory input. However, the present finding of synaptic activation by only slightly subphysiological plasma glucose concentrations further indicates that the dorsal midline thalamus may be involved in the pathogenesis of hypoglycemia-associated autonomic failure in diabetes.
In conclusion, these data suggest that there is a hierarchy among the brain responses to falling plasma glucose concentrations with synaptic activation in the dorsal midline thalamus coinciding with activation of glucose counterregulatory systems as plasma glucose concentrations drift just below physiological levels.
Acknowledgments
This study was supported, in part, by National Institutes of Health (NIH) grants R37 DK27085, MO1 RR00036 (now UL1 RR24992), and P60 DK20579, and by Washington University in St. Louis. A.M.A. was supported, in part, by NIH grant UL1RR024992.
P.E.C. has served as a consultant to Bristol-Myers Squibb/AstraZeneca and Novo Nordisk in the past year. No other potential conflicts of interest relevant to this article were reported.
A.M.A. planned, performed, and conducted the study, analyzed data, and wrote the manuscript. J.R.R., T.H., and T.O.V. analyzed data and wrote the manuscript. W.J.P. and P.E.C. planned the study, analyzed data, and wrote the manuscript. A.M.A. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented as an oral presentation at the 70th Scientific Sessions of the American Diabetes Association, Orlando, Florida, 25–29 June 2010.
The authors acknowledge the assistance of the staff of the Washington University Clinical Research Unit in the performance of this study; the technical assistance of Krishan Jethi, Lennis Lich, the Washington University Clinical Research Unit, Medical School Cyclotron staff, the Center for Clinical Imaging Research, and the East Building Magnetic Resonance Imaging Facility staff for their assistance. Ms. Janet Dedeke, the senior author’s assistant, prepared this manuscript. All are staff at Washington University in St. Louis.
References
- 1.Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes 2008;57:3169–3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cryer PE. Death during intensive glycemic therapy of diabetes: mechanisms and implications. Am J Med 2011;124:993–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skrivarhaug T, Bangstad H-J, Stene LC, Sandvik L, Hanssen KF, Joner G. Long-term mortality in a nationwide cohort of childhood-onset type 1 diabetic patients in Norway. Diabetologia 2006;49:298–305 [DOI] [PubMed] [Google Scholar]
- 4.Cryer PE. The prevention and correction of hypoglycemia. In Handbook of Physiology Section 7, The Endocrine System Volume II, The Endocrine Pancreas and Regulation of Metabolism . Jefferson LS, Cherrington AD, Eds. New York, Oxford University Press, 2001, p. 1057–1092 [Google Scholar]
- 5.Teves D, Videen TO, Cryer PE, Powers WJ. Activation of human medial prefrontal cortex during autonomic responses to hypoglycemia. Proc Natl Acad Sci USA 2004;101:6217–6221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arbelaez AM, Powers WJ, Videen TO, Price JL, Cryer PE. Attenuation of counterregulatory responses to recurrent hypoglycemia by active thalamic inhibition: a mechanism for hypoglycemia-associated autonomic failure. Diabetes 2008;57:470–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teh MM, Dunn JT, Choudhary P, et al. Evolution and resolution of human brain perfusion responses to the stress of induced hypoglycemia. Neuroimage 2010;53:584–592 [DOI] [PubMed] [Google Scholar]
- 8.Towler DA, Havlin CE, Craft S, Cryer P. Mechanism of awareness of hypoglycemia. Perception of neurogenic (predominantly cholinergic) rather than neuroglycopenic symptoms. Diabetes 1993;42:1791–1798 [DOI] [PubMed] [Google Scholar]
- 9.Shah SD, Clutter WE, Cryer PE. External and internal standards in the single-isotope derivative (radioenzymatic) measurement of plasma norepinephrine and epinephrine. J Lab Clin Med 1985;106:624–629 [PubMed] [Google Scholar]
- 10.Wienhard K, Dahlbom M, Eriksson L, et al. The ECAT EXACT HR: performance of a new high resolution positron scanner. J Comput Assist Tomogr 1994;18:110–118 [PubMed] [Google Scholar]
- 11.Herscovitch P, Markham J, Raichle ME. Brain blood flow measured with intravenous H2(15)O. I. Theory and error analysis. J Nucl Med 1983;24:782–789 [PubMed] [Google Scholar]
- 12.Raichle ME, Martin WR, Herscovitch P, Mintun MA, Markham J. Brain blood flow measured with intravenous H2(15)O. II. Implementation and validation. J Nucl Med 1983;24:790–798 [PubMed] [Google Scholar]
- 13.Videen TO, Perlmutter JS, Herscovitch P, Raichle ME. Brain blood volume, flow, and oxygen utilization measured with 15O radiotracers and positron emission tomography: revised metabolic computations. J Cereb Blood Flow Metab 1987;7:513–516 [DOI] [PubMed] [Google Scholar]
- 14.Woods RP, Cherry SR, Mazziotta JC. Rapid automated algorithm for aligning and reslicing PET images. J Comput Assist Tomogr 1992;16:620–633 [DOI] [PubMed] [Google Scholar]
- 15.Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging. New York, Thieme Medical Publishers, 1988 [Google Scholar]
- 16.Ingvar M, Eriksson L, Greitz T, et al. Methodological aspects of brain activation studies: cerebral blood flow determined with [15O]butanol and positron emission tomography. J Cereb Blood Flow Metab 1994;14:628–638 [DOI] [PubMed] [Google Scholar]
- 17.Friston KJ. Commentary and opinion: II. Statistical parametric mapping: ontology and current issues. J Cereb Blood Flow Metab 1995;15:361–370 [DOI] [PubMed] [Google Scholar]
- 18.Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 1996;4:58–73 [DOI] [PubMed] [Google Scholar]
- 19.Mintun MA, Fox PT, Raichle ME. A highly accurate method of localizing regions of neuronal activation in the human brain with positron emission tomography. J Cereb Blood Flow Metab 1989;9:96–103 [DOI] [PubMed] [Google Scholar]
- 20.Raichle ME. Behind the scenes of functional brain imaging: a historical and physiological perspective. Proc Natl Acad Sci USA 1998;95:765–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ongür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex 2000;10:206–219 [DOI] [PubMed] [Google Scholar]
- 22.Price JL. Prefrontal cortical networks related to visceral function and mood. Ann N Y Acad Sci 1999;877:383–396 [DOI] [PubMed] [Google Scholar]
- 23.Bhatnagar S, Huber R, Nowak N, Trotter P. Lesions of the posterior paraventricular thalamus block habituation of hypothalamic-pituitary-adrenal responses to repeated restraint. J Neuroendocrinol 2002;14:403–410 [DOI] [PubMed] [Google Scholar]
- 24.Herman JP, Figueiredo H, Mueller NK, et al. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol 2003;24:151–180 [DOI] [PubMed] [Google Scholar]
- 25.Moga MM, Weis RP, Moore RY. Efferent projections of the paraventricular thalamic nucleus in the rat. J Comp Neurol 1995;359:221–238 [DOI] [PubMed] [Google Scholar]
- 26.Spencer SJ, Fox JC, Day TA. Thalamic paraventricular nucleus lesions facilitate central amygdala neuronal responses to acute psychological stress. Brain Res 2004;997:234–237 [DOI] [PubMed] [Google Scholar]
- 27.Huang H, Ghosh P, van den Pol AN. Prefrontal cortex-projecting glutamatergic thalamic paraventricular nucleus-excited by hypocretin: a feedforward circuit that may enhance cognitive arousal. J Neurophysiol 2006;95:1656–1668 [DOI] [PubMed] [Google Scholar]