Abstract
OBJECTIVE
Elevated serum ferritin has been known to be associated with the prevalence of metabolic syndrome (MetS). However, there was no research to examine whether serum ferritin levels have been actually associated with the prospective development of MetS. Accordingly, we carried out a prospective study to evaluate the longitudinal effects of baseline serum ferritin levels on the development of MetS.
RESEARCH DESIGN AND METHODS
A MetS-free cohort of 18,022 healthy Korean men, who had participated in a medical health checkup program in 2005, was followed until 2010. MetS was defined according to the joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention. Cox proportional hazards models were performed.
RESULTS
During 45,919.3 person-years of follow-up, 2,127 incident cases of MetS developed between 2006 and 2010. After adjusting for multiple covariates, the hazard ratios (95% CI) for incident MetS comparing the second quintile to the fifth quintile of serum ferritin levels versus the first quintile were 1.19 (0.98–1.45), 1.17 (0.96–1.43), 1.36 (1.12–1.65), and 1.66 (1.38–2.01), respectively (P for trend <0.001). These associations were apparent in the clinically relevant subgroup analyses.
CONCLUSIONS
Elevated serum ferritin levels were independently associated with future development of MetS during the 5-year follow-up period.
Iron is a ubiquitous metal of vital importance to the normal physiologic processes of many organisms (1). The serum ferritin concentration reflects iron stores in the body (2).
There have been several studies that showed the significant association between elevated serum ferritin levels and metabolic disorders. In a population study of Finnish men, serum ferritin levels were correlated with fasting serum glucose and insulin concentrations (3). In several other studies, elevated serum ferritin concentrations were reported to be associated with cardiovascular disease (4–6), essential hypertension (7), insulin resistance (8,9), diabetes (10,11), gestational diabetes mellitus (12), and central adiposity (13). In addition, recent studies showed that serum ferritin levels are positively associated with the prevalence of metabolic syndrome (MetS) in western countries (14,15). In the Asian population, some studies reported the relationship between serum ferritin concentrations and the prevalence of MetS (16,17). However, most of the studies have been confined to the cross-sectional nature. To the best of our knowledge, no prospective research has been conducted to evaluate the longitudinal association between baseline serum ferritin levels and the development of MetS. Therefore, we performed this study to assess the longitudinal effects of baseline serum ferritin levels on the development of MetS during a 5-year follow-up period in middle-aged Korean men.
RESEARCH DESIGN AND METHODS
Study design
A prospective cohort study was conducted in order to investigate the association between serum ferritin levels and the development of MetS. Study participants consisted of middle-aged Korean men undergoing a medical health checkup program at the Health Promotion Center of Kangbuk Samsung Hospital, Sungkyunkwan University, Seoul, Korea. The purpose of the medical health checkup program is to promote the health of the employees and to enhance early detection of existing diseases. All employees participate in either annual or biennial health checkup as required by Korea’s Industrial Safety and Health law. Most of the study population is the employees and family members of various companies from all around the country. The costs of the medical examinations are largely paid by their employers. We took advantage of this opportunity to conduct a follow-up study.
Study population
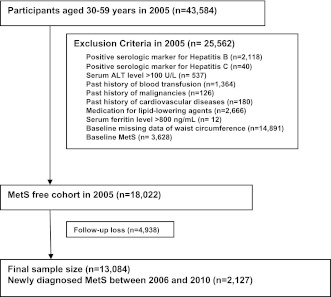
The study participants were a total of 43,584 men, from age 30–59 years, who had visited the Health Promotion Center at Kangbuk Samsung Hospital for a medical checkup in 2005. Among the 43,584 participants, 25,562 were excluded for various reasons: 2,118 had a positive serologic marker for hepatitis B surface antigen; 40 had a positive serologic marker for hepatitis C virus antibody; 537 had serum alanine aminotransferase (ALT) level >100 units/L; 1,364 had a past history of blood transfusion; 126 had a past history of a malignancy; 180 had a past history of cardiovascular disease; 2,666 were receiving medication for lipid-lowering agents; 12 were suspicious to have a history of hemochromatosis based on abnormal values of serum ferritin >800 ng/mL; 14,891 had a baseline missing data of waist circumference (WC); and 3,628 were diagnosed as baseline MetS at initial examinations. The total number of eligible participants was 18,022. We further excluded 4,938 participants who did not attend any follow-up visit between 2006 and 2010. Eventually, 13,084 participants were enrolled in the final analysis and observed for the development of MetS. The total follow-up period was 45,919.3 person-years, and average follow-up period was 3.51 (SD 1.49) person-years. Ethics approvals for the study protocol and analysis of the data were obtained from the institutional review board of Kangbuk Samsung Hospital. Written informed consent was obtained from all subjects (Fig. 1).
Figure 1.
Selection of study participants.
Clinical and laboratory measurements
Study data included a medical history, a physical examination, information provided by a questionnaire, anthropometric measurements, and laboratory measurements. The medical and drug prescription history were assessed by the examining physicians. All of the participants were asked to respond to a health-related behavior questionnaire, which included the topics of alcohol consumption, smoking, and exercise. The questions about alcohol intake included the frequency of alcohol consumption on a weekly basis and the typical amount that was consumed on a daily basis (≥20 g/day). We considered persons reporting that they smoked at the time of the questionnaire to be current smokers. In addition, the participants were asked about the frequency per week of physical activities they engaged in that lasts long enough to produce perspiration such as jogging, bicycling, and swimming (≥1 time/week). Diabetes was defined as fasting serum glucose level of at least 126 mg/dL or the current use of blood glucose–lowering agents. Hypertension was defined as either the current use of antihypertensive medication or as having a measured blood pressure (BP) ≥140/90 mmHg at initial examinations.
Blood samples were collected after >12 h of fasting and drawn from an antecubital vein. Serum levels of aspartate aminotransferase (AST), ALT, and γ-glutamyltransferase (GGT) were measured using Bayer Reagent Packs (Bayer HealthCare, Tarrytown, NY) on an automated chemistry analyzer (ADVIA 1650 Autoanalyzer; Bayer Diagnostics, Leverkusen, Germany). High-sensitivity C-reactive protein (hsCRP) was analyzed by performing particle-enhanced immunonephelomety using the BN System (Dade Behring, Marburg, Germany). Insulin levels were measured with immunoradiometric assays (Biosource, Nivelles, Belgium). Insulin resistance was calculated using the homeostasis model assessment of insulin resistance (HOMA-IR) as described by Matthews et al. (18): fasting serum insulin (μU/ mL) × fasting serum glucose (mg/dL)/22.5. Serum creatinine level was measured using the alkaline picrate (Jaffe) method. The fasting serum glucose was measured using the hexokinase method. Total cholesterol and triglycerides were measured using enzymatic colorimetric tests, LDL cholesterol was measured using the homogeneous enzymatic colorimetric test, and HDL cholesterol was measured using the selective inhibition method (Bayer Diagnostics). Serum levels of ferritin, iron, and total iron-binding capacity (TIBC) were measured by electrochemiluminescence immunoassay using Modular E170 analyzer (Roche Diagnostics, Basel, Switzerland). The BMI was calculated as the weight (kg) divided by the square of the height (m2). The WC was measured in the standing position at the level of umbilicus by a single examiner.
The presence of MetS was made according to the joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention (19). Elevated BP was defined as a systolic or diastolic BP of ≥130/85 mmHg; elevated fasting serum glucose level was defined as ≥100 mg/dL; high serum triglyceride levels were defined as ≥150 mg/dL; low HDL cholesterol levels were defined as <40 mg/dL in men, and elevated WC was defined as >90 cm in men. MetS was defined as the presence of three or more of the above components. Trained nurses obtained sitting BP levels using a standard mercury sphygmomanometer. The first and fifth Korotkoff sounds were used in order to estimate the systolic and diastolic BP. Height and weight were measured after an overnight fast with the shoeless participants wearing a lightweight hospital gown.
Statistical analyses
Data were expressed as means (SD) or medians (interquartile range) for continuous variables and percentages of the number for categorical variables.
One-way ANOVA and χ2 tests were used to analyze the statistical differences among the characteristics of the study participants at the time of enrollment in relation to the quintile groups of serum ferritin levels. Categories of serum ferritin comprised the following quintiles: <61.5, 61.5–85.9, 85.9–113.7, 113.7–155.8, and ≥155.8. The distributions of continuous variables were evaluated, and log transformations were used in the analysis as required.
For incident MetS cases, the time of MetS occurrence was assumed to be the midpoint between the visit at which MetS was first diagnosed and the baseline visit (2005). The person-years were calculated as the sum of follow-up times from the baseline until an assumed time of MetS development or until the final examination of each individual. We used Cox proportional hazards models to estimate adjusted hazard ratios (HRs) and 95% CI for incident MetS comparing the highest four quintiles of baseline serum ferritin versus the lowest quintile. The data were adjusted, first for age alone and then for the multiple covariates. In the multivariate models, we included variables that might confound the relationship between the serum ferritin and MetS, which include age, white blood cell (WBC), GGT, log(hsCRP), HOMA-IR, serum creatinine, TIBC, recent smoking status, alcohol intake, regular exercise, hypertension, and diabetes.
For the linear trends of risk, the number of quintiles was used as a continuous variable and tested on each model. To use the Cox proportional hazards models, we checked the validity of the proportional hazards assumption. Two approaches were used to assess the validity of the proportional hazards assumption. First, the assumption was assessed by log-minus-log-survival function and found to graphically hold. Second, to confirm the validity of the proportional hazards assumption, time-dependent covariate analysis was used. The time-dependent covariate was not statistically significant, suggesting that the proportional hazards assumption is not violated (P = 0.182). P values <0.05 were considered to be statistically significant. Statistical analyses were performed PASW Statistics 18 (SPSS Inc., Chicago, IL).
RESULTS
During 45,919.3 person-years of follow-up, 2,127 (16.3%) incident cases of MetS developed between 2006 and 2010. Compared with analytic cohort (n = 13,084), 4,938 participants not included in analytic cohort were aged >0.2 years (43.7 vs. 43.5) and had a less favorable metabolic profiles at baseline (data not shown).
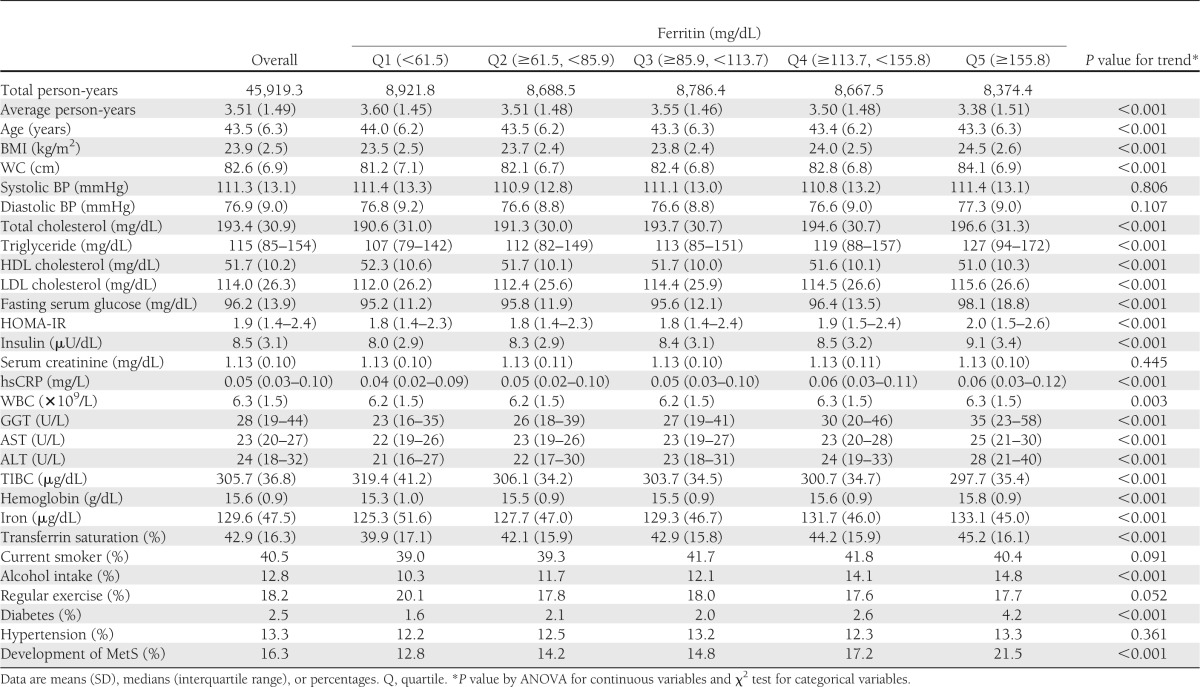
The baseline characteristics of the study participants according to the number of baseline MetS-free components are presented in Supplementary Table 1. The baseline characteristics of the study participants in relation to quintile groups of serum ferritin are presented in Table 1. At baseline, the mean (SD) age and serum ferritin levels of study participants were 43.5 (6.3) years and 112.5 (64.1) mg/dL, respectively. There were clear dose-response relationships between all of the listed variables and quintile groups of serum ferritin levels, with the exception of systolic and diastolic BP, serum creatinine, current smoking status, and hypertension. BMI, WC, total cholesterol, triglycerides, LDL cholesterol, fasting serum glucose, HOMA-IR, insulin, hsCRP, WBC, GGT, AST, ALT, hemoglobin, iron, transferrin saturation, alcohol intake, diabetes, and development of MetS were positively associated with quintile groups of serum ferritin levels, whereas average person-year, age, HDL cholesterol, TIBC, and regular exercise were inversely associated with quintile groups of serum ferritin levels.
Table 1.
Baseline characteristics of participants according to quintile groups of serum ferritin level (N = 13,084)
In contrast to participants without incident MetS, those with incident MetS were slightly older (aged 44.3 vs. 43.4 years) and more likely to have the hypertension and diabetes. As expected, all clinical variables showed statistically significant differences between the two groups (data not shown).
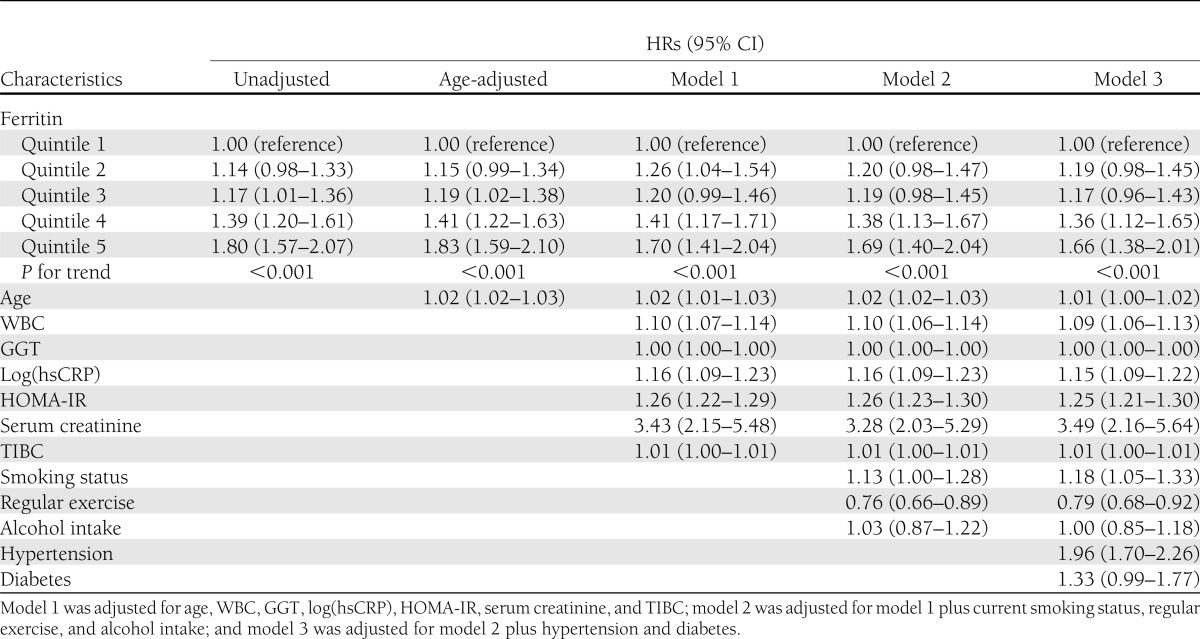
Table 2 shows the HRs and 95% CI for MetS according to the quintile groups of serum ferritin levels. In the unadjusted model, the HRs and 95% CI for MetS comparing the second quintile to the fifth quintile versus the first quintile of serum ferritin were 1.14 (0.98–1.33), 1.17 (1.01–1.36), 1.39 (1.20–1.61), and 1.80 (1.57–2.07), respectively (P for trend <0.001). These associations were also significant even after further adjustments for covariates in model 1, 2, and 3. In Cox proportional hazards models adjusting for age, WBC, GGT, log(hsCRP), HOMA-IR, serum creatinine, TIBC, current smoking status, regular exercise, alcohol intake, hypertension, and diabetes (model 3), the adjusted HRs and 95% CI for MetS across baseline quintile groups of serum ferritin levels were 1.19 (0.98–1.45), 1.17 (0.96–1.43), 1.36 (1.12–1.65), and 1.66 (1.38–2.01), respectively (P for trend <0.001).
Table 2.
HRs and 95% CI for the incidence of the MetS according to quintile groups of serum ferritin level
These associations were apparent in the clinically relevant subgroup analyses of study participants, according to BP <130/85 mmHg, fasting serum glucose <100 mg/dL, triglyceride <150 mg/dL, HDL cholesterol >40 mg/dL, WC <90 cm, AST <75th percentile, HOMA-IR <75th percentile, and GGT <75th percentile (Supplementary Table 2).
CONCLUSIONS
Our recent cross-sectional study showed that high serum ferritin level was independently associated with the prevalence of MetS and its components among healthy Korean men (20). However, whether elevated serum ferritin level is a primary cause or a secondary response of MetS could not be determined from the cross-sectional nature. Therefore, we performed the prospective cohort study described in this study with Cox proportional hazards models. We observed that baseline elevation of serum ferritin level was positively and significantly associated with the development of MetS during 5-year follow-up in initially MetS-free middle-aged Korean men. This association was independent of age, WBC, GGT, log(hsCRP), HOMA-IR, serum creatinine, TIBC, recent smoking status, alcohol intake, regular exercise, hypertension, and diabetes. Furthermore, this association was significant even in the clinically relevant subgroup analyses and in participants with no MetS features at baseline. These results provide novel evidence for a significantly independent association between serum ferritin and future development of MetS. This suggests that high serum ferritin levels may play a causal role in the development of MetS.
Although the pathogenesis between serum ferritin and the development of MetS is not clearly understood, oxidative stress and chronic inflammation with insulin resistance are considered as playing an important role in the development and progression of MetS (21,22). Under oxidative stress, elevated serum ferritin may contribute to cellular or tissue damage leading to insulin dysfunction, such as insulin resistance and abnormal pancreatic β-cell function, through iron toxicity (23,24).
Iron is a transition metal capable of causing oxidative tissue damage by catalyzing the formation of free radicals (25). Through the activation of the stress-sensitive signaling pathway, oxidative stress has been known to increase the activity of NADPH oxidase and simultaneously decrease the antioxidant activity (26–28). Another possible explanation is that chronic oxidative stress has been associated with β-oxidation dysfunction of long chain fatty acids in mitochondria and β-cell dysfunction in the pancreas (29,30). Therefore, insulin resistance mainly resulting from oxidative stress and chronic inflammation plays an important role in the development and progression of MetS.
Several cross-sectional studies have previously shown an association between serum ferritin and MetS and its components (14–17,20). Our results are in line with the previous cross-sectional studies and extend these observations to examine the prospective association between baseline serum ferritin levels and the development of MetS. To the best of our knowledge, no longitudinal research has been done to evaluate the prospective association between baseline serum ferritin levels and the development of MetS. Therefore, we were unable to compare our results with those of other prospective studies. However, there were some other prospective studies comparing the serum ferritin levels and the incident type 2 diabetes (31–33).
When interpreting our results, some limitations should be considered. First, a lot of participants were excluded due to the baseline missing data of WC (n = 14,891). To recover these participants with baseline missing data of WC, we replaced WC with BMI, which is an acceptable surrogate, although not included in the most recent formal MetS definition (19). The results did not change, but they were even strengthened (data not shown). Second, participants were self-selected, so this study may show participant selection bias. Third, we examined only men. Serum ferritin concentrations differ significantly according to sex. Mean serum ferritin levels were preeminently lower in women than in men. This is likely attributable to menstrual iron loss in women (34). Reduced serum ferritin levels may cause lower incidence of MetS in women compared with men. Therefore, in this study, only men were the eligible study participants. These results may not be extrapolated to women, and further studies are needed.
However, the current study is meaningful as a first study to clarify the longitudinal relationship between serum ferritin and the development of MetS.
In conclusion, our findings, which were obtained from large number of cohorts, indicated that serum ferritin may be a predictor for the development of MetS in a 5-year follow-up period, and this association was significant after adjustment for baseline multiple covariates.
Supplementary Material
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
S.K.P. collected the data and reviewed, edited, and wrote the manuscript. J.-H.R. coordinated the study, interpreted the data, contributed to discussion, and wrote the manuscript. M.-G.K. contributed to discussion and reviewed the manuscript. J.-Y.S. interpreted and analyzed the data. J.-H.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-0543/-/DC1.
References
- 1.Heeney MM, Andrews NC. Iron homeostasis and inherited iron overload disorders: an overview. Hematol Oncol Clin North Am 2004;18:1379–1403, ix [DOI] [PubMed] [Google Scholar]
- 2.Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood 2003;101:3359–3364 [DOI] [PubMed] [Google Scholar]
- 3.Tuomainen TP, Nyyssönen K, Salonen R, et al. Body iron stores are associated with serum insulin and blood glucose concentrations. Population study in 1,013 eastern Finnish men. Diabetes Care 1997;20:426–428 [DOI] [PubMed] [Google Scholar]
- 4.Haidari M, Javadi E, Sanati A, Hajilooi M, Ghanbili J. Association of increased ferritin with premature coronary stenosis in men. Clin Chem 2001;47:1666–1672 [PubMed] [Google Scholar]
- 5.Williams MJ, Poulton R, Williams S. Relationship of serum ferritin with cardiovascular risk factors and inflammation in young men and women. Atherosclerosis 2002;165:179–184 [DOI] [PubMed] [Google Scholar]
- 6.Halle M, Konig D, Berg A, Keul J, Baumstark MW. Relationship of serum ferritin concentrations with metabolic cardiovascular risk factors in men without evidence for coronary artery disease. Atherosclerosis 1997;128:235–240 [DOI] [PubMed] [Google Scholar]
- 7.Piperno A, Trombini P, Gelosa M, et al. Increased serum ferritin is common in men with essential hypertension. J Hypertens 2002;20:1513–1518 [DOI] [PubMed] [Google Scholar]
- 8.Sheu WH, Chen YT, Lee WJ, Wang CW, Lin LY. A relationship between serum ferritin and the insulin resistance syndrome is present in non-diabetic women but not in non-diabetic men. Clin Endocrinol (Oxf) 2003;58:380–385 [DOI] [PubMed] [Google Scholar]
- 9.Kim CH, Kim HK, Bae SJ, Park JY, Lee KU. Association of elevated serum ferritin concentration with insulin resistance and impaired glucose metabolism in Korean men and women. Metabolism 2011;60:414–420 [DOI] [PubMed] [Google Scholar]
- 10.Ford ES, Cogswell ME. Diabetes and serum ferritin concentration among U.S. adults. Diabetes Care 1999;22:1978–1983 [DOI] [PubMed] [Google Scholar]
- 11.Shi Z, Hu X, Yuan B, Pan X, Meyer HE, Holmboe-Ottesen G. Association between serum ferritin, hemoglobin, iron intake, and diabetes in adults in Jiangsu, China. Diabetes Care 2006;29:1878–1883 [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Scholl TO, Stein TP. Association of elevated serum ferritin levels and the risk of gestational diabetes mellitus in pregnant women: The Camden study. Diabetes Care 2006;29:1077–1082 [DOI] [PubMed] [Google Scholar]
- 13.Gillum RF. Association of serum ferritin and indices of body fat distribution and obesity in Mexican American men—the Third National Health and Nutrition Examination Survey. Int J Obes Relat Metab Disord 2001;25:639–645 [DOI] [PubMed] [Google Scholar]
- 14.Bozzini C, Girelli D, Olivieri O, et al. Prevalence of body iron excess in the metabolic syndrome. Diabetes Care 2005;28:2061–2063 [DOI] [PubMed] [Google Scholar]
- 15.Jehn M, Clark JM, Guallar E. Serum ferritin and risk of the metabolic syndrome in U.S. adults. Diabetes Care 2004;27:2422–2428 [DOI] [PubMed] [Google Scholar]
- 16.Sun L, Franco OH, Hu FB, et al. Ferritin concentrations, metabolic syndrome, and type 2 diabetes in middle-aged and elderly chinese. J Clin Endocrinol Metab 2008;93:4690–4696 [DOI] [PubMed] [Google Scholar]
- 17.Lee BK, Kim Y, Kim YI. Association of serum ferritin with metabolic syndrome and diabetes mellitus in the South Korean general population according to the Korean National Health and Nutrition Examination Survey 2008. Metabolism 2011;60:1416–1424 [DOI] [PubMed] [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 19.Alberti KG, Eckel RH, Grundy SM, et al. International Diabetes Federation Task Force on Epidemiology and Prevention. Hational Heart, Lung, and Blood Institute. American Heart Association. World Heart Federation. International Atherosclerosis Society. International Association for the Study of Obesity Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–1645 [DOI] [PubMed] [Google Scholar]
- 20.Ryoo JH, Kim MG, Lee DW, Shin JY. The relationship between serum ferritin and metabolic syndrome in healthy Korean men. Diabetes Metab Res Rev 2011;27:597–603 [DOI] [PubMed] [Google Scholar]
- 21.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet 2005;365:1415–1428 [DOI] [PubMed] [Google Scholar]
- 22.Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 2004;114:1752–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson JG, Lindquist JH, Grambow SC, Crook ED, Maher JF. Potential role of increased iron stores in diabetes. Am J Med Sci 2003;325:332–339 [DOI] [PubMed] [Google Scholar]
- 24.Ferrannini E. Insulin resistance, iron, and the liver. Lancet 2000;355:2181–2182 [DOI] [PubMed] [Google Scholar]
- 25.Wolff SP. Diabetes mellitus and free radicals. Free radicals, transition metals and oxidative stress in the aetiology of diabetes mellitus and complications. Br Med Bull 1993;49:642–652 [DOI] [PubMed] [Google Scholar]
- 26.Evans JL, Maddux BA, Goldfine ID. The molecular basis for oxidative stress-induced insulin resistance. Antioxid Redox Signal 2005;7:1040–1052 [DOI] [PubMed] [Google Scholar]
- 27.Ford ES, Mokdad AH, Giles WH, Brown DW. The metabolic syndrome and antioxidant concentrations: findings from the Third National Health and Nutrition Examination Survey. Diabetes 2003;52:2346–2352 [DOI] [PubMed] [Google Scholar]
- 28.Mukherjee SP, Lynn WS. Reduced nicotinamide adenine dinucleotide phosphate oxidase in adipocyte plasma membrane and its activation by insulin. Possible role in the hormone’s effects on adenylate cyclase and the hexose monophosphate shunt. Arch Biochem Biophys 1977;184:69–76 [DOI] [PubMed] [Google Scholar]
- 29.Yao D, Shi W, Gou Y, et al. Fatty acid-mediated intracellular iron translocation: a synergistic mechanism of oxidative injury. Free Radic Biol Med 2005;39:1385–1398 [DOI] [PubMed] [Google Scholar]
- 30.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007;39:44–84 [DOI] [PubMed] [Google Scholar]
- 31.Jehn ML, Guallar E, Clark JM, et al. A prospective study of plasma ferritin level and incident diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Epidemiol 2007;165:1047–1054 [DOI] [PubMed] [Google Scholar]
- 32.Forouhi NG, Harding AH, Allison M, et al. Elevated serum ferritin levels predict new-onset type 2 diabetes: results from the EPIC-Norfolk prospective study. Diabetologia 2007;50:949–956 [DOI] [PubMed] [Google Scholar]
- 33.Jiang R, Manson JE, Meigs JB, Ma J, Rifai N, Hu FB. Body iron stores in relation to risk of type 2 diabetes in apparently healthy women. JAMA 2004;291:711–717 [DOI] [PubMed] [Google Scholar]
- 34.Rushton DH, Dover R, Sainsbury AW, Norris MJ, Gilkes JJ, Ramsay ID. Why should women have lower reference limits for haemoglobin and ferritin concentrations than men? BMJ 2001;322:1355–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.