Abstract
OBJECTIVE
Behavioral interventions targeting “free-living” physical activity (PA) and exercise that produce long-term glycemic control in adults with type 2 diabetes are warranted. However, little is known about how clinical teams should support adults with type 2 diabetes to achieve and sustain a physically active lifestyle.
RESEARCH DESIGN AND METHODS
We conducted a systematic review of randomized controlled trials (RCTs) (published up to January 2012) to establish the effect of behavioral interventions (compared with usual care) on free-living PA/exercise, HbA1c, and BMI in adults with type 2 diabetes. Study characteristics, methodological quality, practical strategies for increasing PA/exercise (taxonomy of behavior change techniques), and treatment fidelity strategies were captured using a data extraction form.
RESULTS
Seventeen RCTs fulfilled the review criteria. Behavioural interventions showed statistically significant increases in objective (standardized mean difference [SMD] 0.45, 95% CI 0.21–0.68) and self-reported PA/exercise (SMD 0.79, 95% CI 0.59–0.98) including clinically significant improvements in HbA1c (weighted mean difference [WMD] –0.32%, 95% CI –0.44% to –0.21%) and BMI (WMD –1.05 kg/m2, 95% CI –1.31 to –0.80). Few studies provided details of treatment fidelity strategies to monitor/improve provider training. Intervention features (e.g., specific behavior change techniques, interventions underpinned by behavior change theories/models, and use of ≥10 behaviour change techniques) moderated effectiveness of behavioral interventions.
CONCLUSIONS
Behavioral interventions increased free-living PA/exercise and produced clinically significant improvements in long-term glucose control. Future studies should consider use of theory and multiple behavior change techniques associated with clinically significant improvements in HbA1c, including structured training for care providers on the delivery of behavioural interventions.
The global prevalence of diabetes is predicted to increase from 171 million individuals (2.8%) in 2000 to 336 million (4.4%) in 2030 (1). Because the increase in prevalence is most marked in younger adults, the disease is expected to inflict a devastating toll on the future working-age population in terms of premature coronary heart disease, amputations, and blindness (2). The main causal risk factor for type 2 diabetes is an imbalance between energy expenditure and energy intake through food consumption (3,4). Drug-based interventions are unlikely to provide the solution to this widespread problem and interventions aimed at increasing energy expenditure through physical activity may provide an effective alternative, because the majority of people with type 2 diabetes are physically inactive when compared with national averages (5).
Physical activity (PA; regular movement such as walking) and exercise (structured activities such as running or cycling), along with diet and medication, are the cornerstones of diabetes management (6). Several reviews (7,8) and meta-analyses (6,9–11) report that increased PA and/or exercise produce a significant improvement in glucose control in people with type 2 diabetes, yielding an average improvement in hemoglobin A1c (HbA1c) of between −0.4% and −0.6%. Despite the clear benefits of increased PA and exercise upon glycemic control, little is known about how clinical care teams should support people with diabetes to achieve and sustain a physically active lifestyle. This evidence-practice gap is seriously hindering the effectiveness of PA and exercise as a therapeutic intervention in routine diabetes care.
Behavioral interventions targeting PA and exercise are heterogeneous in terms of content, implementation, and effectiveness. Interventions differ on a range of dimensions, for example, the theory of behavior change used to underpin them; the behavior change techniques used to encourage change (e.g., goal setting, use of follow-up prompts); and delivery of the intervention (e.g., frequency and duration of contact; one-to-one vs. group delivery). Working around a theory or model of behavior change may assist selecting, sequencing, and communicating relevant behavior change techniques. Techniques, in turn, describe the means of operationalization, e.g., what interventionists do to bring about change, regardless of the use of explicit theory. Despite the benefits of behavior change theory and specific theory-linked behavior change techniques (12,13), historically behavioral interventions have frequently omitted adequate descriptions of the specific theory or model of behavior change used, explicit details of intervention content, and how this was operationalized and evaluated (14), limiting the efficacy of the intervention and replication outside the research setting. Elucidating the theory, content, and delivery of interventions may help to explain the heterogeneity in effect sizes usually observed in systematic reviews and, thereby, to identify what works and what does not, which provides the evidence needed to direct clinical care and research.
Our objective was to conduct a systematic review to answer the following questions: are behavioral interventions more effective than standard clinical care for improving “free-living” PA and exercise and HbA1c in adults with type 2 diabetes in clinical or community settings, and what behavior change theories or theory-linked behavior change techniques (and other features of behavioral interventions) are associated with clinically significant improvements in HbA1c?
RESEARCH DESIGN AND METHODS
This systematic review followed a published protocol (15) and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (16).
Inclusion criteria
Studies were randomized controlled trials (RCTs) of behavioral interventions targeting free-living PA and exercise in adults (18 years or older) with type 2 diabetes (controlled by diet or oral medication or insulin therapy) with a minimum follow-up period of 1 month from baseline. Interventions were delivered in clinical and community settings. Studies also included the primary outcomes: change in level of PA and exercise and change in HbA1c.
Exclusion criteria
Studies were excluded if interventions targeted PA and exercise and diet, although studies were retained if all study participants received a dietary component that was consistent with usual care. Studies were also excluded if they targeted multiple chronic diseases; gestational diabetes or had no focus on engaging in free-living PA and exercise outside of supervised sessions. Studies that included the following components were also excluded: combinations of diet or pharmacological agents with PA and exercise in one arm of the trial; comparisons of pharmacological agents alongside and against PA and exercise; or comparisons of different behavioral interventions targeting PA and exercise that did not include a comparison arm that constituted usual care.
Search strategy
PsycINFO, Medline, CINAHL, EMBASE, Scopus, and the Cochrane Library were searched using a combination of MeSH headings and key words to identify potentially relevant literature (Supplementary Table 1). Searches were completed up to January 23, 2012, and were limited to RCTs published in the English language. Manual searching of reference lists and citation searching of studies fulfilling the eligibility criteria were also conducted.
Selection of studies
Two authors independently screened the titles and abstracts of articles. Articles retained at the first stage were reassessed independently for inclusion by the same two authors using a study selection form, with disagreements resolved via discussion with the review team.
Data extraction
Details on the study population, interventions, comparators, and outcomes were captured using a structured data extraction form. All included studies underwent independent assessment by at least two of the authors (disagreements were resolved via discussion). Corresponding authors of included studies were contacted via e-mail to request additional data when applicable. The Cochrane Collaboration risk of bias tool (17) was used to appraise methodological quality and assess overall risk of bias (low, unclear, or high) within and across studies for each outcome. Data on treatment fidelity were assessed using published guidance (18). Descriptions of intervention content were coded into specific theory-linked behavior change techniques using a reliable and comprehensive taxonomy for intervention techniques targeting PA (19). Behavior change techniques utilized in both intervention and usual care groups were not coded to enable identification of those techniques that could be attributed to changes in outcomes.
Data synthesis
Data on changes in PA and exercise, HbA1c, and BMI were synthesized using meta-analytic techniques (RevMan v5.1, Cochrane IMS). Studies reporting sufficient data to enable calculation of effect sizes were included in meta-analyses. Random effects models were selected to allow for between-group and within-group differences (17). When studies included multiple trial arms, data on each intervention arm compared with the usual care arm were included in meta-analyses. Excessive weightings were controlled in studies with multiple intervention arms, consistent with published guidance (17). Heterogeneity was assessed using I2, with values >50% considered heterogeneous (20). Measures of intervention effects on PA and exercise, HbA1c, and BMI are presented as a function of timing of follow-up measurements: ≥1 month to <6 months (short-term); 6 months (short-term to medium-term); 12 months (medium-term); and 24 months (long-term). Overall measures of effect for interventions represent average effect sizes across these follow-up periods.
To account for variation in the methods used to assess PA and exercise across studies, objective measures (accelerometer [activity counts and/or minutes spent active] and pedometer [steps]) were combined in meta-analyses. Self-reported data on PA and exercise were combined if sufficient information was provided about the content of the measures (i.e., 7-day recall of PA and conversion of activity intensity into MET values or minutes/hours spent active). Sensitivity analyses were undertaken by excluding outlying studies and any with negative ratings on indices of methodological quality.
Moderator analyses were conducted on characteristics of behavioral interventions identified in a minimum of three studies to explore any impact on change in HbA1c (≥−0.30% HbAlc as a cutoff value for a clinically significant improvement). These analyses should be considered exploratory and were undertaken to identify any potential foci of future research and clinical practice.
RESULTS
Nineteen articles reporting 17 RCTs (21–37) fulfilled the review criteria (Supplementary Fig. 1). Two RCTs (27,30) were reported across two articles (38,39). For another four RCTs (21,29,36,37), additional articles were consulted to obtain information on intervention content (40–47).
Eleven RCTs were conducted in Europe (21,22,24–28,30–32,37), two in Australia (23,35), three in North America (33,34,36), and one in Asia (29). Authors of 13 RCTs utilized a theory/model of behavior change to develop and deliver interventions: Transtheoretical Model (29–31,37); Social Cognitive Theory (21,22,27,36); and Precede/Proceed Model (35). Four studies stated that interventions were underpinned by multiple theories/models: Transtheoretical Model and Social Cognitive Theory (34); Cognitive Behavioral Therapy; Motivational Interviewing; and Social Cognitive Theory (24–26).
The studies had a combined total sample size of 1,975. Eight studies included approximately equal numbers of women and men (25,27–31,36,37), whereas nine studies had disproportionate numbers of women (23,32–35) and men (21,22,24,26). Participants were, on average, aged between 51 and 55 (29,33,36,37), 58 and 59 (21,30), 60 and 64 (22,23,26–28,31,32,34,35), or 66 and 70 years (25). One study included participants aged 35 to 75 years (24). Information on time since diagnosis was described in 11 studies (21,22,24–27,29,31,32,34,36). Twelve studies (21–23,25–27,29,31,32,35–37) reported sufficient information on management of type 2 diabetes (diet, oral medication, and/or insulin therapy). A summary of the key characteristics of the 17 RCTs can be found in Supplementary Table 2.
Methodological quality assessment and treatment fidelity
Supplementary Table 3 presents details of methodological quality assessment and overall risk of bias within and across studies for each outcome. Eight studies provided sufficient information to establish the use of adequate randomization sequences (21,25,27,28,30,31,33,34). Six studies provided sufficient detail on the methods used to conceal allocation sequences (21,24,25,30,31,33). Seven studies provided explicit detail on the use of blinding of care providers (22,37) or outcome assessors (21,24,25,31,33). The majority of studies provided sufficient detail to establish the likely absence of selective outcome reporting, incomplete outcome data, and other potential sources of bias.
Twelve studies reported a power calculation (21,22,24–31,33,34), with nine reporting achievement of required sample sizes at final follow-up (21,22,26–31,34). One study had an attrition rate of >20% at final follow-up (36). Eleven studies reported using an intention-to-treat analysis (21,22,24–28,30,31,33,34). The overall risk of bias for HbA1c, self-reported PA, and BMI was graded as “unclear,” and “low” for objectively assessed PA.
The treatment fidelity assessment (Supplementary Table 4) identified that all 17 studies provided sufficient detail to establish the use of treatment fidelity strategies related to study design (e.g., measures taken to ensure length/duration and frequency of contact within intervention groups).
Five studies referred to training of interventionists (21,25,29–31), although only two studies explicitly described strategies for monitoring and improving interventionist training (21,25). Fourteen studies described methods to improve delivery of interventions (21,22,24–27,29–31,33–37) (e.g., providing frequent supervision to interventionists, using scripted intervention protocols, and taking steps to control contamination across intervention and usual care groups). All 17 studies provided sufficient detail to establish use of strategies to monitor and improve the ability of patients to understand the intervention-related cognitive and behavioral skills and strategies to monitor and improve enactment of intervention-related skills in relevant real-life settings (e.g., prompting participants to set goals, self-monitoring of progress, conducting follow-up discussions and telephone calls, and opportunities for participants to review the effect of increased PA on blood glucose levels).
Changes in PA and exercise
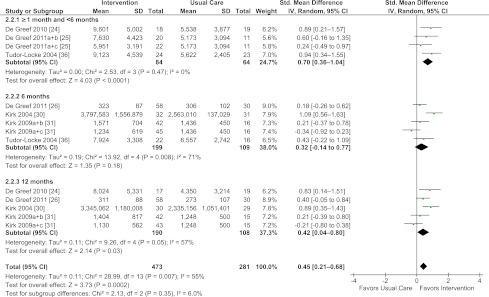
Behavioral interventions (compared with usual care) showed a statistically significant increase in levels of objectively assessed PA and exercise (standardized mean difference [SMD] = 0.45, 95% CI = 0.21–0.68, I2 = 55%) based on data from six studies (24,25,26,30,31,36) (Fig. 1). With the exception of 6 months, this effect was found for the follow-up period ≥1 month to <6 months (SMD = 0.70, CI = 0.36–1.04, I2= 0%) and 12 months (SMD = 0.42, CI = 0.04–0.80, I2 = 57%). Sensitivity analyses (exclusion of one study with a high attrition rate) (36) resulted in a slight decrease in magnitude of the overall effect (SMD = 0.41, CI = 0.15–0.66, I2 = 58%) and at the ≥1 month and <6 months follow-up period (SMD = 0.59, CI = 0.18–1.00, I2= 0%).
Figure 1.
Forest plot for objective PA and exercise. (A high-quality color representation of this figure is available in the online issue.)
Likewise, the 14 RCTs providing self-reported PA and exercise data showed an overall significant positive intervention effect (SMD = 0.79, 95% CI = 0.59–0.98, I2 = 74%) (21–23,25–27,29–35,37) (Supplementary Fig. 2). These effects were maintained across all follow-up periods, with the exception of 24 months.
Changes in HbA1c
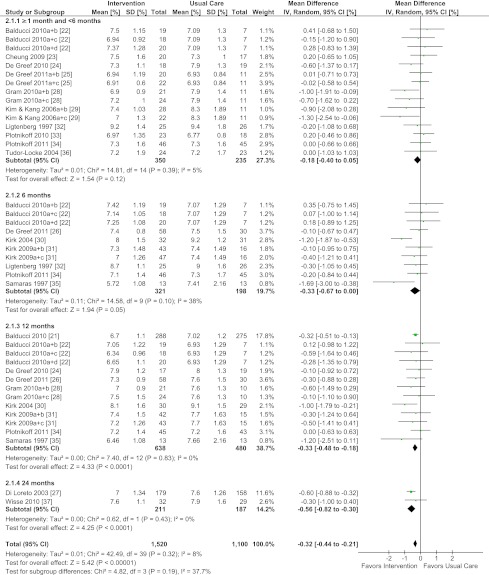
Behavioral interventions (compared with usual care) showed statistically and clinically significant improvements in HbA1c (weighted mean difference [WMD] = −0.32%, 95% CI = −0.44 to –0.21%, I2 = 8%) based on data from 17 studies (21–37) (Fig. 2). With the exception of ≥1 month to <6 months, statistically significant improvements were found across all follow-up periods: 6 months (WMD = −0.33%, CI = −0.67 to 0.00%, I2 = 38%); 12 months (WMD = −0.33%, CI = −0.48 to −0.18%, I2 = 0%); and 24 months (WMD = −0.56%, CI = −0.82 to −0.30%, I2= 0%). Removal of a study with a high attrition rate (36) did not change the conclusions regarding the overall effect.
Figure 2.
Forest plot for HbA1c. (A high-quality color representation of this figure is available in the online issue.)
Changes in BMI
Behavioral interventions targeting PA and exercise (compared with usual care) showed an overall statistically significant reduction in BMI (kg/m2) based on data from 11 studies (21–25,27,28,30,31,33,37) (WMD = −1.05 kg/m2, 95% CI = −1.31 to −0.80, I2 = 2%) (Supplementary Fig. 3). A decrease in BMI was evident across follow-up periods: ≥1 month to <6 months (WMD = −0.75 kg/m2, CI = −1.22 to −0.28, I2 = 0%); 6 months (WMD = −0.77 kg/m2, CI = −1.39 to −0.15, I2= 20%); 12 months (WMD = −1.32 kg/m2, CI = −1.73 to −0.90, I2 = 0%); and 24 months (WMD = −1.52 kg/m2, CI = −2.23 to −0.81, I2= 0%).
Moderators of intervention effects on HbA1c
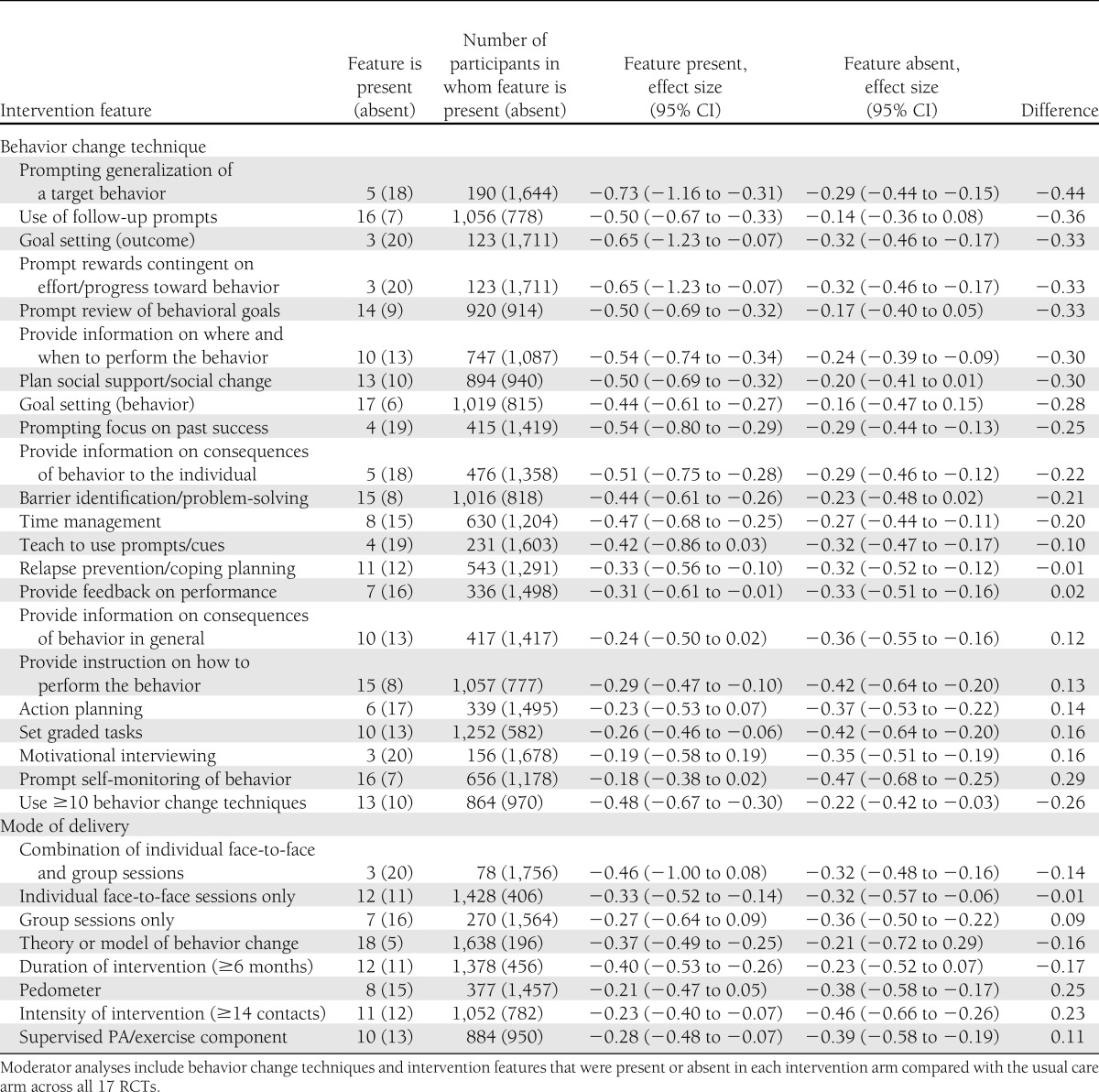
Twenty-five different behavior change techniques were identified across the 17 studies (Supplementary Table 5). The studies used a minimum of two and a maximum of 14 behavior change techniques (median 10, IQR 8).
Exploratory moderator analyses comparing effect size estimates for trials with or without a range of intervention features (i.e., behavior change techniques, modes of delivery, and theory use, Table 1) suggested that effects varied considerably. Although these differences do not equate to statistical significance reflecting the limited power based on the current evidence, these analyses suggest that utilization of 10 different behavior change techniques within behavioral interventions may be associated with clinically significant improvements in HbA1c (≥0.3% HbA1c): prompting generalization of a target behavior; use of follow-up prompts; prompt review of behavioral goals; provide information on where and when to perform PA; plan social support/social change; goal setting (behavior); time management; prompting focus on past success; barrier identification/problem-solving; and providing information on the consequences specific to the individual.
Table 1.
Moderating effect of intervention features on HbA1c (%)
Clinically significant improvements in HbA1c were also suggested for studies utilizing more behavior change techniques (median ≥10), interventions underpinned by a theory or model of behavior change, and durations of ≥6 months. These analyses also suggested that different modes of intervention delivery, interventions utilizing pedometers, interventions of greater intensity (median ≥14 contacts), and inclusion of a supervised PA and exercise component were not associated with clinically significant improvements in HbA1c.
CONCLUSIONS
There is evidence to report that behavioral interventions (with low or unclear risk of bias) targeting increased PA and exercise produce a clinically significant improvement in HbA1c in adults with type 2 diabetes. Specific behavior change techniques may increase the likelihood of clinically significant improvements in HbA1c. Regarding utilization of behavior change techniques, more might be better. Other intervention features such as utilization of theory and intervention duration may also impact upon intervention effectiveness.
A strength of this dataset is that all studies were undertaken in clinical care or community settings, demonstrating potential clinical utility. The major implication of this review is that behavioral interventions have potential to effectively reduce HbA1c in adults with type 2 diabetes in routine clinical care. The reductions in HbA1c of 0.21 to 0.44% are consistent with previous meta-analyses, including a wider collection of PA and exercise studies (6–11). Follow-up periods of <6 months failed to show a significant impact on HbA1c. However, <6 months duration is not sufficient to elicit an observable effect on HbA1c, highlighting that longer behavioral interventions are needed if clinically meaningful changes in HbA1c are desired. The benefits to glycemic control were sustained for up to 24 months (Fig. 2) and were comparable with common long-term drug or insulin therapy (48,49). Potential limitations of this review are the disparate definitions of “usual care” across studies and the possibility of selection and/or performance and detection bias attributable to either absence or lack of reporting on allocation concealment and blinding, respectively. However, blinding of participants and study personnel is inherently problematic in behavioral studies. Publication bias is possible, although an inspection of funnel plots for primary outcomes did not show any substantial asymmetry, indicating a low risk of publication or small study bias.
Critically, all studies reviewed involved supporting adults with diabetes to undertake free-living PA and exercise. Despite the focus on PA and exercise, meta-analyses reported significant heterogeneity for objective (Fig. 1) and self-reported PA (Supplementary Fig. 2). Although there were individual differences in response, the variations in sensitivity and specificity in monitoring PA and exercise between the different objective and self-reported methods observed will have contributed to this heterogeneity. The lack of accurate, standardized, and transparent methods for monitoring PA and exercise remains a significant barrier to accurately determining the efficacy of interventions targeting free-living PA and exercise and should be addressed in future studies. This lack of sensitivity also makes describing what participants are doing difficult, limiting discussion about what type, intensity, and frequency are minimal and optimal to confer benefit. Furthermore, many interventions reported reductions in BMI (Supplementary Fig. 3). Because PA or exercise alone is generally insufficient to incur weight loss (50), this suggests an impact of behavioral interventions targeting PA and exercise on calorie intake, and Hawthorne effects cannot be ruled out. Furthermore, there is a complex relationship between mode and intensity of activity, nutrition, and the behavioral methods used to achieve these that is not addressed in this article. However, the lack of detail about calorie intake or different modes of PA and exercise should not detract from the reported clinical utility of behavioral interventions targeting PA and exercise in terms of long-term glucose control.
The studies identified used a range of behavior change theories and behavior change techniques that may moderate the effectiveness of the behavioral interventions. Although effect sizes found in moderator analyses do not differ significantly from the main findings, they help identify specific candidate behavior change techniques most likely to be effective for future research and as potential foci for clinical practice. Ten behavior change techniques (19) were associated with potential clinically significant improvements in HbA1c: prompting generalization of a target behavior (e.g., once PA is performed in one situation, the individual is encouraged to try it in another); use of follow-up prompts (e.g., telephone calls in place of face-to-face sessions to support maintenance); prompt review of behavioral goals (e.g., review whether PA goals were achieved followed by revisions or adjustments); providing information on where and when to be active (e.g., tips on places and times to access local PA and exercise clubs); plan social support and social change (e.g., encourage individuals to elicit social support from others to help achieve a PA-related goal); goal setting (e.g., supporting individuals to formulate specific, measurable, achievable, relevant, and timely PA-related goals); time management (e.g., making time to be active); prompting focus on past success (e.g., identifying previous successful attempts at PA); barrier identification/problem solving (e.g., identifying potential barriers to PA and ways to overcome them); and providing information on the consequences of PA specific to the individual (e.g., information about the benefits and costs of PA to individuals). This list of behavior change techniques is not definitive and is limited in terms of statistical power by the available body of evidence. More research is needed to determine effectiveness of single or aggregated behavior change techniques in randomized trials and to investigate how clusters of these techniques could be individually tailored for people with diabetes. To enhance reproducibility, attention should also be given to highlighting the utilization of specific behavior change techniques, with reference to a reliable taxonomy (19), when describing intervention content. Because HbA1c is influenced by the type, intensity, and frequency of PA and exercise, it is possible that some of the behavior change techniques were successful in changing behavior, but that the level of PA and exercise achieved was insufficient to improve glycemic control. The limited description of intervention content in studies may also limit the ability to identify the success of a single behavior change technique. As such, caution should be given to dismissing some of the behavior change techniques that, in the current review, either were not identified or were not associated with clinically significant improvements in HbA1c. Exploratory moderator analyses suggested that the following intervention features were associated with clinically significant improvements in HbA1c: underpinning interventions with behavior change theories/models (although no one model appeared to hold benefit over others); utilizing ≥10 behavior change techniques; and intervention duration of ≥6 months.
Further research also is required to determine what professional training enables care providers to effectively deliver behavioral interventions. Five studies reported that individuals delivering the interventions had been trained for this purpose, but only two studies provided information on mode, content, and utilization of strategies for monitoring and improving delivery of training. Professional training is a crucial component of behavioral interventions because it improves treatment fidelity and enhances reproducibility in routine practice. Both the mode of delivery and pathway of care provider training have a significant impact on the cost of delivering the intervention and, as a result, the likelihood of implementation in routine care. Future studies, in addition to describing behavior change theories, behavior change techniques and how these were operationalized also should report on how care providers were trained and aspects of treatment fidelity. This increased clarity will assist in addressing the current evidence-practice gap and will serve to increase the efficacy of PA as a management option in routine diabetes care.
Combined, these data reveal that behavioral interventions targeting increased PA and exercise in clinical care or community settings have significant clinical utility. Although these observations are encouraging, there remains a pressing need for further research to understand how these should be optimized and implemented into routine clinical care.
Supplementary Material
Acknowledgments
This study was funded by the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement Health-F2-2009-241762 for the project FLIP. L.A. is supported by a PhD scholarship from the Newcastle Centre for Brain Ageing and Vitality. M.I.T. is supported by a Senior Fellowship from the National Institute of Health Research. F.F.S. is funded by Fuse, the Centre for Translational Research in Public Health, a UK Clinical Research Collaboration Public Health Research Centre of Excellence.
No conflicts of interest relevant to this article were reported.
L.A., D.F., F.F.S., and M.I.T. conceived the review protocol. F.F.S. and M.I.T. supervised the review. L.A., D.F., A.v.W., and F.F.S. developed the search strategy. L.A. performed the electronic searches. L.A. and D.F. conducted the screening of titles and abstracts and evaluated the eligibility of full text articles. L.A. and M.I.T. assessed included studies for methodological quality and overall risk of bias (within and across studies for each outcome). L.A. and D.F. assessed included studies for treatment fidelity. L.A. conducted the meta-analyses and moderator analyses. All authors provided input to the development of the methods, data extraction, data analyses, and drafting of the manuscript. M.I.T. is the guarantor of this work and, as such, had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Elements of this systematic review were presented as a poster at the Diabetes UK Annual Professional Conference, Glasgow, U.K., 7–9 March 2012.
The authors acknowledge the assistance of Linda Errington, Assistant Librarian, Stephan Dombrowski, Research Associate, and Andy Bryant, Research Assistant (Statistician), from the Faculty of Medical Sciences, Newcastle University, U.K., for guidance with electronic searching and meta-analyses. The authors also thank the corresponding authors of included studies who responded to requests for additional data.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-2452/-/DC1.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–1053 [DOI] [PubMed] [Google Scholar]
- 2.Lazar MA. How obesity causes diabetes: not a tall tale. Science 2005;307:373–375 [DOI] [PubMed] [Google Scholar]
- 3.Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science 2003;299:853–855 [DOI] [PubMed] [Google Scholar]
- 4.Taylor R. Pathogenesis of type 2 diabetes: tracing the reverse route from cure to cause. Diabetologia 2008;51:1781–1789 [DOI] [PubMed] [Google Scholar]
- 5.Morrato EH, Hill JO, Wyatt HR, Ghushchyan V, Sullivan PW. Physical activity in U.S. adults with diabetes and at risk for developing diabetes, 2003. Diabetes Care 2007;30:203–209 [DOI] [PubMed] [Google Scholar]
- 6.Boulé NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA 2001;286:1218–1227 [DOI] [PubMed] [Google Scholar]
- 7.Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C. Physical activity/exercise and type 2 diabetes. Diabetes Care 2004;27:2518–2539 [DOI] [PubMed] [Google Scholar]
- 8.Zanuso S, Jimenez A, Pugliese G, Corigliano G, Balducci S. Exercise for the management of type 2 diabetes: a review of the evidence. Acta Diabetol 2010;47:15–22 [DOI] [PubMed] [Google Scholar]
- 9.Thomas DE, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev 2006;3:CD002968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snowling NJ, Hopkins WG. Effects of different modes of exercise training on glucose control and risk factors for complications in type 2 diabetic patients: a meta-analysis. Diabetes Care 2006;29:2518–2527 [DOI] [PubMed] [Google Scholar]
- 11.Umpierre D, Ribeiro PAB, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA 2011;305:1790–1799 [DOI] [PubMed] [Google Scholar]
- 12.Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council Guidance. BMJ 2008;337:a1655 [DOI] [PMC free article] [PubMed]
- 13.Michie S, Prestwich A. Are interventions theory-based? Development of a theory coding scheme. Health Psychol 2010;29:1–8 [DOI] [PubMed] [Google Scholar]
- 14.Dombrowski SU, Sniehotta FF, Avenell S. Towards a cumulative science of behaviour change: do current conduct and reporting of behavioural interventions fall short of good practice? Psychol Health 2007;22:869–874 [Google Scholar]
- 15.Avery L, Flynn D, van Wersch A, Trenell MI, Sniehotta FF. A systematic review of behaviour change interventions targeting physical activity, exercise and HbA1c in adults with type 2 diabetes. PROSPERO 2011:CRD42011001274,2011. Available from http://www.crd.york.ac.uk/PROSPERO/full_doc.asp?ID=CRD42011001274 Accessed 5 Dec 2011
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JPT, Green S (Eds.). Cochrane handbook for systematic reviews of interventions version 5.0.2, The Cochrane Collaboration, 2009. Available from www.cochrane-handbook.org Accessed 3 March 2011
- 18.Bellg AJ, Borrelli B, Resnick B, et al. Treatment Fidelity Workgroup of the NIH Behavior Change Consortium Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol 2004;23:443–451 [DOI] [PubMed] [Google Scholar]
- 19.Michie S, Ashford S, Sniehotta FF, Dombrowski SU, Bishop A, French DP. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO-RE taxonomy. Psychol Health 2011;26:1479–1498 [DOI] [PubMed] [Google Scholar]
- 20.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balducci S, Zanuso S, Nicolucci A, et al. Italian Diabetes Exercise Study (IDES) Investigators Effect of an intensive exercise intervention strategy on modifiable cardiovascular risk factors in subjects with type 2 diabetes mellitus: a randomized controlled trial: the Italian Diabetes and Exercise Study (IDES). Arch Intern Med 2010;170:1794–1803 [DOI] [PubMed] [Google Scholar]
- 22.Balducci S, Zanuso S, Nicolucci A, et al. Anti-inflammatory effect of exercise training in subjects with type 2 diabetes and the metabolic syndrome is dependent on exercise modalities and independent of weight loss. Nutr Metab Cardiovasc Dis 2010;20:608–617 [DOI] [PubMed] [Google Scholar]
- 23.Cheung NW, Cinnadaio N, Russo M, Marek S. A pilot randomised controlled trial of resistance exercise bands in the management of sedentary subjects with type 2 diabetes. Diabetes Res Clin Pract 2009;83:e68–e71 [DOI] [PubMed] [Google Scholar]
- 24.De Greef K, Deforche B, Tudor-Locke C, De Bourdeaudhuij I. A cognitive-behavioural pedometer-based group intervention on physical activity and sedentary behaviour in individuals with type 2 diabetes. Health Educ Res 2010;25:724–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Greef K, Deforche B, Tudor-Locke C, De Bourdeaudhuij I. Increasing physical activity in Belgian type 2 diabetes patients: a three-arm randomized controlled trial. Int J Behav Med 2011;18:188–198 [DOI] [PubMed] [Google Scholar]
- 26.De Greef KP, Deforche BI, Ruige JB, et al. The effects of a pedometer-based behavioral modification program with telephone support on physical activity and sedentary behavior in type 2 diabetes patients. Patient Educ Couns 2011;84:275–279 [DOI] [PubMed] [Google Scholar]
- 27.Di Loreto C, Fanelli C, Lucidi P, et al. Validation of a counseling strategy to promote the adoption and the maintenance of physical activity by type 2 diabetic subjects. Diabetes Care 2003;26:404–408 [DOI] [PubMed] [Google Scholar]
- 28.Gram B, Christensen R, Christiansen C, Gram J. Effects of nordic walking and exercise in type 2 diabetes mellitus: a randomized controlled trial. Clin J Sport Med 2010;20:355–361 [DOI] [PubMed] [Google Scholar]
- 29.Kim CJ, Kang DH. Utility of a Web-based intervention for individuals with type 2 diabetes: the impact on physical activity levels and glycemic control. Comput Inform Nurs 2006;24:337–345 [DOI] [PubMed] [Google Scholar]
- 30.Kirk A, Mutrie N, MacIntyre P, Fisher M. Effects of a 12-month physical activity counselling intervention on glycaemic control and on the status of cardiovascular risk factors in people with Type 2 diabetes. Diabetologia 2004;47:821–832 [DOI] [PubMed] [Google Scholar]
- 31.Kirk A, Barnett J, Leese G, Mutrie N. A randomized trial investigating the 12-month changes in physical activity and health outcomes following a physical activity consultation delivered by a person or in written form in Type 2 diabetes: Time2Act. Diabet Med 2009;26:293–301 [DOI] [PubMed] [Google Scholar]
- 32.Ligtenberg PC, Hoekstra JBL, Bol E, Zonderland ML, Erkelens DW. Effects of physical training on metabolic control in elderly type 2 diabetes mellitus patients. Clin Sci (Lond) 1997;93:127–135 [DOI] [PubMed] [Google Scholar]
- 33.Plotnikoff RC, Eves N, Jung M, Sigal RJ, Padwal R, Karunamuni N. Multicomponent, home-based resistance training for obese adults with type 2 diabetes: a randomized controlled trial. Int J Obes (Lond) 2010;34:1733–1741 [DOI] [PubMed] [Google Scholar]
- 34.Plotnikoff RC, Pickering MA, Glenn N, et al. The effects of a supplemental, theory-based physical activity counseling intervention for adults with type 2 diabetes. J Phys Act Health 2011;8:944–954 [DOI] [PubMed] [Google Scholar]
- 35.Samaras K, Ashwell S, Mackintosh AM, Fleury AC, Campbell LV, Chisholm DJ. Will older sedentary people with non-insulin-dependent diabetes mellitus start exercising? A health promotion model. Diabetes Res Clin Pract 1997;37:121–128 [DOI] [PubMed] [Google Scholar]
- 36.Tudor-Locke C, Bell RC, Myers AM, et al. Controlled outcome evaluation of the First Step Program: a daily physical activity intervention for individuals with type II diabetes. Int J Obes Relat Metab Disord 2004;28:113–119 [DOI] [PubMed] [Google Scholar]
- 37.Wisse W, Rookhuizen MB, de Kruif MD, et al. Prescription of physical activity is not sufficient to change sedentary behavior and improve glycemic control in type 2 diabetes patients. Diabetes Res Clin Pract 2010;88:e10–e13 [DOI] [PubMed] [Google Scholar]
- 38.Di Loreto C, Fanelli C, Lucidi P, et al. Make your diabetic patients walk: long-term impact of different amounts of physical activity on type 2 diabetes. Diabetes Care 2005;28:1295–1302 [DOI] [PubMed] [Google Scholar]
- 39.Kirk A, Mutrie N, MacIntyre P, Fisher M. Increasing physical activity in people with type 2 diabetes. Diabetes Care 2003;26:1186–1192 [DOI] [PubMed] [Google Scholar]
- 40.Kim CJ, Hwang AR, Yoo JS. The impact of a stage-matched intervention to promote exercise behavior in participants with type 2 diabetes. Int J Nurs Stud 2004;41:833–841 [DOI] [PubMed] [Google Scholar]
- 41.Yoo JS, Hwang AR, Lee HC, Kim CJ. Development and validation of a computerized exercise intervention program for patients with type 2 diabetes mellitus in Korea. Yonsei Med J 2003;44:892–904 [DOI] [PubMed] [Google Scholar]
- 42.Tudor-Locke CE, Myers AM, Rodger NW. Development of a theory-based daily activity intervention for individuals with type 2 diabetes. Diabetes Educ 2001;27:85–93 [DOI] [PubMed] [Google Scholar]
- 43.Tudor-Locke CE, Myers AM, Bell RC, Harris SB, Wilson Rodger N. Preliminary outcome evaluation of the First Step Program: a daily physical activity intervention for individuals with type 2 diabetes. Patient Educ Couns 2002;47:23–28 [DOI] [PubMed] [Google Scholar]
- 44.Tudor-Locke C. Promoting lifestyle physical activity: Experiences with the first step program. Am J Lifestyle Med 2009;3(Suppl):508–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calfas KJ, Long BJ, Sallis JF, Wooten WJ, Pratt M, Patrick K. A controlled trial of physician counseling to promote the adoption of physical activity. Prev Med 1996;25:225–233 [DOI] [PubMed] [Google Scholar]
- 46.van Sluijs EMF, van Poppel MNM, Twisk JWR, Chin A Paw MJ, Calfas KJ, van Mechelen W. Effect of a tailored physical activity intervention delivered in general practice settings: results of a randomized controlled trial. Am J Public Health 2005;95:1825–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balducci S, Zanuso S, Massarini M, et al. Italian Diabetes Exercise Study (IDES) Group The Italian Diabetes and Exercise Study (IDES): design and methods for a prospective Italian multicentre trial of intensive lifestyle intervention in people with type 2 diabetes and the metabolic syndrome. Nutr Metab Cardiovasc Dis 2008;18:585–595 [DOI] [PubMed] [Google Scholar]
- 48.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 49.UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–865 [PubMed] [Google Scholar]
- 50.Miller WC, Koceja DM, Hamilton EJ. A meta-analysis of the past 25 years of weight loss research using diet, exercise or diet plus exercise intervention. Int J Obes Relat Metab Disord 1997;21:941–947 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.